Abstract
We have identified a novel cDNA clone, termed DcCDT1, from Digitaria ciliaris, that confers cadmium (Cd)-tolerance to yeast (Saccharomyces cerevisiae). The gene encodes a predicted peptide of 55 amino acid residues of which 15 (27.3%) are cysteine residues. We found that monocotyledonous plants possess multiple DcCDT1 homologues, for example rice contains five DcCDT1 homologues (designated OsCDT1∼5), whereas dicotyledonous plants, including Arabidopsis thaliana, Brassica rapa, poplar (Populus tremula × Populus alba) and Picea sitchensis, appear to possess only a single homologue. GFP fusion experiments demonstrate that DcCDT1 and OsCDT1 are targeted to both the plant cytoplasmic membranes and cell walls. Constitutive expression of DcCDT1 or OsCDT1 confers Cd-tolerance to transgenic A. thaliana plants by lowering the accumulation of Cd in the cells. The functions of the DcCDT1 family members are discussed in the light of these findings.
Key words: cadmium, cysteine-rich peptide, hypoaccumulation, phytoremediation, tolerance
Cadmium (Cd) is a highly toxic transition metal, and is nonessential for almost all living organisms.1 Therefore, Cd pollution of the earth's environment could potentially cause serious problems for both the global ecosystem and human health.2 Indeed, we have a tragic history brought about by Cd poisoning, with patients suffering kidney failure and unbearable pain in the joints and spine due to bone softening.3,4 Despite the numerous laws that have been enacted to prevent further Cd contamination, yet pollution with heavy metals including Cd is still increasing on a global scale. As the control of Cd contamination of foodstuffs is expected to become extremely severe, remediation of Cd-contaminated soils is an issue that needs to be urgently addressed. One countermeasure strategy is the use of plants in phytoremediation5 which, despite its tremendous potential, still requires vast improvements before it can be promoted as an effective and established technology. Such improvements will necessitate the identification and development of novel and useful ‘molecular resources’. As a step towards this aim, we have screened cDNA libraries derived from natural habitat plants growing in a former mining site and isolated numerous candidate genes that could confer Cd tolerance to Cd-hypersensitive yeast mutant cells.6 Of these genes, we chose DcCDT1 (Digitaria ciliaris cadmium tolerance 1) for further analysis as it encodes a novel 55-amino acid-peptide product containing 15 cysteine (Cys) residues, and because several other Cys- rich peptides are known to function as heavy metal chelators.7,8 Rice plants possess five DcCDT1 homologues, designated OsCDT1∼5, while other monocotyledonous plant species, such as maize and barley, also contain multiple DcCDT1 homologues. In contrast, Arabidopsis thaliana appears to contain only a single DcCDT1 homologue (accession number NM_202281, At1g52827) with an open-reading frame (nucleotide positions 29–178) encoding a 49-amino acid peptide of sequence,
MKAPPQQEMTYYDNVKKRQDEQGCLFATFYALFCCCCCYEKCKCCCCCV,
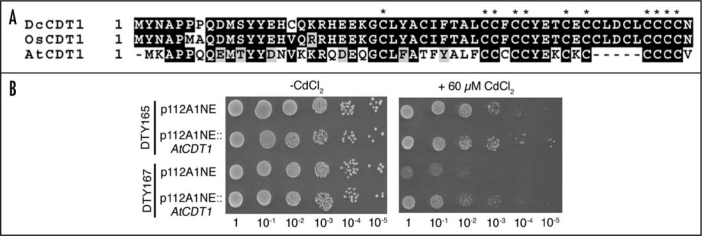
whereas the database predicts a peptide (encoded by an alternative open-reading frame between nucleotides 63–251) consisting of 62-amino acids, MTM SRN GKT NKA AYS QRF TRC SVA VAA TRS ASV VAA AFD FYI CII IST LLS LIV SLA SQL LF, which is of unknown function and shows no similarity to DcCDT1. The 49-amino acid-peptide sequence (here termed AtCDT1) shares a high level of identity with DcCDT1 and OsCDT1, and these all contain 11 conserved Cys residues clustered in their carboxy-distal regions (Fig. 1A). Furthermore, as with DcCDT1 and OsCDT1, constitutive expression of AtCDT1 confers Cd-tolerance to S. cerevisiae (Fig. 1B), confirming that A. thaliana possesses a functional DcCDT1 counterpart. The question thus arises if other dicotyledonous plants also possess homologous gene(s). It appears that they do since we found two other examples, a Populus EST clone (accession number CU225257) and also a Brassica rapa subsp. pekinensis clone (accession number AC189268), that encode DcCDT1 homologues. However, further analyses will be required to determine whether these plants carry a single gene like Arabidopsis or a small gene family as in rice plants.
