Abstract
In order to understand more details about the role of abscisic acid (ABA) in fruit ripening and senescence, six 740 bp cDNAs (LeNCED1, LeNCED2, PpNCED1, VVNCED1, DKNCED1 and CMNCED1) which encode 9-cis-epoxycarotenoid dioxygenase (NCED) as a key enzyme in ABA biosynthesis, were cloned from fruits of tomato, peach, grape, persimmon and melon using an RT-PCR approach. A Blast homology search revealed a similarity of amino acid 85.76% between the NCEDs. A relationship between ABA and ethylene during ripening was also investigated. At the mature green stage, exogenous ABA treatment increased ABA content in flesh, and promoting ethylene synthesis and fruit ripening, while treatment with nordihydroguaiaretic acid (NDGA), inhibited them, delayed fruit ripening and softening. However, ABA inhibited the ethylene synthesis obviously while NDGA promoted them when treated the immature fruit with these chemicals. At the breaker, NDGA treatment cannot block ABA accumulation and ethylene synthesis. Based on the results obtained in this study, it was concluded that ABA plays different role in ethylene synthesis system in different stages of tomato fruit ripening.
Key words: tomato, NCED gene, ABA, ethylene, fruit ripening, peach, grape, persimmon, melon
Introduction
Because the plant hormone abscisic acid (ABA) displays a pattern of change similar to ethylene at late stages of fruit development, it was considered that ABA had a crucial role, perhaps even more crucial role than that of ethylene, in fruit maturation and senescence.1–3 However, had not demonstration of molecular level. To date, the ripening mechanism of climacteric fruit, especially the effect of ethylene, has been well studied,4 while the mechanism involved in the ripening of non-climacteric fruits remains unclear. However, the two types of fruits exhibit the same ripening phenomena in terms of colour and texture, with only a difference in ethylene production. On the other hand, ABA can be considered as the ripening control factor, because the ABA content is very low in unripe fruit but increases during the process of fruit ripening in both climacteric5,6 and non-climacteric fruits.7–10 At present, a relationship between ABA and ethylene during ripening and senescence was indicated in the tomato fruit: (i) the expression of the ABA biosynthetic gene (LeNCED1) occurs before that of ethylene biosynthesis genes; (ii) ABA content also preceded the climacteric increase in ethylene production; (iii) ABA may induce ethylene biosynthesis via the regulation of ACS and ACO gene expression; (iv) exogenous ABA accelerates fruit ripening, and fluridone or NDGA treatment delayed fruit ripening by inhibition of ABA; and (v) ethylene plays a key role in the later stages of fruit ripening.11 Also, in our experimental of peach and grape fruits, the potential contribution of ABA was analyzed, in relation to ethylene, in the induction of fruit ripening in both species. The results demonstrated: (1) PpNCED1 and VVNCED1 initiated ABA biosynthesis at the beginning of fruit ripening in peach and grape, respectively, (2) ABA accumulation preceded the climacteric raise in ethylene production, (3) Exogenous ABA stimulated ethylene production and accelerated fruit ripening, (4) Inhibition of ABA synthesis by Fluridone or NDGA suppressed ethylene production and delayed fruit ripening, and (5) ethylene plays a important role in the later stage of fruit ripening. Together, these evidences indicate that ABA may be a trigger hormonal stimuli inducing ethylene production and consequently initiating the ripening process.12
In this study, we analyzed the influence of ABA on the ethylene biosynthesis system in tomato with the fruits of immature, mature green and break stage, which would help to understand the mechanism of ABA involved in ethylene physiology and fruit ripening physiology and to enrich the study of mechanism of interaction of plant hormone.
Plant Material and Chemical Treatments
Tomato (Lycopersicon esculentum cv. Jia Bao) plants were grown in a greenhouse under the standard cultural conditions (25/20°C, relative humidity 70%). The tomato fruits were used for chemical treatment at different days (stages) after anthesis: 30 d (immature); 40 d (mature green), 44 d (break stage). Immediately after harvest, fruits were divided into 3 groups and used for following treatments: ABA (100 µM, group 1) (GIBCO BRL), NDGA (100 µM, group 2), Control (CK, distilled water, group 3). The fruits were treated with 0.5 ml each of solutions or distilled water, respectively. Each solution was injected into the fruit from pedicle with medical injector. Then the treated fruits were incubated (stored) at 30°C and 75% RH for 0 d, 2 d, 4 d, 6 d and 8 d, respectively.
RNA Extraction, RT-PCR and Sequencing
Total RNA was extracted from 10 g flesh using the hot borate method.14 The NCEDs cloning and sequencing were carried using the methods described in Zhang et al.12
Results
Cloning and sequencing of NCED genes from fruits.
