Abstract
The basal forebrain (BF) contains a diffuse array of cholinergic and non-cholinergic neurons that project to the cerebral cortex and basolateral nuclear complex of the amygdala (BLC). Previous studies have shown that the GABAergic subpopulation of non-cholinergic corticopetal BF neurons selectively innervates cortical interneurons. Although several investigations in both rodents and primates have indicated that some BF neurons projecting to the BLC are non-cholinergic, there have been no studies that have attempted to identify the neurochemical phenotype(s) of these neurons. The present study combined Fluorogold retrograde tract tracing with immunohistochemistry for two markers of BF GABAergic neurons, parvalbumin (PV) or glutamic acid decarboxylase (GAD), to determine if a subpopulation of BF GABAergic cells projects to the BLC. Injections of Fluorogold confined to the rat BLC, and centered in the basolateral nucleus, produced extensive retrograde labeling in the ventral pallidum and substantia innominata regions of the BF. Although the great majority of retrogradely labeled neurons were not double-labeled, about 10% of these neurons, located mainly along the ventral aspects of the fundus striati and globus pallidus, exhibited immunoreactivity for PV or GAD. The results of this investigation contradict the long-held belief that there is no extra-amygdalar source of GABAergic inputs to the BLC, and indicate that the cortex-like BLC, in addition to the cortex proper, receives inhibitory inputs from the basal forebrain.
Keywords: ventral pallidum, substantia innominata, inhibitory, retrograde tract tracing, Fluorogold, immunohistochemistry
The basal forebrain (BF) contains a diffuse array of corticopetal cholinergic and non-cholinergic neurons that exhibit a topographical organization (Mesulam et al., 1983; Woolf, 1991; Gritti et al., 1997, 2003; Henny and Jones, 2008). These BF corticopetal neurons are important for attention and learning, and have been implicated in several neurological diseases, including Alzheimer’s disease (Everitt and Robbins, 1997). In addition to projecting to the cortex, the caudolateral two-thirds of the BF, including the substantia innominata and ventral pallidum, contains neurons that project to the cortex-like basolateral nuclear complex of the amygdala (BLC; Carlsen et al., 1985; Zaborzsky et al., 1986). The BLC, especially the basolateral nucleus proper, receives one of the densest cholinergic innervations in the central nervous system in both rodents and primates (Ben-Ari et al., 1977; Hellendall et al., 1986; Amaral and Bassett, 1989). However, approximately 5% of amygdalopetal BF neurons in primates (Kordower et al., 1989) and 20–25% of these neurons in rodents (Carlsen et al., 1985; Zaborzsky et al., 1986) are non-cholinergic.
One important subtype of non-cholinergic BF neuron that has been extensively studied is the GABAergic corticopetal neuronal population (Freund and Buzsaki, 1996; Semba, 2000; Sarter and Bruno, 2002). These BF cells, which can be identified using either GABAergic markers or antibodies to the calcium-binding protein parvalbumin (Freund, 1989; Gritti et al., 2003), innervate primarily interneurons in both the hippocampus and neocortex (Freund and Gulyas, 1991; Freund and Meskenaite, 1992; Freund and Buzsaki, 1996). Via this innervation BF GABAergic cells disinhibit assemblies of cortical pyramidal cells and act together with cholinergic BF neurons to generate rhythmic oscillatory activity in the hippocampus that is important for mnemonic function (Freund and Buzsaki, 1996; Toth et al., 1997; Buzsaki, 2002; Borhegyi et al., 2004).
To date there have been no studies that have attempted to identify the neurochemical phenotype of the non-cholinergic amygdalopetal BF neurons. The present study combined retrograde tract tracing with immunohistochemistry for two markers of BF GABAergic neurons, parvalbumin (PV) or glutamic acid decarboxylase (GAD), to identify a subpopulation of these cells that project to the BLC. The results of this investigation contradict the long-held belief (Le Gal LaSalle et al., 1978) that there is no extra-amygdalar source of GABAergic inputs to the BLC.
EXPERIMENTAL PROCEDURES
Eight adult male Sprague-Dawley rats (250–350g; Harlan, Indianapolis, IN) received bilateral injections of Fluorogold (FG) into the BLC to determine if parvalbumin-positive or GAD-positive neurons in the basal forebrain project to the amygdala. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Use and Care Committee (IACUC) of the University of South Carolina. All experiments were conducted in a manner that minimized suffering and the number of animals used.
PV/FG experiments
Rats were anesthetized with sodium pentobarbital (50 mg/kg) and placed in a stereotaxic head holder (Stoelting, Wood Dale, IL) for injections of 2% FG (Fluorochrome, LLC; Denver, Co) into the basolateral amygdalar nuclear complex (BLC) using coordinates obtained from an atlas of the rat brain (Paxinos and Watson, 1997). Bilateral iontophoretic injections of FG in saline were made via glass micropipettes (40 μm inner tip diameter) using a Midgard high voltage current source set at 2.5–3.0 μA (7 s on, 7 s off, for 30–45 min). Micropipettes were left in place for 10 minutes to prevent FG from flowing up the pipette track. After a 5 day survival, rats were perfused with 4.0% paraformaldehyde. Following perfusion, brains were removed and postfixed for 3.5 hours in 4.0% paraformaldehyde. Brains were sectioned on a vibratome at a thickness of 50 μm in the coronal plane and processed for immunohistochemistry.
