Abstract
Circumnutation is a helical organ movement widespread among plants. It is variable due to a different magnitude of trajectory (amplitude) outlined by the organ tip, duration of one cycle (period), circular, elliptical, pendulum-like or irregular shape and clock- and counterclockwise direction of rotation. Some of those movement parameters are regulated by circadian clock and show daily and infradian rhythms. Circumnutation is influenced by light, temperature, chemicals and can depend on organ morphology. The diversity of this phenomenon is easier to see now that the digital time-lapse video method is developing fast. Whether circumnutation is an endogenous action, a reaction to exogenous stimuli or has a combined character has been discussed for a long time. Similarly, the relationship between growth and circumnutation is still unclear. In the mechanism of circumnutation, epidermal and endodermal cells as well as plasmodesmata, plasma membrane, ions (Ca2+, K+ and Cl−), ion channels and the proton pump (H+ATPase) are engaged. Based on these data, the hypothetical electrophysiological model of the circumnutation mechanism has been proposed here. In the recent circumnutation studies, gravitropic, auxin, clock and phytochrome mutants are used and new functions of circumnutation in plants' life have been investigated and described.
Key words: circumnutation, Helianthus annuus, plant movement, elongation, growth, ultradian rhythm, circadian rhythm, time-lapse video, ion channels
Introduction
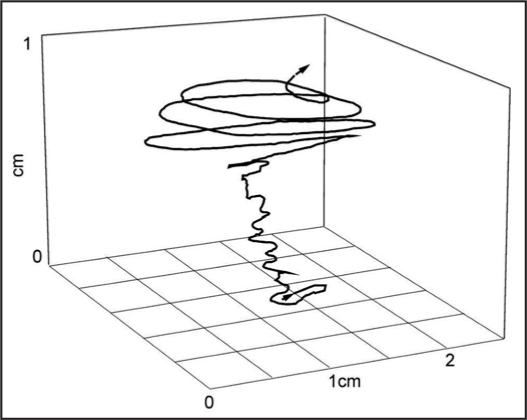
The sessile life-style, but not lack of movements, is a crucial difference between plants and animals. When observed over a sufficiently long period, i.e., from several minutes to many hours (using time-lapse video method), plants appear to be vigorous and sensitive organisms. They are capable of moving various organs: movements of leaves, shoots, tendrils, flower petals and roots are widely known.1,2 Movements in plants are most frequently regarded as a result of action of an environmental stimulus, e.g., light (phototropism, photonasties), temperature (thermonasties), or gravity (gravitropism). However, besides these movements, plants have a widespread ability of autonomous, endogenous movement without apparent stimuli. Circumnutations are one type of such movements, called nutations. Circumnutations (Latin circus for “circle”, nutatio for “sway”) are independent, autonomous movements of plant organs (e.g., the hypocotyl, coleoptile, epicotyl, stem, shoot, tendril, petiole or root), the tip of which outlines a circle, full ellipsis, pendulum-like shape or irregular zigzags within a several minute- to several hour-long period. Due to elongation of the organ, a series of single circumnutations produce a more or less regular helix (Fig. 1).
Figure 1.
Example of circumnutations of Helianthus annuus stem tip during one day in the diurnal (lower part) and nocturnal periods (upper part).
Widespread Occurrence of Circumnutations
Circumnutations are widespread in the plant kingdom. Charles Darwin was one of the first scientists who described circumnutation movements extensively. In his work The Power of Movement in Plants,3 Darwin presented circumnutations in numerous plant species and claimed that all the other movements, i.e., geotropism or sleep movements are a modification of the basic circumnutation movement. Descriptions of numerous species were included in the studies of French physiologists: Arnal, Dutrochet and Tronchet, and in the 50ies compiled by Baillaud,4 who presented circumnutations in many plant species and described their trajectories (shapes and direction) and temporal characteristics. There are well-known circumnutations of tendrils and roots in Pisum, tendrils in Passiflora and Sicyos, coleoptiles of rye (Triticum) and oats (Avena), shoots of beans (Phaseolus) and Cuscuta, Ipomoea, Carthamus,5,6 hypocotyls in Helianthus annuus,7 tulip (Tulipa) petiole8 and circumnutations in Arabidopsis thaliana.9 Apart from their prevalence, circumnutations are noteworthy for being typical of young, growing parts of plant organs.
The widespread occurrence of circumnutations and their marked differentiation indicate a variety of plants' behavior in various environmental conditions. The circumnutation parameters presented below demonstrate the complexity and variability of circumnutations depending on endogenous and exogenous factors.
Parameters of Circumnutations—The Amplitude, Period, Shape, Direction
The amplitude of circumnutations.
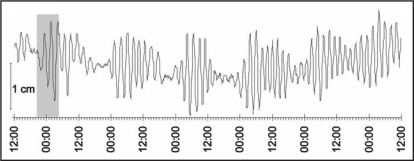
The amplitude of circumnutations differs in various plant species and, in a particular species, it does not depend on changes in the length of the circumnutating organ, which was observed in Arabidopsis thaliana.9 Changes in circumnutation amplitude have repeatedly been shown to be evoked by various stimuli. In Phaseolus, the radius of the shoot movement is approximately 10 cm; administration of gibberellins increased the circumnutation amplitude noticeably, whereas treatment with a growth inhibitor (e.g., AMO 1618) and with Li+ ions decreased it.10,11 Brown12 reports that the amplitude of oscillations in 4–7-day-old sunflower hypocotyl is 7.36 mm and may fall to 2.77 mm in the microgravity conditions of the circumterrestrial orbit. A change in the amplitude may also be evoked by the action of gravity in the range 1–3 g.13 Administration of Li+ ions affects the circumnutation of Helianthus annuus hypocotyls.14 Figure 2 presents variability of the circumnutation amplitude in the sunflower stem induced by exogenous (light/dark transition) and endogenous (circadian clock) factors.
Figure 2.
The amplitude and period of circumnutations in the stem of a three-week old sunflower in the conditions of constant light. The circumnutation period is about 130 min. The grey rectangle denotes darkness.
The period of circumnutations—ultradian rhythm.
