Abstract
Proving allelopathic chemical interference is a daunting endeavor, in that production and movement of a phytotoxin from a donor plant to a receiving plant must be demonstrated in the substrate in which the plants grow, which is usually a complex soil matrix. The soil levels or soil flux levels of the compound generated by the donor must be proven to be sufficient to adversely affect the receiving plant. Reports of (-)-catechin to be the novel weapon used by Centaurea stoebe (spotted knapweed) to invade new territories are not supported by the paper featured in this Addendum, nor by papers produced by two other laboratories. These papers find that (-)-catechin levels in soil in which C. stoebe grows are orders of magnitude below levels that cause only minor growth effects on reported sensitive species. Furthermore, the claim that (-)-catechin acts as a phytotoxin through causing oxidative damage is refuted by the fact that the molecule is a strong antioxidant and is quickly degraded by extracellular root enzymes.
Key words: allelochemical, allelopathy, catechin, chemical interference, phytotoxin, spotted knapweed
Introduction
Negative effects of one plant on another due to the phytotoxicity of chemicals produced by the aggressor plant is the most commonly claimed form of allelopathy, sometimes termed chemical interference. For many years, both scientists and non-scientists have used allelopathy to explain the negative actions of certain plant species on others.1 However, allelopathy research has often been fraught with controversy over whether claims of allelopathy are valid. For example, the high profile paper in which Muller et al.2 claimed vegetation patterning around aromatic shrubs was due to the release of phytotoxic volatile compounds was later disputed by Bartholomew,3 who demonstrated that the phenomenon could also be explained by animal activity around the shrubs. Some ecologists such as Harper4 have dismissed allelopathy as an unlikely and usually weak phenomenon when it occurs. Nevertheless, the number of papers on this topic has expanded substantially in recent years, and the research described in a significant number of these papers has enlisted cutting edge methods to provide a more robust proof of allelopathy.
Recently, there have been several studies supporting the view that root-exuded phytotoxins are involved in allelopathy. Phytotoxins identified include sorgoleone in Sorghum species,5–7 m-tyrosine in Festuca rubra,8,9 momilactone B in rice,10,11 and (-)-catechin in Centaurea stoebe (spotted knapweed).12–15 In the latter example, once again, papers have appeared which seriously question the results.16–20 Our addendum is written to expand the commentary from one of those dissenting papers.20
Proving Allelopathy
Proof of allelopathy requires demonstration of production of a phytotoxin(s) or a prophytotoxin(s) by a donor species, movement of the compound(s) and/or its phytotoxic derivatives(s) to a target plant at concentrations proven to cause harm to the target species, and measurement of a harmful effect to the target plant caused by the compound(s) in the environment containing both the donor and target plants. The task is simplified if the molecular target site of the phytotoxin is known and there is evidence that the target site is affected during chemical interference between species in the field. Allelopathy can be due to release of phytotoxins from live plants or to release of phytotoxins from decaying plant material. In the case of prophytotoxins, the compound produced by the donor plant is converted to a more phytotoxic compound by a process in the soil.21 The interactions of compounds in soil (e.g., by direct interactions with soil components or metabolic conversion) are potentially complex. Finally, there is the potential of antagonistic or synergistic interactions with other compounds. To unequivocally prove allelopathy in a natural setting is usually a daunting task.
The recent interest in C. stoebe as an allelopathic invasive plant began with a study in which Ridenour and Callaway22 grew C. stoebe with a common North American native plant, Festuca idahoensis. Based on the finding that F. idahoensis grown with C. stoebe and activated charcoal were 85% larger than F. idahoensis with C. stoebe without activated charcoal, they concluded that a significant amount of the ability of C. stoebe to inhibit the growth of F. idahoensis was associated with allelopathy. Activated charcoal is sometimes used in allelopathy studies to sequester putative allelochemicals while leaving resource competition intact. The following year, Bais et al.13 used a bioassay-guided approach, and reported (-)-catechin as a potent allelochemical of C. stoebe. In this paper and others,12,14,15,23–26 high levels of this compound were reported from either plants growing in liquid media or from soils collected from C. stoebe infestations.
