Abstract
Though emotion conveys memory benefits, it does not enhance memory equally for all aspects of an experience nor for all types of emotional events. In this review, I outline the behavioral evidence for arousal’s focal enhancements of memory and describe the neural processes that may support those focal enhancements. I also present behavioral evidence to suggest that these focal enhancements occur more often for negative experiences than for positive ones. This effect of valence appears to arise because of valence-dependent effects on the neural processes recruited during episodic encoding and retrieval, with negative affect associated with increased engagement of sensory processes and positive affect leading to enhanced recruitment of conceptual processes.
Introduction
“There seems something more speakingly incomprehensible in the powers, the failures, the inequalities of memory, than in any other of our intelligences.”
As captured in this quotation from Jane Austen’s Mansfield Park, memory is both resolute and fragile. We are left with durable and lasting traces of many events and yet we can forget other events just moments after their occurrence. Even when we retain memories of past events, they never are exact reproductions of those initial experiences. We remember some pieces of an event but forget others, and the event details we recall often are shaped by our current mindset and molded by thoughts and experiences that have occurred between the original event and the moment of remembering.
Though we are not always aware of our memories’ errors, most of us would not be surprised to learn that memory is not perfect. Many marital squabbles arise due to inconsistencies in how a past event is remembered, and nearly everyone has, at one time or another, struggled to remember when they were last in a particular location or why the person across the room looks familiar. However, many of us nevertheless share the intuition that there are some moments in our lives that have been indelibly preserved: perhaps a wedding day, or the day a baby was brought home from the hospital. William James wrote that “some events are so emotional as to leave a scar upon the cerebral tissues” (James, 1890/1998), capturing this intuition that although memory is not always perfect, sometimes a memory can accurately preserve a moment in time.
This belief in the durability of emotional memories – a term that is often used as short-hand to denote memories for events that elicited an emotional response at the time of their occurrence – is closely related to the concept of a “flashbulb memory,” a phrase coined by Brown and Kulik (1977). These authors argued that when a highly surprising event occurs, a special memory mechanism takes over, causing the moment to be recorded with picture-perfect accuracy. When they asked people, fourteen years after the assassination of J.F.K., to report details such as where they were when they learned of the assassination, how they learned the news, what they were doing at the time, and how the news impacted them, nearly everyone recalled these details confidently. Though these memory reports could not be retrospectively checked for accuracy, people’s beliefs that the information was retained vividly and accurately gave rise to the proposal that emotional memories may differ from nonemotional ones in terms of the details retained. Many studies have replicated Brown and Kulik’s (1977) original finding. People vividly recall natural disasters (Bahrick, Parker, Fivush, & Levett, 1998) or injuries that they experienced (Peterson & Bell, 1996; Peterson & Whalen, 2001), and even years later, people can remember the context in which they learned about assassinations (Christianson, 1989; Colgrove, 1889; Winograd & Killinger, 1983), terrorist attacks (Budson et al., 2004; Budson et al., 2007; Paradis et al., 2004; Pezdek, 2003; Smith et al., 2003; Wolters & Goudsmit, 2005), space shuttle explosions (Bohannon, 1988; Kensinger, Krendl, & Corkin, 2006; Neisser & Harsch, 1992), or the start of a war (Bohn & Berntsen, 2007; Tekcan, 2001).
Despite their subjective vividness, however, even emotional memories are subject to distortion. Compelling evidence for inaccuracies within emotional memories has come from studies that measure the consistency with which people report details such as where they were, or what they were doing, when they learned that an event occurred. If these details were retained accurately, then people should report exactly the same details at each retelling. In reality, however, people’s accounts of these details change over time: Someone initially may state that he learned of the Challenger explosion from a friend but six months later may note that he learned of the explosion from a television broadcast (e.g., Neisser & Harsch, 1992). Often individuals retain high confidence in the accuracy of the reported details despite recounting different details each time. In fact, there can be little correlation between people’s confidence in their memories and the consistency with which they remember event details (Neisser & Harsch, 1992; Schmidt, 2004; Schmolck et al., 2000; Talarico & Rubin, 2003). Recent evidence has suggested that people can be biased to endorse negative items as “old,” falsely claiming that they’ve studied negative items that in reality are novel (Dougal & Rotello, 2007). In fact, some studies have suggested that memories for emotional experiences may seem subjectively vivid yet hold little accurate detail (Sharot et al., 2004; Talarico & Rubin, 2003). These data have led to the suggestion that the hallmark of an emotional memory may be the subjective vividness with which it is remembered rather than the accuracy with which the event is retained (e.g., Dougal & Rotello, 2007; Sharot et al., 2004; Talarico & Rubin, 2003).
In the context of this renewed discussion regarding the effects of emotion on memory accuracy, this review will emphasize the importance of considering both the type of detail and the quality of the affective experience when attempting to understand how emotion influences memory. Like others (e.g., Buchanan & Adolphs, 2002; Reisberg & Heuer, 2004; Mather, 2007), I will argue that emotion enhances memory for some, but not all, details of an experience. I will present neuroimaging evidence to suggest that at least some of these focal effects of emotion on memory for detail arise from the way in which affective-attentional processes are engaged during the encoding of arousing experiences. In particular, I will present evidence to suggest that engagement of emotion processing regions (including the amygdala and orbitofrontal cortex) during memory encoding increases the likelihood that emotional events are remembered but does not enhance memory for all details of those emotional experiences. I also will argue that although positive arousing and negative arousing experiences are more likely to be remembered than neutral ones, when it comes to remembering the details of those emotional experiences, valence is a critical factor. Negative affect, in particular, is more likely to lead to focal memory enhancements, whereas positive affect often conveys little benefit to memory accuracy. These differential effects of valence on memory seem critically tied to the types of processes that are recruited during the initial encoding of an emotional experience and that are re-engaged during the event’s retrieval. At the end of the review, I will return to the issue of the imperfect mapping between a person’s beliefs about the validity of their memories and the accuracy for those memories, suggesting some future avenues for research that may help to elucidate the basis for this intriguing disconnect.
Emotional Arousal Leads to Focal Memory Enhancements
It has long been known that experiences that elicit arousal are more likely to be remembered than experiences that do not evoke an emotional response. This emotional memory enhancement has been demonstrated across a range of paradigms and using a variety of stimuli (e.g., Bradley et al., 1992; Cahill & McGaugh, 1995; Kensinger et al., 2002). These enhancements are particularly pronounced for events that elicit arousal (e.g., Anderson et al., 2006 Buchanan et al., 2004; Kensinger & Corkin, 2003; Talmi & Moscovitch, 2004), and it is believed that the release of stress hormones may play an important role in modulating these mnemonic influences. In particular, it has been proposed that arousal-mediated enhancement of memory may occur when there is both an arousal-related enhancement in noradrenergic activation, leading to interactions between the basolateral nucleus of the amygdala and other regions important for sensory and mnemonic processing, and also the release of glucocorticoids (reviewed by McGaugh, 2004; Wolf, 2008). Though it might have been assumed that such effects would be too sluggish to modulate memory on a trial-by-trial basis, evidence is accumulating to suggest that arousal-mediated enhancement is likely to occur even when there is a relatively rapid fluctuation between emotional and neutral stimuli. For example, even when emotional and neutral stimuli are intermixed on a study list and are presented for a relatively short duration (e.g., a few seconds), arousal-related responses, such as galvanic skin conductance, are strong predictors of later memory (e.g., Anderson et al., 2006), and noradrenergic blockade can remove the effects of emotion on memory (e.g., Strange & Dolan, 2007).
In order for a previous event to be remembered, at least three memory phases must occur successfully. First, the event must be recorded by sensory registers and encoded. Second, the event must be consolidated into a stable and lasting representation. Third, the event must be retrieved. There is evidence to indicate that when an experience elicits an arousal response, there are emotion-specific processes that are engaged at each of these stages, enhancing the likelihood that information is encoded, consolidated, and retrieved. In brief, information eliciting arousal is more likely to be detected and attended (reviewed by Dolan & Vuilleumier, 2003; Kensinger, 2004; MacLeod & Matthews, 2004), increasing the likelihood that the information is encoded. Arousing information also appears to be consolidated into memory more effectively than nonarousing information, as evidenced by the fact that the mnemonic benefit for arousing information (as compared to nonemotional information) tends to increase with longer retention delays. In other words, while nonarousing information is readily forgotten, once encoded, arousing information seems more likely to be established into a durable memory (LaBar & Phelps, 1998). Once stored, arousing information also may be more likely to be retrieved, though there is less conclusive evidence regarding how emotion influences retrieval processes (see review by Buchanan, 2007). Thus, when information is arousing, it is not remembered simply because of the engagement of the same sorts of processes that would enhance memory for more mundane experiences (e.g., enhanced semantic or autobiographical elaboration, additional rehearsal), but rather because of the engagement of processes not typically recruited unless an experience evokes an emotional reaction.
At a neural systems level, the memory enhancement seems to occur because, once active, regions within the affect processing system (e.g., the amygdala and the orbitofrontal cortex) modulate the processing of regions that facilitate the encoding of sensory detail (e.g., regions of the fusiform gyrus) and the consolidation of memory (e.g., the hippocampal formation; see Figure 1). There is extensive evidence that such modulation occurs in animals (reviewed by McGaugh, 2004), and there is increasing support for a modulatory influence in humans as well. For example, neuroimaging studies have revealed that during the processing of emotional information, there are correlations between the strength of activity in the amygdala and in the hippocampus (e.g., Kensinger & Corkin, 2004), and the strength of these correlations can correspond with the magnitude of the mnemonic boost for emotional information (e.g., Richardson et al., 2004). There also are often correlations between the amount of activity in the amygdala and the fusiform gyrus (a region important for higher-level visual processing; Iidaka et al., 2001; Vuilleumier et al., 2004), and these interactions boost the likelihood that visual details are encoded into memory (e.g., Kensinger, Garoff-Eaton, & Schacter, 2007; Talmi et al., 2008). Neuroimaging studies, many investigating the retrieval of emotional autobiographical memories, have suggested that the amygdala may modulate retrieval processes as well (reviewed by Buchanan, 2007), perhaps facilitating the mnemonic search process (Daselaar et al., 2007). For example, during retrieval, there appears to be synchrony between the activity in the amygdala, the hippocampus, and the fusiform gyrus (e.g., Kensinger & Schacter, 2007; Smith et al., 2006). There also is increased strength of connectivity between the amygdala and the hippocampus during the retrieval of emotional information, modulated by activity within the orbitofrontal cortex (Smith et al., 2006). These modulations may lead to an enhanced ability to retrieve the details associated with an episode.
