Abstract
Aquarium fishes are becoming increasingly important because of their value in biomedical research and the ornamental fish trade, and because many have become threatened or endangered in the wild. This review summarizes the current status of sperm cryopreservation in three fishes widely used in biomedical research: zebrafish, medaka, and live-bearing fishes of the genus Xiphophorus, and will focus on the needs and opportunities for future research and application of cryopreservation in aquarium fish. First, we summarize the basic biological characteristics regarding natural habitat, testis structure, spermatogenesis, sperm morphology, and sperm physiology. Second, we compare protocol development of sperm cryopreservation. Third, we emphasize the importance of artificial fertilization in sperm cryopreservation to evaluate the viability of thawed sperm. We conclude with a look to future research directions for sperm cryopreservation and the application of this technique in aquarium species.
Keywords: sperm cryopreservation, fish models, zebrafish, medaka, Xiphophorus, review
1. Introduction
With the development of genomic research conserved and species-specific genetic and molecular mechanisms can be identified, and comparative studies among vertebrate species are becoming commonplace for human biomedical research. As the largest class of vertebrates, bony fishes offer almost unlimited versatility for research. Generally, model fish require features such as small body size, high fecundity, ease of culture, and most importantly, specific characteristics for particular research topics. Currently, the most widely used fish models are zebrafish Danio rerio (Driever et al., 1994; Kari et al., 2007), medaka Oryzias latipes (Wittbrodt et al., 2002), live-bearing fishes of the genus Xiphophorus (Walter and Kazianis, 2001), mummichog Fundulus heteroclitus (Atz, 1986; Burnett et al., 2007) and the pufferfish Fugu rubripes (Elgar et al., 1996). With extensive studies using these fish models, tens of thousand of specific strains and lines have been created, and are currently housed worldwide as live animals in resource centers, such as the Zebrafish International Resource Center (University of Oregon, Eugene, OR) which holds around 1080 inbred, transgenic, knockout and mutant strains, the University of Georgia (Athens, GA), which holds several inbred and transgenic medaka lines, and the Xiphophorus Genetic Stock Center (Texas State University, San Marcos, TX), which holds 61 inbred lines (for 50 to more than 100 generations) of 24 species. Preservation of the genetic resources of these and other valuable fishes presents significant and urgent challenges. Gamete (sperm and egg) or embryo cryopreservation is a useful approach to address these challenges.
Ideally, a conservation program should include the preservation of sperm, eggs, or embryos and larvae to secure the revival of species or strains. Currently, cryopreservation techniques in fish are mostly applied to sperm. Cryopreservation has not been successful for eggs and early embryos because of their large size, high lipid content, polar organization (Blesbios and Labbe, 2003), and membrane impermeability (Hagedorn et al., 1998). Sperm cryopreservation in fish was first reported more than 50 years ago (Blaxter, 1953). Since then, more than 200 species of fish have been studied (Rana, 1995; Tiersch and Mazik, 2000). However, most research has focused on large-bodied aquaculture species, such as salmonids, carps, and catfishes, and only several studies have addressed aquarium fishes (Tiersch, 2001), which are characterized by small body sizes, and a sperm availability limited to microliters. Consequently, sperm cryopreservation in these fishes presents challenges, such as in experimental design, gamete collection, and artificial fertilization, especially in live-bearing fishes.
Overall, zebrafish, medaka, and Xiphophorus fishes are grouped (somewhat artificially) as aquarium fishes, but they possess some distinct differences. For example, they occur naturally in two habitats: strict freshwater (zebrafish and Xiphophorus) (Hawkins et al., 2001) and brackish-water-accommodated freshwater (medaka) (Inoue and Takei, 2002), and they have two reproduction modes: external fertilization (zebrafish and medaka) and internal fertilization (Xiphophorus). We intend for this review to provide readers with an overview of sperm cryopreservation in these important biomedical fish models, and to serve as a template for research on other aquarium fishes.
