Abstract
Objectives
To evaluate the relationship of hormone (ERα, ERβ, PR) and growth factor receptor (IGF1R, HER2) expression with disease progression in uterine carcinosarcoma.
Study Design
Immunohistochemistry was performed on tissue arrays using standard methodology. Differences between groups were evaluated by the Wilcoxon rank-sum test. Interactions between tumor stage and receptor expression were determined by linear trend analysis.
Results
Compared to normal endometrium, carcinosarcomas exhibited low ERα and PR expression (all P<0.01), but overexpressed ERβ (P=0.02). ERβ expression increased in advanced stage disease (P=0.02). IGF1R expression was lower in carcinosarcoma compared with normal endometrium (P=0.01). HER2 expression was elevated and increased with disease progression (P<0.01).
Conclusions
In uterine carcinosarcoma, ERβ expression is elevated and increases with disease progression, whereas ERα and PR are suppressed. HER2 expression is increased while IGF1R is lower than in normal endometrium. These data support a potential role for ERβ in disease progression via crosstalk with HER2.
Keywords: carcinosarcoma, estrogen receptor, growth factor receptor, progesterone receptor, uterine neoplasm
Introduction
Carcinosarcoma of the uterus is a highly aggressive tumor composed of mixed malignant epithelial and mesenchymal components.1, 2 These tumors are relatively uncommon, accounting for only 4-9% of all uterine cancers, but are associated with disproportionately higher mortality rates compared with other corpus malignancies.3 Compared to endometrioid uterine neoplasms, carcinosarcomas are also more likely to metastasize to the lung and lymph nodes.3
The etiopathogenesis of carcinosarcoma is poorly understood. Two major theories have been proposed regarding the mechanism of tumor formation. The “collision” or “multiclonal” hypothesis posits that the epithelial and mesenchymal components are distinct coexisting populations with different cells of origin. The alternative “monoclonal” hypothesis suggests that carcinosarcoma arises from a single multipotent stem cell that differentiates along epithelial and mesenchymal pathways. Subsequent evaluation of X chromosome inactivation and other genetic analyses support a common clonal origin of both histologic components, at least in the majority of cases. 4-8 On a molecular level, growth factor receptors involved in AKT signaling pathways including human epidermal growth factor receptor 2 (HER2) and epidermal growth factor receptor (EGFR) were found to be aberrantly expressed in carcinosarcomas, and growth factor receptor overexpression was associated with increased AKT activation.9-11
Carcinosarcomas and endometrial carcinomas share similar epidemiological risk factors including advanced age, obesity, and exposure to exogenous estrogen. An increased incidence of carcinosarcoma has been observed among patients exposed to selective estrogen receptor modulators (SERMs). 12-18 These observations support a potential role of estrogen signaling in the development of this tumor.
Estrogens influence the growth, differentiation, and function of reproductive tissues, including the uterus. Ligand binding to the estrogen receptor (ER) leads to a variety of downstream effects, which occur via its transcriptional activity (genomic signaling) as well as via activation of cytosolic signaling pathways (nongenomic signaling). However, many of the activities of estrogen in human tissues, including activity in the absence of ER, remained inexplicable. A second ER known as ERβ was cloned a decade ago. An important aspect of this discovery is that many of the diverse functions of estrogen in normal tissues and tumors can now be divided into those mediated by ERβ, and the original estrogen receptor, now known as ERα. Expression of the two estrogen receptors has been studied in human reproductive tissues, in which cell-specific localization of the ER subtypes has been demonstrated.19 The cell and tissue-specific agonist/antagonist effects of 17β-estradiol (E2) and SERMs are mediated by the ER subtype expression as well as the recruitment of coactivators and corepressors to target promoters. 20-22 In addition, growth factor receptors such as the insulin-like growth factor receptor (IGF1R) and epidermal growth factor receptor (EGFR) family members mediate nongenomic estrogen signaling pathways, as well as resistance to SERMs.23, 24
To date, investigation of the ER signaling pathways involved in carcinosarcoma has been limited. Our group recently reported that ERα positivity was associated with a reduced risk of death in carcinosarcoma patients.25 ERα, but not ERβ or growth factor receptor expression data, was available for analysis in the prior study. We have identified only two reports addressing ER subtype expression in carcinosarcomas. In one study, ER subtype expression was evaluated at the mRNAlevel in seven carcinosarcomas, and altered ERα/ERβ ratios were observed compared to normal endometrium.26 In the second study, two patients who developed carcinosarcoma after prolonged tamoxifen therapy were found to have tumors expressing undetectable protein levels of ERα, but high levels of ERβ.27 Based upon these observations, we hypothesized that ERβ may play a crucial modulation of carcinosarcoma progression via cross talk with the growth factor receptors HER2 and IGF1R. Therefore, the objective of this pilot study was to determine the estrogen receptor subtype expression in uterine carcinosarcoma, and to perform an exploratory analysis of pathologic and clinical correlates of ERα and ERβ expression.