Figure 1. Anatomy of an emotional memory.
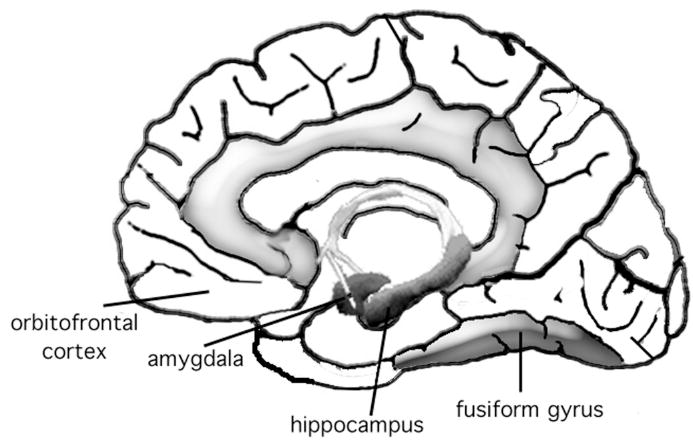
The memory boost for emotional information seems to occur because affective processing regions (e.g., the amygdala and the orbitofrontal cortex) modulate the processing of regions that facilitate encoding of sensory detail (fusiform gyrus) and memory consolidation (hippocampal formation).
These behavioral and neuroimaging results converge on the conclusion that memory for emotional events can benefit from the engagement of emotion-specific processes. However, the neuroimaging data emphasize that the emotion-specific processes do not replace the standard memory network. Rather, activity within emotion processing regions seems to functionally modulate the memory network that supports learning and retrieval of all experiences (even those void of emotion) and the visual processing regions that support the encoding of any event’s sensory details (see reviews by Phelps, 2004; LaBar & Cabeza, 2006).
These studies have provided evidence for memory-enhancing properties of emotional arousal; when an event is emotionally arousing, it is more likely to be remembered. However, if we examine not just whether an event is remembered, but also what types of details people remember about that event, then the literature suggests that emotional arousal does not enhance memory across-the-board and for all types of event details. Rather, emotional arousal appears to be associated with memory-narrowing effects. There are a number of related theories suggesting that the effects of emotional arousal on memory may be best characterized by focal enhancements (e.g., Buchanan & Adolphs, 2003; Reisberg & Heuer, 2004; Mather, 2007). Though the theories differ from one another in important ways, they all share the central tenet that some aspects of an emotional experience are remembered well because of their arousing nature, while other elements may receive no mnemonic benefit and in fact may be more likely to be forgotten.
The most widely discussed theory of arousal’s narrowing effects on memory arose from Easterbrook’s (1959) proposal that arousal restricts the focus of attention, causing a person to notice information that elicits arousal but to fail to process other information. Though Easterbrook’s proposal was about attention focusing, proof of the concept has been derived from studies in which memory for “central” event aspects, directly tied to the emotion elicitor, is compared to memory for “peripheral” aspects, removed from the source of the emotion. Across a range of studies, researchers have demonstrated an emotion-induced memory trade-off, whereby individuals remember the central emotional content of a stimulus but often forget the other details (see Buchanan & Adolphs, 2002; Reisberg & Heuer, 2004 for reviews). For example, after studying an image of a car accident on a street, participants tend to have good memory for the car accident but poor memory for the street. In fact, their memory for the street can be worse if they saw a car accident on the street than if they saw a nonemotional version of the scene, such as a taxi driving down the street. These sorts of trade-offs can occur not only for information presented in close spatial proximity to an emotional item but also for information presented in temporal proximity: For instance, after seeing an arousing word, participants are less likely to remember the word that immediately follows it (Hurlemann et al., 2005).
Focal enhancements for emotional information occur not only when multiple items are shown in close temporal or spatial proximity, but also when memory is queried for multiple episodic details associated with a single arousing item’s presentation. For example, when a person is presented with an image of a snake and is asked to decide whether it depicts a living thing or whether it would fit in a shoebox, participants are quite good at remembering what the snake looked like but they do not remember which decision they were asked to make about the snake (Kensinger et al., 2007). More generally, there seem to be some types of details that are reliably enhanced by emotion, including the perceptual details of a word (such as its font) or object (such as its shape, color, or orientation; Doerksen & Shimamura, 2001; Kensinger & Corkin, 2003; Kensinger, Garoff-Eaton, & Schacter, 2006; D’Argembeau & Van der Linden, 2004; MacKay et al., 2004), well as the item’s spatial location (D’Argembeau & Van der Linden, 2004; MacKay & Ahmetzanov, 2005; Mather & Nesmith, in press). By contrast, other details such as the temporal order in which an emotional item was presented, or the decision made about an item (Cook, Hicks, & Marsh, 2007; Kensinger & Schacter, 2006a; Kensinger, Garoff-Eaton, & Schacter, 2007), are not remembered more reliably for emotional items than for nonemotional ones.
Based on the available evidence, my working hypothesis has been that arousal (and, as I will discuss later, arousal accompanying a negative emotion in particular) enhances memory for “intrinsic” item features details but not “extrinsic” contextual details (see Kensinger & Schacter, 2006; Kensinger, 2007). This hypothesis can accommodate the central/peripheral trade-offs (where what is “intrinsic” is the emotional item whereas what is “extrinsic” is the information spatially, temporally, or conceptually removed from that item), and it also is consistent with the literature examining the effect of arousal on “source memory,” or the ability to remember the context in which a piece of information was learned (Johnson, Hashtroudi, & Lindsay, 1993). The types of source details that have been reliably enhanced by emotion tend to be those that are integral to our ability to process the information, such as the sensory features associated with the information’s presentation.
This “intrinsic” vs. “extrinsic” dissociation is conceptually related to Mather’s (2007) proposed distinction between memory binding for within-item features, which she purports is enhanced by arousal, and memory binding for between-item features, which she proposes receives no benefit from arousal. In most instances, what Mather would label a “within-object” feature I would label “intrinsic,” and what she would consider “between-object” I would consider to be “extrinsic.” However, I do not think there is a perfect mapping between our terminologies. For example, I would include as “extrinsic” characteristics even those event qualities that are not items per se (e.g., temporal order, the decision made about an item) and more generally, I do not consider the “intrinsic” vs. “extrinsic” distinction to be tied to object processing or even to the visual domain. I also conceive of “intrinsic” and “extrinsic” as dimensions that relate to how event features are processed in relation to the emotional aspect of an event rather than to fixed properties of the stimuli. For instance, if emotional and neutral words were presented sequentially and one at a time, but together formed a sentence (e.g., the - man - abused - children), I would expect memory to be enhanced for all of the words when compared to a word sequence that conveyed no emotional meaning. By contrast, if emotional and neutral words were presented in sequence but did not form a coherent statement, I would expect memory to be enhanced only for the emotional words within the stream (and see Kensinger et al., 2002 for some evidence to support this hypothesis). Thus, what is “intrinsic” vs. “extrinsic” need not be a fixed stimulus property but rather may be manipulated based on how the information is interpreted and processed.
Though there are conceptual differences between the central/peripheral, intrinsic/extrinsic, and within- vs. between-item binding theories, at the present time, I do not think there are sufficient data to adjudicate between these alternate theories (and they may not be mutually exclusive) or to elucidate the boundary conditions in which each may operate. Therefore, I want to focus on the conviction shared by each of these theories: that emotion leads to focal enhancements in memory and that these focal effects arise because of the way in which arousing information is attended and bound during encoding and consolidation. In the section below, I will review some of the behavioral and neuroimaging studies that have begun to shed light on the processes leading to these focal effects.
The Processes Leading to Arousal’s Focal Memory Enhancements
It is well known that arousing items can capture and sustain attention (reviewed by Dolan & Vuilleumier, 2003; MacLeod & Matthews, 2004), and it makes sense that if attention is devoted toward the processing of details that are intrinsic to an arousing item, this could leave fewer cognitive resources for the processing of other event details. Although changes in attention allocation have long been theorized to explain the focal effects of emotion on memory for detail (e.g., Easterbrook, 1959), only recently have studies begun to address this hypothesis empirically.
Behavioral evidence for a role of attentional factors during encoding has come from studies indicating that the trade-offs in memory are far more likely to occur when there is an object that “grabs” attention than when there is only thematically-induced emotion, not tied to any particular aspect of a scene or story. For example, Laney et al (2004) found that when participants listened to a story about either date rape or a successful first date, participants who heard about the date rape showed better memory for all aspects of the story than individuals who heard about the first date, with no tradeoff elicited. This finding suggests that trade-offs may occur only for the subset of emotional experiences in which there is an “attention magnet” (a term used by Laney et al., 2004). Without such a magnet, the enhancing effects of emotion may be more widespread (see Reisberg & Heuer, 2004 for more discussion).
Additional behavioral evidence that the trade-off may be tied to the strength of the emotional “attention magnet” comes from studies that have manipulated participants’ encoding tasks, altering how their attention is directed toward the scenes. The logic behind these studies is that if the focal effects arise due to attention focusing during encoding, then it should be possible to alter the types of details that are remembered well by changing the way in which participants are asked to process the information. Indeed, my colleagues and I found that when we gave young adults intentional encoding instructions, informing them that they should remember all aspects of the scenes because their memory would be tested later, their ability to remember background details (e.g., the street) became as good as their ability to remember the negative details (e.g., the accident; Kensinger, Piguet, Krendl, & Corkin, 2005). A similar dissipation of emotion-induced trade-off effects can occur if participants are asked to focus upon the visual details of the scene, describing the scene so that an artist could reproduce it acurately. With this type of focused encoding task, young adults became just as good at remembering the background details of a negative scene than of a neutral scene (Kensinger, Garoff-Eaton, & Schacter, 2007).
These behavioral data suggest that the way in which the information is attended to and encoded has important consequences for what details later are remembered. In order to more directly examine what encoding processes may lead to these focal effects, my colleagues and I asked participants to undergo a functional magnetic resonance imaging (fMRI) scan. While in the fMRI scanner, we asked participants to view positive, negative, and neutral items. Participants made one of two decisions about each item. For some items, they decided whether the it was animate, and for other items they determined whether it was something commonly encountered. Later, outside of the scanner, participants saw some images that were exactly the same as the studied image, other images that shared the same theme as the studied image but differed in visual details (e.g., a different image of a snake) and other items that were unrelated to any studied image. Participants had to indicate whether each item was the “same” as the studied item, was “similar” but not identical to the studied item, or was “new” (Kensinger & Schacter, 2007). By looking at how well participants could distinguish “same” from “similar” exemplars, we could examine how emotional valence influenced participants’ ability to remember the precise visual details of an object (Kensinger, Garoff-Eaton, & Schacter, 2007). Using a subsequent-memory paradigm (reviewed by Paller & Wagner, 2002; see Figure 2), the neural activity during the encoding of each item was sorted based upon whether that item was later remembered with visual specificity, remembered without visual specificity, or forgotten. Neural activity during encoding also was sorted based on later memory for the decision made about the item (as a function of whether the decision was remembered correctly or incorrectly).