In various plant species, the period of circumnutations may take from several minutes to several hours. Variable period oscillations may occur constitutively in the same plant. The period of oscillations is variable and depends on morphological features in the plant (e.g., plant variety or type of circumnutating organ) and also on environmental conditions, i.e., temperature, light or gravity. It may also change in response to gravitational, mechanical or chemical stimuli (e.g., ethylene, lithium or aluminium).
In Sicyos and Passiflora, a phenomenon called Fünfphasenbewegungen can be observed.5 The same tendril displayed two periods of movement (in a mutual ratio 1:5): one of the periods when the movement occurred in the same plane, and the other when the tendril moved in a plane that was perpendicular to the first one. Phaseolus epicotyls5 display short-period and low-amplitude oscillations (27 min/15°C; 12 min/27°C) called micronutations, which overlap circumnutations. This suggests two separate oscillation mechanisms in Phaseolus. In decapitated maize roots (Zea mays L. cv. Aussie Gold),15,16 there are circumnutations and micronutations correlated with H+ fluxes exhibiting oscillations with a period of approximately 90 min and 7 min in the elongation zone. This confirms the existence of two oscillation systems. The oscillation period of H+ fluxes and root movements is identically modified by external pH changes.17 The circumnutation period in Triticum coleoptiles6 typically reaches 150 min, but in a certain percentage of plants or in special conditions it may be shorter by a half (approximately 70–80 min). A similar situation is observed in Phaseolus.6 Roblin6 observed in Mimosa that the circumnutation period in the pulvinus increases alongside with the amplitude increase. The period may be dependent on the length of the circumnutating organ, which was observed in partially immobilized Passiflora tendrils or shoots.6 In numerous species, the circumnutation period is 4-times longer than the lag phase time of geotropic reaction.6 The period in Helianthus and Phaseolus may be dependent on the circumnutation direction.6
The period in tulip petioles reaches ca. 4 hours.8 In Arabidopsis thaliana seedlings,9 nutation periods range from 15 min to ca. 24 hr. Two types of oscillations have been distinguished: SPN—short period nutations (20–60 min long) and LPN—long period nutations (1–8 hrs, with a maximum occurrence between 95–200 min). SPN circumnutation period (in the range of 20°–30°C Q10 = 2) becomes shorter as the temperature rises; it increases together with plant's age. Circumnutations were particularly slow in red light and in Landsberg erecta race. In constant, bright, white light, circumnutations changed accordingly to silence periods, which was correlated with the growth rate, and had a circadian character.
The period of ultradian rhythm may undergo changes induced by mechanical stress/stimulus, for instance, rubbing or touching, as in the case of the shoots of pole bean Phaseolus vulgaris L. cv. Kentucky Wonder.18 Short mechanical stress, e.g., 10-fold application of rubbing, resulted in an increase of the mean period from 1.4 to 2.0 hr. A similar effect is observed at 5-minute-long application of temperature stimuli of high (45°C) or low (0°C) temperature. The period, however, returns to the initial value after one cycle. In our laboratory, we have observed one lengthened circumnutation cycle after harmful, burning stimulation of the leaf.19 In Phaseolus, acupuncture insertion of two needles on the opposite sides of the stem shortened the circumnutation period of one cycle.20
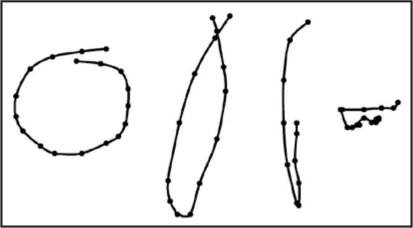
Shape of circumnutations.
The top-view circumnutations trajectory of the tip organ most frequently assumes the elliptical shape, although there are also circular, pendulum-like and irregularly zigzag-shaped circumnutations (Fig. 3). In Phaseolus, circular and elliptical circumnutations are predominant.10 In Arabidopsis thaliana seedlings,9 the shape of circumnutations may vary from circular to elliptical or pendulum-like. The pendulum-like (linear) form of circumnutations may be a transient form accompanying a change in the direction. Another characteristic rosette-like shape of circumnutations (Fig. 4) was observed by Baillaud4 in Ipomea, Passiflora and Bryopsis and by Hejnowicz and Sievers8 in Tulipa.
Figure 3.
Examples of circumnutation shapes in a three-week old sunflower plant. The circumnutation trajectory of the tip organ most frequently assumes the elliptical shape, although there are also circular, pendulum-like and irregularly zigzag-shaped circumnutations.
Figure 4.
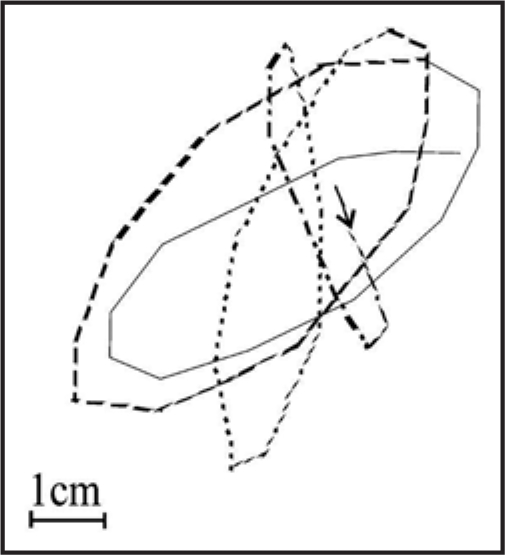
Example of rosette-like shape of circumnutation trajectory in a three-week old sunflower plant.
The direction of circumnutations.
The direction of circumnutations may be clockwise (cw) or counterclockwise (ccw) depending on the species and moment of observation. Phaseolus shoots display ccw movement,10 likewise the shoots of Glycine soya and G. max.21 In Arabidopsis thaliana seedlings,9 the direction of SPN circumnutations is usually of the cw type, possibly changeable, whereas the LPN circumnutations are usually ccw.