Materials and Methods
Patient selection
This study was approved by the Montefiore Office of Research and Sponsored Programs and Office of Institutional Board Review and was classified as exempt per federal regulations 45 CFR 46.101 (b). Twenty-four patients treated for uterine carcinosarcoma at a single institution between 1995 and 2003 were identified and clinicopathologic data was abstracted from medical records. A de-identified tissue microarray (TMA) was constructed utilizing triplicate to quadruplicate cores of representative areas of tumor from early (FIGO Stage I/II) and advanced (FIGO Stage III/IV) patients, including both primary and where available, metastatic sites. The study pathologist reviewed hematoxylin and eosin stained sections of the tissue cores. Control tissues on the TMA included normal endometrium from patients undergoing hysterectomy for benign indications, normal liver, and normal kidney.
Immunohistochemistry
Tissue sections (4 μm) were deparaffinized, rehydrated, and quenched in hydrogen peroxide. Antigen retrieval was performed in DakoCytomation Target Retrieval Solution, 95 C for 45 minutes. Primary antibodies used were as follows: Estrogen Receptor α, Clone 1D5 (DAKO), Estrogen Receptor β, Clone 14C8 (GeneTex), Progesterone Receptor, Clone PgR 636 (DAKO), IGF-1 Receptor (Cell Signaling), HER2 HercepTest Kit (DAKO). Standard operating procedures were used for ERα, PR, and HER2, using an automated stainer. For ERβ and IGF1R, the optimal blocking and dilutions were determined using prostate and endometrium tissue sections prior to staining of the arrays manually. Secondary antibody and detection was performed using the Envision+ Polymer System (DAKO). For each antibody the interpretation of the immunostaining of both the epithelial and stromal components was performed by the study pathologist (M.L.) who was blinded to the clinical data. For ERα, ERβ, and PR, H-scores [the product of staining intensity (0,1+, 2+, 3+) and the percentage of cells with positive staining (0-100%)] were calculated. IGF1R and HER2 were analyzed as dichotomous variables; IGF1R expression was deemed positive if at least 10% of cells demonstrated membrane staining with intensity of at least 2+28, and HER2 expression was considered positive if specific membrane staining of at least 1+ was present.
Statistical Analysis
The categorical data was summarized by computing frequency distributions for each group and differences in variable distributions were evaluated using the Chi-squared statistic. Standard descriptive statistics (mean, median, standard deviation, range) were used to summarize H-scores and continuous variables. The association of clinicopathologic variables and stage was evaluated using Chi-squared analysis and Fisher’s exact methods as appropriate. For ERα, ERβ, and PR, mean H-scores for early and advanced stage CS were compared using the Wilcoxon rank-sum test. For IGF1R and HER2, linear trend was determined by evaluating the significance of linear association of positive/negative receptor staining across stage.
Results
Clinicopathologic variables
Early stage and advanced stage patients were similar in age, BMI, and racial distribution (Table 1). In patients with advanced stage disease, there was a trend toward increased frequency of serous or clear cell histology of the epithelial component (P=0.09). Deep myometrial invasion and lymphovascular invasion were common in both groups. Among patients with advanced stage diseaes, 46% had adnexal metastases and 56% had nodal metastases.