When Blair et al.16 independently attempted to use the extraction techniques reported in the previously mentioned studies, they discovered that the liquid extraction protocol did not work because catechin is not soluble in the reported extraction solvent (hexane), and extraction efficiencies from soil with their methanol technique were consistently low (0 to 17%) from a variety of soil types. Furthermore, when Blair et al.16,17 developed more reliable techniques, they found catechin levels from liquid media to be two orders of magnitude lower than previously reported and three orders of magnitude lower, if found at all, from field sites infested with C. stoebe. Later, Lau et al.25 reported that using activated carbon can cause experimental artifacts, either decreasing or increasing growth of plants. Such findings led Blair et al.16–18 to call into question the role of catechin as an ecologically important allelochemical of C. stoebe. Other papers provide rigorous evidence that the claims of strong phytotoxicity of (-)-catechin in either an aqueous medium or soil are unfounded.19,20
Our most recent paper20 strongly supports the view that neither enantiomer of catechin is likely to be involved in allelopathy of any species and questions whether (-)-catechin could act as a toxin through generation of oxidative stress, as claimed by Bais et al.12 We found (-) and (+)-catechin to have similar, weak phytotoxicities, with little or no phytotoxicity in soils at concentrations far exceeding those reported in any of the above papers. Furthermore, we confirmed that it is a strong antioxidant, virtually ruling out that it could harm plants by causing oxidative stress. One could hypothesize that catechin is only substantially active when in the presence of some other component of C. stoebe. However, dramatic synergism between any allelochemicals has thus far not been rigorously proven (discussed by Duke et al.20). The half-life of catechin in soil is increased by the presence of other phenolic compounds, but it is still short and essentially inactive.19
In the Figure 1, we provide further evidence that catechins are rapidly converted by extracellular enzymes of plant roots into colored products. Tharayil et al.19 suggested that these products are generated by extracellular peroxidases and that they accumulate on the root exterior, as shown in our paper.20 Peroxidases have been known for many years to be present in root exudates.27 We found that root browning was not associated with toxicity.20
Figure 1.
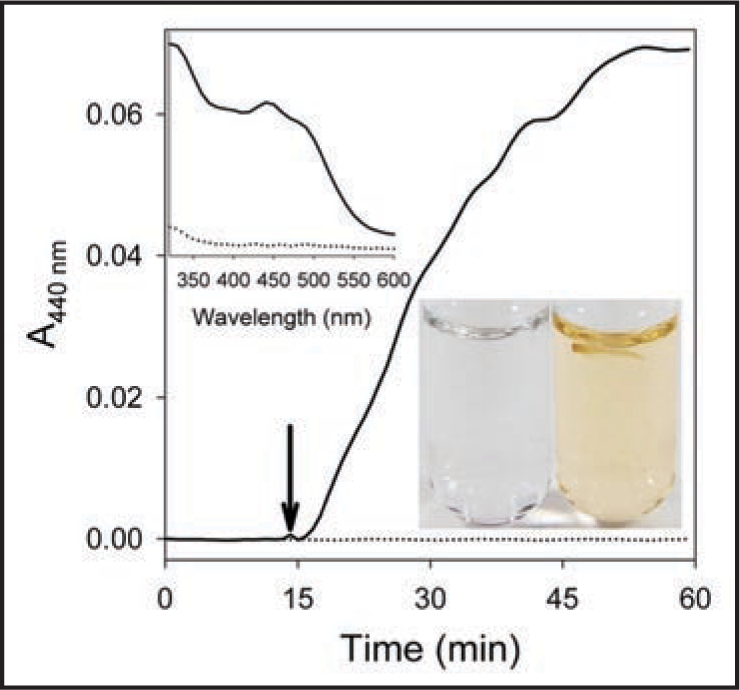
Enzymatic conversion of (±)-catechin to colored products by extracellular lettuce root enzymes. A 2-day-old lettuce seedling root tip (5 mm) was placed on top of a 1 mM (±)-catechin solution in 5 mM MES buffer (see arrow at 15 min on the time course ——). Absorbance was measured at 440 nm over 1 h in a cuvette at 25°C. The base line is the catechin solution without the root tip (……). The upper left inset shows the absorption spectrum of the solution before (……) and after (——) incubation with the root tip. The lower right inset shows 1 mM (±)-catechin after incubation for 1 h with (right) or without (left) a lettuce root tip in test tubes (notice the root floating on top of the solution). Without the root material, (±)-catechin will also oxidize, but over a much longer period of time. Similar results were obtained when an intact seedling was placed in the solution.
Catechin might be converted to a more phytotoxic and stable compound by soil microbes that were lost or inactive in our studies with native range soils in which C. stoebe had grown. However, the soils that we used had not been autoclaved, and incubating the catechin in moist soil for 14 days before planting the target species had no effect on the results. Furthermore, none of the known catechin derivatives that were evaluated in our paper20 and that of Tharayil et al.19 were significantly phytotoxic.