A one-in-four series of sections through the basal forebrain and amygdala were incubated in a cocktail of a rabbit polyclonal PV antibody (1:2000; donated by Dr. Kenneth Baimbridge, University of British Columbia) and a guinea pig polyclonal antibody to FG (1:3000; donated by Dr. Lothar Jennes, University of Kentucky) overnight at 4° C. After incubation in the primary antibody cocktail, sections were rinsed in 3 changes of PBS (10 min each), and then incubated in a cocktail of goat anti-rabbit Alexa-568 and goat-anti-guinea pig Alexa-488 labeled secondary antibodies (1:400; Molecular Probes, Eugene, OR) for 3 hrs at room temperature. Sections were then rinsed in 3 changes of PBS (10 min each) and mounted on glass slides using Vectashield mounting medium (Vector Laboratories, Burlingame, CA). In addition to the series of sections processed for immunofluorescence, a second series of adjacent sections was mounted on gelatinized slides, dried overnight, dehydrated in ethanol, stained with cresyl violet to identify nuclear borders, and coverslipped with Permount (Fisher Scientific, Pittsburgh, PA).
Sections for immunofluorescence were examined with a Bio-Rad MRC-1024 confocal laser scanning system equipped with an argon-krypton laser attached to a Nikon Eclipse E800M microscope. Fluorescence of Alexa 488 (green) and Alexa 568 (red) dyes was analyzed using filter configurations for sequential excitation/imaging via 488 nm and 568 nm channels. Digital images were adjusted for brightness and contrast using Photoshop 6.0 software. In each of the confocal immunofluorescence cases some control sections were processed with one of the two primary antibodies omitted. In all cases only the color of the corresponding secondary fluorescent antibody was observed, and only on the appropriate channel. These results indicated that the secondary antibodies were specific for rabbit or mouse IgGs and that there was no “crosstalk” between the red and green channels (Wouterlood et al., 1998).
In cases with successful injections confined to the BLC, all immunofluorescence sections through the BF and amygdala were examined, and PV+/FG+ double-labeled neurons were plotted on drawings of atlas sections (Paxinos and Watson, 1997). In one case (case 22L) photomontages of confocal images were made at approximately 0.5 mm intervals at the main levels of the basal forebrain that exhibited double-labeled cells (bregma +0.2 to bregma −1.4). These montages were overlaid with size-matched drawings of the adjacent Nissl-stained section at each level to map the exact locations of PV+/FG+ double-labeled neurons and FG+ single-labeled neurons in the BF at these levels.
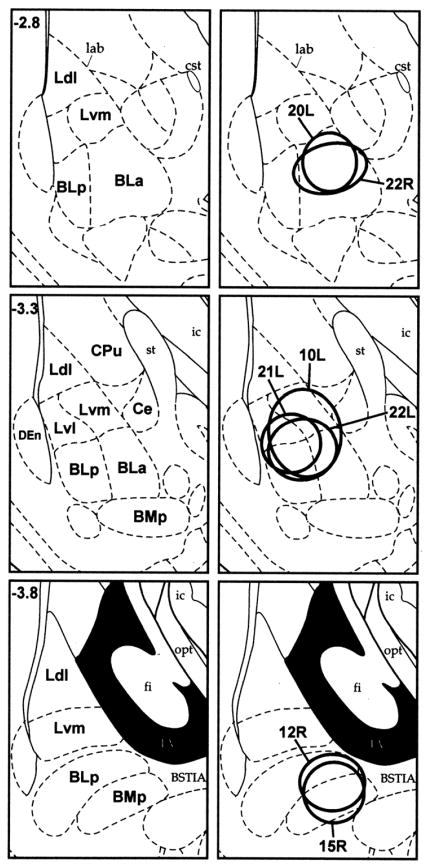
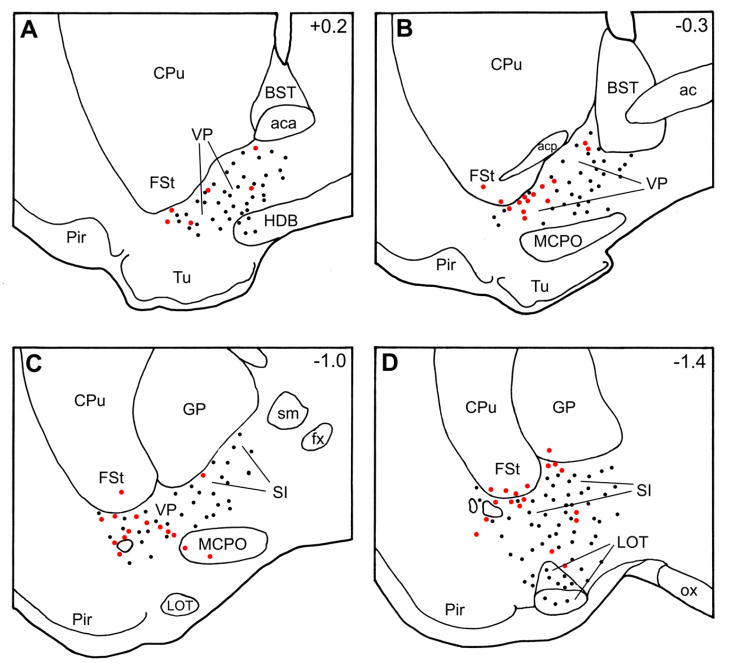
In order to estimate the percentage of FG+ neurons in the BF that were also PV+, cell counts of single-labeled FG+ neurons and double-labeled PV+/FG+ neurons in the substantia innominata/ventral pallidal (SI/VP) region were made in five representative cases: two cases with FG injections at rostral levels of the BLC (cases 20L and 22R), two cases with FG injections at middle levels of the BLC (cases 21L and 22L), and one case with a FG injection at a caudal level of the BLC (case 12R; Fig. 1). Since the majority of double-labeled PV+/FG+ neurons were located between bregma levels −0.3 and −1.8, in each case cell counts were performed in the three sections that were closest to the bregma −1.0 level (i.e., approximately midway between −0.3 and −1.8, at the junction of the SI with the VP; see Fig. 3).