Figure 2. The subsequent-memory paradigm.
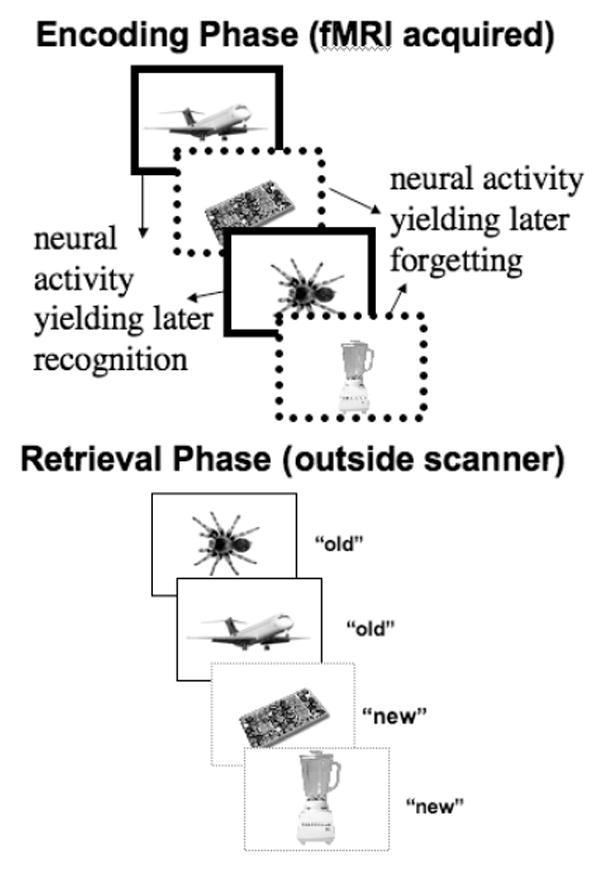
The neural activity during encoding is sorted based upon whether the items are later recognized (correctly called “old”) or later forgotten (incorrectly called “new”). Regions whose activity is enhanced during the encoding of items later recognized (solid lines) compared to later forgotten (dotted lines) are those regions implicated in the successful encoding of information.
These neuroimaging analyses revealed that amygdala activity showed no correspondence with memory for the decision made about the item. Rather, amygdala activity was equally high during the encoding of all negative items that people later remembered studying, regardless of whether they were correct or incorrect about what decision they had made about the item. By contrast, amygdala activity showed a strong correspondence to memory for visual detail, as did activity within the fusiform gyrus. Activity in each of these regions was highest during the encoding of negative items that were remembered with accurate visual detail (i.e., same items accurately given a “same” response) and was less during the encoding of negative items that were remembered without visual detail (i.e., same items inaccurately given a “similar” response). There was a strong correlation between the level of activity in the right amygdala and in the right fusiform gyrus during the encoding of negative objects that would later be remembered with specific visual detail (i.e., later given a “same” response), suggesting that interactions between these regions are important for modulating the effect of negative emotion on the visual specificity of object memory (Figure 3). These results highlight the fact that the relation between amygdala activity during encoding and memory for event details may depend on the particular type of detail that is assessed. Emotion does not enhance memory for all aspects of an encoding episode, and amygdala engagement at encoding does not ensure that all details will be accurately remembered. Rather, amygdala engagement during encoding can lead some aspects of an experience to be remembered well but can result in other aspects of an experience being forgotten.
Figure 3. Sensory-mnemonic correlations.
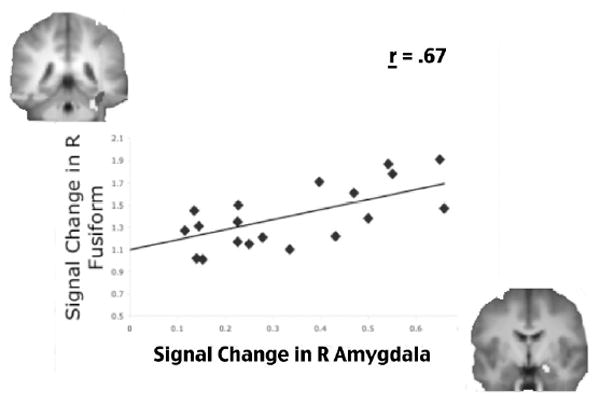
During the encoding of negative items that would later be remembered with specific visual detail, participants showed a robust correlation between the amount of activity in the right amygdala (x-axis) and the amount of activity in the right fusiform gyrus (y-axis). Each diamond represents one participant. Data from those published in Kensinger, Garoff-Eaton, & Schacter, 2007.
Beyond the amygdala, there was a broader affective-attentional network whose activity increased the likelihood of remembering a negative item’s visual details but decreased the likelihood of remembering the task performed with the item. These regions included the orbitofrontal cortex, ventral striatum, and anterior cingulate gyrus, regions that have been implicated in the prioritized processing of emotional stimuli (Vuilleumier et al., 2001) and in the motivational processing of affective stimuli (e.g., Robbins & Everitt, 1996; Schultz, 2000). The revelation of this network fits well with the hypothesis that at least some of the focal effects of emotion on memory arise from the prioritized processing and attentional focusing on arousing items during encoding. It makes sense that if participants are focused on the intrinsic attributes of an item (e.g., its visual features) they may fail to encode other details associated with the item’s presentation (e.g., the decision made about the item). In other words, activity within this affective attentional network may serve to focus and guide encoding processes, assuring that intrinsic details of negative items are encoded, but this focusing may come at the cost of encoding more extrinsic elements. Thus, these neuroimaging data suggest that it may be the same neural processes that lead to emotional enhancements that also lead to poorer memory for other types of details. The more affectively focused a person is during encoding, the more likely they are to remember some, but not all, aspects of an emotional experience.
The conclusion that heightened affective focus leads to memory trade-offs fits well with the behavioral data described earlier. Asking participants to describe the visual details of a scene rather than to determine whether they want to approach the scene, in essence manipulates how affectively focused participants are during the encoding episode. When they were affectively focused – deciding whether to approach a potentially threatening scene – they showed a robust memory trade-off, whereas when they were guided to be non-affectively focused – describing the scene for an artist - the magnitude of the trade-off was reduced (Kensinger, Garoff-Eaton, & Schacter, 2007).
Memory Accuracy Depends on the Valence of the Emotion Elicited by the Event
Though the theories described thus far have focused primarily on the arousal level of the stimuli, increasing evidence indicates that even when arousal is controlled, the valence of emotion elicited by an event (whether it is positive or negative) can influence the detail with which that event is remembered. In the section below, I will review evidence to suggest that focal enhancements may occur more readily for negative information, while positive information often is not remembered with more detail than neutral information. It is important to keep in mind that both negative and positive events are more likely to be remembered than nonemotional ones; what I am suggesting may vary with valence is not the ability to remember that an event happened (e.g., that you attended a funeral or a wedding) but rather the ability to remember the particular details of the event (e.g., what the church looked like, where you sat during the service). In addition to presenting evidence to suggest that these types of episodic details may be remembered more readily for negative items, I also will describe recent neuroimaging data to suggest that these effects of valence on memory accuracy may be critically tied to the way in which negative and positive information is processed during encoding.
Negative Events are Remembered with More Accurate Detail than Positive Events
The research comparing memory for negative and positive events has begun to reveal a fundamental influence of valence on memory accuracy. Negative information often is remembered with a greater sense of vividness than positive information. People often claim that they “remember” the details of negative events, whereas they are more likely to only “know” that a positive event occurred, without remembering the details (e.g., Bless & Schwarz, 1999; Dewhurst & Parry, 2000; Ochsner, 2000). Negative items also are more likely to be remembered with visual detail than are positive items. For example, people are more accurate in deciding which snake or grenade they saw than which gown or cake they saw (Kensinger, Garoff-Eaton, & Schacter, 2007). People also are quite good at knowing whether they saw or only imagined negative items, whereas they are more likely to confuse imagined items for perceived ones if those items are positive (Kensinger, O’Brien, et al., 2007). These studies all suggest that negative valence conveys a greater benefit upon memory for detail than does positive valence.
Researchers have been sensitive to the fact that stimulus characteristics aside from valence (e.g., item distinctiveness, arousal, semantic clustering, personal relevance) could contribute to these effects and are aware that it is difficult (if not impossible) to equate positive and negative stimuli on all of these dimensions. With these concerns in mind, particularly compelling evidence for an effect of valence on memory accuracy has come from three studies examining whether the valence of a person’s response to an event outcome (finding the outcome positive or negative) affects how accurately that person remembers the event’s details. Because it is the perception of the event outcome, and not the event itself, that varies in these studies, this type of design minimizes the likelihood that differences aside from event valence will confound the comparison. For example, individuals who find the event negative or positive tend to find the event to be similarly distinctive, surprising, personally important, and arousing, reducing concerns that differences caused by these factors are masquerading as effects of valence.
These studies, examining how a person’s response to an event outcome can influence memory, have converged on the conclusion that positive emotion can be associated with a stronger disconnect between memory confidence and memory vividness, and with a greater propensity for memory errors, than negative emotion. Levine and Bluck (2004) asked participants who had strong opinions about the verdict in the O.J. Simpson trial to take a recognition memory test about events that had occurred during the trial. They found that individuals who were pleased about the verdict were more liberal in accepting that something had occurred; these individuals endorsed more fictitious events from the trial than those who were displeased about the verdict. Kensinger & Schacter (2006) asked Red Sox fans and Yankees fans to report what they remembered about the final game of the 2004 American League Championship series, in which the Red Sox defeated the Yankees. In this study, the Red Sox fans, who were elated with the outcome of the game, showed more memory inconsistencies and were more likely to be overconfident in their memories than were Yankees fans who were devastated by the game outcome (see Figure 4). Bohn & Berntsen (2007) asked Germans who found the fall of the Berlin Wall to be either a highly positive or highly negative event to recount details related to the event and to report on the vividness of their memories for the event. They found that participants who viewed the event as highly positive reported more vivid memories for the event than participants who viewed the event as highly negative; however, the positive group had less accurate memory for detail than the negative group. Thus, across all three studies, positive emotion was associated with a greater propensity for memory distortion than negative emotion.
