Abstract
Plant organogenesis generally involves three basic processes: cell division, cell expansion and cell differentiation. Endoreduplication, a process of genome replication without intervening mitosis, often occurs during cell expansion and cell differentiation. The switch from the mitotic cell cycle to the endocycle, however, is still poorly understood in plants. We have recently demonstrated that FIZZY-RELATED2 (FZR2) is a factor controlling endoreduplication in Arabidopsis. fzr2 mutants lacked gross morphological defects but showed a general decrease of endoploidy level in trichomes and other leaf cells, while expression of FZR2 under constitutive or tissue specific promoters induced extra or ectopic endoreduplication in all tissues examined. We also showed that decrease of leaf cell size in fzr2 mutants could be compensated by increased cell proliferation. In this addendum, we discuss additional phenotypes of FZR2 misexpression, including apparent mosaic leaf sectors in which local cell overexpansion due to 35S::FZR2 appears to be compensated by reduced cell expansion in neighboring tissues.
Key words: Arabidopsis, fizzy-related, CCS52A1, endoreduplication, embryo, mosaic analysis, compensation mechanism
Plants begin vegetative development as single zygotes and reach final sizes million-fold bigger, with complex tissues and cell types. During development, plants undergo three basic processes: cell division, cell expansion and cell differentiation. While cell division is dependent on mitotic cell cycle, cell expansion and cell differentiation are often coupled with a modified cell cycle called endoreduplication.1 Endoreduplication enables a cell to increase its ploidy by replicating its genome without subsequent chromosomal and cellular division. This endocycle, widespread in eukaryotes but especially common in plants, may provide individual cells with the gene-expression capacity to reach larger sizes.2 In the well-studied dicotyledon model Arabidopsis thaliana, endoreduplication occurs in most of the differentiated cell types, such as trichoblasts and trichomes, or cells with very high metabolic activity, such as the endosperm.3 The switch from mitotic cycles to endocycles requires cells to start another round of DNA replication without intervening mitosis. Therefore, a cell must induce re-entry into S-phase after G1-phase while inhibiting M-phase. The regulatory mechanisms mediating the G1 to S transition in endocycles share components of the mitotic pathways,4 with the CDK/CYCLIN B complex influencing DNA replication.5 Much evidence in yeast, fly and plants has pointed to the involvement of a WD-40 protein, Fizzy-Related/Ccs52, in triggering the switch to endoreduplication by controlling the degradation of Cyclin B.6,7
Using reverse genetics in Arabidopsis, we investigated FIZZY-RELATED2 loss-of-function mutants.8 fzr2 plants showed reduced endoreduplication and cell size in both pavement cells and trichomes.8 When FZR2 was misexpressed with the CMV 35S promoter, transgenic plants showed a range of phenotypes such as retarded growth, supernumerary trichome branches and distorted roots, with ectopic endoreduplication induced in all examined tissues. When expressed under control of the petal- and stamen-specific APETELA3 promoter, FZR2 caused great increases in the cell and nuclear sizes of petal and stamen cells, which normally endocycle little or not at all in Arabidopsis.8
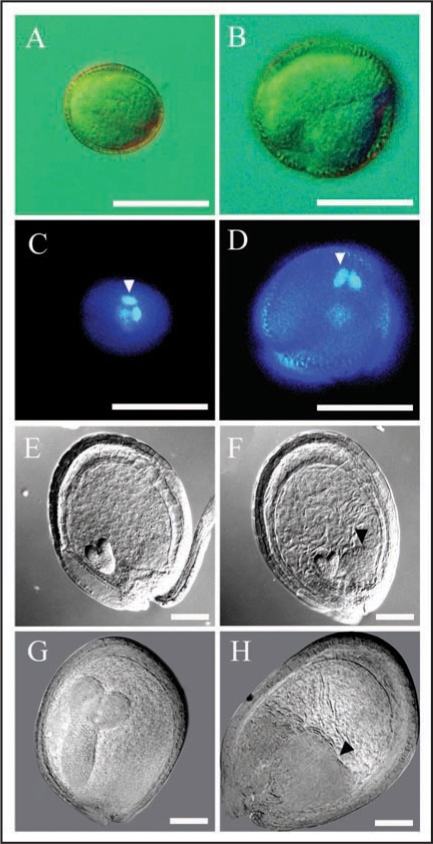
Since AP3 also drives gene expression in pollen, and pollen mother cells undergo two rounds of meiosis to generate haploid sperm cells,9 the effects of FZR2 expression on male gametogenesis seemed particularly interesting. Microscopic analysis showed larger pollen grains in AP3::FZR2 plants relative to wildtype, whereas DAPI staining revealed a concomitant increase in sperm cell nuclear size (Fig. 1A–D). These results suggested that endoreduplication had been induced in these pollen grains. Although these polyploid sperm cells proceeded through double fertilization, the corresponding embryos failed to complete development. Examination of cleared embryos with Nomarski microscopy showed that about half of them stopped growth at the torpedo stage (Fig. 1G and H), possibly due to abnormal endosperm development. When endosperm cellularization was completed in wildtype seeds (Fig. 1E), there were only 2 to 3 bubble-like structures at the chalazal poles of developing AP3::FZR2 seeds (Fig. 1F). This phenotype was similar to that of developing seeds derived from fertilization of a diploid plant with pollen from an hexaploid plant,10 further supporting the conclusion that AP3::FZR2 sperm cells underwent endoreduplication.