Fig. 1.
Fluorogold injection sites in cases with injections confined to the BLC. Injections into the left or right amygdala (L or R) are indicated after each case number. Injections are plotted at bregma levels −2.8, −3.3 and −3.8 of the atlas by Paxinos and Watson, 1997. Abbreviations: BLa, anterior basolateral nucleus; BLp, posterior basolateral nucleus; BMp, posterior basomedial nucleus; Ce, central nucleus; CPu, caudate-putamen; DEn, dorsal endopiriform nucleus; Ldl, dorsolateral subdivision of the lateral nucleus; Lvl, ventrolateral subdivision of the lateral nucleus; Lvm, ventromedial subdivision of the lateral nucleus.
Fig. 3.
Plots of single-labeled FG+ neurons (black dots) and double-labeled PV+/FG+ neurons (red dots) at four levels of the basal forebrain arranged from rostral (A) to caudal (D) in case 22L. FG+ neurons in surrounding areas, such as the piriform cortex (Pir) and hypothalamus are not plotted on this diagram. Bregma levels are indicated in the upper right corner of each section. Note that single-labeled FG+ neurons (black dots) are plotted from one section whereas double-labeled PV+/FG+ neurons (red dots) are plotted from 3 sections so that the spatial distribution of the latter cells can be better appreciated. Abbreviations: ac, anterior commissure; aca, anterior limb of the anterior commissure; acp, posterior limb of the anterior commissure; BST, bed nucleus of the stria terminalis; CPu, caudate-putamen; fx, fornix; FSt, fundus striati; GP, globus pallidus; HDB, nucleus of the horizontal limb of the diagonal band; LOT, nucleus of the lateral olfactory tract; MCPO, magnocellular preoptic nucleus; ox, optic chiasm; Pir, piriform cortex; SI, substantia innominata; sm, stria medullaris; Tu, olfactory tubercle; VP, ventral pallidum.
GAD/FG experiments
Two rats received bilateral injections of FG into the BLC as described above. After a four day survival, rats were anesthetized and received bilateral injections of colchicine into the lateral ventricles via a microsyringe (50 μg colchicine dissolved in 5 μl distilled water on each side). One day later, rats were perfused, and brains postfixed and sectioned as described above. A one-in-four series of sections through the basal forebrain was collected and incubated in a cocktail of a rabbit polyclonal GAD67 antibody (1:5000; Chemicon International, Temecula, CA) and a guinea pig polyclonal antibody to FG (1:3000; donated by Dr. Lothar Jennes, University of Kentucky) overnight at 4° C. Sections were then processed and examined as described above for the PV/FG experiments, but no cell counts or plots were performed.
RESULTS
PV/FG experiments
Bilateral FG injections into the amygdala in 6 rats produced 7 injection sites that were confined to various portions of the BLC (Figs. 1, 2A). In all cases there was little or no injectate located along the pipette track in the overlying caudate-putamen (Fig. 2A). These seven injections, and others that involved the BLC along with adjacent nuclei, resulted in extensive retrograde labeling in the ventral pallidum and substantia innominata of the basal forebrain. Some unsuccessful injection attempts resulted in no injection site due to presumptive clogging of the pipette. In other cases injections missed the amygdala because the pipette tip went into the lateral ventricle caudal to the central amygdalar nucleus, or through the amygdala to reach the subarachnoid space ventral to the amygdala. Little or no FG retrograde labeling was seen in the basal forebrain (BF) on the side of the failed injection in these cases, indicating that the BF projection to the BLC is mainly ipsilateral (see also Poulin et al., 2006). In one case (15L) the pipette went through the BLC to reach the ventrally-adjacent posterolateral cortical amygdalar nucleus, and the injection site was confined to the latter nucleus; very few retrogradely labeled cells (1–2 per section), and no PV+/FG+ neurons, were seen in the BF in this case. The finding that all of the injections that missed the BLC produced little or no BF retrograde labeling indicates that the large number of BF retrogradely labeled cells seen with BLC injections is not due to injectate spreading up the pipette track to involve the overlying caudate-putamen or cortex, since the pipette tracks in all of the missed injections went through these overlying regions.