2. Characteristics of zebrafish, medaka, and Xiphophorus
2.1. Habitat
The natural environment can influence reproductive modes and traits. Zebrafish, a strict freshwater species, naturally occurs in slow or still freshwater systems (rivers, small stream, pools and rice paddies) in a range extending from Pakistan in the west to Myanmar (Burma) in the east, and from Nepal in the north to the Indian state of Karnataka in the south (Engeszer et al., 2007). Fishes of the genus Xiphophorus naturally live in backwaters in Mexico, Guatemala, Belize and Hondura along the Gulf coast of Mexico (Kallman, 2001), and are also strict freshwater species. Medaka is a euryhaline species, distributed widely in freshwater of China, Japan, and Korea (Naruse et al., 1993; Naruse, 1996), and also found in brackish water (Miyamoto et al., 1986). Unlike zebrafish and Xiphophorus fishes, medaka can be acclimated from freshwater to brackish and even sea water, and can reproduce in fresh water and brackish water (Inoue and Takei, 2002).
2.2. Reproduction
Zebrafish, medaka, and Xiphophorus fishes are all dioecious. Zebrafish reproduce by external fertilization. Eggs and sperm are released into environmental water to fertilize and develop, and fry can hatch out at about 24 h after fertilization at 26-28 °C (Laale, 1977).
In medaka, the male and female participate in mating behavior before spawning, eggs are expelled as a cluster attached to the belly of the female, and become attached to floating water plants. Fry hatch out after 7-10 d at 26 °C (Yamamoto, 1975a). Although fertilization is usually external, it has been reported that internal fertilization and development of eggs may occur in extremely rare cases (Amemiya and Murayama, 1931). Eggs fertilized within the female are shed together with ripe ova at the next spawning (usually the next day).
For Xiphophorus fishes, the reproduction is internal fertilization. Males are distinguished from females by the secondary sexual organ, a modified anal fin called the gonapodium, and other phenotypes such as body color and in some species, a sword-like tail. After copulating with the male, female Xiphophorus can store sperm for months and subsequently produce broods at approximately 30-d intervals for 4-5 months without the presence of a male. The intervals between mating and the first brood are irregular and can vary between 26 and 90 d (Tavolga, 1949).
2.3. Body size and availability of testis
Zebrafish, medaka, and Xiphophorus are all small-bodied fishes with body lengths of less than 5 cm, and the availability of sperm is limited to several microliters. For zebrafish, the accumulated data of 64 mature males (6-8 months old) in our laboratory showed that for body lengths of 3.1 ± 0.1 cm (mean ± SD), and body weights of 0.484 ± 0.077 g, the testis dissected weighed 7.0 ± 2.5 mg. Correlation analysis showed that body weight, body length, and testis weight were significantly correlated with each other (P ≤ 0.015) and the coefficient of determination was 0.4327 between body weight and body length, 0.0914 between standard length and testis weight, and 0.5105 between body weight and testis weight (Figure 1, column A).
Figure 1.
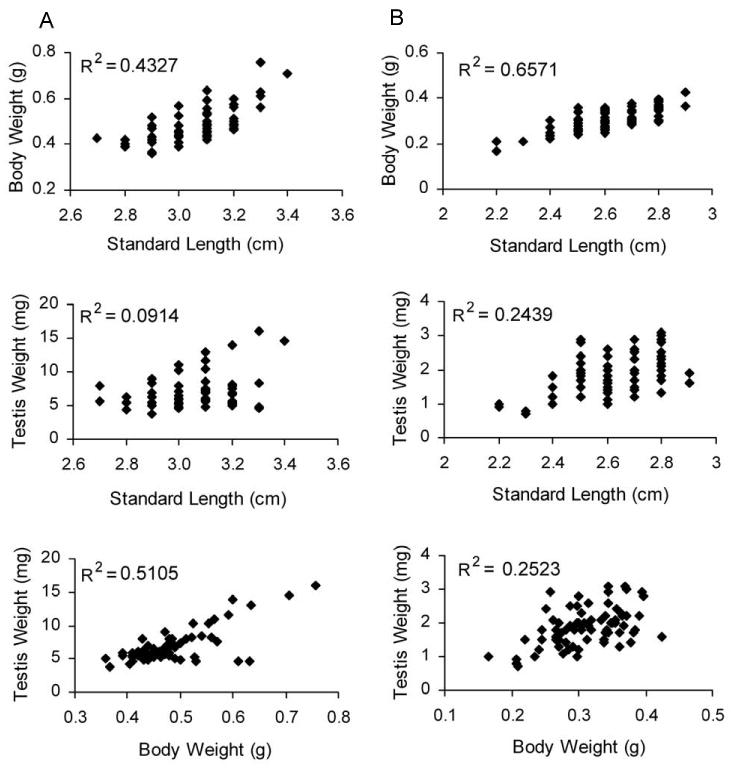
Body weight (mean ± SD), standard length, testis weight, and their correlations for males of zebrafish (N = 64, column A) and medaka (N = 74, column B).