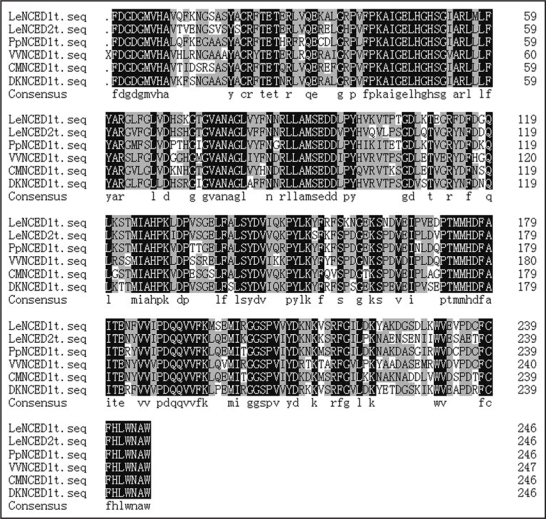
Using the RT-PCR approach, we were able to obtain one band of PCR product for fruit of tomatoes, peaches, grapes, persimmons and melons. Nucleotide sequence lengths generated for tomatoes (LeNCED1, Z97215; LeNCED2, EU912387), peaches (PpNCED1, EF625684; PpNCED2, EU912386), grapes (VVNCED1, EF625685), persimmon (DKNCED1, EU925812) and melon (CMNCED1, EU180589) were 740 bp, respectively. These cDNAs differed in the sequence of a 740 bp fragment. A BLAST homology search revealed that the deduced amino acid sequence of above mentioned six NCEDs have 85.76% identity (Fig. 1).
Figure 1.
The cloning of LeNCED1, LeNCED2, PpNCED1, VVNCED1, DKNCED1 and CMNCED1 from the fruits of tomato, peach, grape, persimmon and melon.
Effects of ABA and NDGA on the ethylene production in different ripening stage of tomato.
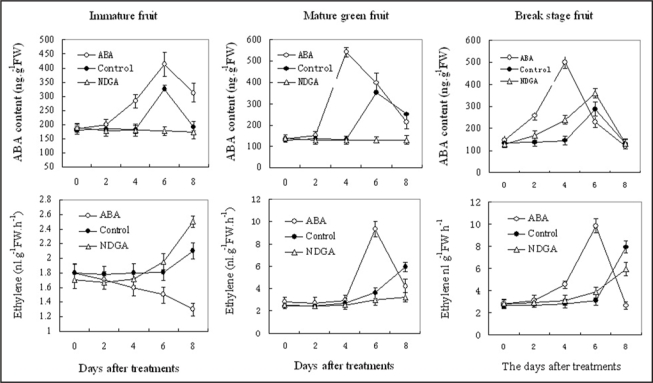
The changes in ABA content and ethylene production after treatment by exogenous ABA or NDGA, respectively, were shown in Figure 2. At the mature green stage, when the fruits were treated with exogenous ABA, ABA in fruits increased rapidly from 2 to 4 days, the peak of ABA level was found 2 d earlier than that in the control fruits. On the contrary, in fruits treated with NDGA, the ABA was completely blocked. On the other hand, ethylene increased remarkably in the fruits treated with exogenous ABA from 4 d, and reached to the peak at 6 d after treatments. The treatment of fruits with NDGA significantly decreased ABA levels and inhibited the fruit softening. The experiment results indicated that ABA treatment induced ethylene synthesis.
Figure 2.
Effects of exogenous 100 µM ABA and 100 µM NDGA treatment on ABA levels and ethylene production in different ripening stages. Each point in A represents the mean of three replications and vertical bars represent ±SE.
The ethylene production of the fruits sampled from the immature tomato fruit was effectively inhibited with application of 100 µM ABA. On the contrary, the ethylene release from the fruits treated with 100 µM NDGA increased significantly. At the break stage, ABA accumulation and ethylene production cannot were blocked when fruits were treated with 100 µM NDGA (Fig. 3).
Figure 3.