In a particular plant, the change from one direction into another one may proceed spontaneously or be stimulus-evoked. The change in the direction is induced by gravitational and touch stimuli.22 In Arabidopsis thaliana, rotations of the root tip and changes in the rotation direction caused by a touch stimulus were observed.23 Changes in the direction may be induced by applying an injurious stimulus, as in the case of burning of the leaf tip in sunflower.19 In Helianthus annuus, changes in the direction may be evoked by a geotropic stimulus;6 in the microgravity conditions of the circumterrestial orbit, the direction of circumnutations changes more frequently than on the earth.12 Johnsson22 shows changes in the direction in the sunflower accompanied by a change in the amplitude and frequency of circumnutations. The study of Israelsson and Johnsson7 revealed that 60% of the sunflower population studied circumnutated in the ccw direction, and 25%-cw; the others displayed pendulum-like movement.
Daily, Circadian and Infradian Variability of Circumnutation Parameters
Despite the great variability of the above mentioned circumnutation parameters, certain regularity is manifested by their diurnal, circadian and infradian rhythms. The length of the amplitude/trajectory in Helianthus annuus plants exhibits diurnal (24 h) and infradian (several and more days long) fluctuations. Also, changes in the period length in the conditions of cyclic diurnal illumination display a diurnal rhythm.24,25 Moreover, in sunflower plants, changes of trajectory length, period and shape of circumnutations have a circadian (close to 24 h) rhythm.26 The circadian character of circumnutations was described in the hypocotyl of Arabidopsis thaliana seedlings.9 Based on a genetic analysis (using toc-1, elf3 mutants and the wild-type Arabidopsis plants) Niinuma et al.27 revealed circadian regulation of circumnutation speed in inflorescence stems, with period length close to 24 h.
Light and Circumnutations
The influence of light on circumnutations is relatively little known. In Arabidopsis thaliana,9 circumnutations occurred less frequently in green light (only 1/3 of the plants circumnutated, compared to as many as 3/4 of circumnutating plants in white light); they were less regular and had a smaller amplitude. There were mainly LPN1 recorded and more numerous ultrashort oscillations (USPNs ultrashort SPNs) were observed. Their period was by a half shorter than SPNs and they were ccw directed. In constant red light, circumnutations were particularly short; mainly LPN1 of a ca. 200 min period were observed and their direction was the same as in white light. It is plausible that the phytochrom affects circumnutations and stimulates LPNs. In bright white light (ca. 2,300 lux), almost all the Arabidopsis plants circumnutated displaying a circadian character, which was less visible at the intensities 160 and 10.5 lux. In Arabidopsis hypocotyl, the phototropic response to unilateral blue light illumination overlapped nutations.28 The red light-induced inhibition of circumnutation was demonstrated in dark-grown Oryza sativa coleoptiles, and phytochrome A null mutants were used to investigate the participation of phythochromes in the circumnutation mechanism.29
Temperature and Circumnutations
The circumnutation period is strongly dependent on temperature. Between ca. 10°C to ca. 35°C, the period is shortened together with the temperature rise in the hypocotyls of Helianthus,7 Phaseolus, Triticum, Pisum and Ipomoea.5
Organ Morphology and Circumnutations
Dependence between the tip and leaves of Pisum sativum green seedlings and circumnutations30 has been determined. By removing the shoot tip and leaves, inhibition of circumnutations was obtained. In etiolated Pisum seedlings,31 dependence of nutations movements on the apical hook was observed; in Avena a relation between the arrangement of the long axis of circumnutations and the coleoptile flattening axis was reported.7
Models of Circumnutation Mechanism
Since the end of the 18th century, there have been various conflicting concepts of the nature of the mechanism that generates circumnutations. Three concepts can be distinguished: one—circumnutations are of endogenous origin, the second—circumnutations are a result of earth gravity, thus they are exogenous, and the third and most recent idea—circumnutations stem from both endogenous and exogenous processes.6,12,22,32–34
Endogenous Nature of Circumnutations—An Inner Oscillator.
Charles Darwin,3 the author of the concept of endogenous character of circumnutations, published already in 1880, described circumnutations in numerous plant species and claimed that they were endogenous and other types of movement were their modification. His view of the endogenous nature of circumnutations is still valid nowadays. In this model, earth gravity does not play a crucial and decisive role in generating circumnutations. Studies on sunflower circumnutations in the circumterrestial orbit, which excluded the significance of gravity in generating circumnutations, contributed to the revival of Darwin's model,12 in which the inner oscillator is an indispensable element in generating circumnutations.
Exogenous Nature of Circumnutations—The “Overshoot” Theory.
Circumnutations are generated by an external stimulus, i.e., earth gravity. This theory, postulated by Baranetzky in 1883, gave rise to long-standing experiments and discussion about the significance of gravity in the generation of circumnutations.7 Theoretical divagations and calculations were undertaken in an attempt to mathematically describe circumnutations of 4–5 day-old Helianthus annuus hypocotyl.7,35–37 A key element in this model is the constant ratio of circumnutation period to the geotropic lag-phase, equalling 4, and the overshoot phenomenon in the hypocotyl, facilitating constant gravitropic response and gravitropic feedback-loop between the positions of the hypocotyl. The gravity stimulus induces changes in the statolith position in the cells of a circumnutating organ, and subsequently, in the auxin gradient. This results in asymmetrical growth and bending of the organ and in changes in the position of the statoliths in space, which evokes a new, subsequent geotropic stimulation and repetition of the cycle.5 In experiments with the use of the clinostat and centrifuge as well as the microgravity in the circumterrestial orbit, it was repeatedly demonstrated that changes in gravity affected the intensity, period and amplitude of circumnutations.7,12,13 Recent investigations of Arabidopsis thaliana and Pharbitis nil mutants scr i pgm (having defective differentiation of endodermal cells) reveal that gravitropic response is an essential component in plant shoot circumnutation.38–40
Circumnutations as the Result of Actions of Endo- and Exogenous Factors. A combined model.