For medaka, a summary of 74 mature males (∼6 months old) showed for standard body lengths of 2.6 ± 0.2 cm (mean ± SD), and body weight of 0.311 ± 0.052 g, the testis dissected weight was 1.9 ± 0.6 mg. Correlation analysis showed that body length, body weight, and testis weight were positively correlated to each other (P ≤ 0.000), and the coefficient of determination was 0.6571 between body weight and body length, 0.2493 between standard length and testis weight, and 0.2523 between body weight and testis weight (Figure 1, column B).
For Xiphophorus helleri, a summary for 45 males showed that for body standard lengths of 3.20 ± 0.25 cm, and body weights of 0.630 ± 0.168 g, the testis weight was 9.18 ± 5.49 mg (Huang et al., 2004b). For Xiphophorus couchianus, a total 66 males (Xc strain) showed that for body lengths of 2.0 ± 0.2 cm (mean ± SD), and body weights of 0.184 ± 0.052 g, the testis weight was 3.1 ± 1.0 mg (Yang et al., in review). Correlation analysis showed that body length, body weight, and testis weight were positively correlated to each other (P ≤ 0.044), and the coefficient of determination was 0.6852 between body weight and standard length, 0.1069 between standard length and testis weight, and 0.1275 between body weight and testis weight (Yang et al., in review).
2.4. Testis morphology
Gonadal and gamete morphology of fishes has been studied at anatomical or histological levels to identify annual reproduction cycle and length of breeding season for decades, and have been used for analysis of evolution and phylogeny in bony fishes (Jamieson, 1991; Parenti and Grier, 2004). For zebrafish, medaka and Xiphophorus fishes, their testis structures fall into three different types. Zebrafish possess what has been characterized as the anastomosing tubular type (Maack and Segner, 2003), which is widely found throughout primitive teleost taxa (Parenti and Grier, 2004). Medaka posses the type characterized as the restricted lobular testis (Grier, 1976), in which spermatogonia distribute into the distal ends of lobules. Xiphophorus fishes possess the type characterized as the restricted lobular testis (Grier et al., 1980), and the spermatozoa are formed into spermatozeugmata (“sperm bundles”), a specialized structure with spermatid nuclei oriented outward toward the sertoli cells.
2.5. Sperm motility activation
Fish spermatozoa are usually immotile in the testis and seminal plasma in most species studied (Cosson, 2004). Naturally, sperm are activated during spawning to fertilize eggs. For most fishes with external fertilization, osmolality is a dominant factor for activating sperm (Morisawa and Suzuki, 1980). In general, for freshwater fish species, sperm motility is initiated by exposure to hypotonic solutions; for marine species, sperm motility is initiated by exposure to hypertonic solutions (Morisawa and Suzuki, 1980). These activation modes match the environment where spermatozoa function during spawning. For example, zebrafish inhabit freshwater, and sperm motility can be activated by exposure to hypotonic solutions (Yang et al., 2007a).
Although they inhabit freshwater, as a euryhaline fish medaka show a mode for activation of sperm motility different from the typical freshwater fishes. Sperm motility in medaka could be initiated by distilled water without electrolytes (25 mOsmol/kg) and by Hanks' balanced salt solution with an osmolality spanning from 92 to 686 mOsmol/kg, a range including hypotonic, isotonic, and hypertonic osmolalities (Yang and Tiersch, in review). Upon activation, the sperm could remain continuously motile for as long as one week. These sperm characteristics are distinct from those in most freshwater and marine fishes whether they are external or internal fertilizers, and are potentially representative of many euryhaline fishes.
As viviparous fishes, Xiphophorus possess a completely different mechanism for activation of sperm motility. Within the testis, sperm are compacted into spermatozeugmata and are immotile. When suspended in electrolyte solutions isotonic to seminal plasma, the sperm become motile, but in either hypertonic or hypotonic solutions, the sperm are quiescent (Morisawa and Suzuki, 1980; Yang et al., 2006). This mode of motility activation has been described in Xiphophorus species (Huang et al., 2004a; Yang et al., 2006) and mosquitofish Gambusia affinis (Morisawa and Suzuki, 1980), but may not be representative of all viviparous fishes, such as the redtail splitfin Xenotoca eiseni of the family Goodeidae (our unpublished data). This is because viviparity has evolved independently in a variety of groups (e. g. Long et al., 2008), thus presenting a diverse array of adaptations to address the problem of internal fertilization (Ryan, 1998; DeMarais and Oldis, 2005).