Figure 1.
DcCDT1 and its homologues are a highly-conserved, Cys-rich family of proteins and the Arabidopsis homologue, AtCDT1, confers Cd-tolerant to yeast cells. (A) Amino acid alignment of DcCDT1, OsCDT1 and AtCDT1. OsCDT1 is one of the rice DcCDT1 homologues, and AtCDT1 is the Arabidopsis thaliana-derived 49-amino acid-peptide. Identical and conserved amino acid residues are highlighted in black and gray backgrounds, respectively. Conserved Cys residues are marked with an asterisk. (B) Constitutive expression of the nucleotide sequence encoding AtCDT1 confers Cd tolerance to yeast cells. S. cerevisiae strain, DTY165 (genotypic markers MATα, ura3-52, leu2-3,-112, his3-delta200, trp1-delta901, lys2-801, suc2-delta9), and its ycf16,17-mutant strain, DTY167 (genotypic markers MATα, ura3-52, leu2-3,-112, his3-delta200, trp1-delta901, lys2-801, suc2-delta9, ycf1::hisG), cells were transformed with the control vector p112A1NE or p112A1NE::AtCDT1.18 The transformants obtained were grown in yeast liquid culture media, and the cell density of the cultures then adjusted to an optical density of 1.0 at 600 nm (OD600). Aliquots of 5 µl of the serial dilutions (1, 10−1, 10−2, 10−3, 10−4 and 10−5) were then plated onto Synthetic Dropout (SD)-Trp media without CdCl2 (left) or containing 60 µM CdCl2 (right).
Several different plant components contribute to heavy metal homeostasis and detoxification. Among these are the low molecular mass (4–14 kDa) metallothioneins that contain a high ratio of Cys residues, and the small peptides known as phytochelatins that have the general structure, (γ-Glu-Cys)n-Gly.7–10 Other Cys-rich plant proteins involved in Cd tolerance and detoxification have also been reported.11,12 However, compared to all these, DcCDT1 and its homologues are unique and distinctive in both their peptide lengths (49–60 amino acids) and arrangement of Cys residues in the CL-(Y/F)-A-(C/T)-X5-CC-(F/C)-CCYE-(T/K)-C-(E/K)-C( CLDCL or delete)-CCCC consensus sequence.
As with other non-essential heavy metals, Cd can be detoxified by a variety of mechanisms, including secretion, compartmentalization, or chelation by metal ligands.13–16 DcCDT1 and OsCDT1 confer Cd-tolerance to both yeast and Arabidopsis via a reduction in their cellular Cd contents. Both proteins also appear to be localized to the plant cell surface, including the cell walls, as judged by our GFP-fusion experiments. Based on these findings, we propose several possible functions for this novel peptide family. In one mechanism, the DcCDT1 family proteins chelate Cd at the cellular surface and prevent further Cd entry into the cells. In an alternative mechanism, the intracellular-formed DcCDT1-Cd complex is secreted out from the cells via an unknown mechanism. Induced expression of DcCDT1 in 109Cd-preloaded cells may well allow us to distinguish between these two possibilities.
A final question that needs to be addressed is whether the genes encoding this family of proteins can be potentially useful genetic resources for phytoremediation. OsCDT1, one of the five rice DcCDT1 homologues, reduces the Cd contents of yeast and Arabidopsis cells. Therefore, from the viewpoint of food safety, this protein family may be useful since constitutive root-specific expression of the genes may contribute to reduced Cd accumulation in the edible plant parts. Alternatively, silencing of all DcCDT1 homologues in rice could plausibly result in the hyperaccumulation of Cd and the use of these plants in phytoremediation strategies.
Acknowledgements
We thank Dr. Dennis J. Thiele (Duke University Medical Center) for kindly providing the yeast strains and vector. This work was supported, in part, by a Grant-in-aid (Hazardous Chemicals) from the Ministry of Agriculture, Forestry, and Fisheries of Japan (HC-05-11130, to T.K., E.K. and S.Y.) and a grant from the Gakucho-project of Akita Prefectural University (to S.Y.).