Nowadays circumnutations are more readily presumed to be induced by both the inner oscillator and gravity.6,22,32 Following experiments in the circumterrestial orbit, which excluded the indispensability of gravity in generation of circumnutations, a growth-affected symplastic-communication control model was elaborated.33 In this model, distortions in plasmodesma development in the growing tissue lead to asymmetry in the symplastic transport of growth substances, thus producing organ bending and self-sustaining oscillations.33 Here, both endogenous factors and gravity play an important role in the circumnutation mechanism. Gravitropism and nutations may be controlled independently,31,28 which emphasizes the key role of the inner oscillator, while gravity may merely modulate circumnutations. According to Hejnowicz and Sievers,8 the inner oscillator may be entrained by the gravitropic feedback, which is visible in the characteristic, predictable ratio between circumnutation period and the lag phase of the gravitropic response, equalling 5. In numerous plant species, the circumnutation period is 4-fold longer than the lag phase of the gravitropic reaction.6
Circumnutations and the Growth Rate
The detailed relationship between circumnutations and growth is still unclear.6,22,41 The dependence between growth rate and circumnutations was studied in Periploca graeca shoots,6 where circumnutations took place above the threshold value of growth, i.e., ca. 0.5 mm/h. In Arabidopsis thaliana seedlings9 SPN circumnutations require the minimal growth rate of 0.05 mm/h, whereas LPNs occur when the growth rate is slow. This is a general observation; however, in Arabidopsis thaliana there is no strict, proportional dependence between changes in growth rate and changes in circumnutation period.22 The circadian character of circumnutations in the hypocotyl of Arabidopsis thaliana seedlings9 is strictly related to the circadian character of hypocotyl growth. In constant white light, circumnutations occurred alternately with the silent phases, which was correlated with changes in the growth rate. During intensive growth periods, the greatest amplitude SPNs were observed, while LPNs occurred at the time of a lower growth rate. No circumnutations took place at low growth rate. The circadian circumnutations had the period of 26.2 h.
Interesting observations of Helianthus hypocotyl growth were obtained by Berg and Peacock.42 With the use of lanolin-coated resin beads positioned along the hypocotyls and with a video technique, they observed changes in the placement of the beads on the hypocotyls surface accompanying the movements. These observations indicated dependence between the growth zone and the bending zone. In circumnutating bean hypocotyls (Phaseolus multiflorus), basipetal movement of the bending was observed,5 which testifies to movement of the zone of enhanced growth along the organ. These observations indicate that growth has a local character, and zones of enhanced growth may move. Our experiments in Helanthus annuus indicate that the disturbances of the growth rate are accompanied by changes in circumnutation parameters but we have also seen that there is no simple quantitative relation between growth rate and circumnutation rate.43
There is a possibility of separation of growth from circumnutations. In Phaseolus, after application of LiCl, growing, although not circumnutating shoots were obtained.10 Removal of the wheat coleoptile tip (Triticum vulgare) resulted in arrest of circumnutations, which, however, resumed after its renewal or application of IAA.6 In green pea seedlings (Pisum sativum),30 after removal of growth tip, circumnutations were several-fold smaller; they increased when the remaining shoot was treated with IAA. This indicates participation of auxins in the circumnutation mechanism.
Changes in cell volume are undoubtedly fundamental in the generation of circumnutations.
It is still not explicit if they are only:
irreversible volume increase resulting in elongation growth
cell shrink/swollen (contraction/relaxation) phenomena
a combination of both phenomena
It should be stressed that in Phaseolus vulgaris,44 epidermis cells in the bending, mobile part of the shoot, the initial length of which ranges from 60–120 µm, display partially reversible changes in the length of the cells (by ca. 10 µm) during shoot growth. The ability of growing epidermal cells to contraction is transient and is displayed in the motor zone of the shoot. The length changes are of a rhythmical character and their period is equal to the period of circular movement. This fact is worth emphasizing because it indicates that apart from stomatal and pulvinal motor cells also epidermal cells are capable of transient contraction.
Cellular and Molecular Basis for Circumnutations
To make circumnutations possible, the cells around the circumnutating organ must create a highly coordinated and phase-synchronised group, so to say “a motor tissue”. Which tissues are involved in circumnutations? The epidermis is a tissue attributed with the properties of releasing protons into the cell-external matrix,8,45 thus it has essential properties for the acid mechanism of cell growth. Moreover, in sunflower, the epidermis is vital in the hypocotyl growth control.46 In Phaseolus vulgaris, it is epidermal cells that display partially reversible changes in the length of the bending, mobile part of the shoot.44 The endodermal cells (statocytes with amyloplasts functioning as statoliths) also play an important role in circumnutations, as revealed by scr mutants of Arabidopsis and Pharbitis nil.38,39 Plasmodesmata, by which cells create a continuous system (symplast) facilitating intercellular communication, are equally important. Brown' circumnutation model33 was based on symplastic communication. A great number of plasmodesmata were present in the motor zone in Phaseolus shoot. Due to the parallel arrangement of cellulose fibrils and plication of the cell wall, the cells were to some extent flexible and could contract and expand.11
Nowadays, while constructing a model of the circumnutation mechanism, one must take into account a number of physiological aspects, for instance, turgor changes, ion content fluctuations or the level of growth regulators.22 A wave of cell turgor changes proceeding along the bean shoot was observed.11
Ion channels, which facilitate ion and water fluxes, are an important element in the circumnutation mechanism. Their participation is confirmed in experiments on ion channel blockers inducing changes in the amplitude and period. In Phaseolus, the circumnutation period was extended after application of Gd+ i La3+ ions, which are blockers of various types of Ca2+ ion channels.10,47 The circumnutation period in Helianthus hypocotyl and Phaseolus shoot was lengthened in the presence of Li+ ions, which may indirectly affect the concentration of cytoplasmatic Ca2+.10,14
Participation of K+ channels in the circumnutations of Phaseolus shoots is also plausible, since there is a relation between shoot bending and varied content of K+ ions in the concave and convex sides of the organ.10,48 A strong correlation between H+ and Ca2+ fluxes and circumnutations and micronutations was observed in Zea mays roots.15,16 In circumnutating tulip petioles, strand pH changes related to a different growth intensity involved in circumnutation generation were observed.8 The involvement of ions and ion channels in circumnutations is indirectly visible in electrical potential changes occurring in Ipomoea purpurea tendrils, where the oscillation period overlaps the nutation period.22 Oscillations of the electrical potential related to auxin oscillations in Zea mays coleoptiles were also reported.22 The role of auxins in nutational movements was studied in etiolated and green Pisum sativum seedlings.30,31
Therefore, Ca2+ and K+ ion channels and H+-ATPase play a vital role in circumnutations. Due to the character of circumnutations, these are probably voltage- gated K+ channels, inward (K+in) channels activated with hyperpolarization, outward (K+out) channels activated with depolarization, stretch-activated K+ and Ca2+ channels, ligand-gated K+ channels, e.g., with auxins and light-activated K+ channels.49 Equally important, probably, are anion channels, which participate in the hypocotyl elongation and osmoregulation in stomatal cells, that is, processes controlled by auxins and blue light.50
H+-ATPase and Ca2+, K+ and Cl− channels are a part of the oscillation system in the contraction/expansion model in the motor cell of the Desmodium motorium pulvinus, proposed by Engelmann.51,52 The rhythmical cell volume changes are induced by contraction and expansion processes in the motor cell. The proton pump constantly works; K+ ions passively enter the cell, water is taken up thanks to the osmotic activity of the ions, the cell expands, the stretch-activated Ca2+ channels cause depolarization, the small potential opens the outward K+ and Cl− channels, the massive ion flux is accompanied with loss of water and the cell contracts. Such a system, being a feedback loop, may be treated as an inner oscillator indispensable in the circumnutation mechanism.