Figure 4. Event Valence Affects Memory Consistency.
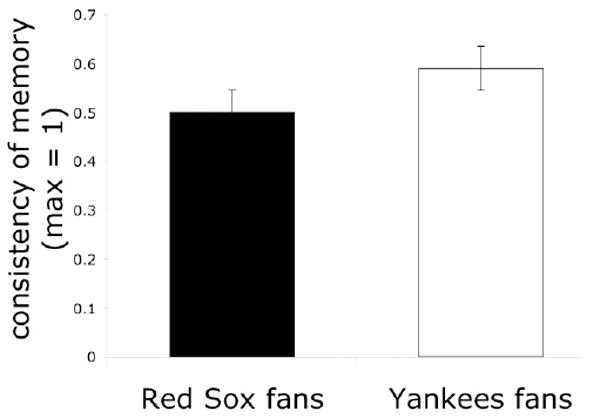
Yankees fans, displeased with the outcome of the game, remembered event details more consistently than Red Sox fans. Consistency refers to the overlap in detail provided at two different points in time: within one week of the game and 23-27 weeks later. Data from Kensinger & Schacter (2006).
Similar effects of valence have been demonstrated using mood induction procedures within the laboratory. When participants are induced into a positive mood, they tend to be more liberal in endorsing items as having been seen before, and they are more likely to falsely claim that related (but novel) items have been studied than are participants in neutral or negative moods (Bless et al., 1996; Storbeck & Clore, 2005). This increase in reconstructive memory errors likely arises because individuals in a happy mood process information in a more schematic or heuristic fashion, while individuals in a negative mood are more likely to focus on the specific details of information (e.g., Bless et al., 1996; Storbeck & Clore, 2005).
Valence may Influence both Encoding and Retrieval Processes
Although these studies suggest a striking effect of valence on memory accuracy, they cannot speak to the memory stage at which valence is exerting its influence. As noted at the start of this review, there is a “core” emotional memory network, consisting of interactions between the amygdala, hippocampus, and orbitofrontal cortex, that seems critically engaged whenever information elicits arousal, irrespective of valence (subset of regions depicted in Figure 1). Studies typically find no effect of valence on the relation between amygdala activity at encoding and subsequent memory performance (e.g., Dolcos et al., 2004b; Dougal et al., 2007; Kensinger & Schacter, 2006, Kensinger & Schacter, 2008), and a few studies have suggested that amygdala-hippocampal connections may be strengthened primarily when information elicits a strong arousal response (e.g., Kensinger & Corkin, 2004; Anderson et al., 2006). Similarly, activity within the orbitofrontal cortex tends to correspond with successful encoding of both positive and negative information, so long as that information is arousing (Mickley & Kensinger, in press; but see Schutter & van Honk, 2006 for evidence that laterality effects within orbitofrontal cortex may vary as a function of the valence of the to-be-remembered information).
While the regions constituting this arousal-dependent emotional memory network are widely accepted (reviewed by Phelps, 2004; LaBar & Cabeza, 2006), it has been less clear whether there also are valence-dependent neural processes that diverge during encoding, consolidation, or retrieval phases of memory. Though the behavioral data make clear that people can remember more accurate details of negative as compared to positive events, there is no easy way to know at which phase(s) of memory valence is exerting its influence. Fortunately, neuroimaging methods provide a means to examine this issue.
In one study (Mickley & Kensinger, in press), participants studied positive arousing, negative arousing, and neutral items. After a short delay, participants performed a recognition memory task. They were asked to indicate whether they vividly “remembered” an item, “knew” it had been presented but did not remember any episodic details of its presentation, or believed that the item was “new.” The remember/know procedure allows researchers to distinguish recognition responses that are supported by memory for episodic detail (signified by a “remember” response) from those responses that are made on the basis of item familiarity in the absence of remembered episodic detail (indicated by a “know” response; see Jacoby, 1991; Mandler, 1980; Yonelinas, 2002 review for discussion about this paradigm and the cognitive processes underlying the two types of responses). This study revealed that, during encoding, negative items that were later “remembered” recruited temporo-occipital regions associated with sensory processing more than positive or neutral items that later were “remembered.” There were no regions disproportionately recruited during the encoding of positive items that would later be “remembered.” By contrast, the encoding of positive information later “known” recruited regions associated with conceptual and self-referential processing (e.g., the cingulate gyrus and bilateral frontal and parietal areas) to a greater extent than negative or neutral items that were later “known” (Figure 5). These results emphasize that some of the influences of valence on memory arise because of differences in the processes recruited during encoding. The additional recruitment of sensory processes during the encoding of negative items may allow these items to be vividly “remembered,” whereas enhanced conceptual and self-referential processing of positive information may yield feelings of familiarity but not memory for episodic detail.
Figure 5. Valence affects the neural encoding processes that correspond with later “remembering” (upper panel) versus later “knowing” (bottom panel).
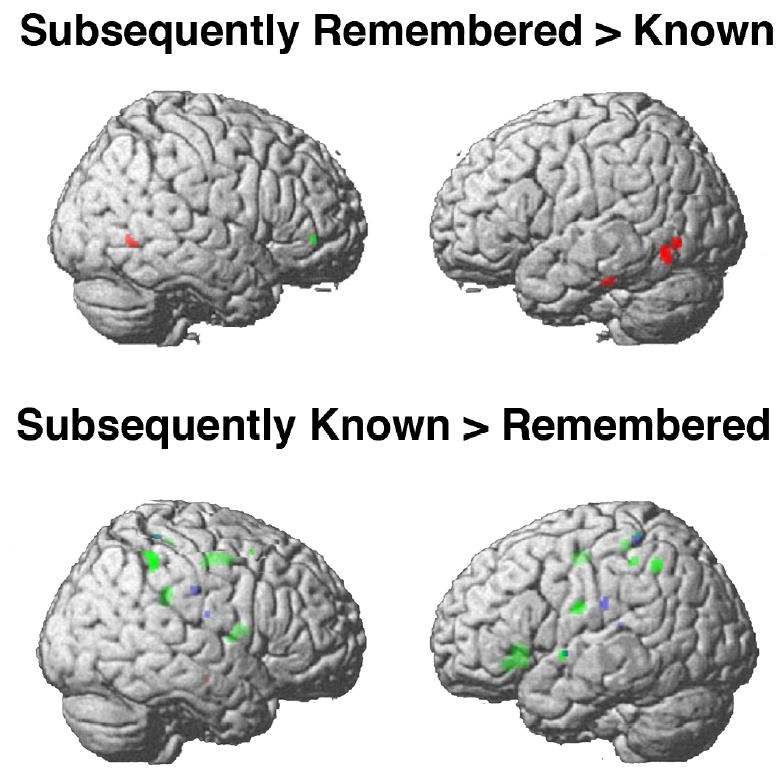
Red regions are those that show the correspondence for negative items but not positive ones, green regions are those that show the correspondence for positive items but not negative ones, and blue regions are those that show the correspondence for both negative and positive items. Data from Mickley & Kensinger (2008).
Though this study suggested that there was a link between the way in which participants encoded negative and positive information and the likelihood that they could later remember episodic details linked to those items’ presentations, it could not inform which details participants were remembering. A “remember” response could have signified memory for any number of details about the negative items’ presentations. It also was possible that participants were just biased to say that they “remembered” negative items, but that those memories were not actually associated with any more details than their memories of positive items.
Given the evidence outlined in the prior section, my colleagues and I hypothesized that negative emotion would convey a particular benefit on memory for intrinsic item details, such as the visual details of an item. This hypothesis was in keeping with the extant behavioral data, and it also was consistent with the finding that activity in sensory regions corresponded with the later “remembering” of negative items, suggesting that much of what people “remember” about those items may be the sensory details that were encoded. As a more direct way to explore this possibility, Daniel Schacter and I (Kensinger & Schacter, 2008) asked participants to undergo an fMRI scan as they viewed images that evoked pleasant or unpleasant emotions. We assessed participants’ memories for the visual specifics of the images, using the same/similar distinction described earlier. By looking at how well participants could distinguish “same” from “similar” exemplars, we could examine how emotional valence influenced participants’ ability to remember the precise visual details of an object. Consistent with prior behavioral studies (Kensinger, Garoff-Eaton, & Schacter, 2006, 2007), participants were better able to discriminate “same” from “similar” images when the images elicited negative emotion than when they elicited positive emotion.
At a neural level, the effects of valence on memory accuracy once again were related to differences in the processes engaged during the initial encoding of information. Consistent with the idea that there is a core emotional memory network that is not modulated by emotional valence, amygdala and orbitofrontal activity corresponded with successful encoding for both positive and negative items (see also Kensinger & Schacter, 2006; Figure 6). Importantly, however, there also were effects of valence on the processes engaged during encoding. The successful encoding of negative information resulted in disproportionate activity within sensory processing regions including the occipital (visual) cortex and along the fusiform gyrus (a region specialized for processing high-level features of objects and faces and for encoding those stimuli; e.g., Bernstein et al., 2002; Garoff et al., 2005; Kirchhoff et al., 2000; Kuskowski & Pardo, 1999). By contrast, the encoding of positive information was tied to disproportionate recruitment of lateral prefrontal and temporal regions that often have been implicated in the processing of semantic or conceptual information (see Dobbins & Wagner, 2005; Poldrack et al., 1999; Figure 7).
Figure 6. Amygdala activity corresponds with successful encoding for positive and negative items, but not for neutral items.
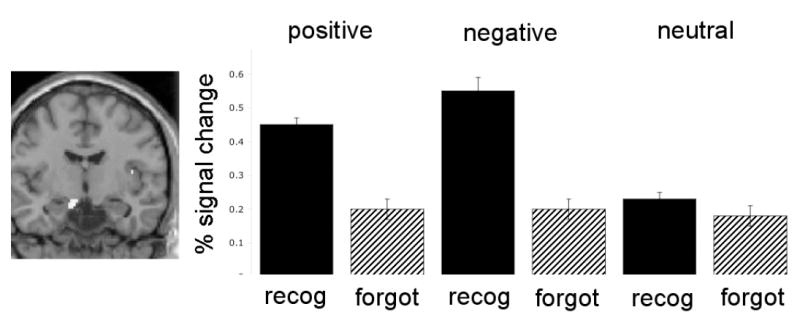
For positive and negative items, amygdala activity is higher during the encoding of items later recognized (recog) than during the encoding of items later forgotten (forgot). Data from Kensinger & Schacter (2008).
Figure 7. Valence influences the neural processes that are related to subsequent memory.