3. Development of protocols for sperm cryopreservation
Cryopreservation is a technique involving a series of steps including sample collection, sperm extension, cryoprotectant selection, cooling, storage, thawing, and viability detection (Tiersch, 2000). Development of protocols for sperm cryopreservation requires suitable choices at each step and consideration of the interactions among the factors. The success of cryopreservation can be demonstrated by fertilization and production of live offspring. Due to the multiple steps and their interactions, errors at each step can accumulate and lead to considerable losses of viable cells. Thus, careful attention should be given to the numerous details at each step, and care should be taken to reduce or eliminate sources of uncontrolled variation (Leibo, 2000). Protocols of sperm cryopreservation can vary because of species-specific differences in sperm size, shape, and biochemical characteristics. Development of protocols for sperm cryopreservation has been studied over the past 25 years for zebrafish, medaka, and Xiphophorus fishes (Table 1). We review and compare below the current status of sperm cryopreservation in these fishes.
Table 1.
Summary of protocols for sperm cryopreservation of zebrafish Danio rerio, medaka Oryzias latipes and Xiphorphorus fishes
| Extender | Cryoprotectant | Packaging | Cooling rate | Thawing method | Fertilization | Reference |
|---|---|---|---|---|---|---|
| Zebrafish | ||||||
| Ginsburg | 8.3% Methanol | Capillary | 16 °C/min from 4 to -35 °C | Room temperature | 51 ± 36%a | Harvey et al. 1982 |
| BSMIS | 10% DMA | Capillary | Placement on dry ice for 30 min | Dilution with 20× volume of room temperature extender | 9-14% | Morris et al. 2003 |
| Ginsburg | 8.3% Methanol | Cryovials | Placement on dry ice for 20 min | 33 °C for 8 s | 28 ± 18% | Draper et al. 2004 |
| HBSS | 8% Methanol | French straw | 10 °C /min from 5 to -80 °C | 40 °C for 5 s | 33 ± 20% | Yang et al. 2007 |
| Medaka | ||||||
| Fetal bovine serum | 10% DMF | Cryovials | Placement in vapor of liquid nitrogen for 10 or 20 min | 30 °C for 0.5-1 min, dilution in 2× of BSS solution | 96-100% | Aoki et al. 1997 |
| 0.6 M sucrose | 10% DMSO | Capillary | Placement on dry ice for 20 min | Holding the capillary between fingers | 85% | Krone and Wittbrodt 1997 |
| Xiphophorus helleri | ||||||
| HBSS 240-300 | 10% DMSO | French straw | 45 °C /min from 5 to -80 °C | 40 °C for 7 s | 29 ± 8%b | Huang et al. 2004 |
| HBSS300 | 14% glycerol | French straw | 20-35 °C/min from 5 to -80 °C | 40 °C for 7 s | 77 ± 3% b | Huang et al. 2004 |
| HBSS310 HBSS500 |
14% glycerol | French straw | 25 °C /min from 5 to -80 °C | 40 °C for 5 s | 1-3 spawned of 15 females | Yang et al. 2007 |
| Xiphophorus couchianus | ||||||
| HBSS 240-300 | 14% glycerol | French straw | 25 °C /min from 5 to -80 °C | 40 °C for 7 s | 78±3% b | Huang et al. 2004 |
| HBSS500 | 14% glycerol | French straw | 25 °C /min from 5 to -80 °C | 40 °C for 5 s | 2 spawned of 15 females | Yang et al. in review |
This number is not actual value, it is relative to the hatchery control which is 71 ± 5%; thus the actual rate was: 0.71*51 = 36%.
Post-thaw motility only, no fertilization data available.
3.1. Sperm collection
Sperm of aquarium fishes can be collected by stripping or by crushing of dissected testis. Due to their small body size, the availability of sperm by stripping is limited to 1-2 μl. For Xiphophorus fishes, stripped sperm samples are a mixture of single sperm cells, and broken and intact bundles (our unpublished observations). For zebrafish and medaka, the stripped samples are composed of highly concentrated single cells. Collection by stripping of sperm samples avoids the killing of valuable fish, and individual males can be sampled repeatedly. However, to maximize the volume of sperm available, especially to allow experimental replication, crushing of dissected testis has been used for sperm collection in most published studies.