ER/PR expression in normal endometrium vs. carcinosarcoma
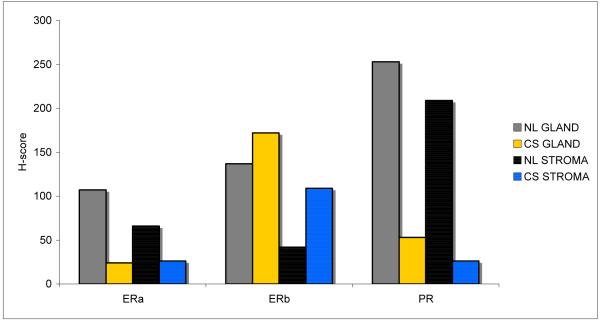
In carcinosarcoma, ERα and PR expression were suppressed in both the epithelial and stromal compartments, with 5- to 10-fold lower mean H-scores compared with normal endometrium (all P<0.01). In contrast, ERβ was highly expressed in the carcinosarcoma specimens. In particular, the stromal compartment of carcinosarcoma demonstrated a significantly higher mean H-score for ERβ compared with normal endometrial stroma (P=0.02). (Fig 1)
Figure 1.
Glandular and stromal hormone receptor expression in normal endometrium and carcinosarcoma. NL=normal; CS=carcinosarcoma; GLAND=glandular/epithelial tissue; STROMA=stromal/mesenchymal tissue.
ER/PR expression in early stage vs. advanced stage carcinosarcoma
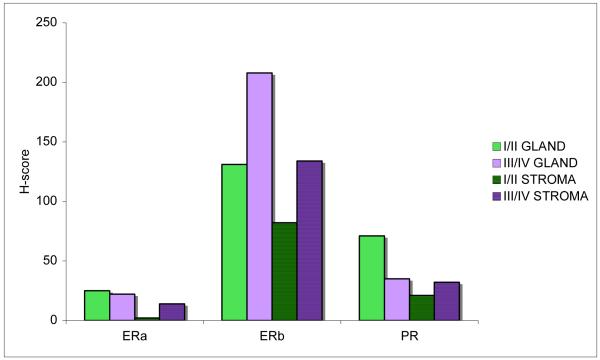
ERα and PR expression remained low in the epithelial and stromal compartments of early (Stage I/II) and advanced (Stage III/IV) disease. Stromal ERα expression was significantly lower in early compared with late stage carcinosarcoma, P=0.048. (Fig 2) In the epithelial compartment of advanced stage carcinosarcoma, there was a suggested trend toward a further suppression of PR expression, compared with early stage disease, P=0.11. When evaluating the PR expression within the advanced stage group, a notable absence of PR expression was observed in the primary tumors of patients with distant metastases, i.e. Stage 4 patients, which represented a significant difference compared with patients without distant metastatic disease, P=0.02. With regard to ERβ, advanced stage carcinosarcoma had higher expression than early stage disease, though this difference was only statistically significant for the glandular component, P=0.02. (Fig 4)
Figure 2.
Comparison of early and advanced stage carcinosarcoma with regard to glandular and stromal hormone receptor expression. I/II=FIGO Stage I or II (early); III/IV=FIGO Stage III or IV (advanced); GLAND=glandular/epithelial tissue; STROMA=stromal/mesenchymal tissue.
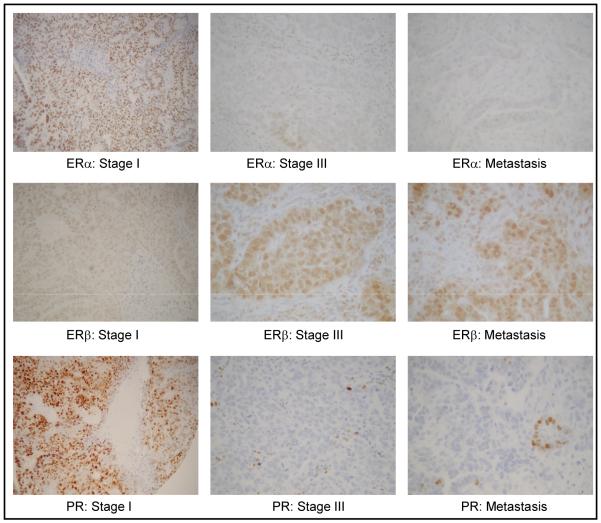
Figure 4.
Representative photographs of immunohistochemical staining for ERα, ERβ, and PR in early stage and advanced stage carcinosarcoma, and metastatic sites.