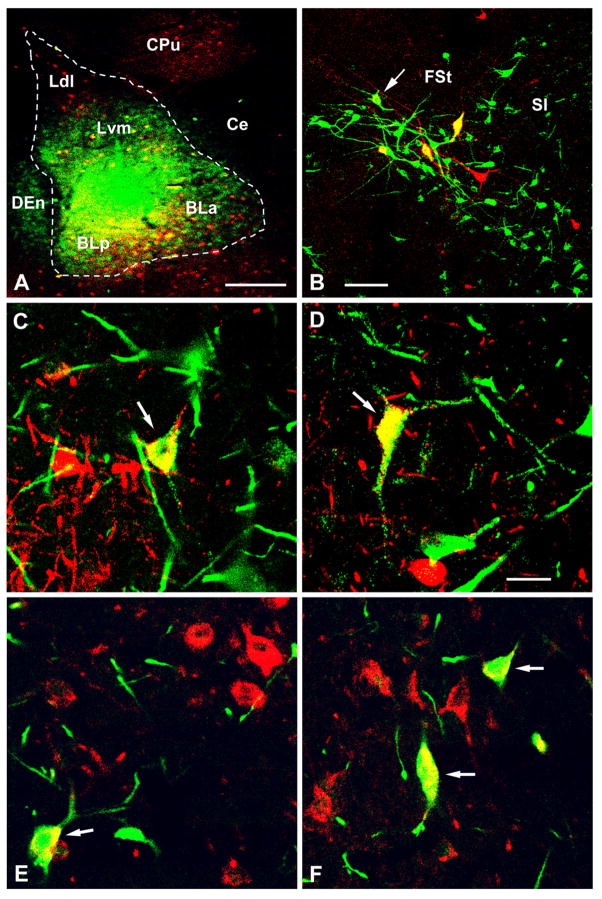
Fig. 2.
A) Injection site in case 22L, located at the bregma −3.3 level (compare with Fig. 1). Section is stained for FG (green) and PV (red). Left is lateral in this and the other plates of this figure. See Figure 1 for abbreviations. B) FG+ neurons (green), PV+ neurons (red), and three double-labeled PV+/FG+ neurons (yellow) at the bregma −1.4 level. In addition, there is another double-labeled FG+ neuron with light PV labeling (arrow). Most of the double-labeled neurons at this level were located along the ventral border of the fundus striati (FSt), lateral to the substantia innominata (SI) (compare with Fig. 3D). C-D) Higher power views of FG+ neurons (green), PV+ neurons (red), and double-labeled PV+/FG+ neurons (yellow, arrows) in the ventral pallidum (C) and substantia innominata (D).). E-F) FG+ neurons (green), GAD+ neurons (red), and double-labeled FG+/GAD+ neurons (yellow, arrows) in the ventral pallidum (E) and substantia innominata (F). Scale bars = 500 μm in A, 100μm in B, and 25 μm in D (C, E, and F are at the same magnification as D).
All injections involving the BLC produced extensive FG retrograde labeling in the BF (Figs. 2B-D, 3), and the pattern of labeling appeared to be fairly similar in all of these cases. Most FG+ neurons in the BF (about 25–40 per section) were located in the substantia innominata (SI) and ventral pallidum (VP) at bregma levels +0.2 to −1.8, consistent with previous studies (Ottersen, 1980; Carlsen et al., 1985). Approximately equal numbers of PV+ neurons in the BF were also observed at these levels. Some of these PV+ neurons were intermingled with the FG+ neurons, but at rostral levels of the ventral pallidum there was a tendency for there to be non-overlapping clusters of FG+ versus PV+ neurons.
Most double-labeled PV+/FG+ neurons (usually 1–5 per section) were observed in the SI and VP (Figs. 2B-D, 3). Many of these PV+/FG+ cells were located fairly close to the ventral aspects of the fundus striati and globus pallidus (Fig. 2B, 3). There was no obvious difference in the morphology of single-labeled FG+ neurons, single-labeled PV+ neurons, and double-labeled FG+PV+ neurons (Figs. 2B-D). Most of these neurons had somata that were about 15–25 μm in diameter, and had 3–4 sparsely-branched dendrites (Figs. 2B-D). Double-labeled PV+/FG+ neurons constituted 4–13% of the total population of FG+ neurons in the SI/VP region (Table 1).
Table 1.
Cell counts of single-labeled FG+ neurons and double-labeled PV+/FG+ neurons in the substantia innominata/ventral pallidal region in five representative cases with injections of FG confined to the BLC. In each case counts were made from the three sections nearest to the bregma −1.0 level (mean ± standard deviation).