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/8272
References
- 1.Lane TW, Saito MA, George GN, Pickering IJ, Prince RC, Morel FMM. A cadmium enzyme from a marine diatom. Nature. 2005;435:42. doi: 10.1038/435042a. [DOI] [PubMed] [Google Scholar]
- 2.Järup L. Hazards of heavy metal contamination. Br Med Bull. 2003;68:67–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 3.Aoshima K, Teranishi H, Kasuya M, Hagino N. Pathogenesis and pathophysiology of Itai-itai disease: a report of two cases and its clinical study (in Japanese) Jpn J Int Med. 1984;73:838–848. [PubMed] [Google Scholar]
- 4.Ellis KJ, Morgan WD, Zanzi I, Yasumura S, Cohn SH. Critical concentration of cadmium in human renal cortex: dose-effect studies in cadmium smelter workers. J Toxicol Env Hlth. 1981;7:691–703. doi: 10.1080/15287398109530012. [DOI] [PubMed] [Google Scholar]
- 5.Ensley BD. Rationale for use of phytoremediation. In: Raskin I, Ensley BD, editors. Phytoremediation of toxic metals. Using plants to clean up the environment. John Wiley & Sons, Inc; 2000. pp. 3–11. [Google Scholar]
- 6.Riesmeier JW, Willmitzer L, Frommer WB. Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J. 1992;11:4705–4713. doi: 10.1002/j.1460-2075.1992.tb05575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clemens S, Kim EJ, Neumann D, Schroeder JI. Tolerance to toxic metals by a gene family of phytochelatin synthases from plant and yeast. EMBO J. 1999;18:3325–3333. doi: 10.1093/emboj/18.12.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cobbett CS, Goldsbrough PB. Mechanisms of metal resistance: phytochelatins and metallothioneins. In: Raskin I, Ensley BD, editors. Phytoremediation of toxic metals. Using plants to clean up the environment. John Wiley & Sons, Inc; pp. 247–269. [Google Scholar]
- 9.Clemens S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie. 2006;88:1707–1719. doi: 10.1016/j.biochi.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Guo WJ, Meetam M, Goldsbrough PB. Examining the specific contributions of individual Arabidopsis metallothioneins to copper distribution and metal tolerance. Plant Physiol. 2008;146:1697–1706. doi: 10.1104/pp.108.115782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song W-Y, Martinoia E, Lee J, Kim D, Kim D-Y, Vogt E, et al. A novel family of cys-rich membrane proteins mediates cadmium resistance in Arabidopsis. Plant Physiol. 2004;135:1027–1039. doi: 10.1104/pp.103.037739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki N, Yamaguchi Y, Koizumi N, Sano H. Functional characterization of a heavy metal binding protein CdI19 from Arabidopsis. Plant J. 2002;32:165–173. doi: 10.1046/j.1365-313x.2002.01412.x. [DOI] [PubMed] [Google Scholar]
- 13.Hall JL. Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot. 2002;53:1–11. [PubMed] [Google Scholar]
- 14.Choi YE, Harada E, Wada M, Tsuboi H, Morita Y, Kusano T, Sano H. Detoxification of cadmium in tobacco plants: formation and active excretion of crystals containing cadmium and calcium through trichomes. Planta. 2001;213:45–50. doi: 10.1007/s004250000487. [DOI] [PubMed] [Google Scholar]
- 15.Domínguez-Solís JR, López-Martín MC, Ager FJ, Ynsa MD, Romero LC, Gotor C. Increased cysteine availability is essential for cadmium tolerance and accumulation in Arabidopsis thaliana. Plant Biotechnol J. 2004;2:469–476. doi: 10.1111/j.1467-7652.2004.00092.x. [DOI] [PubMed] [Google Scholar]
- 16.Shitan N, Horiuchi K, Sato F, Yazaki K. Bowman-birk proteinase inhibitor confers heavy metal and multiple drug tolerance in yeast. Plant Cell Physiol. 2007;48:193–197. doi: 10.1093/pcp/pcl051. [DOI] [PubMed] [Google Scholar]
- 17.Li ZS, Szczypka M, Lu YP, Thiele DJ, Rea PA. The yeast cadmium factor protein (YCF1) is a vacuolar glutathione S-conjugate pump. J Biol Chem. 1996;271:6509–6517. doi: 10.1074/jbc.271.11.6509. [DOI] [PubMed] [Google Scholar]
- 18.Guthrie C, Fink RG. Guide to yeast genetics and molecular and cell biology, Part B, Methods in Enzymology. Vol. 350. Academic Press; 2002. pp. 18–19. [Google Scholar]