Hypothetical, Electrophysiological Model of Circumnutations
A model based on Engelman's hypothesis51 for Desmodium motorium pulvinus motor cells may be proposed as a model of an ultradian oscillator involved in circumnutations. This could also be a model explaining the mechanisms and ionic bases for electrical potential changes accompanying circumnutations. Briefly, the bending zone of the circumnutating organ may be regarded as the motor tissue and its cells may be referred to as motor cells. Circumnutations are most frequently ellipsoidal, so the cells on the opposite side of the organ (along the circumnutation long axis on the concave and convex sides), would display an analogous function as the flexor and extensor during the shrinkage and swelling in pulvinus and undergo a transient depolarization and hyperpolarization. An oscillator composed of H+ATPase and ion channels in the cell membrane is the main element in this model. Energy in the form of ATP availability is necessary.
Electrical oscillations are driven by the negative feedback loop between H+, K+, Cl−, Ca2+ ion fluxes through the plasma membrane, where voltage-gated ion channels, e.g., K+, play a key role. In the feedback, where there is additional cell contraction and extension, an essential role is undoubtedly played by ion channels (for instance, Ca2+ channels whose opening and closing is a turning point of the loop) activated or de-activated by cell membrane tension. Electrical oscillations may be identical with the oscillations of the cell volume, when ion (K+) fluxes are followed by water or when Ca2+ influx into the cell induces cytoskeletal filament contraction, which results in water efflux.53,54
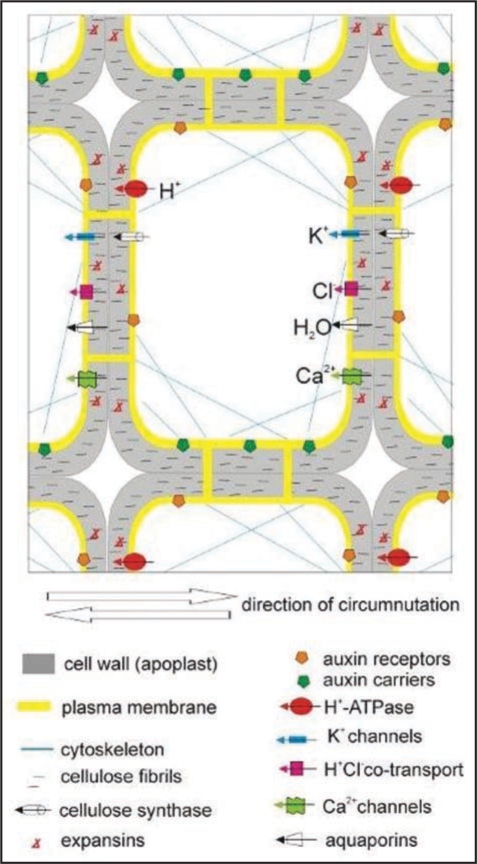
Epidermal cells as well as parenchymal—subepidermal cells are involved in generation of circumnutations.22,46 Therefore, epidermal and parenchymal cells might function as motor cells. Figure 5 presents a scheme of a hypothetical cell, i.e., a part of the stem motor tissue, with marked elements constituting the oscillator and elements involved in circumnutations.
Figure 5.
A scheme of a hypothetical cell that is a part of the stem motor tissue with marked elements constituting the oscillator and elements involved in circumnutations. It is constructed on the basis of the oscillator model in Desmodium motorium (Engelmann 1996). The model contains numerous elements of the oscillator, indispensable in generation of circumnutations; certain elements are responsible for cell growth and ability of the electrical-hydraulic signal to propagate around the stem. Description of co-operation of individual elements is found in the text.
The sequence of events during circumnutations might be as follows: H+ ATPase continuously works and hyperpolarizes the cell membrane, which results in opening inward, voltage (hyperpolarization)—gated K+ channels; next Cl− ions are transported inwardly through a co-transport with H+ ions. Influx of K+ and Cl− ions causes an increase in the osmotic potential, which facilitates water influx into the cell, for instance, through aquaporins. The water influx results in an enlarged cell and stretched cell membrane, which may cause opening of mechanosensitive Ca2+ channels and Ca2+ ions influx, thus leading to cell membrane depolarization. Depolarization may open outward K+ ion channels, which lowers the osmotic potential inside the cell and induces water efflux. The increase in the cell volume will then be inhibited. By releasing H+ ions into the cell wall, the proton pump acidifies the environment, thus activating expansins which trigger off “crawling” of the cell wall. Moreover, the positive charge outside the cell membrane enhances cellulose synthase activity, which promotes cell wall growth.55 Increased turgor, in turn, facilitates the exocytosis processes, namely, enlargement of the cell membrane surface and externalization of hemicelluloses. Ca2+ ion influx at the maximal cell turgor may activate a number of cascades related to Ca2+-dependent protein kinases; it may also stimulate the activity of cytoskeleton elements. The cytoskeleton dynamics may contribute to the perpendicular arrangement of cellulose fibrils against the cell long axis, as in the case of fast growing cells, and may be essential in the polar auxin transport. The effect of gravity on circumnutations may proceed through a system of graviperception, based both on the statoliths-cytoskeleton model of Sievers et al.56 and on the tension- and pressure-sensitive cell membraneextracellular matrix model proposed by Wayne and Staves.57 Both models take into account the possibility of opening of ion channels induced by directional changes related to gravity changes. The hypothetical, electrophysiological model of the circumnutation mechanism is an attempt to include the biggest number of factors participating in circumnutations, known from literature.