| Case | Single-labeled FG+ Neurons (Per Section) | Double-labeled PV+/FG+ Neurons (Per Section) | Percentage of FG+ Neurons that were also PV+(Total from 3 Sections) |
|---|---|---|---|
| 12R | 32.0 ± 1.0 | 1.3 ± 0.6 | 4.0% (4/100) |
| 20L | 30.3 ± 8.7 | 2.3 ± 1.2 | 7.1% (7/98) |
| 21L | 31.3 ± 4.9 | 3.3 ± 1.5 | 9.6% (10/104) |
| 22L | 37.6 ± 6.4 | 4.3 ± 2.1 | 10.3% (13/126) |
| 22R | 37.7 ± 5.0 | 5.7 ± 1.5 | 13.1% (17/130) |
GAD/FG experiments
Bilateral FG injections into the amygdala in 2 rats produced 3 injection sites that were confined to the BLC, and centered in the basolateral nucleus. In all 3 cases there was little or no injectate located along the pipette track in the overlying caudate-putamen. FG retrograde labeling was similar to that described for the PV/FG experiments. GAD staining of basal forebrain neurons varied from very light to moderate, but was significantly less intense than interneuronal staining in the cortex and BLC. GAD+/FG+ double-labeled neurons were observed in the vicinity of single-labeled FG+ neurons in the ventral pallidum (Fig. 2E) and substantia innominata (Fig. 2F).
DISCUSSION
The distribution of retrogradely-labeled cells in the basal forebrain (BF) in the present study, with most neurons located in the substantia innominata (SI) and ventral pallidum (VP), closely matches that seen in previous retrograde tract tracing studies of the amygdala (Ottersen, 1980; Carlsen et al., 1985). Our results are also consistent with previous studies which have shown that this projection mainly terminates in the BLC (Ottersen, 1980). Investigations combining retrograde tract tracing with immunohistochemistry for choline acetyltransferase (ChAT) have demonstrated that 20–25% of BF neurons projecting to the rat basolateral nucleus are non-cholinergic (Carlsen et al., 1985; Zaborzsky et al., 1986). Virtually all PV+ neurons in the SI/VP are GABAergic (and vice versa), and these PV+ GABAergic neurons are not ChAT+ (Gritti et al., 2003). Therefore, the retrogradely-labeled PV+ and GAD+ neurons seen in the present study undoubtedly represent the same non-cholinergic GABAergic cell type, and would appear to constitute about half of the non-cholinergic amygdalopetal BF neurons seen in previous retrograde tract tracing studies of the amygdala (Carlsen et al., 1985; Zaborzsky et al., 1986). The present investigation is the first anatomical study to document the existence of an extrinsic GABAergic innervation of the BLC, and is consistent with the findings of a previous electrophysiological study which reported monosynaptic inhibitory responses in the BLC from stimulation of the SI/VP region, but not from stimulation of the thalamus or cortex (Mello et al., 1992).
Similar to the BLC, both the hippocampus (Köhler et al., 1984) and neocortex (Fisher et al., 1988; Gritti et al., 1997, Henny and Jones, 2008) receive GABAergic BF inputs. Retrograde tract tracing studies suggest that about 30% of BF neurons projecting to the hippocampus and neocortex are GABAergic (Köhler et al., 1984; Gritti et al., 1997). A recent anterograde tract tracing study of the prefrontal cortex demonstrated that 52% of axonal varicosities of BF origin were GABAergic, 19% were cholinergic, and 15% were glutamatergic (Henny and Jones, 2008). The GABAergic component of the BF projection to the BLC observed in the present study (about 10% of amygdalopetal neurons) is smaller that that exhibited by corticopetal BF systems. The finding that 20–25% of amygdalopetal BF neurons are non-cholinergic (Carlsen et al., 1985; Zaborzsky et al., 1986) suggests that about 10–15% of amygdalopetal BF neurons use other transmitters, perhaps including glutamate as in the neocortex (Henny and Jones, 2008). It is also of interest that about one-third of the cholinergic BF neurons projecting to the BLC contain vesicular glutamate transporter 3 (VGLUT3), suggesting that the axons of these neurons may release both acetylcholine and glutamate (Poulin et al., 2006).
In both the neocortex and hippocampus PV+/GABAergic axons of basal forebrain origin are characterized by clustered large varicosities that form multiple “climbing fiber-like” synaptic contacts with the somata and dendrites of GABAergic interneurons (Type 1 axons), whereas the BF non-GABAergic axons (Type 2 axons) have smaller varicosities that mostly avoid cortical interneurons (Freund and Antal, 1988; Freund, 1989; Freund and Meskenaite, 1992). All types of interneurons investigated in the rat and monkey hippocampus receive a robust innervation by Type 1 GABAergic axons from the septum region of the basal forebrain, including interneurons containing parvalbumin, calbindin, calretinin, somatostatin, vasoactive intestinal peptide, neuropeptide Y, or CCK (for a review see Freund and Buzsaki, 1996). In the neocortex there was a differential innervation of particular interneuronal subpopulations by Type 1 BF axons (Freund and Gulyas, 1991; Freund and Meskenaite, 1992). Recent tract tracing studies in our laboratory, with injections of anterograde tracers into the SI/VP portion of the BF, have labeled both Type 1 and Type 2 fibers in the BLC, primarily in the basolateral nucleus (unpublished observations). These preparations were dual-labeled for anterograde tracers and interneuronal markers, and many interneurons, especially PV+ neurons, in the basolateral nucleus received multiple contacts from the clustered large varicosities of Type 1 axons. These findings suggest the possibility that the amygdalopetal PV+/GAD+ neurons in the BF identified in the present study innervate interneurons in the cortex-like basolateral nucleus, analogous to the innervation pattern seen in the cortex proper (see Carlsen and Heimer, 1988, and McDonald, 1992, for reviews of the cortex-like nature of the BLC). The results of the present study, in conjunction with these preliminary observations, are consistent with an electrophysiological study that found evidence for inhibition of presumptive inhibitory neurons in the BLC upon stimulation of the SI/VP region (Mello et al., 1992).