3.2. Extender selection
Dilution of sperm in extender solution is necessary for cryopreservation. The role of the extender is to retain the functional capability and fertilizing ability of sperm by controlling the pH, osmolality, ion concentration, and in some cases, the supply of energy. An understanding of sperm activation and motility is necessary to formulate extender solutions. Usually, extenders are salt-balanced buffers with certain pH and osmolality to prevent sperm motility activation. Although, many extenders have been reported for sperm cryopreservation, there is not always an observed difference in post-thaw motility (Stoss and Holtz, 1981).
For zebrafish, osmolality is the dominant factor to control sperm motility activation as stated above. Once activated by hypotonic osmolality, sperm have a short burst of motility (30 sec to 5 min) (Yang et al., 2007a). Thus, sperm need to be held in extender that is isotonic to the plasma osmolality (∼300 mOsmol/kg) to inhibit activation. In zebrafish, three different extenders (Ginsburg, BSMIS, HBSS) have been reported to for use with sperm cryopreservation (Appendix), and all of them functioned well to retain fertility of post-thaw sperm (Harvey et al., 1982; Morris et al., 2003; Draper et al., 2004; Yang et al., 2007a). With respect to composition, Ginsburg extender contains a specific brand of powdered skim milk (Harvey et al., 1982; Draper et al., 2004) which is not readily available worldwide, and impedes the observation of sperm motility and morphology, especially after thawing. The other extenders, HBSS and BSMIS, which do not contain milk, are more suitable choices for sperm cyropreservation of zebrafish.
Medaka sperm have a long swimming duration upon activation (Yang and Tiersch, in review). Therefore, extender is not necessarily required to inhibit sperm motility. Sperm suspended in HBSS at osmolalities ranging from 92 to 373 mOsmol/kg remained continuously motile for 7 days (Yang and Tiersch, in review). Therefore, HBSS can be a candidate extender for medaka sperm across this range of osmolalities. In artificial insemination of medaka eggs, a balanced salt solution (BSS, see Appendix) with an osmolality of 230 mOsmol/kg was routinely used to retain the fertility of the gametes (Iwamatsu, 1983; The office of NBRP Medaka, 2007). For sperm cryopreservation, different extenders have been used and have yielded comparable post-thaw sperm motility and fertility, including BSS (The office of NBRP Medaka, 2007), fetal bovine serum (Aoki et al., 1997), 0.6 M sucrose (Krone and Wittbrodt, 1997), and HBSS with an osmolality of 350 mOsmol/kg (our unpublished data). Systematic investigation is needed to test whether different osmolalities and composition of extenders can produce differences in sperm viability during sperm cryopresrevation.
For Xiphophorus fishes, motility can be activated by isotonic solutions (∼310 mOsmol/kg), rather than hypotonic or hypertonic, and upon activation sperm can remain continuously motile for as long as one week (Huang et al., 2004b; Yang et al., 2006). Based on these characteristics, HBSS at 310 mOsmol/kg was used as the extender for sperm cryopreservation, and high motility was obtained for cryopreserved X. helleri sperm after thawing (58 ± 7%) (Huang et al., 2004c) and X. couchianus (78 ± 3%) (Huang et al., 2004a). Alternatively, HBSS at an osmolality of 500 mOsmol/kg was also used as extender for X. helleri because sperm immobilized at this osmolality could be re-activated by changing into isotonic osmolality (Yang et al., 2006). Sperm of X. helleri extended in HBSS at an osmolality of 500 mOsmol/kg were also cryopreserved, and high motility (∼55%) was obtained after thawing (Yang et al., 2006). It was hypothesized that osmotically immobilizing sperm prior to freezing by use of hypertonic HBSS at 500 mOsmol/kg could conserve sperm energy capacity, and consequently could provide an advantage for internal fertilization compared to sperm cryopreserved using isotonic HBSS, especially if the sperm were to be stored prior to cryopreservation or insemination. Through artificial insemination, sperm cryopreserved at 310 and 500 mOsmol/kg each HBSS produced verified (hybrid) offspring (Yang et al., 2007b).