Figure 1.
Comparisons of pollen grain sizes, nuclear sizes and embryo development among wildtype (WT, left: A, C, E and G) and AP3::FZR2 lines (right: B, D, F and H). (A and B) Micrographs of representative pollen grains. (C and D) DAPI staining of representative pollen grain. Arrowheads in (C and D) indicate the enlarged nuclei of sperm cells. (E and F) Micrographs of heart-stage embryos. (G and H) Micrographs of torpedo-stage embryos. Arrowheads in (F and H) indicate the abnormal endosperms. In (E–H), seeds were cleared with Hoyer solution and viewed using Nomarski optics. Scale bars represent 10 µm in (A–D), and 100 µm in (E–H).
Another interesting result of this study was the different manner in which stamens and petals were altered by AP3::FZR2 expression. While petal cells showed extreme increases in size and decreases in numbers, the organs became disrupted, losing their characteristic laminar shape. Conversely, AP3::FZR2 stamens maintained their cylindrical shape, despite becoming wider at the organ level and composed of larger cells.8 This discrepancy in the severity of petal and stamen organ-level phenotypes may be because the two tissues respond differently to FZR2 misexpression, or because the shapes of these two organs place unique constraints on the effects of cell overgrowth. Like these stamens, roots and stems of 35S::FZR2 plants also retained normal shape despite severe distortion of internal tissue architecture.8 Perhaps a cylindrical organ is maintained more easily due to the dynamics of biophysical forces. It is also possible that the morphogenesis of a filamentous structure makes more use of intercellular communication than a laminar structure, so the cell proliferation and cell expansion are more strictly regulated by non-cell autonomous signals such as protein movement via plasmodesmata to provide additional positional information.11 The regulatory contribution of these additional signals may override the effects of FZR2 ectopic expression.
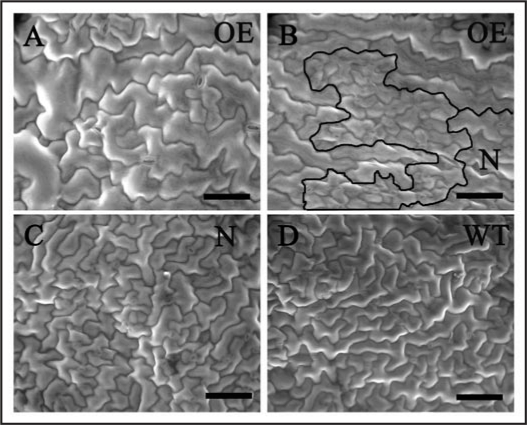
Finally, the most intriguing phenotype found in fzr2-1 mutant was that the overall leaf size showed no significant difference compared with wildtype, although the average cell was smaller. This suggests that proliferation is enhanced to generate more cells in response to the decreased average cell size. A mechanism called compensation is postulated to coordinate cell proliferation and cell expansion to attain proper organ size.12 For example, mutations or transgenes that cause decreases in leaf cell proliferation can be compensated by extra leaf cell expansion, such that the organ approaches normal size.13 Little is known, however, about how organs and cells respond to local perturbations of cell sizes. In a subset of 35S::FZR2 transgenic plants, the expression of FZR2 was silenced at the whole plant level, but some groups of cells escaped silencing. These sectors showed FZR2 overexpression phenotypes such as over-branched trichomes and giant pavement cells, whereas nearby sections of the same leaf contained normal-sized pavement cells and 3- or 4-branch trichomes. These mosaic sectors provided an opportunity to observe how compensation works even within an organ. Inside the sectors were overgrown pavement cells typical of some FZR2 overexpression lines (Fig. 2A). Away from the sectors, the pavement cells were wildtype in appearance (Fig. 2C and D). At the sector boundary, however, a strip of very small cells formed (Fig. 2B). The smaller cell size at the border may have came about to compensate for the abnormally large cells within the sector, although it is unclear whether this decrease in cell size was followed reduced endoreduplication or simple space limitation.