ER/PR expression in paired primary and metastatic site tumors
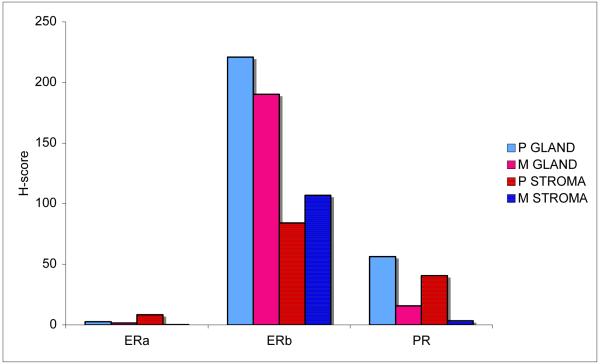
For a subset of Stage III/IV cases, paired primary and metastatic site tumors were included on the tissue array. The immunohistochemical profiles were similar between paired primary and metastatic site tumors.(Fig 3) ERα and PR were further depressed in metastatic lesions, although the small numbers preclude a formal test of statistical significance. In contrast, ERβ expression was maintained at similar, high levels in primary and metastatic sites. (Fig 4)
Figure 3.
ERα, ERβ, and PR glandular and stromal expression in paired primary and metastatic carcinosarcoma. P=primary; M=metastatic; GLAND=glandular/epithelial tissue; STROMA=stromal/mesenchymal tissue.
Growth factor receptor expression
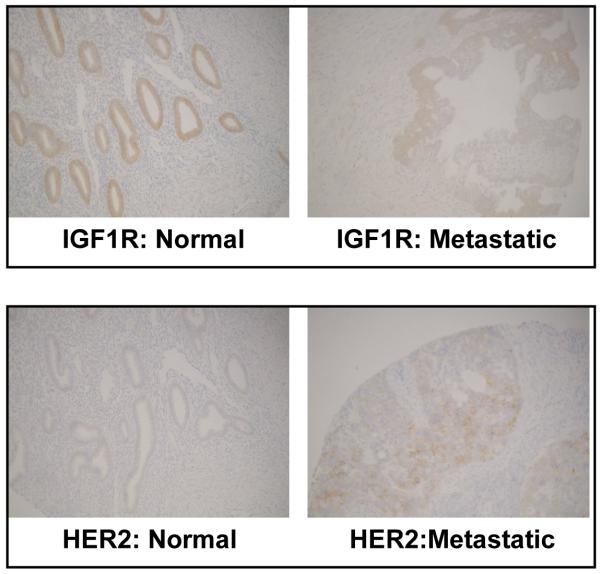
Total IGF1R expression was lower in carcinosarcoma (33% tested positive for IGF1R using standard criteria28) compared with normal endometrium (88% positive; P=0.01). (Fig 5)
Figure 5.
Representative photographs of IGF1R and HER2 immunoreactivity in normal endometrium and metastatic carcinosarcoma.
None of the normal endometrial samples had positive HER2 expression. In contrast, 65% of the tumors expressed HER2 that was localized to the cell membrane (P<0.01). (Fig 5) The percentage of patients with HER2 expression increased with advancing stage of disease, albeit the formal test for linear trend did not attain statistical significance (P=0.16).
Selective estrogen receptor modulator (SERM) exposure
In the study cohort, one patient had prior tamoxifen exposure, and an additional patient had prior raloxifene exposure. Both patients had Stage I disease. For the patient with prior tamoxifen exposure, only partial data was available (due to lack of epithelial component representation in tissue cores from this patient). The staining pattern for the stromal compartment of this tumor was as follows: ERα negative, ERβ positive, PR negative, IGF1R positive, HER2 negative. For the patient with prior raloxifene exposure, the staining pattern for the epithelial/stromal compartments were as follows: ERα negative/negative, ERβ positive/positive, PR positive/positive, IGF1R positive/negative, and HER2 positive/negative.
Comment
To our knowledge, this study is the first to evaluate ER subtype expression in a cohort of patients with carcinosarcoma using tissue array methodology. The results indicate that ERβ is increased in carcinosarcoma stroma compared with normal endometrium stroma, and its expression further increases in advanced disease, while ERα and PR are suppressed. In addition, HER2 expression is elevated, while IGF1R expression is significantly decreased in carcinosarcoma.
In the two patients with a history of SERM exposure, the overall expression pattern of hormone and growth factor receptors was similar to our study population as a whole. Both patients were negative for ERα, but positive for ERβ. These findings are concordant with a prior report of ER subtype expression in two patients who developed carcinosarcoma while taking tamoxifen.27 Combining both series, all four SERM-associated tumors were negative for ERα, but positive for ERβ. Our finding of high ERβ expression in carcinosarcoma and SERM-associated carcinosarcoma is interesting, and contrasts with the role of ERβ as an antiproliferative signaling molecule in many tissue types.29 For example, in prostate tissue, ERβ acts to counteract the mitogenic effect of ERα signaling. Further laboratory investigation is underway to evaluate the function of ERβ in uterine carcinosarcoma, and to assess whether ERβ could be acting via novel distinct mechanisms in this tumor type.