If future studies demonstrate the GABAergic nature of the BF Type 1 axons contacting interneurons in our unpublished studies, this would suggest that the amygdalopetal BF PV+/GAD+ neurons observed in the present investigation are actually involved in disinhibition, rather than inhibition, of pyramidal cells in the BLC, similar to what is seen in the hippocampus (Toth et al., 1997). Electrophysiological studies have shown that long-term potentiation (LTP) in the lateral nucleus of the BLC is tightly controlled by local GABAergic interneurons, and that dopamine facilitates LTP by causing disinhibition of pyramidal cells (Bissiere et al., 2003). Disinhibition generated by the activation of amygdalopetal BF PV+/GAD+ neurons could presumably have the same effect on LTP in the basolateral nucleus, potentially facilitating contextual fear conditioning dependent on hippocampal inputs to this nucleus (Maren and Fanselow, 1995). Interestingly, ultrastructural studies have shown that prefrontal afferents to the SI/VP portion of the basal forebrain form asymmetric (presumably excitatory) synapses with PV+ GABAergic neurons, but not with ChAT+ cholinergic neurons (Záborszky et al., 1997). These findings suggest that the prefrontal cortex might regulate synaptic plasticity involved in emotional learning by activating the amygdalopetal BF PV+/GAD+ neurons identified in the present study.
The results of this investigation, along with previous tract tracing studies, demonstrate that the reciprocal interconnections between the basal forebrain and amygdala are very complex, and involve synaptic interactions of specific neuronal subpopulations. The BLC, which receives sensory inputs from the cerebral cortex and thalamus (McDonald, 1998), has light projections to the SI/VP portions of the BF that, in part, innervate cholinergic neurons via asymmetrical (presumably excitatory) synapses (Jolkkonen et al., 2002; Záborszky et al., 1984). The BLC also has projections to two amygdalar nuclei, the central nucleus and intercalated nuclei, which have strong projections to the BF. The lateral portions of the central nucleus have projections to the substantia innominata that mainly target non-cholinergic neurons, but also synapse with a distinct subpopulation of small cholinergic neurons, primarily via symmetrical (presumably inhibitory) synapses (Jolkkonen et al., 2002; Gastard et al., 2002; Loopuijt and Zahm, 2006). The rostral intercalated nuclei have strong GABAergic projections to the substantia innominata that form symmetrical synapses with non-GABAergic, presumptive cholinergic neurons (Paré and Smith, 1994). In the opposite direction, amygdalopetal projections from the cholinergic BF neurons in the SI/VP region bn mainly target the BLC, where they form synapses with both pyramidal projection neurons and interneurons (Carlsen et al., 1985; Muller et al., 2008). As suggested above, the PV+ GABAergic amygdalopetal BF neurons identified in the present study most likely innervate interneurons in the BLC. The extensively-branched axonal arborizations of BLC interneurons (McDonald, 1982, 1992; Millhouse and deOlmos, 1983; Rainnie et al., 2006) would presumably amplify the effects of the relatively small number of GABAergic BF neurons projecting to the BLC. While it is difficult to predict the emergent properties of these complex BF-amygdalar networks, previous studies of BF-hippocampal networks suggest that they may be critical for the generation of rhythmic oscillatory activity involved in mnemonic function (Freund and Buzsaki, 1996; Toth et al., 1997; Buzsaki, 2002; Borhegyi et al., 2004). Similar oscillations in the BLC play an important role in emotional arousal and emotional memory (Pape et al., 2005; Paré and Collins, 2000; Paré et al., 2002).
Acknowledgments
The authors are grateful to Dr. Kenneth Baimbridge (University of British Columbia, Vancouver, Canada) and Dr. Lothar Jennes (University of Kentucky, Lexington, Kentucky) for their generous donations of the antibodies to PV and FG, respectively. We would also like to thank Dr. Jay Muller (University of South Carolina School of Medicine) for comments on an earlier draft of this manuscript. This work was supported by NIH grant R01NS38998.