Because of the limited availability of sperm from aquarium fishes, dilution of samples with extender is useful to maximize the volume available for subsequent analysis. Therefore, dilution ratios of testis weight (mg) : HBSS volume (μl) of 1:50, 1:100, 1:200, 1:400, 1:800, and 1:1600 were evaluated with sperm of X. helleri. Motility of samples diluted at 1:50, 1:100 and 1:200 was found to be not significantly different before freezing or after thawing, but declined significantly at ratios of higher than 1:200 (Huang et al., 2004b). For zebrafish and medaka, no similar evaluation of dilution ratio has been reported, but ratios of testis weight (mg) : HBSS (μl) of 1: 50-80 were used for sperm cryopreservation, and high motility (%) and fertility (%) were obtained in thawed sperm (Yang et al., 2007a). Extreme dilution of samples has been found to reduce sperm motility in animals such as mammals (Harrison et al., 1978), rainbow trout (Scott and Baynes, 1980; Billard, 1983) and oysters (Paniagua-Chavez et al., 1998), and remains a topic for study in aquarium fishes.
3.3. Cryoprotectant selection
In sperm cryopreservation, cryoprotectants are additives necessary for protection against freezing damage due to intracellular ice crystal formation and excessive dehydration. Usually cryoprotectants are grouped into two categories: permeating cryoprotectants (e.g., dimethyl sulfoxide (DMSO), methanol, and glycerol) and non-permeating cryoprotectants (e.g., egg yolk, milk, and proteins). A variety of cryoprotectants have been evaluated for different species (Full et al., 2004), and selections are usually determined experimentally. Theoretically, the higher the concentrations of cryoprotectant, the better the protection to sperm cells should be during cryopreservation. However, high concentration of cryoprotectants can be toxic or lethal to sperm cells. The optimum concentration should be a value which balances these two effects. Usually, concentrations of 5 to 20% were chosen for experiments with sperm cryopreservation in aquarium fish.
For zebrafish, the toxicity of DMSO, N,N-dimethyl acetamide (DMA), methanol, and glycerol at concentrations of 5, 10, and 15% were evaluated with sperm cells. Glycerol was the most toxic, and was eliminated for sperm cryopreservation. The other three chemicals were used for sperm cryopreservation, and analysis of post-thaw motility showed that methanol at a concentration of 8% was the best choice (Yang et al., 2007a). This was also the choice in two earlier studies (Harvey et al., 1982; Draper et al., 2004). In addition, DMA (10%) was used as cryoprotectant for zebrafish sperm (Morris et al., 2003), but the fertilization level after thawing (9-14%) was lower than that observed (28-51%) when methanol was used as cryoprotectant (Harvey et al., 1982; Draper et al., 2004; Yang et al., 2007a).
For medaka, 10% DMSO and 10% dimethylformamide (DMF) were used to cryopreserve sperm, and after thawing yielded motility (78-100%) and hatching (82-100%), comparable to fresh sperm (Aoki et al., 1997; Krone and Wittbrodt, 1997). However, systematic comparison with other cryoprotectants has not been performed.
For Xiphophorus, DMSO, DMF, N-dimethyl acetamide, glycerol, propylene glycol, methanol, and sucrose were evaluated as cryoprotectants, each with final concentrations of 6% and 10% (v/v). The results indicated that DMSO and glycerol were suitable cryoprotectants, and further evaluation of these two cryoprotectants at different concentrations showed that glycerol was better than DMSO in retaining motility and prolonging storage time for X. helleri sperm after thawing, and the effective concentration for glycerol was 14% (Huang et al., 2004c). Also, glycerol showed the best results for cryopreservation of X. couchianus sperm with a concentration of 14% (Huang et al., 2004a), and for osmotically immobilized sperm from X. helleri (Yang et al., 2006; Yang et al., 2007b).
After mixing with sperm, cryoprotectants require time for equilibration to penetrate the cells. This is a dynamic process depending on the permeability of sperm cells, cryoprotectants, and their concentrations. For zebrafish, based on toxicity analysis, a 10-min equilibration time was chosen (Yang et al., 2007a). For medaka, no specific equilibration time was reported, with sperm going directly to the freezing process after mixing with the cryoprotectants (Aoki et al., 1997; Krone and Wittbrodt, 1997). For Xiphophoprus, equilibration times of 10, 20, 30, 60, and 120 min were evaluated for sperm of X. helleri and X. couchianus. An equilibration time of less than 30 min yielded the highest post-thaw motility in each species, but there was no consistent difference across equilibration times ranging from 10 to 120 min (Huang et al., 2004a; Huang et al., 2004b).