Mutants in Research on the Circumnutation Mechanism
Recently, there appeared reports on circumnutation distortions in mutants defective in graviresponse (pgm1, scr2-1), auxin response pathways (axr2-1), circadian clock (toc1 and elf3) and red light reception (phyA). Gravitropic mutants (pgm1, scr2-1) of Arabidopsis thaliana and Pharbitis nil were defective in circumnutations so there was a link between circumnutation and gravitropism.38–40 Investigations of auxin-resistant axr2-1 mutant of Arabidopsis supported the notion that auxin participates in circumnutation.39 In Oryza sativa coleoptiles, phy A mutants that cannot express phyA were investigated to clarify the involvement of phytochromes in red light induced circumnutation inhibition.29 In Arabidopsis inflorescence stem investigations of elf3 and toc1 mutants demonstrated that circumnutation is one of the outputs controlled by circadian clock.27,58
Function of Circumnutation Movement in Plant's Life
In climbing plants, the function of nutation movements is obvious and consists in seeking mechanical support by nutating shoots and tendrils, as elaborated by Darwin.3 Recent studies are carried out on variation in circumnutation of twining vine to examine how different circumnutation behaviors influence the vines' exploration and exploitation of support host.59 Yet, there are plants which do not need a support but their organs do circumnutate. A prevailing hypothesis of the functions of circumnutation movements proposed by Schuster and Engelman9 suggests that circumnutations protect stability of the hypocotyl during elongation growth, otherwise, the growth-related, simultaneous loosening of the cell wall in all the cells surrounding the organ destabilize it. Studies demonstrating circumnutation inhibition without suppression of growth after administration of aluminum are noteworthy, too.60 The inhibition and disturbances of circumnutations could probably be an early phase of response to stress, prior to inhibition of the elongation of the plant. In Oryza sativa, root circumnutation plays an important role in establishment of seedlings on flooded and soft soil.61,62 Therefore, there is mounting evidence that circumnutations have specific ecological functions and are not merely a way of growth.
Conclusions and Perspectives
Circumnutations are a complex phenomenon, a compilation of growth processes and intercellular communication involving the biological clock. Circumnutations are controlled by an ultradian oscillator of a broad period range (from a few minutes to several hours) as well as by a circadian oscillator. Probably, it is the mechanism of growth and cell volume changes that is the site of clock regulations. Due to their endogenous character, circumnutations may be modified by internal and external factors and may serve as markers of plant behavior. In animals, the main parameter that defines behavior is motor activity. Circumnutations may be regarded as its analogue and, thus, as manifestation of plant behavior. Endogenous circumnutation movements are an indication of the action of an inner hydraulic system and may be a good model for future studies on the electro-hydraulic signalling mechanism, which seems to be a plant integrating system. It may be expected that, thanks to the fast developing time-lapse video method, numerous well-known mutants related to the above-mentioned circumnutation factors will prove helpful in further research on the circumnutation mechanism. Besides study on the molecular mechanism, future investigation should also concern the wide description of the variable circumnutation parameters which reflect the inner mechanism.
Abbreviations
- LPN
long period nutations
- SPN
short period nutations
- cw
clockwise
- ccw
counterclockwise
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/8293
References
- 1.Haupt W, Feinleib ME. Physiology of movements. In: Pirson A, Zimmermann MH, editors. Encyclopedia of plant physiology. Berlin: Springer-Verlag; 1979. [Google Scholar]
- 2.Mancuso S, Shabala S. Rhythms in plants: phenomenology, mechanisms and adaptative significance. Springer; 2007. [Google Scholar]
- 3.Darwin C, Darwin F. The power of movement in plants. London: John Murray; 1880. [Google Scholar]
- 4.Baillaud L. Mouvements autonomes des tiges, vrilles et autres organes à l'exception des organes volubiles et des feuilles. In: Ruhland W, editor. Handbuch der Pflanzenphysiologie 17/2. Berlin: Springer-Verlag; 1962. pp. 562–634. (Ger). [Google Scholar]
- 5.Johnsson A, Heathcote D. Experimental evidence and models on circumnutations. Z Pflanzenphysiol. 1973;70:371–405. [Google Scholar]
- 6.Johnsson A. Circumnutation. In: Haupt W, Feinleib E, editors. Encyclopedia of plant physiology, N S, vol 7, Physiology of Movements. Berlin: Springer; 1979. pp. 627–646. [Google Scholar]
- 7.Israelsson D, Johnsson A. A theory for circumnutations in Helianthus annuus. Physiol Plant. 1967;20:957–976. [Google Scholar]
- 8.Hejnowicz Z, Sievers A. Proton efflux from the outer layer of the peduncle of tulip in gravitropism and circumnutation. Bot Acta. 1995;108:7–13. [Google Scholar]
- 9.Schuster J, Engelmann W. Circumnutations of Arabidopsis thaliana seedlings. Biol Rhythm Res. 1997;28:422–444. [Google Scholar]
- 10.Millet B, Badot PM. The revolving movement mechanism in Phaseolus; New approaches to old questions. In: Greppin H, Degli Agosti R, Bonzon M, editors. Vistas on Biorhythmicity. University of Geneva; 1996. pp. 77–98. [Google Scholar]