Abbreviations
- ac
anterior commissure
- aca
anterior limb of the anterior commissure
- acp
posterior limb of the anterior commissure
- BF
basal forebrain
- BLa
anterior basolateral nucleus
- BLp
posterior basolateral nucleus
- BLC
basolateral nuclear complex of the amygdala
- BMp
posterior basomedial nucleus
- BST
bed nucleus of the stria terminalis
- Ce
central nucleus
- ChAT
choline acetyltransferase
- CPu
caudate-putamen
- DEn
dorsal endopiriform nucleus
- FG
Fluorogold
- FSt
fundus striati
- fx
fornix
- GABA
gamma aminobutyric acid
- GAD
glutamic acid decarboxylase
- GP
globus pallidus
- HDB
nucleus of the horizontal limb of the diagonal band
- Ldl
dorsolateral subdivision of the lateral nucleus
- LOT
nucleus of the lateral olfactory tract
- Lvl
ventrolateral subdivision of the lateral nucleus
- Lvm
ventromedial subdivision of the lateral nucleus
- MCPO
magnocellular preoptic nucleus
- ox
optic chiasm
- PBS
phosphate buffered saline
- Pir
piriform cortex
- PV
parvalbumin
- SI
substantia innominata
- sm
stria medullaris
- Tu
olfactory tubercle
- VP
ventral pallidum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaral DG, Bassett JL. Cholinergic innervation of the monkey amygdala: an immunohistochemical analysis with antisera to choline acetyltransferase. J Comp Neurol. 1989;281:337–361. doi: 10.1002/cne.902810303. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Zigmond RE, Shute CC, Lewis PR. Regional distribution of choline acetyltransferase and acetylcholinesterase within the amygdaloid complex and stria terminalis system. Brain Res. 1977;120:435–444. doi: 10.1016/0006-8993(77)90397-3. [DOI] [PubMed] [Google Scholar]
- Bissière S, Humeau Y, Lüthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci. 2003;6:587–592. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]
- Borhegyi Z, Varga V, Szilágyi N, Fabo D, Freund TF. Phase segregation of medial septal GABAergic neurons during hippocampal theta activity. J Neurosci. 2004;24:8470–8479. doi: 10.1523/JNEUROSCI.1413-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Carlsen J, Záborszky L, Heimer L. Cholinergic projections from the basal forebrain to the basolateral amygdaloid complex: a combined retrograde fluorescent and immunohistochemical study. J Comp Neurol. 1985;234:155–167. doi: 10.1002/cne.902340203. [DOI] [PubMed] [Google Scholar]
- Carlsen J, Heimer L. The basolateral amygdaloid complex as a cortical-like structure. Brain Res. 1988;441:377–380. doi: 10.1016/0006-8993(88)91418-7. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Buchwald NA, Hull CD, Levine MS. GABAergic basal forebrain neurons project to the neocortex: the localization of glutamic acid decarboxylase and choline acetyltransferase in feline corticopetal neurons. J Comp Neurol. 1988;272:489–502. doi: 10.1002/cne.902720404. [DOI] [PubMed] [Google Scholar]
- Freund TF. GABAergic septohippocampal neurons contain parvalbumin. Brain Res. 1989;478:375–381. doi: 10.1016/0006-8993(89)91520-5. [DOI] [PubMed] [Google Scholar]
- Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Freund TF, Gulyás AI. GABAergic interneurons containing calbindin D28K or somatostatin are major targets of GABAergic basal forebrain afferents in the rat neocortex. J Comp Neurol. 1991;314:187–199. doi: 10.1002/cne.903140117. [DOI] [PubMed] [Google Scholar]
- Freund TF, Meskenaite V. Gamma-Aminobutyric acid-containing basal forebrain neurons innervate inhibitory interneurons in the neocortex. Proc Natl Acad Sci U S A. 1992;89:738–742. doi: 10.1073/pnas.89.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gastard M, Jensen SL, Martin JR, 3rd, Williams EA, Zahm DS. The caudal sublenticular region/anterior amygdaloid area is the only part of the rat forebrain and mesopontine tegmentum occupied by magnocellular cholinergic neurons that receives outputs from the central division of extended amygdala. Brain Res. 2002;957:207–222. doi: 10.1016/s0006-8993(02)03513-8. [DOI] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Mancia M, Jones BE. GABAergic and other noncholinergic basal forebrain neurons, together with cholinergic neurons, project to the mesocortex and isocortex in the rat. J Comp Neurol. 1997;383:163–177. [PubMed] [Google Scholar]
- Gritti I, Manns ID, Mainville L, Jones BE. Parvalbumin, calbindin, or calretinin in cortically projecting and GABAergic, cholinergic, or glutamatergic basal forebrain neurons of the rat. J Comp Neurol. 2003;458:11–31. doi: 10.1002/cne.10505. [DOI] [PubMed] [Google Scholar]
- Hellendall RP, Godfrey DA, Ross CD, Armstrong DM, Price JL. The distribution of choline acetyltransferase in the rat amygdaloid complex and adjacent cortical areas, as determined by quantitative micro-assay and immunohistochemistry. J Comp Neurol. 1986;249:486–498. doi: 10.1002/cne.902490405. [DOI] [PubMed] [Google Scholar]
- Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci. 2008;27:654–670. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolkkonen E, Miettinen R, Pikkarainen M, Pitkänen A. Projections from the amygdaloid complex to the magnocellular cholinergic basal forebrain in rat. Neuroscience. 2002;111:133–149. doi: 10.1016/s0306-4522(01)00578-4. [DOI] [PubMed] [Google Scholar]