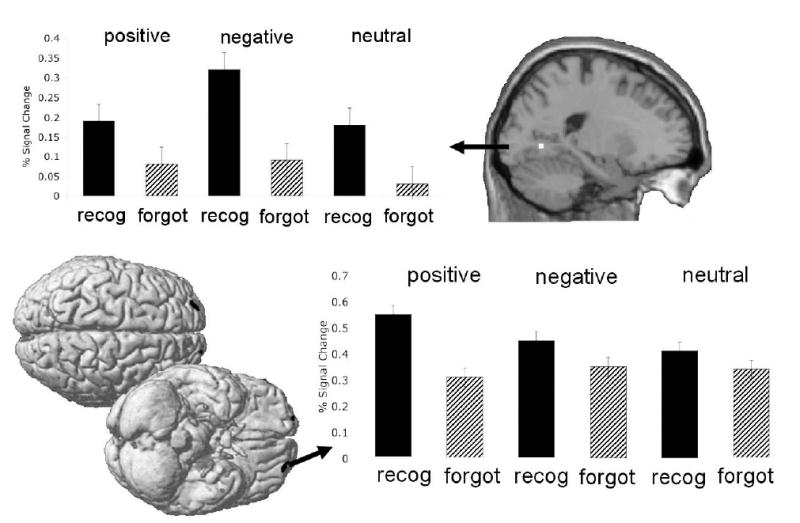
The fusiform gyrus was disproportionately associated with the encoding of negative information (upper panel), while activity in fronto-temporal regions was associated with the encoding of positive information (lower panel). Data are from the young adults reported in Kensinger & Schacter, 2008.
These findings are generally quite consistent with those of Mickley & Kensinger (in press), supporting the conclusion that recruitment of valence-specific processes during encoding leads to differences in the amount of detail with which emotional information is remembered. Individuals may later remember the specific sensory details associated with a negative item’s presentation (e.g., “a green and black snake with yellow eyes”) but only the gist of the positive item’s presentation (e.g., “a cake”) because they engage more sensory processing during the encoding of negative information and more semantic or conceptual processing during the encoding of positive information. More generally, this valence-dependent effect on encoding processes is consistent with the proposal that negative valence leads individuals to focus attention on local details whereas positive valence leads to a broadening of attention and to a focus on heuristics (e.g., Fredrickson & Branigan, 2005; Gasper & Clore, 2002; Rowe et al., 2007). The types of neural processes recruited during the encoding of negative versus positive items would be entirely consistent with this type of valence-dependent influence on processing strategy.
Of course, demonstrating a role for encoding does not rule out a role for consolidation or retrieval processes in influencing emotional memory. Studies have not thoroughly investigated the effect of valence on memory consolidation, but neuroimaging evidence has begun to suggest that valence may exert important influences on retrieval processes. A few studies have revealed differences in the neural processes recruited during the retrieval of positive as compared to negative events, with positive events recruiting frontal regions (those associated with conceptual and semantic processing) and negative events recruiting more posterior sensory regions (Markowitsch, Vandekerckhove, Lanfermann, & Russ, 2003; Piefke, Weiss, Ziles, Markowitsch, and Fink, 2003). It also appears that interactions between emotion processing regions and sensory processing regions (e.g., between the amygdala and fusiform gyrus) may be stronger during the retrieval of negative information than during the retrieval of positive information (E.A. Kensinger, unpublished data). These connections between emotion processing and sensory processing regions may occur both when an emotional cue is used to trigger a memory (e.g., when a person sees the snake that frightened them earlier) and also when a neutral cue is used to elicit the memory (e.g., when a person sees a face that had been presented in an emotional context; Smith et al., 2006).
These studies reveal that there is a link between negative emotion and sensory processing, and between positive emotion and conceptual processing, and that this link exists both during memory encoding as well as during memory retrieval. Neuroimaging studies have demonstrated that processes recruited during retrieval can sometimes reflect the recapitulation of processes engaged during an encoding episode (e.g., Kahn et al., 2004; Wheeler et al., 2000; Nyberg et al., 2000; Vaidya et al., 2002). In other words, when we remember an event, we bring online those regions that we initially recruited to process that event. Given this tendency for recapitulation, it would follow that there should be overlap in the effects of valence on encoding- and retrieval-related processes. It also is well known that retrieval is most successful when there is a match between the processes engaged during encoding and those engaged during retrieval (e.g., Craik & Lockhart, 1972). Therefore, it makes sense that the way in which people orient toward information at encoding (i.e., in a perceptual vs. conceptual manner) would have downstream effects on the types of retrieval processes or retrieval cues that would be most effective in guiding memory retrieval and on the types of information that would be retrieved.
Concluding Remarks and Directions for Further Inquiry
This review has emphasized the importance of considering both the arousal and the valence of affective experiences when examining how those experiences will be remembered. Though there has been extensive evidence for arousal-mediated enhancement of memory (e.g., McGaugh, 2004; Phelps, 2004), with positive and negative arousing events more likely to be remembered than nonarousing ones, when it comes to remembering the details of emotional events, there appear to be many instances in which the focal enhancements are stronger for negative experiences than for positive ones. The extant data suggest that negative arousal may lead to focal enhancements because of valence-dependent engagement of sensory processes, leading negative affect to focus attention on intrinsic details and positive affect to increase the likelihood that the gist of an event is remembered but that its details are forgotten.
Though research is moving us closer to understanding emotion’s effects on memory accuracy, there are still a number of fundamental questions that remain unanswered. In this section I will briefly describe two avenues of further inquiry that I think will be particularly fruitful. The first highlights the importance of taking an individual-differences approach when examining the effects of emotion on memory accuracy. The second returns us to where we started this review, asking why there may be disconnects between what people accurately remember about emotional experiences and what information they believe their memories contain.
Memory Accuracy Depends on Individual Differences in how an Event is Experienced
Although groups of individuals tend to remember some details of emotional events better than others, not everyone remembers the same details of an experience. The sections above already have described some ways in which individual differences in experience can impact emotion’s effects on memory for detail. Individuals who find an event to be negative are more likely to retain accurate details of the event than those who find an event to be positive. Individuals who are focused on encoding particular details (e.g., because of intentional encoding instructions, or because of an encoding task that focuses them on those details) also tend to remember those details better than individuals who do not attempt to overcome the attention capture by the intrinsically negative aspects of an experience. But there are a number of other aspects of event experience that can influence what people remember about an event. For one, being an actor in an event can influence the details that are remembered about that event. For instance, although people around the world remember the terrorist attacks of September 11, 2001, individuals living in New York tend to retain more event details about the day (e.g., when the second plane hit the building) than autobiographical details (e.g., what they were doing when they first learned of the attacks). In contrast, individuals further removed from the event locus (those living in California or Hawaii) retain more autobiographical details than event details (Pezdek, 2003; see also Luminet et al., 2004; Smith, Bibi, & Sheard, 2003; Tekcan et al., 2003). Separation of even just a few miles can influence how an event is remembered. Sharot, Martorella, Delgado, and Phelps (2007) queried New York City residents about the terrorist attacks of September 11, 2001. Participants who had been close to the World Trade Center reported more vivid and detailed memories than individuals who were further away, and the individuals who were close to the World Trade Center showed greater left amygdala activation during recall of events from September 11 than did individuals who were further away. These findings suggest that a person’s involvement with an event can be a critical factor influencing the extent of emotion-specific processing evoked during retrieval of the event memories. Note that these findings can be consistent with the proposal that negative emotion is enhancing memory for “intrinsic” details; what a person processes as intrinsic may vary based upon personal experience. For someone in New York, the intrinsic ties to the emotional event may be the event details themselves (e.g., when the plane hit the building); by contrast, for individuals further away, the intrinsic details may be tied to how they learned about the attacks.
Another important factor relates to the resources that an individual has available to devote toward event processing. Individuals who can devote only limited cognitive resources to event processing (either because they are performing a secondary task or because they are individuals with relatively poor cognitive control ability) tend to show much larger emotion-related memory trade-offs than people who can devote more substantial cognitive resources to event processing. With limited resources, people tend to retain the intrinsic emotional aspects of the event but not the nonemotional contextual details. Thus, after studying a picture of a snake in a forest, they may remember exactly what the snake looked like, but they will almost never remember the forest (Waring et al., in press).
These findings fit well with evidence that attention may be focused relatively automatically on the negative aspects of events (and see Dolan & Vuilleumier, 2003 for evidence), thereby boosting memory for those aspects even when attentional resources are limited. In contrast, flexible allocation of attention may be essential in order for event details more extrinsic to the emotional aspects to be recorded and remembered. This fact is likely to explain why the way in which attention is focused during encoding can have such a large impact on the types of details that are remembered about emotional events: Although there are some details that may always be remembered well (perhaps intrinsic details of the negative items), other details may only be recorded and retrieved when attention is directly devoted toward their processing.
The way in which cognitive resources are devoted toward event processing may also be closely tied to individual differences in personality or anxiety level. For example, people higher in anxiety tend to focus more automatically on negative event details, causing them to remember those details better (e.g., Ferguson et al, 2007; MacLeod & Matthews, 2004). However, they have a harder time remembering the contextual details, plausibly because they cannot flexibly deploy their attention away from the emotional aspects and toward nonemotional event details (Waring et al., in press). People higher in neuroticism also are more likely to dwell on the negative, and tend to have better memory for negative elements than nonemotional or positive elements of presented information (e.g., Chan et al., 2007). These results emphasize the importance of considering individual differences when examining the ways in which emotion impacts memory, as the degree to which memory is enhanced, or narrowed, likely it not the same across all individuals.
There are likely to be a multitude of other factors that can influence the way in which resources are devoted toward information processing. Individuals who find information particularly self-relevant may deploy resources differently than individuals who find information to be unconnected to their self-concept (discussed in Schacter, Gutchess, & Kensinger, in press), and individuals in a powerless position may process information differently from individuals with power over a situation (e.g., Guinote, 2007). Individuals who are engaging in emotion regulation strategies also may process and remember different types of event details than those who are not attempting to regulate their affective reactions to events (Richards & Gross, 2000). A person’s gender can also influence how well emotional events are remembered (Cahill, 2003; Hamann & Canli, 2004) as can a person’s perspective about the finite (or infinite) nature of their future (Carstensen et al., 1999; Mather & Carstensen, 2005). As research continues to delve into the complexities of emotion’s effects on memory accuracy, it will be important for these types of individual differences to be considered so that broad effects of emotion (i.e., those that impact nearly everyone’s memory) can be distinguished from those that may impact only a subset of individuals or that may arise under only a constrained set of circumstances.