Impacts of the Catechin Debate on Allelopathy as a Field of Study
Our paper20 strongly supports the view of others16–19 that (-)-catechin is not an allelochemical. This very weakly phytotoxic compound is found in exceedingly low concentrations, if at all, in the soil in which producing plants are found.17 Furthermore, it is very unstable in soil and in water, and its derivatives do not appear to be significantly phytotoxic. We hope that the findings that challenge the view that (-)-catechin is an allelochemical do not discourage others from initiating or continuing research in this intriguing area of chemical ecology. Indeed, there are several well-documented cases of allelopathy in the recent literature such as that of momilactone-B in rice which has been corroborated by more than one laboratory.10,11 We believe that understanding allelopathic interactions between plant species can help in understanding the success of some invasive species and vegetation patterns in many ecosystems. Furthermore, allelopathy research has more than academic value, as it promises to be useful in generating weed-fighting crop varieties to reduce the use of synthetic herbicides.28
Acknowledgements
We thank Robert Johnson for his technical assistance.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/8273
References
- 1.Willis RJ. The historical bases of the concept of allelopathy. J History Biol. 1985;18:71–103. [Google Scholar]
- 2.Muller CH, Muller WH, Haines BL. Volatile growth inhibitors produced by aromatic shrubs. Science. 1964;143:471–473. doi: 10.1126/science.143.3605.471. [DOI] [PubMed] [Google Scholar]
- 3.Bartholomew B. Bare zone between California shrub and grassland communities: The role of animals. Science. 1970;170:1210–1212. doi: 10.1126/science.170.3963.1210. [DOI] [PubMed] [Google Scholar]
- 4.Harper JL. Population Biology of Plants. London: Academic Press; 1977. p. 892. [Google Scholar]
- 5.Einhellig FA, Souza IF. Phytotoxicity of sorgoleone found in grain sorghum root exudates. J Chem Ecol. 1992;18:1–11. doi: 10.1007/BF00997160. [DOI] [PubMed] [Google Scholar]
- 6.Dayan FE. Factors modulating the levels of the allelochemical sorgoleone in Sorghum bicolor. Planta. 2006;224:339–346. doi: 10.1007/s00425-005-0217-5. [DOI] [PubMed] [Google Scholar]
- 7.Baerson SB, Dayan FE, Rimando AM, Nanayakkara NPD, Liu CJ, Schröder J, et al. A functional genomics investigation of allelochemical biosynthesis in Sorghum bicolor root hairs. J Biol Chem. 2008;283:3231–3247. doi: 10.1074/jbc.M706587200. [DOI] [PubMed] [Google Scholar]
- 8.Bertin C, Paul RN, Duke SO, Weston LA. Laboratory assessment of the allelopathic effects of fine leaf fescue. J Chem Ecol. 2003;29:1919–1937. doi: 10.1023/a:1024810630275. [DOI] [PubMed] [Google Scholar]
- 9.Bertin C, Weston LA, Huang T, Jander G, Owens T, Meinwald J, Schroeder FC. Grass roots chemistry: meta-Tyrosine, an herbicidal nonprotein amino acid. Proc Natl Acad Sci USA. 2007;104:16964–16969. doi: 10.1073/pnas.0707198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong CH, Li HB, Hu F, Xu XH, Wang P. Allelochemicals released by rice roots and residues in soil. Plant Soil. 2006;288:47–56. [Google Scholar]
- 11.Kato-Noguchi H. Allelopathic substance in rice root exudates: Rediscovery of momilactone B as an allelochemical. J Plant Physiol. 2004;161:271–276. doi: 10.1078/0176-1617-01188. [DOI] [PubMed] [Google Scholar]
- 12.Bais HP, Vepacedu R, Gilroy S, Callaway RM, Vivanco JM. Allelopathy and exotic plant invasion: from molecules and genes to species interactions. Science. 2003;301:1377–1380. doi: 10.1126/science.1083245. [DOI] [PubMed] [Google Scholar]
- 13.Bais HP, Walker HS, Stermitz FR, Hufbauer RA, Vivanco JM. Enantiomeric-dependent phytotoxic and antimicrobial activity of (±)-catechin. A rhizosecreted racemic mixture from spotted knapweed. Plant Physiol. 2002;128:1173–1179. doi: 10.1104/pp.011019. [DOI] [PubMed] [Google Scholar]