3.4. Packaging of samples for freezing
In sperm cryopreservation, packaging of samples for freezing and storage is important to standardize the cooling rate, and to assure sample identification. Currently, several different kinds of containers have been used for aquarium fishes such as plastic cryovials, glass tubes and ampules, and plastic straws. The different materials and shapes of these containers result in different heat transfer properties during freezing and thawing. Even for the same style of container, differences can exist with products from different manufacturer producers, which can result in variation of cooling or thawing rates. Therefore, it is necessary to standardize the packaging method to ensure that protocols will be repeatable especially in different laboratories.
For the aquarium fishes in this review, the small volumes of sperm available limited the choices for sample packaging. In zebrafish and medaka, glass capillary tubes or cryovials were first employed in sperm cryopreservation (Harvey et al., 1982; Aoki et al., 1997; Krone and Wittbrodt, 1997; Morris et al., 2003; Draper et al., 2004). Recently, to standardize protocols with potential for automation at high throughput, French straws were chosen for sperm packaging with the smallest commercially available volume (0.25 ml), and a more standardized protocol was developed with results comparable to previous studies (Yang et al., 2007a). For Xiphophorus fishes, French straws (0.25 ml) were used for sample packaging in all studies (Huang et al., 2004a; Huang et al., 2004c; Yang et al., 2006; Yang et al., 2007b). Compared to capillary tubes or cryovials, the use of French straws has the following advantages: potential for use with automated straw filling and sealing equipment, sample identification by permanent printing of alpha-numeric labels or barcodes on straws, sample biosecurity by complete sealing of the straw, and standardization of the cooling process.
3.5. Cooling rate selection
Cooling rate is a crucial factor in sperm cryopreservation because it affects the osmotic and pH balance of intracellular and extracellular solutions during freezing. Theoretically, with an excessively slow cooling rate, osmotic equilibrium is maintained, and much of the freezable water leaves the cell resulting in excessive dehydration; with an excessively fast cooling rate, little or no freezable water leaves the cell, and thus large intracellular crystals can form, causing damage to the cell. Ideally, a balanced situation allows survival when the cooling rate is fast enough to minimize the time of exposure to concentrated solutions and yet is slow enough to minimize the amount of intracellular ice formation. Optimum cooling rates vary with different cryoprotectants and the physiology of sperm cells from different species, and can be determined empirically by experimentation, or predicted by theoretical calculation using techniques such as differential scanning calorimetry (DSC). This technique can be used to estimate water permeability (Lp) at subzero temperatures and the activation energy of that process, and these values can be used to compute the amount of water loss in cells as a function of cooling rate and temperature, and pridict the optimum cooling rate from such plots (Devireddy et al., 1998). The actual cooling process for aquarium fishes has been accomplished by use of the following methods: placement on dry ice, suspension in liquid nitrogen vapor, or controlled cooling with a programmable freezer. Dry ice and liquid nitrogen vapor are inexpensive and can be used in field situations, but the cooling rates are difficult to quantify and control. In contrast, programmable freezers offer high levels of control and reproducibility, but are expensive and difficult to use in the field.
For zebrafish, the cooling methods that have been reported are placement on dry ice (Harvey et al., 1982; Morris et al., 2003; Draper et al., 2004) and a programmable freezer (Yang et al., 2007a). With 8% methanol as the cryoprotectant and HBSS as extender, the suitable cooling rate was identified as 10 °C/min (Yang et al., 2007a), and with Ginsburg buffer plus powdered milk as extender 16 °C/min was selected (Harvey et al., 1982).
For medaka, the cooling rate was controlled by use of liquid nitrogen vapor (Aoki et al., 1997) or dry ice (Krone and Wittbrodt, 1997) without quantification. In our unpublished studies, cooling rate, controlled by a programmable freezer, was found to be a sensitive factor for determining post-thaw motility of medaka sperm. A change of as small as 5 °C/min in the cooling rate resulted in a significant change in post-thaw motility (unpublished data).
For Xiphophorus, cooling rate was controlled by use of a programmable freezer. The results showed that 20 to 30 °C per min was optimum when sperm were cryopreserved with 14% glycerol (Huang et al., 2004a; Huang et al., 2004c). The optimal value predicted for cooling rate by differential scanning calorimetry agreed with the empirical results in X. helleri (Huang et al., 2004a; Huang et al., 2004c; Thirumala et al., 2005), but not in X. maculatus for which the cooling rate was predicted as 47 °C/min (Pinisetty et al., 2005).