- 11.Millet B, Melin D, Badot PM. Circumnutation in Phaseolus vulgaris I. Growth, osmotic potential and ultrastructure in the free-moving part of the shoot. Physiol Plant. 1988;72:133–138. [Google Scholar]
- 12.Brown A, Chapman DK, Lewis RF, Venditti Circumnutation of sunflower hypocotyls in satelite orbit. Plant Physiol. 1990;94:233–238. doi: 10.1104/pp.94.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zachariassen E, Johnsson A, Brown AH, Chapman DK, Johnson-Glebe C. Influence of g-force on the circumnutations of sunflower hypocotyls. Physiol Plant. 1987;70:447–452. doi: 10.1111/j.1399-3054.1987.tb02841.x. [DOI] [PubMed] [Google Scholar]
- 14.Zachariassen E, Johnsson A. Effects of lithum ions on the circumnutations of Helianthus hypocotyls. Physiol Plant. 1988;72:147–152. [Google Scholar]
- 15.Shabala SN, Newman IA. Proton and calcium flux oscillations in the elongation region correlate with root nutation. Physiol Plant. 1997;100:917–926. [PubMed] [Google Scholar]
- 16.Shabala SN, Newman IA. Root nutation modelled by two ion flux-linked growth waves around the root. Physiol Plant. 1997;101:770–776. [Google Scholar]
- 17.Shabala SN, Newman IA, Morris J. Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiol. 1997;113:111–118. doi: 10.1104/pp.113.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AndersonBernadas C, Cornelissen G, Turner CM, Koukkari WL. Rhythmic nature of thigmomorphogenesis and thermal stress of Phaseolus vulgaris L shoots. J Plant Physiol. 1997;151:575–580. [Google Scholar]
- 19.Stolarz M, Dziubinska H, Krupa M, Buda A, Trebacz K, Zawadzki T. Disturbances of stem circumnutations evoked by wound-induced variation potentials in Helianthus annuus L. Cell Moll Biol Lett. 2003;8:31–40. [PubMed] [Google Scholar]
- 20.Hou TZ, Li MD. Experimental evidence of a plant meridian system 5. Acupuncture effect on circumnutation movements of shoots of Phaseolus vulgaris L. pole bean. Am J Chin Med. 1997;25:253–261. doi: 10.1142/S0192415X97000287. [DOI] [PubMed] [Google Scholar]
- 21.Adolfson KA, Sothern RB, Koukkari WL. Ultradian movements of shoots of two species of soybeans Glycine soja (Sieb. and Zucc.) and Glycine max (L.) Merr. Chronobiol Int. 1998;15:1–11. doi: 10.3109/07420529808998664. [DOI] [PubMed] [Google Scholar]
- 22.Johnsson A. Circumnutations: results from recent experiments on Earth and in space. Planta. 1997;203:141–158. doi: 10.1007/pl00008103. [DOI] [PubMed] [Google Scholar]
- 23.Okada K, Shimura Y. Reversible root tip rotation in Arabidopsis seedlings induced by obstacle—touching stimulations. Science. 1990;250:274–276. doi: 10.1126/science.250.4978.274. [DOI] [PubMed] [Google Scholar]
- 24.Buda A, Zawadzki T, Krupa M, Stolarz M, Okulski W. Daily and infradian rhythms of circumnutation intensity in Helianthus annuus. Physiol Plant. 2003;119:582–589. [Google Scholar]
- 25.Charzewska A. The rhythms of circumnutation in higher plants. In: Teixeira da Silva JA, editor. Floriculture ornamental and plant biotechnology: Advances and topical issues. Vol. 1. Department of Horticulare. Kagawa University; 2006. pp. 268–275. [Google Scholar]
- 26.Charzewska A, Zawadzki T. Circadian modulation of circumnutation length period and shape in Helianthus annuus. J Plant Growth Regul. 2006;25:324–331. [Google Scholar]
- 27.Niinuma K, Someya N, Kimura M, Yamaguchi I, Hamamoto H. Circadian rhythm of circumnutation in inflorescence stems of Arabidopsis. Plant Cell Physiol. 2005;46:1423–1427. doi: 10.1093/pcp/pci127. [DOI] [PubMed] [Google Scholar]
- 28.Orbovic V, Poff KL. Interaction of light and gravitropism with nutation of hypocotyls of Arabidopsis thaliana seedlings. Plant Growth Reg. 1997;23:141–146. doi: 10.1023/a:1005853128971. [DOI] [PubMed] [Google Scholar]
- 29.Yoshihara T, Iino M. Circumnutation of rice coleoptiles: its occurrence regulation by phytochrome, and relationship with gravitropism. Plant Cell Environ. 2005;28:134–146. doi: 10.1111/j.1365-3040.2004.01249.x. [DOI] [PubMed] [Google Scholar]
- 30.Tepper HB, Yang RL. Influence of the shoot tip and leaves on circumnutation in green pea seedlings. Bot Acta. 1996;109:502–505. doi: 10.1111/j.1438-8677.1996.tb00603.x. [DOI] [PubMed] [Google Scholar]
- 31.Britz SJ, Galston AW. Physiology of movements in stems of seedling Pisum sativum L. cv Alaska. Plant Physiol. 1982;70:1401–1404. doi: 10.1104/pp.70.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnsson A, Jansen C, Engelmann W, Schuster J. Circumnutations without gravity: a two-oscillator model. J Grav Physiol. 1999;6:9–12. [PubMed] [Google Scholar]
- 33.Brown AH. Circumnutations: from Darwin to space flights. Plant Physiol. 1993;101:345–348. doi: 10.1104/pp.101.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mugnai S, Azzarello E, Masi E, Pandolfi C, Mancuso S. Nutation in plants. In: Mancuso S, Shabala S, editors. Rhythms in plants: phenomenology, mechanisms and adaptative significance. Springer; 2007. pp. 77–90. [Google Scholar]
- 35.Andersen H, Johnsson A. Entrainment of geotropic oscillations in hypocotyl of Helianthus annuus—an experimental and theoretical investigation I. Geotropic movement initiated by one single geotropic stimulation. Physiol Plant. 1972;26:44–51. [Google Scholar]
- 36.Andersen H, Johnsson A. Entrainment of geotropic oscillations in hypocotyl of Helianthus annuus—an experimental and theoretical investigation II. Geotropic movements due to periodically repeated stimulations. Physiol Plant. 1972;26:52–61. [Google Scholar]