In our study, HER2 membrane expression was noted in 65% of tumors. In addition, the frequency of HER2 expression increased with advancing stage, wherein 82% of Stage III/IV tumors exhibited HER2 positivity. Although we did not specifically evaluate HER2 amplification, the low-moderate staining intensity of 1-2+ that we observed is not typically associated with HER2 amplification. These findings are concordant with a recently published study, which found HER2 amplification by FISH in only one of 30 (3%) CS tumors.30 The HER pathway is an important mediator of non-genomic estrogen signaling and tamoxifen resistance. In laboratory studies, overexpression of HER2 in a breast cancer cell line resulted in increased cross-talk, phosphorylation and activation of the ER and the EGFR/HER2 receptors, the signaling molecules AKT and ERK 1,2 mitogen-activated protein kinase (MAPK), and coactivator AIB1 in response to estrogen or tamoxifen treatment.24, 31
While IGF signaling may play a role in the pathogenesis of endometrial hyperplasia and endometrioid carcinoma, we observed low IGF1R expression in carcinosarcoma. In an immunohistochemical study of endometrioid cancer, IGF1R was overexpressed and demonstrated increased activation during the progression from normal endometrium to hyperplasia and carcinoma.28 However, in a recent prospective study, our group found that circulating free IGF-1 levels were significantly lower in patients who subsequently developed endometrial cancer.32 Consistent with these findings, we found IGF1R expression to be significantly lower in carcinosarcoma specimens compared with normal endometrium. However, it is possible that tumors with low total IGF1R expression may nevertheless exhibit aberrant activation of IGF signaling, if the receptor is highly activated. A future objective is to evaluate the functional activity of IGF receptors in this disease by measuring levels of phosphorylated receptors, ligand expression, and downstream activation of signaling molecules such as AKT and MAP kinases.
In summary, the present study is, to our knowledge, the largest and most comprehensive evaluation of hormonal signaling in uterine carcinosarcoma. The immunohistochemical approach permitted independent evaluation of receptor expression and localization in epithelial and stromal tissues. In addition, utilization of tissue array methodology facilitated evaluation of multiple receptors in a high-throughput manner. However, a limitation of this study is the small number of patients, due to the rarity of this disease, which precluded multivariate and survival analyses. In addition to including cases from collaborating institutions, we intend to evaluate the response to hormone and growth factor stimulation and withdrawal using carcinosarcoma cell line and xenograft models33. These experimental models will more clearly delineate the dependency of carcinosarcoma cells on estrogen and growth factor receptor ligands. We are also evaluating novel drug combinations in preclinical models using cytotoxic chemotherapy in combination with antiestrogens and growth factor receptor inhibitors. These studies should facilitate the development of rational strategies for prevention and treatment of this lethal disease.
Acknowledgements
We thank Dr. Harriet O. Smith for discussion and editorial expertise, Dr. Changcheng Zhu and Dr. Jaya Sunkara for immunohistochemistry advice, Dr. Dwayne Breining and Dr. DingMing Yang for pathology expertise and construction of the tissue microarray, and Dr. Mimi Kim and the Biostatistics Shared Resource of the Albert Einstein Cancer Center for statistical consultation.
Dr. Huang is an NCI-NICHD Scholar of the Reproductive Scientist Development Program supported by NIH grant #5K12HD00849.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Condensation
In uterine carcinosarcomas, expression of ERβ and HER2 are elevated and correlated with disease progression, whereas ERα, PR, and IGF1R are suppressed.