- Köhler C, Chan-Palay V, Wu JY. Septal neurons containing glutamic acid decarboxylase immunoreactivity project to the hippocampal region in the rat brain. Anat Embryol (Berl) 1984;169:41–44. doi: 10.1007/BF00300585. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Bartus RT, Marciano FF, Gash DM. Telencephalic cholinergic system of the New World monkey (Cebus apella): morphological and cytoarchitectonic assessment and analysis of the projection to the amygdala. J Comp Neurol. 1989;279:528–545. doi: 10.1002/cne.902790403. [DOI] [PubMed] [Google Scholar]
- Le Gal LaSalle G, Paxinos G, Ben-Ari Y. Neurochemical mapping of GABAergic systems in the amygdaloid complex and bed nucleus of the stria terminalis. Brain Res. 1978;155:397–403. doi: 10.1016/0006-8993(78)91037-5. [DOI] [PubMed] [Google Scholar]
- Loopuijt LD, Zahm DS. Synaptologic and fine structural features distinguishing a subset of basal forebrain cholinergic neurons embedded in the dense intrinsic fiber network of the caudal extended amygdala. J Comp Neurol. 2006;498:93–111. doi: 10.1002/cne.21044. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Neurons of the lateral and basolateral amygdaloid nuclei: a Golgi study in the rat. J Comp Neurol. 1982;212:293–312. doi: 10.1002/cne.902120307. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cell types and intrinsic connections of the amygdala. In: Aggleton JP, editor. The amygdala. New York: Wiley-Liss; 1992. pp. 67–96. [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Mello LE, Tan AM, Finch DM. Convergence of projections from the rat hippocampal formation, medial geniculate and basal forebrain onto single amygdaloid neurons: an in vivo extra- and intracellular electrophysiological study. Brain Res. 1992;587:24–40. doi: 10.1016/0006-8993(92)91425-e. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6) Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Millhouse OE, DeOlmos J. Neuronal configurations in lateral and basolateral amygdala. Neuroscience. 1983;10:1269–1300. doi: 10.1016/0306-4522(83)90112-4. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Cholinergic innervation of pyramidal cells and interneurons in the rat basolateral amygdala. Program No 129.4 2008 Neuroscience Meeting Planner; Washington, DC: Society for Neuroscience; 2008. Online. [Google Scholar]
- Nickerson Poulin A, Guerci A, El Mestikawy S, Semba K. Vesicular glutamate transporter 3 immunoreactivity is present in cholinergic basal forebrain neurons projecting to the basolateral amygdala in rat. J Comp Neurol. 2006;498:690–711. doi: 10.1002/cne.21081. [DOI] [PubMed] [Google Scholar]
- Ottersen OP. Afferent connections to the amygdaloid complex of the rat and cat: II. Afferents from the hypothalamus and the basal telencephalon. J Comp Neurol. 1980;194:267–289. doi: 10.1002/cne.901940113. [DOI] [PubMed] [Google Scholar]
- Pape HC, Narayanan RT, Smid J, Stork O, Seidenbecher T. Theta activity in neurons and networks of the amygdala related to long-term fear memory. Hippocampus. 2005;15:874–880. doi: 10.1002/hipo.20120. [DOI] [PubMed] [Google Scholar]
- Paré D, Smith Y. GABAergic projection from the intercalated cell masses of the amygdala to the basal forebrain in cats. J Comp Neurol. 1994;344:33–49. doi: 10.1002/cne.903440104. [DOI] [PubMed] [Google Scholar]
- Paré D, Collins DR. Neuronal correlates of fear in the lateral amygdala: multiple extracellular recordings in conscious cats. J Neurosci. 2000;20:2701–2710. doi: 10.1523/JNEUROSCI.20-07-02701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Collins DR, Pelletier JG. Amygdala oscillations and the consolidation of emotional memories. Trends Cogn Sci. 2002;6:306–314. doi: 10.1016/s1364-6613(02)01924-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Mania I, Mascagni F, McDonald AJ. Physiological and morphological characterization of parvalbumin-containing interneurons of the rat basolateral amygdala. J Comp Neurol. 2006;498:142–161. doi: 10.1002/cne.21049. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. The neglected constituent of the basal forebrain corticopetal projection system: GABAergic projections. Eur J Neurosci. 2002;15:1867–1873. doi: 10.1046/j.1460-9568.2002.02004.x. [DOI] [PubMed] [Google Scholar]
- Semba K. Multiple output pathways of the basal forebrain: organization, chemical heterogeneity, and roles in vigilance. Behav Brain Res. 2000;115:117–141. doi: 10.1016/s0166-4328(00)00254-0. [DOI] [PubMed] [Google Scholar]
- Tóth K, Freund TF, Miles R. Disinhibition of rat hippocampal pyramidal cells by GABAergic afferents from the septum. J Physiol. 1997;500:463–474. doi: 10.1113/jphysiol.1997.sp022033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol. 1991;37:475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]
- Záborszky L, Léránth C, Heimer L. Ultrastructural evidence of amygdalofugal axons terminating on cholinergic cells of the rostral forebrain. Neurosci Lett. 1984;52:219–225. doi: 10.1016/0304-3940(84)90165-4. [DOI] [PubMed] [Google Scholar]
- Záborszky L, Heimer L, Eckenstein F, Leranth C. GABAergic input to cholinergic forebrain neurons: an ultrastructural study using retrograde tracing of HRP and double immunolabeling. J Comp Neurol. 1986;250:282–295. doi: 10.1002/cne.902500303. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Gaykema RP, Swanson DJ, Cullinan WE. Cortical input to the basal forebrain. Neuroscience. 1997;79:1051–1078. doi: 10.1016/s0306-4522(97)00049-3. [DOI] [PubMed] [Google Scholar]