- 37.Johnsson A, Israelsson D. Aplication of theory for circumnutations to geotropic movements. Physiol Plant. 1968;21:282–291. [Google Scholar]
- 38.Kitazawa D, Hatakeda Y, Kamada M, Fujii N, Miyazawa Y, Hoshino A, et al. Shoot circumnutation and winding movements require gravisensing cells. Proc Natl Acad Sci USA. 2005;102:18742–18747. doi: 10.1073/pnas.0504617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hatakeda Y, Kamada M, Goto N, Fukaki H, Tasaka M, Suge H, et al. Gravitropic response plays an important role in the nutational movements of the shoots of Pharbitis nil and Arabidopsis thaliana. Physiol Plant. 2003;118:464–473. [Google Scholar]
- 40.Kiss JZ. Up, down and all around: How plants sense and respond to environmental stimuli. Proc Natl Acad Sci USA. 2006;103:829–830. doi: 10.1073/pnas.0510471102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baskin TI. Ultradian growth oscillations in organs: Physiological signal or noise? In: Mancuso S, Shabala S, editors. Rhythms in plants: phenomenology, mechanisms and adaptative significance. Springer; 2007. pp. 63–76. [Google Scholar]
- 42.Berg AR, Peacock K. Growth patterns in nutating and nonnutating sunflower (Helianthus annuus) hypocotyls. Am J Bot. 1992;79:77–85. [Google Scholar]
- 43.Stolarz M, Król E, Dziubinska H, Zawadzki T. Complex relathionship between growth and circumnutations in Helianthus annuus stem. Plant Signal Behav. 2008;6:376–380. doi: 10.4161/psb.3.6.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caré AF, Nefed'ev L, Bonnet B, Millet B, Badot PM. Cell elongation and revolving movement in Phaseolus vulgaris L. twining shots. Plant Cell Physiol. 1998;39:914–929. [Google Scholar]
- 45.Gruszecki WI, Trebacz K, Iwaszko E. Application of very small force measurements in monitoring the response of sunflower to weak blue light. J Photoch Photobiol B. 2002;66:141–147. doi: 10.1016/s1011-1344(02)00234-8. [DOI] [PubMed] [Google Scholar]
- 46.Hejnowicz Z, Rusin A, Rusin T. Tensile tissue stress affects the orientation of cortical microtubules in the epidermis of sunflower hypocotyl. J Plant Gowth Regul. 2000;19:31–44. doi: 10.1007/s003440000005. [DOI] [PubMed] [Google Scholar]
- 47.Giboz D, Badot PM, Millet B. Involvement of calcium ions in circumnutation of twining shoots. Biol Cell. 1995:102. [Google Scholar]
- 48.Comparot S, Giboz D, Badot PM. Possible involvement of ion channels in the cell water relations in the moving part of twining shoots. Colloque SBCF-ATIPE/CNRS Biologie du Developpement. 1995:97. [Google Scholar]
- 49.Król E, Trebacz K. Ways of ion channel gating in plant cells. Ann Bot. 2000;86:449–469. [Google Scholar]
- 50.Barbier-Brygoo H, Vinauger M, Colcombet J, Ephritikhine G, Frachisse JM, Maurel C. Anion channels in higer plants: functional characterization, molecular structure and physiological role. Biochim Biophys Acta. 2000;1465:199–218. doi: 10.1016/s0005-2736(00)00139-5. [DOI] [PubMed] [Google Scholar]
- 51.Engelmann W. Leaf movement rhythms as hands of biological rhythms. In: Greppin H, Degli Agosti R, Bonzon M, editors. Vistas on Biorhythmicity. University of Geneva; 1996. pp. 51–76. [Google Scholar]
- 52.Engelmann W, Johnsson A. Rhythms in organ movement. In: Lumsden PJ, Millar AJ, editors. Biological rhythms and photoperiodism in plants. Oxford, Washington: Bios Scientific Publishers; 1998. pp. 35–50. [Google Scholar]
- 53.Cooper MS. Intercellular signalling in neuronal-glial networks. BioSystems. 1995;34:65–85. doi: 10.1016/0303-2647(94)01450-l. [DOI] [PubMed] [Google Scholar]
- 54.Huang RF, Wang XC, Lou CH. Cytoskeletal inhibitors suppress the stomatal opening of Vicia faba L. induced by fusicoccin and IAA. Plant Sci. 2000;156:65–71. doi: 10.1016/s0168-9452(00)00240-5. [DOI] [PubMed] [Google Scholar]
- 55.Davies E. Action potentials as multifunctional signals in plants: a unifying hypothesis to explain apparently disparate wound responses. Plant Cell Environ. 1987;10:623–631. [Google Scholar]
- 56.Sievers A, Buchen B, Hodick D. Gravity sensing in tip-growing cells. Trends Plant Sci. 1996;8:273–279. doi: 10.1016/1360-1385(96)10028-5. [DOI] [PubMed] [Google Scholar]
- 57.Wayne R, Staves MP. A down to earth model of gravisensing or Newton's law of gravitation from the apple's perspective. Physiol Plant. 1996;98:917–921. [PubMed] [Google Scholar]
- 58.Niinuma K, Nakagawa M, Calvino M, Mizoguchi T. Dance of plants with circadian clock. Plant Biotechnology. 2007;24:87–97. [Google Scholar]
- 59.Larson K. Circumnutation behavior of an exotic honeysuckle vine and its native congener: influence on clonal mobility. Amer J Bot. 2000;87:533–538. [PubMed] [Google Scholar]
- 60.Hayashi Y, Nishiyama H, Tanoi K, Ohya T, Nihei N, Tanioka K, Nakanishi TM. An aluminium influence on root circumnutation in dark revealed by a new super-HARP (High-gain Avalanche Rushing Amorphous Photoconductor) camera. Plant Cell Physiol. 2004;45:351–356. doi: 10.1093/pcp/pch042. [DOI] [PubMed] [Google Scholar]
- 61.Inoue N, Arase T, Hagiwara M, Amnao T, Hyashi T, Ikeda R. Ecological significance of root tip rotation for seedling establishment of Oryza sativa L. Ecol Res. 1999;14:31–38. [Google Scholar]
- 62.Minorsky PV. Circumnutation. Plant Physiol. 2003;132:1779–1780. doi: 10.1104/pp.900085. [DOI] [PMC free article] [PubMed] [Google Scholar]