Disconnects between Emotional Memory Accuracy and Confidence
At the outset of this review, I described how people sometimes hold tight to memories that are inaccurate. Emotional memories are at least as subject to this type of accuracy-confidence disconnect as nonemotional memories, and in fact some research has suggested that overconfidence and recognition memory bias may occur more often for emotional memories than for memories of more mundane experiences (e.g., Schmolck et al., 2000; Windmann & Kutas, 2002; Dougal & Rotello, 2007; see also Jonsson et al., 2005; Hertz, 2000 for an interesting extension to odor-cued memories). Though there may be any number of contributors to this effect, a good place to begin may be with an examination of the effects of emotion on metamemory processes, or the beliefs that people hold about their own memories. Indeed, there is some evidence to suggest that emotion may affect a person’s assumptions about what details they will be able to remember about an event. For instance, many studies of eyewitness memory have suggested that there can be dissociations between an individual’s confidence that they will be able to select a perpetrator from a lineup and their actual recognition ability when they make their selection (e.g., Busey et al., 2000; Clark & Tunnicliff, 2001).
Given this evidence for disconnects between what a person believes they will remember and what information their emotional memories actually contain, a particularly worthwhile line of investigation may relate to the effects of emotion on “feeling-of-knowing” metamemory decisions. Feeling-of-knowing refers to participants’ predictions about the likelihood that they will be able to recognize information when they have failed to recall that information (Hart, 1965, 1967). These decisions often are made on the basis of two types of information: partial retrieval of information (Koriat, 1993; Koriat & Levy-Sadot, 2001) and cue familiarity (Metcalfe et al., 1993). Reliance on partial retrieval of information refers to the fact that if participants remember some nonspecific or incomplete pieces of information about an encoding event, they will be more likely to assume that they will be able to remember other aspects of that encoding event when given additional cues. Reliance on cue familiarity refers to participants’ tendencies to use the familiarity of a retrieval cue to predict the likelihood that they will later recognize the target information: Participants tend to believe that if the cue is highly familiar, the information will be more likely to be recognized than if the cue is relatively unfamiliar.
Emotion would be expected to enhance each of these factors. First, because negative emotion enhances memory for some details, participants should be more likely to remember some details of an emotional item’s presentation than of a neutral item’s presentation. This partial recollection may lead participants to believe that they will be able to remember other details of an emotional item’s presentation, though this is likely a faulty assumption. In other words, if I retain a very vivid memory of a gun that was pointed at me, this may lead me to believe that I will be able to recognize the perpetrator who held the gun. Second, previous research has demonstrated that emotional information – and particularly negatively emotional information - tends to be associated with a greater fluency of processing, and that participants often misinterpret that fluency as reflecting a recent encounter with the stimulus (e.g., Bargh et al., 1992; Kitayama, 1990; Whittlesea, 1993; Whittlesea & Williams, 2000; Windmann & Kutas, 2001). It is plausible that, because of this perceived cue familiarity, participants would be more likely to have inflated confidence in their ability to remember event details tied to that item’s presentation.
It is important to note that feeling-of-knowing is only one type of metamemory decision, and it likely is supported by distinct processes from other types of metamemory assessments, such as reports of memory confidence. Though the ability to tease apart the processes supporting accurate memory retrieval and metamemory assessments continues to be a challenge, with the help of cleverly-designed behavioral studies and the use of neuroimaging and neuropsychology methods, researchers are making progress (e.g., Chua et al., 2006; Kikyo et al., 2002; Pannu & Kaszniak, 2005; Schnyer et al., 2005). To my knowledge, however, this research has all been conducted with stimuli void of emotional content. It seems that there is much to be learned by adapting these metamemory methods for use with emotional stimuli, to understand not only how emotion affects the details remembered about an event, but also how emotion affects our self-awareness of our memories’ content and the monitoring processes that we engage when retrieving information.
Acknowledgments
Preparation of this manuscript was supported by NIH grant MH080833 and by NSF grant BCS0542694. I thank Lisa Feldman Barrett, Hiram Brownell, Angela Gutchess, and Daniel Schacter for helpful discussion.
References
- Anderson AK, Yamaguchi Y, Grabski W, Lacka D. Emotional memories are not all created equal: evidence for selective memory enhancement. Learning and Memory. 2006;13:711–718. doi: 10.1101/lm.388906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrick LE, Parker JF, Fivush R, Levitt M. The effects of stress on young children’s memory for a natural disaster. Journal of Experimental Psychology: Applied. 1998;4:308–331. doi: 10.1037/1076-898X.12.3.142. [DOI] [PubMed] [Google Scholar]
- Bargh JA, Chaiken S, Govender R, Pratto F. The generality of the attitude activation effect. Journal of Personality and Social Psychology. 1992;62:893–912. doi: 10.1037//0022-3514.62.6.893. [DOI] [PubMed] [Google Scholar]
- Bernstein LJ, Beig S, Siegenthaler AL, Grady CL. The effect of encoding strategy on the neural correlates of memory for faces. Neuropsychologia. 2002;40:86–98. doi: 10.1016/s0028-3932(01)00070-7. [DOI] [PubMed] [Google Scholar]
- Bless H, Schwarz N. Sufficient and necessary conditions in dual process models: The case of mood and information processing. In: Chaiken S, Trope Y, editors. Dual process theories in social psychology. New York: Guilford Press; 1999. pp. 423–440. [Google Scholar]
- Bless H, Clore GL, Schwarz N, Golisano V, Rabe C, Wolk M. Mood and the use of scripts: Does a happy mood really lead to mindlessness? Journal of Personality and Social Psychology. 1996;71:665–679. doi: 10.1037//0022-3514.71.4.665. [DOI] [PubMed] [Google Scholar]
- Bohannon JN. Flashbulb memories for the space shuttle disaster: a tale of two theories. Cognition. 1988;29:179–196. doi: 10.1016/0010-0277(88)90036-4. [DOI] [PubMed] [Google Scholar]
- Bohn A, Berntsen D. Pleasantness bias in flashbulb memories: positive and negative flashbulb memories of the fall of the Berlin Wall among East and West Germans. Memory and Cognition. 2007;35:565–577. doi: 10.3758/bf03193295. [DOI] [PubMed] [Google Scholar]
- Brown R, Kulik J. Flashbulb memories. Cognition. 1977;5:73–99. [Google Scholar]
- Buchanan TW, Adolphs R. The role of the human amygdala in emotional modulation of long-term declarative memory. In: Moore S, Oaksford M, editors. Emotional Cognition: From Brain to Behavior. Amsterdam: John Benjamins Publishing; 2002. pp. 9–34. [Google Scholar]
- Buchanan TW. Retrieval of emotional memories. Psychonomic Bulletin. 2007;133:761–779. doi: 10.1037/0033-2909.133.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26:307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- Budson AE, Simons JS, Sullivan AL, Beier JS, Soloman PR, Scinto LF, et al. Memory and emotions for the September 11, 2001, terrorist attacks in patients with Alzheimer’s disease, patients with mild cognitive impairment, and healthy older adults. Neuropsychology. 2004;18:315–327. doi: 10.1037/0894-4105.18.2.315. [DOI] [PubMed] [Google Scholar]
- Budson AE, Simons JS, Waring JD, Sullivan AL, Hussoin T, Schacter DL. Memory for the September 11, 2001, terrorist attacks one year later in patients with Alzheimer’s disease, patients with mild cognitive impairment, and healthy older adults. Cortex. 2007;43:875–888. doi: 10.1016/s0010-9452(08)70687-7. [DOI] [PubMed] [Google Scholar]
- Busey T, Tunnicliff J, Loftus G, Loftus E. What’s good for the goose isn’t always what’s good for the gander: Confidence and accuracy depend on different information. Psvchonomic Bulletin & Review. 2000;7:26–48. doi: 10.3758/bf03210724. [DOI] [PubMed] [Google Scholar]
- Cahill L. Sex- and hemisphere-related influences on the neurobiology of emotionally influenced memory. Progress in Neuropsyhopharmacology and Biological Psychiatry. 2003;27:1235–1241. doi: 10.1016/j.pnpbp.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. A novel demonstration of enhanced memory associated with emotional arousal. Conscious Cognition. 1995;4:410–421. doi: 10.1006/ccog.1995.1048. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously: A theory of socioemotional selectivity. American Psychologist. 1999;54:165–181. doi: 10.1037//0003-066x.54.3.165. [DOI] [PubMed] [Google Scholar]
- Chan SW, Goodwin GM, Harmer CJ. Highly neurotic never-depressed students have negative biases in information processing. Psychological Medicine. 2007;37:1281–1291. doi: 10.1017/S0033291707000669. [DOI] [PubMed] [Google Scholar]
- Christianson SA. Flashbulb memories: Special, but not so special. Memory and Cognition. 1989;17:435–43. doi: 10.3758/bf03202615. [DOI] [PubMed] [Google Scholar]
- Chua EF, Schacter DL, Rand-Giovannetti E, Sperling RA. Understanding metamemory: neural correlates of the cognitive process and subjective level of confidence in recognition memory. Neuroimage. 2006;29:1150–1160. doi: 10.1016/j.neuroimage.2005.09.058. [DOI] [PubMed] [Google Scholar]
- Clark S, Tunnicliff JL. Selecting lineup foils in eyewitness identification experiments: Experimental control and real world simulation. Law and Human Behavior. 2001;25:199–216. doi: 10.1023/a:1010753809988. [DOI] [PubMed] [Google Scholar]
- Colgrove FW. Individual memories. American Journal of Psychology. 1899;10:228–255. [Google Scholar]
- Cook GI, Hicks JL, Marsh RL. Source monitoring is not always enhanced for valenced material. Memory and Cognition. 2007;35:222–230. doi: 10.3758/bf03193443. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Lockhart RS. Levels of processing: A framework for memory research. Journal of Verbal Learning and Verbal Behavior. 1972;11:671–684. [Google Scholar]
- D’Argembeau A, van der Linden M. Influence of affective meaning on memory for contextual information. Emotion. 2004;4:173–188. doi: 10.1037/1528-3542.4.2.173. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Rice HJ, Greenberg DL, Cabeza R, LaBar KS, Rubin DC. The spatiotemporal dynamics of autobiographical memory: Neural correlates of recall, emotional intensity, and reliving. Cerebral Cortex. 2007 doi: 10.1093/cercor/bhm048. [DOI] [PubMed] [Google Scholar]
