Abstract
During stomatal closure, nitric oxide (NO) operates as one of the key intermediates in the complex, abscisic acid (ABA)-mediated, guard cell signaling network that regulates this process. However, data concerning the role of NO in stomatal closure that occurs in turgid vs. dehydrated plants is limited. The data presented demonstrate that, while there is a requirement for NO during the ABA-induced stomatal closure of turgid leaves, such a requirement does not exist for ABA-enhanced stomatal closure observed to occur during conditions of rapid dehydration. The data also indicate that the ABA signaling pathway must be both functional and to some degree activated for guard cell NO signaling to occur. These observations are in line with the idea that the effects of NO in guard cells are mediated via a Ca2+-dependent rather than a Ca2+-independent ABA signaling pathway. It appears that there is a role for NO in the fine tuning of the stomatal apertures of turgid leaves that occurs in response to fluctuations in the prevailing environment.
Key words: abscisic acid, nitrate reductase, nitric oxide, stomatal apertures, guard cells
Nitric oxide (NO) acts as a key signal that regulates many of the responses of plants to environmental stresses1 and is an important intermediate in the abscisic acid (ABA) signal transduction pathway which controls stomatal apertures.2 Prior studies have shown that ABA-induced stomatal closure in Arabidopsis thaliana is dependent on guard cell nitrate reductase (NR)-mediated NO synthesis.3 More specifically, of the two NR isoforms encoded by the genes NIA1 and NIA2, ABA-induced stomatal closure is dependent on the synthesis of NO by the NIA1-encoded isoform, NR1.2 However, until now, there has been a lack of comparative data detailing the degree to which this is required as turgid plants subsequently dehydrate during periods of drought.
In our original paper4 we showed that while the removal of guard cell NR1-associated NO prevented ABA-induced stomatal closure in turgid leaves, NO removal, either by scavenging with 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) or as a consequence of loss-of-function mutation of NIA1 (nia1::Ds), did very little to reduce the ABA-enhanced stomatal closure observed in leaves undergoing rapid dehydration. Plants of the NR1-lacking mutant, nia1::Ds, were not obviously any more wilty than those of their wild-type Landsberg background when watering was withheld and must, therefore, be able to regulate the amount of water they lose through transpiration during periods of drought. It is, therefore, intriguing that the responses of turgid guard cells to NO require the ABA signal transduction pathway to be both functional and to some degree engaged.4 The stomata of the turgid leaves of neither the ABA deficient, lossof- function mutant, aba1-1, nor the ABA insensitive mutant, abi1-1, close in response to the application of either NO via the donor sodium nitroprusside (SNP) or the nitrite substrate of NR-mediated NO synthesis.4 Thus, the inference is that stomatal closure is regulated by both NO-dependent and -independent mechanisms of ABA signaling that operate in a manner dependent on the prevailing environmental conditions. Indeed this observation aligns well with those concerning the involvement of Ca2+ in guard cell ABA signaling. For some time the ABA-dependent signaling of NO in guard cells has been thought to involve an elevation of intracellular Ca2+ which then alters the activities of Ca2+-dependent K+ and Cl− ion channels.5 Certainly, NO does affect cellular Ca2+ mobilisation6 and a requirement for altered Ca2+ flux for normal guard cell responses to NO has been shown in Vicia faba.7 In guard cells, ABA signaling is known to operate via both Ca2+-dependent8 and pH-dependent9/Ca2+-independent pathways.10–13 NO accumulates in ABA-treated guard cells of the abi1-1 mutant, but this does not result in stomatal closure.4 This is consistent with the concept that, in these cells, NO signals via the Ca2+-dependent ABA signaling pathway which is impaired in this mutant.14 Exactly how this occurs is still unclear, but may involve S-nitrosylation of thiol groups within Ca2+-dependent K+ ion channels.15
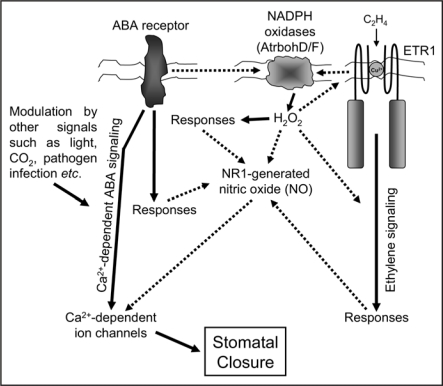
It is also interesting that the accumulation and signaling of NO in Arabidopsis guard cells is dependent on the prior synthesis of H2O2 by the NADPH oxidase isoforms AtrbohD/F16 and that this generation of H2O2 is in turn dependent on the normal ethylene-sensing function of the ethylene receptor encoded by ETR1.17 Neither the histidine kinase function of ETR1 nor the downstream components of the ethylene signaling pathway appear to be required for the guard cell accumulation of H2O2 to occur per se, but do appear to be required for the synthesised H2O2 to be effective. Thus, in turgid guard cells the production of H2O2 and the subsequent synthesis and signaling of NO may constitute points of convergence of the ethylene and ABA signaling pathways. Figure 1 illustrates our current thinking with regard to the integration of these different signals in guard cells.