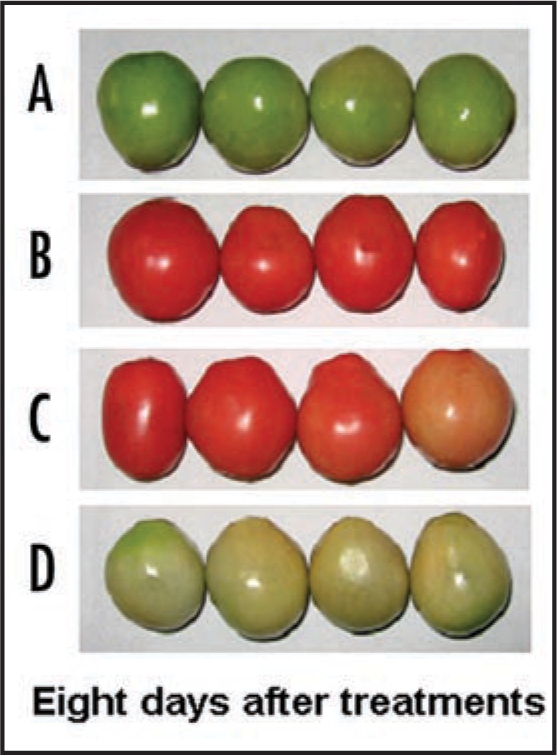
Changes of skin color in tomato fruits treated by exogenous 100 µM ABA or 100 µM NDGA solution. The tomato fruits were harvested and treated by 0.5 ml/fruit each of 100 µM ABA or 100 µM NDGA or 0.5 ml/fruit of distilled water (control). (A) Immature green fruits were treated by 100 µM ABA; (B) Mature green fruits were treated by distilled water; (C) Breaker fruits were treated by 100 µM NDGA; (D) Mature green fruits were treated by 100 µM NDGA.
Addendum to: Zhang M, Yuan B, Leng P. The role of ABA in triggering the ethylene biosynthesis and ripening of tomato fruit. J Exp Bot. 2009 doi: 10.1093/jxb/erp026. In press.
and
Zhang M, Leng P, Zhang GL, Li XX. Cloning and functional analysis of 9-cis-epoxycarotenoid dioxygenase (NCED) genes encoding a key enzyme during abscisic acid biosynthesis from peach and grape fruits. J Plant Physiol. 2009 doi: 10.1016/j.jplph.2009.01.013. In press.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/8542
References
- 1.Giovannoni J. Molecular biology of fruit maturation and ripening. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:725–749. doi: 10.1146/annurev.arplant.52.1.725. [DOI] [PubMed] [Google Scholar]
- 2.Giovannoni J. Genetic regulation of fruit development and ripening. Plant Cell. 2004;16:170–180. doi: 10.1105/tpc.019158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodrigo MJ, Marcos JF, Alferez F, Mallent M, Zacarias L. Characterization of Pinalate, a novel Citrus sinensis mutant with a fruit-specific alteration that results in yellow pigmentation and decreased ABA content. J Exp Bot. 2003;54:727–738. doi: 10.1093/jxb/erg083. [DOI] [PubMed] [Google Scholar]
- 4.Alexander L, Grierson D. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J Exp Bot. 2002;53:2039–2055. doi: 10.1093/jxb/erf072. [DOI] [PubMed] [Google Scholar]
- 5.Vendrell M, Buesa C. Relationship between abscisic acid content and ripening of apples. Acta Hort. 1989;258:389–396. [Google Scholar]
- 6.Buesa C, Dominguez M, Vendrell M. Abscisic acid effects on ethylene production and respiration rate in detached apple fruits at different stages of development. Revista Espanola de cienciay Tenologia de Alimentos. 1994;34:495–506. [Google Scholar]
- 7.Inaba A, Ishida M, Sobajima Y. Changes in endogenous hormone concentration during berry development in relation to the ripening of Delaware grape. J Japn Soc Hort Sci. 1976;45:245–252. [Google Scholar]
- 8.Kojima K. Distribution and change of endogenous IAA and ABA in asparagus spear and orange fruit. Chem Reg Plant. 1996;31:68–71. [Google Scholar]
- 9.Kondo S, Inoue K. Abscisic acid and 1-aminocyclopropane-1-carboxylic acid (ACC) content during growth of ‘Satohnishiki’ cherry fruit, and the effect of ABA and ethephon application on fruit quality. J Hort Sci. 1997;72:221–227. [Google Scholar]
- 10.Kondo S, Tomiyama A. Changes of free and conjugated ABA in the fruit of Satohnishiki sweet cherry and the ABA metabolism after application of (s)-(+)-ABA. J Hort Sci Biotechnol. 1998;73:467–472. [Google Scholar]
- 11.Zhang M, Yuan B, Leng P. The role of ABA in triggering the ethylene biosynthesis and ripening of tomato fruit. J Exp Bot. 2009 doi: 10.1093/jxb/erp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang M, Leng P, Zhang G, Li X. Cloning and functional analysis of 9-cis-epoxycarotenoid dioxygenase (NCED) genes encoding a key enzyme during abscisic acid biosynthesis from peach and grape fruits. J Plant Physiol. 2009 doi: 10.1016/j.jplph.2009.01.013. In press. [DOI] [PubMed] [Google Scholar]
- 13.Wan CY, Wilkins TA. A modified hot borate method significantly enhances the yield of high-quality RNA from cotton (Gossypium hirsutum L.) Anal Biochem. 2004;223:7–12. doi: 10.1006/abio.1994.1538. [DOI] [PubMed] [Google Scholar]