- Dewhurst SA, Parry LA. Emotionality, distinctiveness, and recollective experience. European Journal of Cognitive Psychology. 2000;12:541–551. [Google Scholar]
- Dobbins IG, Wagner AD. Domain-general and domain-sensitive prefrontal mechanisms for recollecting events and detecting novelty. Cerebral Cortex. 2005;15:1768–1778. doi: 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Doerksen S, Shimamura A. Source memory enhancement for emotional words. Emotion. 2001;1:5–11. doi: 10.1037/1528-3542.1.1.5. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Vuilleumier P. Amygdala automaticity in emotional processing. Annals of the New York Academy of Sciences. 2003;985:348–355. doi: 10.1111/j.1749-6632.2003.tb07093.x. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. Neuroimage. 2004a;23:64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004b;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Dougal S, Phelps EA, Davachi L. The role of medial temporal lobe in item recognition and source recollection of emotional stimuli. Cognitive, Affective, and Behavioral Neuroscience. 2007;7:233–242. doi: 10.3758/cabn.7.3.233. [DOI] [PubMed] [Google Scholar]
- Dougal S, Rotello CM. “Remembering” emotional words is based on response bias, not recollection. Psychonomic Bulletin and Review. 2007;14:423–429. doi: 10.3758/bf03194083. [DOI] [PubMed] [Google Scholar]
- Easterbrook JA. The effects of emotion on cue utilization and the organization of behavior. Psychological Review. 1959;66:183–201. doi: 10.1037/h0047707. [DOI] [PubMed] [Google Scholar]
- Ferguson E, Moghaddam NG, Bibby PA. Memory bias in health anxiety is related to the emotional valence of health-related words. Journal of Psychosomatic Research. 2007;62:263–274. doi: 10.1016/j.jpsychores.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Branigan C. Positive emotions broaden the scope of attention and thought-action repertoires. Cognition & Emotion. 2005;19:313–332. doi: 10.1080/02699930441000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff RJ, Slotnick SD, Schacter DL. The neural origins of specific and general memory: The role of the fusiform cortex. Neuropsychologia. 2005;43:847–859. doi: 10.1016/j.neuropsychologia.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Gasper K, Clore GL. Attending to the big picture: Mood and global vs. local processing of visual information. Psychological Science. 2002;13:34–40. doi: 10.1111/1467-9280.00406. [DOI] [PubMed] [Google Scholar]
- Guinote A. Power affects basic cognition: Increased attentional inhibition and flexibility. Journal of Experimental Social Psychology. 2007;43:685–697. [Google Scholar]
- Hamann S, Canli T. Individual differences in emotion processing. Current Opinion in Neurobiology. 2004;14:233–238. doi: 10.1016/j.conb.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Hart JT. Memory and the feeling-of-knowing experience. Journal of Educational Psychology. 1965;56:208–216. doi: 10.1037/h0022263. [DOI] [PubMed] [Google Scholar]
- Hart JT. Memory and the memory monitoring process. Journal of Verbal Learning and Behavior. 1967;6:685–691. [Google Scholar]
- Hurlemann R, Hawellek B, Matusch A, Kolsch H, Wollersen H, Madea B, Vogeley K, Maier W, Dolan RJ. Noradrenergic modulation of emotion-induced forgetting and remembering. Journal of Neuroscience. 2005;25:6343–6349. doi: 10.1523/JNEUROSCI.0228-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iidaka T, Omori M, Murata T, Kosaka H, Yonekura Y, Okada T, Sadato N. Neural interaction of the amygdala with the prefrontal and temporal cortices in the processing of facial expressions as revealed by fMRI. Journal of Cognitive Neuroscience. 2001;13:1035–1047. doi: 10.1162/089892901753294338. [DOI] [PubMed] [Google Scholar]
- Jacoby LL. A process dissociation framework: Separating automatic from intentional uses of memory. Journal of Memory and Language. 1991;30:513–541. [Google Scholar]
- James W. The principles of psychology. In: Wozniak RH, editor. Thoemmes Press – Classics in Psychology. London: Thoemmes Continuum; 18901998. [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychological Bulletin. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Jonsson FU, Olsson H, Olsson MJ. Odor emotionality affects the confidence in odor naming. Chemical Senses. 2005;30:29–35. doi: 10.1093/chemse/bjh254. [DOI] [PubMed] [Google Scholar]
- Kahn I, Davachi L, Wagner AD. Functional-neuroanatomic correlates of recollection: implications for models of recognition memory. Journal of Neuroscience. 2004;24:4172–4180. doi: 10.1523/JNEUROSCI.0624-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA. Remembering emotional experiences: The contribution of valence and arousal. Reviews in the Neurosciences. 2004;15:241–251. doi: 10.1515/revneuro.2004.15.4.241. [DOI] [PubMed] [Google Scholar]
- Kensinger EA. How negative emotion affects memory accuracy: Behavioral and neuroimaging evidence. Current Directions in Psychological Science. 2007;16:213–218. [Google Scholar]
- Kensinger EA, Brierley B, Medford N, Growdon JH, Corkin S. Effects of normal aging and Alzheimer’s disease on emotional memory. Emotion. 2002;2:118–134. doi: 10.1037/1528-3542.2.2.118. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Memory enhancement for emotional words: Are emotional words more vividly remembered than neutral words? Memory and Cognition. 2003;31:1169–1180. doi: 10.3758/bf03195800. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Two routes to emotional memory: Distinct neural processes for valence and arousal. Proceedings of the National Academy of Sciences, USA. 2004;101:3310–3315. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Memory for specific visual details can be enhanced by negative arousing content. Journal of Memory and Language. 2006;54:99–112. [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Effects of emotion on memory specificity in young and older adults. Journal of Gerontology: Psychological Sciences. 2007a;62:P208–P215. doi: 10.1093/geronb/62.4.p208. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Effects of emotion on memory specificity: Memory trade-offs elicited by negative visually arousing stimuli. Journal of Memory and Language. 2007b;56:575–591. [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. How negative emotion enhances the visual specificity of a memory. Journal of Cognitive Neuroscience. 2007c;19:1872–1887. doi: 10.1162/jocn.2007.19.11.1872. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Gutchess AH, Schacter DL. Effects of aging and encoding instructions on emotion-induced memory trade-offs. Psychology and Aging. 2007;22:781–795. doi: 10.1037/0882-7974.22.4.781. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Krendl AC, Corkin S. Memories of an emotional and a nonemotional event: Effects of aging and delay interval. Experimental Aging Research. 2006;32:23–45. doi: 10.1080/01902140500325031. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, O’Brien J, Swanberg K, Garoff-Eaton RJ, Schacter DL. The effects of emotional content on reality-monitoring performance in young and older adults. Psychology and Aging. 2007;22:752–764. doi: 10.1037/0882-7974.22.4.752. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Piguet O, Krendl AC, Corkin S. Memory for contextual details: Effects of emotion and aging. Psychology and Aging. 2005;20:241–250. doi: 10.1037/0882-7974.20.2.241. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Amygdala activity is associated with the successful encoding of item, but not source, information for positive and negative stimuli. Journal of Neuroscience. 2006a;26:2564–2570. doi: 10.1523/JNEUROSCI.5241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Reality monitoring and memory distortion: Effects of negative, arousing content. Memory and Cognition. 2006b;34:251–260. doi: 10.3758/bf03193403. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. When the Red Sox shocked the Yankees: Comparing negative and positive memories. Psychonomic Bulletin and Review. 2006c;13:757–763. doi: 10.3758/bf03193993. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Remembering the specific visual details of presented objects: Neuroimaging evidence for effects of emotion. Neuropsychologia. 2007;45:2951–2962. doi: 10.1016/j.neuropsychologia.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Neural processes supporting young and older adults’ emotional memories. Journal of Cognitive Neuroscience. 2008;7:1–13. doi: 10.1162/jocn.2008.20080. [DOI] [PubMed] [Google Scholar]
- Kern RP, Libkuman TM, Otani H, Holmes K. Emotional stimuli, divided attention, and memory. Emotion. 2005;5:408–417. doi: 10.1037/1528-3542.5.4.408. [DOI] [PubMed] [Google Scholar]
- Kikyo H, Ohki K, Miyashita Y. Neural correlates for feeling-of-knowing: an fMRI parametric analysis. Neuron. 2002;36:177–186. doi: 10.1016/s0896-6273(02)00939-x. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. Journal of Neuroscience. 2000;20:6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama S. Interaction between affect and cognition in word perception. Journal of Personality and Social Psychology. 1990;58:209–217. doi: 10.1037//0022-3514.58.2.209. [DOI] [PubMed] [Google Scholar]
- Koriat A. How do we know that we know? The accessibility model of the feeling-of-knowing. Psychological Review. 1993;100:609–639. doi: 10.1037/0033-295x.100.4.609. [DOI] [PubMed] [Google Scholar]
- Koriat A, Levy-Sadot R. The combined contributions of the cue-familiarity and accessibility heuristics to feelings of knowing. Journal of Experimental Psychology: Learning Memory, and Cognition. 2001;27:34–53. [PubMed] [Google Scholar]
- Kuhlmann S, Wolf OT. Arousal and cortisol interact in modulating memory consolidation in healthy young men. Behavioral Neuroscience. 2006;120:217–223. doi: 10.1037/0735-7044.120.1.217. [DOI] [PubMed] [Google Scholar]
- Kuskowski MA, Pardo JV. The role of the fusiform gyrus in successful encoding of face stimuli. Neuroimage. 1999;9:599–610. doi: 10.1006/nimg.1999.0442. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Neuroscience Reviews. 2006;7:54–56. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Phelps EA. Arousal-mediated memory consolidation: Role of the medial temporal lobe in humans. Psychological Science. 1998;9:490–493. [Google Scholar]
- Laney C, Campbell HV, Heuer F, Reisberg D. Memory for thematically arousing events. Memory and Cognition. 2004;32:1149–1159. doi: 10.3758/bf03196888. [DOI] [PubMed] [Google Scholar]
- Levine LJ, Bluck S. Painting with broad strokes: Happiness and the malleability of event memory. Cognition and Emotion. 2004;18:559–574. [Google Scholar]
- Luminet O, Curci A, Marsh EJ, Wessel I, Constantin T, Gencoz F, Yogo M. The cognitive, emotional, and social impacts of the September 11 attacks: group differences in memory for the reception context and the determinants of flashbulb memory. Journal of General Psychology. 2004;131:197–224. doi: 10.3200/GENP.131.3.197-224. [DOI] [PubMed] [Google Scholar]
- MacKay DG, Ahmetzanov MV. Emotion, memory and attention in the taboo Stroop paradigm: An experimental analogue of flashbulb memories. Psychological Science. 2005;16:25–32. doi: 10.1111/j.0956-7976.2005.00776.x. [DOI] [PubMed] [Google Scholar]