Figure 1.
Signal crosstalk during NO-regulated stomatal closure in turgid leaves of Arabidopsis thaliana. In turgid leaves ABA-induced stomatal closure is dependent on the synthesis of H2O2 by various NADPH oxidases and the subsequent synthesis of nitric oxide (NO) by nitrate reductase 1 (NR1).16 NO signals via a Ca2+-dependent ABA signaling pathway,4 possibly by the S-nitrosylation of Ca2+-dependent ion channels.15 Numerous environmental signals modulate this response and determine the actual stomatal apertures observed.4,19 Interestingly the ABA-induced accumulation of H2O2 in turgid guard cells also appears to depend on the ability of the ethylene receptor, ETR1, to perceive ethylene and although not actually required for the ABA-induced accumulation of this reactive oxygen species, downstream components of the ethylene signal transduction pathway also seem to be required for H2O2 to signal its presence in these cells.17,21 Thus, the synthesis and signaling of H2O2 and NO in turgid guard cells may constitute points of convergence of ABA and ethylene signaling. However, numerous question remain concerning how the activities of the NADPH oxidases and NR1 are regulated and whether or not this occurs via the direct regulation of the extant proteins or via increased transcription as a result of ABA and ethylene signaling. Additionally, questions remain as to exactly how H2O2 induces NR1-mediated NO synthesis and whether this is either by the direct modulation of protein activity or as a result of downstream signaling. For example, H2O2 may signal in its own right or may mediate the activity of components of the ethylene signal transduction chain leading to an increase in NIA1 transcription. Future studies aim to clarify these questions.
One of the questions that remains is that if NO is not involved in stomatal closure during conditions of rapid dehydration, what exactly is its role in mediating guard cell movements? Turgid guard cells accumulate NO during the light to dark transition18 and scavenging NO or preventing its synthesis, as in the nia1::Ds mutant, prevents the darkinduced closure from occurring.4 Thus, it would seem that, in this context, NO has a physiological role in mediating guard cell movements in well hydrated plants. However, stomatal closure does not always occur in the dark and many other factors dictate whether or not this happens.19 High levels of CO2 applied in the form of the bicarbonate ion, for example, induce the sequential accumulation of H2O2 and NO in guard cells during stomatal closure.20 Presumably, the degree of closure that occurs in the dark is dependent on the balance of multiple input signals. On reflection this makes sense considering the seasonal changes in the growing environment that occur in temperate regions. Currently, it seems as though NO acts to mediate this balance and does so by signaling via a Ca2+-dependent ABA signal transduction pathway. The focus of future studies will then be to determine exactly how such signaling “crosstalk” operates and which proteins are specifically modified by NO during the stomatal closure that is thus regulated.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/8545
References
- 1.Neill S, Barros R, Bright J, Desikan R, Hancock J, Harrison J, et al. Nitric oxide, stomatal closure and abiotic stress. J Exp Bot. 2008;59:165–176. doi: 10.1093/jxb/erm293. [DOI] [PubMed] [Google Scholar]
- 2.Wilson ID, Neill SJ, Hancock JT. Nitric oxide synthesis and signaling in plants. Plant Cell Environ. 2008;31:622–631. doi: 10.1111/j.1365-3040.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- 3.Desikan R, Griffiths R, Hancock J, Neill SJ. A new role for an old enzyme: nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2002;99:16314–16318. doi: 10.1073/pnas.252461999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribeiro DM, Desikan R, Bright J, Confraria A, Harrison J, Hancock JT, et al. Differential requirement for NO during ABA-induced stomatal closure in turgid and wilted leaves. Plant Cell Environ. 2008;59:165–176. doi: 10.1111/j.1365-3040.2008.01906.x. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Mata C, Gay R, Sokolovski S, Hills A, Lamattina L, Blatt MR. Nitric oxide regulates K+ and Cl− channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc Natl Acad Sci USA. 2003;100:1116–1121. doi: 10.1073/pnas.1434381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamotte O, Courtois C, Dobrowolska G, Besson A, Pugin A, Wendehenne D. Mechanisms of nitric oxide induced increase of free cytosolic Ca2+ concentration in Nicotiana plumbaginifolia cells. Free Rad Biol Med. 2006;40:1369–1376. doi: 10.1016/j.freeradbiomed.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Sokolovski S, Hills A, Gay R, Garcia-Mata C, Lamattina L, Blatt MR. Protein phosphorylation is a prerequisite for intracellular Ca2+ release and ion channel control by nitric oxide and abscisic acid in guard cells. Plant J. 2005;43:520–529. doi: 10.1111/j.1365-313X.2005.02471.x. [DOI] [PubMed] [Google Scholar]
- 8.Grabov A, Blatt MR. A steep dependence of inward-rectifying potassium channels on cytosolic free calcium concentration increase evoked by hyperpolarization in guard cells. Plant Physiol. 1999;119:277–288. doi: 10.1104/pp.119.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grabov A, Blatt MR. Parallel control of the inward-rectifier K+ channel by cytosolic free Ca2+ and pH in Vicia guard cells. Planta. 1997;201:84–95. [Google Scholar]
- 10.Allan AC, Fricker MD, Ward JL, Beale MH, Trewavas AJ. Two transduction pathways mediate rapid effects of abscisic acid in Commelina guard cells. Plant Cell. 1994;6:1319–1328. doi: 10.1105/tpc.6.9.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romano LA, Jacob T, Gilroy S, Assmann SM. Increases in cytosolic Ca2+ are not required for abscisic acid-inhibition of inward K+ currents in guard cells of Vicia faba L. Planta. 2000;211:209–217. doi: 10.1007/s004250000286. [DOI] [PubMed] [Google Scholar]
- 12.Outlaw WH. Integration of cellular and physiological functions of guard cells. Crit Rev Plant Sci. 2003;22:503–529. [Google Scholar]
- 13.Israelsson M, Siegel RS, Young J, Hashimoto M, Iba K, Schroeder JI. Guard cell ABA and CO2 signaling network updates and Ca2+ sensor priming hypothesis. Curr Opin Plant Biol. 2006;9:654–663. doi: 10.1016/j.pbi.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong F, Leung J, Grabov A, Brearley J, Giraudat J, Blatt MR. Sensitivity to abscisic acid of guard-cell K+ channels is suppressed by abi1-1, a mutant Arabidopsis gene encoding a putative protein phosphatase. Proc Natl Acad Sci USA. 1995;92:9520–9524. doi: 10.1073/pnas.92.21.9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sokolovski S, Blatt MR. Nitric oxide block of outward-rectifying K+ channels indicates direct control by protein nitrosylation in guard cells. Plant Physiol. 2004;136:4275–4284. doi: 10.1104/pp.104.050344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bright J, Desikan R, Hancock JT, Neill SJ. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006;45:113–122. doi: 10.1111/j.1365-313X.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- 17.Desikan R, Last K, Harret-Williams R, Tagliavia C, Harter K, Hooley R, et al. Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. Plant J. 2006;47:907–916. doi: 10.1111/j.1365-313X.2006.02842.x. [DOI] [PubMed] [Google Scholar]
- 18.She XP, Song XG, He JM. Role and relationship of nitric oxide and hydrogen peroxide in light/dark-regulated stomatal movement in Vicia faba. Acta Bot Sinica. 2004;46:1292–1300. [Google Scholar]
- 19.Caird MA, Richards JH, Donovan LA. Night-time stomatal conductance and transpiration in C3 and C4 plants. Plant Physiol. 2007;143:4–10. doi: 10.1104/pp.106.092940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolla VA, Raghavendra AS. Nitric oxide is a signaling intermediate during bicarbonate-induced stomatal closure in Pisum sativum. Physiol Plant. 2007;130:91–98. [Google Scholar]
- 21.Desikan R, Horák J, Chaban C, Mira-Rodado V, Witthöft J, Elgass K, et al. The histidine kinase AHK5 integrates endogenous and environmental signals in Arabidopsis guard cells. PLoS One. 2008;3:2491. doi: 10.1371/journal.pone.0002491. [DOI] [PMC free article] [PubMed] [Google Scholar]