References
- 1.Silverberg SG, Major FJ, Blessing JA, et al. A Gynecologic Oncology Group pathologic study of 203 cases Carcinosarcoma (malignant mixed mesodermal tumor) of the uterus. Int J Gynecol Pathol. 1990;9:1–19. doi: 10.1097/00004347-199001000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Bitterman P, Chun B, Kurman RJ. The significance of epithelial differentiation in mixed mesodermal tumors of the uterus. A clinicopathologic and immunohistochemical study. Am J Surg Pathol. 1990;14:317–28. doi: 10.1097/00000478-199004000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Amant F, Cadron I, Fuso L, et al. Endometrial carcinosarcomas have a different prognosis and pattern of spread compared to high-risk epithelial endometrial cancer. Gynecol Oncol. 2005;98:274–80. doi: 10.1016/j.ygyno.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 4.de Brito PA, Silverberg SG, Orenstein JM. Carcinosarcoma (malignant mixed mullerian (mesodermal) tumor) of the female genital tract: immunohistochemical and ultrastructural analysis of 28 cases. Hum Pathol. 1993;24:132–42. doi: 10.1016/0046-8177(93)90291-n. [DOI] [PubMed] [Google Scholar]
- 5.Gorai I, Yanagibashi T, Taki A, et al. Uterine carcinosarcoma is derived from a single stem cell: an in vitro study. Int J Cancer. 1997;72:821–7. doi: 10.1002/(sici)1097-0215(19970904)72:5<821::aid-ijc19>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Iwasa Y, Haga H, Konishi I, et al. Prognostic factors in uterine carcinosarcoma: a clinicopathologic study of 25 patients. Cancer. 1998;82:512–9. [PubMed] [Google Scholar]
- 7.Watanabe M, Shimizu K, Kato H, et al. Carcinosarcoma of the uterus: immunohistochemical and genetic analysis of clonality of one case. Gynecol Oncol. 2001;82:563–7. doi: 10.1006/gyno.2001.6307. [DOI] [PubMed] [Google Scholar]
- 8.McCluggage WG. Uterine carcinosarcomas (malignant mixed Mullerian tumors) are metaplasticcarcinomas. Int J Gynecol Cancer. 2002;12:687–90. doi: 10.1136/ijgc-00009577-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Amant F, Vloeberghs V, Woestenborghs H, et al. ERBB-2 gene overexpression and amplification in uterine sarcomas. Gynecol Oncol. 2004;95:583–7. doi: 10.1016/j.ygyno.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 10.Livasy CA, Reading FC, Moore DT, Boggess JF, Lininger RA. EGFR expression and HER2/neu overexpression/amplification in endometrial carcinosarcoma. Gynecol Oncol. 2006;100:101–6. doi: 10.1016/j.ygyno.2005.07.124. [DOI] [PubMed] [Google Scholar]
- 11.Rice LW, Stone RL, Xu M, et al. Biologic targets for therapeutic intervention in endometrioid endometrial adenocarcinoma and malignant mixed mullerian tumors. Am J Obstet Gynecol. 2006;194:1119–26. doi: 10.1016/j.ajog.2005.12.020. discussion 1126-8. [DOI] [PubMed] [Google Scholar]
- 12.Hubalek M, Ramoni A, Mueller-Holzner E, Marth C. Malignant mixed mesodermal tumor after tamoxifen therapy for breast cancer. Gynecol Oncol. 2004;95:264–6. doi: 10.1016/j.ygyno.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 13.Deligdisch L, Kalir T, Cohen CJ, de Latour M, Le Bouedec G, Penault-Llorca F. Endometrial histopathology in 700 patients treated with tamoxifen for breast cancer. Gynecol Oncol. 2000;78:181–6. doi: 10.1006/gyno.2000.5859. [DOI] [PubMed] [Google Scholar]
- 14.Evans MJ, Langlois NE, Kitchener HC, Miller ID. Is there an association between long-term tamoxifen treatment and the development of carcinosarcoma (malignant mixed Mullerian tumor) of the uterus? Int J Gynecol Cancer. 1995;5:310–313. doi: 10.1046/j.1525-1438.1995.05040310.x. [DOI] [PubMed] [Google Scholar]
- 15.Cohen I. Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol Oncol. 2004;94:256–66. doi: 10.1016/j.ygyno.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 16.Swerdlow AJ, Jones ME. Tamoxifen treatment for breast cancer and risk of endometrial cancer: a case-control study. J Natl Cancer Inst. 2005;97:375–84. doi: 10.1093/jnci/dji057. [DOI] [PubMed] [Google Scholar]