- MacKay DG, Shafto M, Taylor JK, Marian DE, Abrams L, Dyer JR. Relations between emotion, memory, and attention: evidence from taboo stroop, lexical decision, and immediate memory tasks. Memory and Cognition. 2004;32:474–488. doi: 10.3758/bf03195840. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A. Selective memory effects in anxiety disorders: An overview of research findings and their implications. In: Reisberg D, Hertel P, editors. Memory and Emotion. New York: Oxford University Press; 2004. pp. 155–185. [Google Scholar]
- Mandler G. Recognizing: The judgment of previous occurrence. Psychological Review. 1980;87:252–271. [Google Scholar]
- Markowitsch HJ, Vandekerckhove MM, Laufermann H, Russ MO. Engagement of lateral and medial prefrontal areas in the ecphory of sad and happy autobiographical memories. Cortex. 2003;39:643–665. doi: 10.1016/s0010-9452(08)70858-x. [DOI] [PubMed] [Google Scholar]
- Mather M. Emotional arousal and memory binding: An object-based framework. Perspectives on Psychological Science. 2007;2:33–52. doi: 10.1111/j.1745-6916.2007.00028.x. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: the positivity effect in attention and memory. Trends in Cognitive Science. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mather M, Nesmith K. Arousal-enhanced location memory for pictures. Journal of Memory and Language. doi: 10.1016/j.jml.2007.01.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Metcalfe J, Schwartz BL, Joaquim SG. The cue-familiarity heuristic in metacognition. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1993;19:851–864. doi: 10.1037//0278-7393.19.4.851. [DOI] [PubMed] [Google Scholar]
- Mickley KR, Kensinger EA. Emotional valence influences the neural correlates associated with remembering and knowing. Cognitive, Affective, and Behavioral Neuroscience. 2008;8:143–152. doi: 10.3758/cabn.8.2.143. [DOI] [PubMed] [Google Scholar]
- Neisser U, Harsch N. Phantom flashbulbs: false recollections of hearing the news about Challenger. In: Winograd E, Neisser U, editors. Affect and accuracy in recall: Studies of ‘flashbulb’ memories. New York: Cambridge University Press; 1992. pp. 9–31. [Google Scholar]
- Nyberg L, Habib R, McIntosh AR, Tulving E. Reactivation of encoding-related brain activity during memory retrieval. Proceedings of the National Academy of Sciences, USA. 2000;97:11120–11124. doi: 10.1073/pnas.97.20.11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN. Are affective events richly “remembered” or simply familiar? The experience and process of recognizing feelings past. Journal of Experimental Psychology: General. 2000;129:242–261. doi: 10.1037//0096-3445.129.2.242. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends in Cognitive Sciences. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Pannu JK, Kaszniak AW. Metamemory experiments in neurological populations: a review. Neuropsychology Review. 2005;15:105–130. doi: 10.1007/s11065-005-7091-6. [DOI] [PubMed] [Google Scholar]
- Paradis CM, Solomon LZ, Florer F, Thompson T. Flashbulb memories of personal events of 9/11 and the day after for a sample of New York City residents. Psychological Reports. 2004;95:304–310. doi: 10.2466/pr0.95.1.304-310. [DOI] [PubMed] [Google Scholar]
- Peterson C, Bell M. Children’s memory for traumatic injury. Child Development. 1996;67:3045–3070. [PubMed] [Google Scholar]
- Peterson C, Whalen N. Five years later: Children’s memory for medical emergencies. Applied Cognitive Psychology. 2001;15:17–24. [Google Scholar]
- Pezdek K. Event memory and autobiographical memory for the events of September 11, 2001. Applied Cognitive Psychology. 2003;17:1033–1045. [Google Scholar]
- Phelps EA. Human emotion and memory: Interactions of the amygdala and hippocampal complex. Current Opinion in Neurobiology. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Zilles K, Markowitsch HJ, Fink GR. Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain. 2003;126:650–668. doi: 10.1093/brain/awg064. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Selco SL, Field JE, Cohen NJ. The relationship between skill learning and repetition priming: Experimental and computational analyses. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1999;25:208–235. doi: 10.1037//0278-7393.25.1.208. [DOI] [PubMed] [Google Scholar]
- Reisberg D, Heuer F. Memory for emotional events. In: Reisberg D, Hertel P, editors. Memory and emotion. New York: Oxford University Press; 2004. pp. 3–41. [Google Scholar]
- Richards JM, Gross JJ. Emotion regulation and memory: The cognitive costs of keeping one’s cool. Journal of Personality and Social Psychology. 2000;79:410–424. doi: 10.1037//0022-3514.79.3.410. [DOI] [PubMed] [Google Scholar]
- Richardson MP, Strange B, Dolan RJ. Encoding of emotional memories depends on the amygdala and hippocampus and their interactions. Nature Neuroscience. 2004;7:278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Current Opinion in Neurobiology. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Rowe G, Hirsh JB, Anderson AK. Positive affect increases the breadth of attentional selection. Proceedings of the National Academy of Sciences. 2007;104:383–388. doi: 10.1073/pnas.0605198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Dodson CS. Misattribution, false recognition and the sins of memory. Philosophical Transactions of the Royal Society of London: Biological Sciences. 2001;356:1385–1393. doi: 10.1098/rstb.2001.0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Gutchess AH, Kensinger EA. Specificity of memory: Implications for individual and collective remembering. Bridging Individual Memory and Collective Remembering: Conceptual Foundations 2006 [Google Scholar]
- Schmidt SR. Autobiographical memories for the September 11th attacks: Reconstructive errors and emotional impairment of memory. Memory and Cognition. 2004;32:443–454. doi: 10.3758/bf03195837. [DOI] [PubMed] [Google Scholar]
- Schmolck H, Buffalo EA, Squire LR. Memory distortions develop over time: Recollections of the O.J. Simpson trial verdict after 15 and 32 months. Psychological Science. 2000;11:39–45. doi: 10.1111/1467-9280.00212. [DOI] [PubMed] [Google Scholar]
- Schnyer DM, Nicholls L, Verfaellie M. The role of VMPC in metamemorial judgments of content retrievability. Journal of Cognitive Neuroscience. 2005;17:832–846. doi: 10.1162/0898929053747694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Multiple reward systems in the brain. Nature Reviews Neuroscience. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, van Honk J. Increased positive emotional memory after repetitive transcranial magnetic stimulation over the orbitofrontal cortex. Journal of Psychiatry and Neuroscience. 2006;31:101–104. [PMC free article] [PubMed] [Google Scholar]
- Sharot T, Delgado MR, Phelps EA. How emotion enhances the feeling of remembering. Nature Neuroscience. 2004;7:1376–1380. doi: 10.1038/nn1353. [DOI] [PubMed] [Google Scholar]
- Sharot T, Martorella EA, Delgado MR, Phelps EA. How personal experience modulates the neural circuitry of memories of September 11. Proceedings of the National Academy of Sciences, USA. 2007;104:389–394. doi: 10.1073/pnas.0609230103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MC, Bibi U, Sheard DE. Evidence for the differential impact of time and emotion on personal and event memories for September 11, 2001. Applied Cognitive Psychology. 2003;17:1047–1055. [Google Scholar]
- Smith AP, Stephan KE, Rugg MD, Dolan RJ. Task and content modulate amygdala-hippocampal connectivity in emotional retrieval. Neuron. 2006;49:631–638. doi: 10.1016/j.neuron.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Storbeck J, Clore GL. With sadness comes accuracy; with happiness, false memory: Mood and the false memory effect. Psychological Science. 2005;16:785–791. doi: 10.1111/j.1467-9280.2005.01615.x. [DOI] [PubMed] [Google Scholar]
- Talarico JM, Rubin DC. Confidence, not consistency, characterizes flashbulb memories. Psychological Science. 2003;14:455–461. doi: 10.1111/1467-9280.02453. [DOI] [PubMed] [Google Scholar]
- Talmi D, Moscovitch M. Can semantic relatedness explain the enhancement of memory for emotional words? Memory and Cognition. 2004;32:742–751. doi: 10.3758/bf03195864. [DOI] [PubMed] [Google Scholar]
- Talmi D, Anderson AK, Riggs L, Caplan JB, Moscovitch M. Immediate memory consequences of the effect of emotion on attention to pictures. Learning and Memory. 2008;15:172–182. doi: 10.1101/lm.722908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekcan AI. Flashbulb memories for a negative and a positive event: news of Desert Storm and acceptance to college. Psychological Reports. 2001;88:323–331. doi: 10.2466/pr0.2001.88.2.323. [DOI] [PubMed] [Google Scholar]
- Tekcan AI, Ece B, Gülgöz S, Er N. Autobiographical and event memory for 9/11: Changes across one year. Applied Cognitive Psychology. 2003;17:1057–1066. [Google Scholar]
- Vaidya CJ, Zhao M, Desmond JE, Gabrieli JD. Evidence for cortical encoding specificity in episodic memory: memory-induced re-activation of picture processing areas. Neuropsychologia. 2002;40:2136–2143. doi: 10.1016/s0028-3932(02)00053-2. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: An event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distinct influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Waring JD, Payne JD, Schacter DL, Kensinger EA. Impact of individual differences upon emotion-induced memory trade-offs. Cognition and Emotion. doi: 10.1080/02699930802618918. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Petersen SE, Buckner RL. Memory’s echo: vivid remembering reactivates sensory-specific cortex. Proceedings of the National Academy of Sciences, USA. 2000;97:11125–11129. doi: 10.1073/pnas.97.20.11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittlesea BWA. Illusions of familiarity. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1993;19:1235–1253. [Google Scholar]
- Whittlesea BWA, Williams LD. The source of feelings of familiarity: The discrepancy-attribution hypothesis. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26:547–565. doi: 10.1037//0278-7393.26.3.547. [DOI] [PubMed] [Google Scholar]
- Windmann S, Kutas M. Electrophysiological correlates of emotion-induced recognition bias. Journal of Cognitive Neuroscience. 2001;13:577–592. doi: 10.1162/089892901750363172. [DOI] [PubMed] [Google Scholar]
- Winograd E, Killinger WA., Jr Relating age at encoding in early childhood to adult recall: development of flashbulb memories. Journal of Experimental Psychology: General. 1983;112:413–422. [Google Scholar]
- Wolf OT. The influence of stress hormones on emotional memory: Relevance for psychopathology. Acta Psychologica. 2008;127:513–531. doi: 10.1016/j.actpsy.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Wolters G, Goudsmit JJ. Flashbulb and event memory of September 11, 2001: Consistency, confidence and age effect. Psychological Report. 2005;96:605–619. doi: 10.2466/pr0.96.3.605-619. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]