Figure 2.
Comparisons of cell sizes inside and outside of a mosaic sector. (A) Scanning electron microscope graphs of epidermal cells from a mosaic sector of 35S::FZR2. (B) Epidermal cells at boundary region between mosaic sector (OE) and surrounding normal cells (N). Black lines highlight the band of smaller cells. (C) Normal epidermal cells outside the mosaic sector in the same leaf (N). (D) Epidermal cells from wildtype plants (WT). Scale bar represents 100 µm.
By studying fzr2 mutants and misexpression lines, we showed that FZR2 is necessary and sufficient to induce endoreduplication in various cell types. Our observation that cells increase proliferation to compensate the decreased cell size in fzr2 mutants provides important evidence that cell proliferation and cell expansion are closely interconnected to regulate organ development in Arabidopsis. Further experiments such as mosaic analysis are needed to further elucidate the compensation mechanism.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/8480
References
- 1.Nagl W. DNA endoreduplication and polyteny understood as evolutionary strategies. Nature. 1976;261:614–615. doi: 10.1038/261614a0. [DOI] [PubMed] [Google Scholar]
- 2.Larkins BA, Dilkes BP, Dante RA, Coelho CM, Woo YM, Liu Y. Investigating the hows and whys of DNA endoreduplication. J Exp Bot. 2001;52:183–192. [PubMed] [Google Scholar]
- 3.Sugimoto-Shirasu K, Roberts K. “Big it up”: endoreduplication and cell-size control in plants. Curr Opin Plant Biol. 2003;6:544–553. doi: 10.1016/j.pbi.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez C, Ramirez-Parra E, Castellano MM, del Pozo JC. G(1) to S transition: more than a cell cycle engine switch. Curr Opin Plant Biol. 2002;5:480–486. doi: 10.1016/s1369-5266(02)00301-1. [DOI] [PubMed] [Google Scholar]
- 5.John PCL, Qi R. Cell division and endoreduplication: doubtful engines of vegetative growth. Trends Plant Sci. 2008;13:121–127. doi: 10.1016/j.tplants.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Cebolla A, Vinardell JM, Kiss E, Olah B, Roudier F, Kondorosi A, et al. The mitotic inhibitor ccs52 is required for endoreduplication and ploidy-dependent cell enlargement in plants. EMBO J. 1999;18:4476–4484. doi: 10.1093/emboj/18.16.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang GW, Yu HT, Kirschner MW. Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol Cell. 1998;2:163–171. doi: 10.1016/s1097-2765(00)80126-4. [DOI] [PubMed] [Google Scholar]
- 8.Larson-Rabin Z, Li Z, Masson PH, Day CD. FZR2/CCS52A1 expression is a determinant of endoreduplication and cell expansion in Arabidopsis. Plant Physiol. 2009;149:874–884. doi: 10.1104/pp.108.132449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mccormick S. Male Gametophyte development. Plant Cell. 1993;5:1265–1275. doi: 10.1105/tpc.5.10.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott RJ, Spielman M, Bailey J, Dickinson HG. Parent-of-origin effects on seed development in Arabidopsis thaliana. Development. 1998;125:3329–3341. doi: 10.1242/dev.125.17.3329. [DOI] [PubMed] [Google Scholar]
- 11.Petricka JJ, Benfey PN. Root layers: complex regulation of developmental patterning. Curr Opin Genet Dev. 2008;18:354–361. doi: 10.1016/j.gde.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsukaya H. Organ shape and size: a lesson from studies of leaf morphogenesis. Curr Opin Plant Biol. 2003;6:57–62. doi: 10.1016/s1369526602000055. [DOI] [PubMed] [Google Scholar]
- 13.De Veylder L, Beeckman T, Beemster GTS, Krols L, Terras P, Landrieu I, et al. Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis. Plant Cell. 2001;13:1653–1667. doi: 10.1105/TPC.010087. [DOI] [PMC free article] [PubMed] [Google Scholar]