- 14.Perry GL, Thelen GC, Ridenour WM, Weir TL, Callaway RM, Pashke MW, Vivanco JM. Dual role for an allelochemical: (±)-catechin from Centauria maculosa roots exudates regulates conspecific seedling establishment. J Chem Ecol. 2005;93:1126–1135. [Google Scholar]
- 15.Weir TL, Bais HP, Vivanco JM. Intraspecific and interspecific interactions mediated by a phytotoxin (-)-catechin, secreted by the roots of Centaurea maculosa (spotted knapweed) Chem Ecol. 2003;29:2397–2412. doi: 10.1023/a:1026313031091. [DOI] [PubMed] [Google Scholar]
- 16.Blair AC, Hanson BD, Brunk GR, Marrs RA, Westra P, Nissen SJ, Hufbauer RA. New techniques and findings in the study of a candidate allelochemical implicated in invasion success. Ecol Lett. 2005;8:1039–1047. [Google Scholar]
- 17.Blair AC, Nissen SJ, Brunk GR, Hufbauer RA. A lack of evidence for an ecological role of the putative allelochemical (±)-catechin in spotted knapweed invasion success. J Chem Ecol. 2006;32:2327–2331. doi: 10.1007/s10886-006-9168-y. [DOI] [PubMed] [Google Scholar]
- 18.Blair AC, Weston LA, Nissen SJ, Brunk GR, Hufbauer RA. The importance of analytical techniques in allelopathy studies with the reported allelochemical catechin as an example. Biol Invasions. 2009 In press. [Google Scholar]
- 19.Tharayil N, Bhowmik P, Xing B. Bioavailability of allelochemicals as affected by companion compounds in soil matrices. J Agric Food Chem. 2008;56:3706–3713. doi: 10.1021/jf073310a. [DOI] [PubMed] [Google Scholar]
- 20.Duke SO, Blair AC, Dayan FE, Johnson RD, Meepagala KM, Cook D, Bajsa J. Is (-)-catechin a novel weapon of spotted knapweed (Centaurea stoebe)? J Chem Ecol. 2009;35:141–153. doi: 10.1007/s10886-008-9587-z. [DOI] [PubMed] [Google Scholar]
- 21.Macías FA, Chinchilla N, Varela RM, Oliveros-Bastidas A, Marín D, Molinillo JMG. Structure-activity relationship studies of benzoxazinones and related compounds. Phytotoxicity on Echinochloa crus-galli (L.) P. Beauv. J Agric Food Chem. 2005;53:4373–4380. doi: 10.1021/jf0502911. [DOI] [PubMed] [Google Scholar]
- 22.Ridenour WM, Callaway RM. The relative importance of allelopathy in interference: the effects of an invasive weed on a native bunchgrass. Oecologia. 2001;126:444–450. doi: 10.1007/s004420000533. [DOI] [PubMed] [Google Scholar]
- 23.Weir TL, Bais HP, Stull VJ, Callaway RM, Thelen GC, Redenhour WM, et al. Oxalate contributes to the resistance of Gaillardia grandiflora and Lupinus sericeus to a phytotoxin produced by Centaurea maculosa. Planta. 2006;223:785–795. doi: 10.1007/s00425-005-0192-x. [DOI] [PubMed] [Google Scholar]
- 24.Thelen CC, Vivanco JM, Newingham B, Good W, Bais HP, Landres P, et al. Insect herbivory stimulates allelopathic exudation by an invasive plant and the suppression of natives. Ecol Lett. 2005;8:209–217. [Google Scholar]
- 25.Bais HP, Walker TS, Kennan AJ, Stermitz FR, Vivanco JM. Structure-dependent phytotoxicity of catechins and other flavonoids: Flavonoid conversions by cell-free protein extracts of Centaurea maculosa (spotted knapweed) roots. J Agric Food Chem. 2003;51:897–901. doi: 10.1021/jf020978a. [DOI] [PubMed] [Google Scholar]
- 26.Lau JA, Puliafica KP, Kopshever JA, Stelzer H, Jarvis EP, Schwarzländer M, et al. Inference of allelopathy is complicated by effects of activated carbon on plant growth. New Phytol. 2008;178:412–423. doi: 10.1111/j.1469-8137.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 27.Chrispeels MJ. Biosynthesis, intracellular transport and secretion of extracellular macromolecules. Annu Rev Plant Physiol. 1976;27:19–38. [Google Scholar]
- 28.Duke SO. Weeding with transgenes. Trends Biotechnol. 2003;21:192–195. doi: 10.1016/S0167-7799(03)00056-8. [DOI] [PubMed] [Google Scholar]