- 17.McCluggage WG, Abdulkader M, Price JH, et al. Uterine carcinosarcomas in patients receiving tamoxifen. A report of 19 cases. Int J Gynecol Cancer. 2000;10:280–284. doi: 10.1046/j.1525-1438.2000.010004280.x. [DOI] [PubMed] [Google Scholar]
- 18.Goldman NA, de Los Angeles MM, Jones JG, Goldberg GL. Malignant mixed mullerian tumor of the uterus in a patient taking raloxifene. Obstet Gynecol. 2005;105:1278–80. doi: 10.1097/01.AOG.0000160480.55365.a8. [DOI] [PubMed] [Google Scholar]
- 19.Pelletier G, El-Alfy M. Immunocytochemical localization of estrogen receptors alpha and beta in the human reproductive organs. J Clin Endocrinol Metab. 2000;85:4835–40. doi: 10.1210/jcem.85.12.7029. [DOI] [PubMed] [Google Scholar]
- 20.Shang Y. Molecular mechanisms of oestrogen and SERMs in endometrial carcinogenesis. Nat Rev Cancer. 2006;6:360–8. doi: 10.1038/nrc1879. [DOI] [PubMed] [Google Scholar]
- 21.Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295:2465–8. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- 22.Girault I, Lerebours F, Amarir S, et al. Expression analysis of estrogen receptor alpha coregulators in breast carcinoma: evidence that NCOR1 expression is predictive of the response to tamoxifen. Clin Cancer Res. 2003;9:1259–66. [PubMed] [Google Scholar]
- 23.Song RX, Zhang Z, Chen Y, Bao Y, Santen RJ. Estrogen signaling via a linear pathway involving insulin-like growth factor I receptor, matrix metalloproteinases, and epidermal growth factor receptor to activate mitogen-activated protein kinase in MCF-7 breast cancer cells. Endocrinology. 2007;148:4091–101. doi: 10.1210/en.2007-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shou J, Massarweh S, Osborne CK, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–35. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 25.Huang GS, Chiu LG, Gebb JS, et al. Serum CA125 predicts extrauterine disease and survival in uterine carcinosarcoma. Gynecol Oncol. 2007;107:513–7. doi: 10.1016/j.ygyno.2007.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jazaeri AA, Nunes KJ, Dalton MS, Xu M, Shupnik MA, Rice LW. Well-differentiated endometrial adenocarcinomas and poorly differentiated mixed mullerian tumors have altered ER and PR isoform expression. Oncogene. 2001;20:6965–9. doi: 10.1038/sj.onc.1204809. [DOI] [PubMed] [Google Scholar]
- 27.Zafrakas M, Kostopoulou E, Dragoumis K, Mikos T, Papadimas J, Bontis J. Expression of estrogen receptors alpha and beta in two uterine mesenchymal tumors after prolonged tamoxifen therapy. Report of two cases. Eur J Gynaecol Oncol. 2004;25:530–3. [PubMed] [Google Scholar]
- 28.McCampbell AS, Broaddus RR, Loose DS, Davies PJ. Overexpression of the insulin-like growth factor I receptor and activation of the AKT pathway in hyperplastic endometrium. Clin Cancer Res. 2006;12:6373–8. doi: 10.1158/1078-0432.CCR-06-0912. [DOI] [PubMed] [Google Scholar]
- 29.Koehler KF, Helguero LA, Haldosen LA, Warner M, Gustafsson JA. Reflections on the discovery and significance of estrogen receptor beta. Endocr Rev. 2005;26:465–78. doi: 10.1210/er.2004-0027. [DOI] [PubMed] [Google Scholar]
- 30.Cimbaluk D, Rotmensch J, Scudiere J, Gown A, Bitterman P. Uterine carcinosarcoma: immunohistochemical studies on tissue microarrays with focus on potential therapeutic targets. Gynecol Oncol. 2007;105:138–44. doi: 10.1016/j.ygyno.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Massarweh S, Osborne CK, Creighton CJ, et al. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008;68:826–33. doi: 10.1158/0008-5472.CAN-07-2707. [DOI] [PubMed] [Google Scholar]
- 32.Gunter MJ, Hoover DR, Yu H, et al. A Prospective Evaluation of Insulin and Insulin-like Growth Factor-I as Risk Factors for Endometrial Cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:921–9. doi: 10.1158/1055-9965.EPI-07-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulten HJ, Wolf-Salgo J, Grundker C, Gunawan B, Fuzesi L. Characterization of a newly established uterine carcinosarcoma cell line featuring the sarcomatous phenotype of the tumor in vitro. Int J Gynecol Cancer. 2008;18:339–44. doi: 10.1111/j.1525-1438.2007.01004.x. [DOI] [PubMed] [Google Scholar]