3.6. Storage of frozen samples
Holding of frozen samples in liquid nitrogen (-196 °C) in a storage dewar is a standard method for cryogenic storage of samples from aquarium fishes. During storage, important considerations are identification, potential contamination, and inventory of frozen samples. The use of plastic or French straws for packaging, especially the newer forms with high safety and durability, offers the advantages of permanent labeling by printer, and complete sealing of the straws which minimizes or prevents transfer of materials (e. g. sperm cells or bacteria) among samples stored in the same dewar (Morris, 2005).
3.7. Thawing of frozen samples
Theoretically, the process of thawing is the reverse of freezing, and thus the damage that can occur during cooling also can occur during warming, primarily through formation of ice crystallization between -40 °C and 0 °C (Leung, 1991). Thus, it is usually desirable to thaw cryopreserved samples rapidly to minimize the period of crystal propogation (termed “recrystallization”). Currently, systematic evaluation of thawing rates has not been reported for sperm cryorpeservation of zebrafish, medaka, or Xiphophorus, probably because of the limited sample volumes. Generally, for frozen samples packaged in 0.25-ml French straws, a 5-sec exposure within a 40 °C water bath is practical and yields suitable motility and fertility after thawing in zebrafish and Xiphophorus fishes (Huang et al., 2004a; Huang et al., 2004c; Yang et al., 2006; Yang et al., 2007a; Yang et al., 2007b). For frozen samples packaged in capillary tubes or cryovials in zebrafish and medaka, the thawing process has been performed by leaving samples at room temperature (Harvey et al., 1982), diluting with room temperature buffer (Aoki et al., 1997; Morris et al., 2003), holding within a 33 °C water bath (Draper et al., 2004), or holding within the fingers (Krone and Wittbrodt, 1997).
4. Viability analysis of cryopreserved sperm
The purpose of cryopreservation is to obtain viable sperm which retain their fertility. Examination of the viability of cryopreserved sperm generally includes evaluation of morphology, membrane integrity, motility, ability to bind oocytes, and fertilization. Motility is the most widely used assay, but fertilization is considered to be the most informative.
Artificial insemination is necessary to test the fertility of cryopreserved sperm. This process includes a series of steps: egg collection, holding of eggs prior to fertilization, thawing of cryopreserved sperm, mixing of the sperm and eggs, activation of the gametes, fertilization confirmation, hatching of fertilized eggs, and offspring harvest and identification. For species with internal fertilization such as the Xiphophorus fishes, this process involves more complicated techniques such as the injection of sperm (2-4 μl) into the female reproductive tract and pregnancy confirmation. Factors related to females such as egg quality can also determine fertilization success. Therefore, development of standardized protocols for fertilization assays including collection and holding of eggs and sperm need be included in protocol development for sperm cryopreservation.
For zebrafish and medaka, artificial fertilization protocols have been established with fresh sperm, and can be directly modified to provide fertilization analysis of cryopreserved sperm (Yamamoto, 1975b; Westerfield, 1995). Eggs can be collected daily by squeezing of females or dissection (for medaka), held in isotonic buffer to retain fertility, and then be mixed with a sperm suspension for fertilization. For zebrafish, after mixing of sperm and eggs, fresh water needs to be added to activate gametes for fertilization, but for medaka, this is not necessary because fertilization can occur in isotonic buffer as stated above. Fertilization and hatching are determined by assessing the percentage of developing embryos or hatched fry.
For Xiphophorus fishes, artificial insemination with cryopreserved sperm must consider points such as the use of virgin females (because female Xiphophorus can store sperm for successive broods), proper injection volume and technique, pregnancy monitoring for as long as 90 days, and confirmation of paternal contribution to offspring. Previous work has shown that centrifugation did not reduce post-thaw motility (Dong et al., 2007b), and thus removal of the cryoprotectant from thawed sperm by washing is feasible although it may not be necessary (Yang et al., 2007b). Also, social interactions among females are another factor to be considered because they can influence maturation and brood timing (Earley, 2006). Thus, based on these considerations, hybrid offspring produced through artificial insemination with cryopreserved sperm from X. helleri, were used for verification of paternity in the first report for viviparous fishes (Yang et al., 2007b). Following the same protocols, live young have been produced with cryopreserved sperm from X. couchianus (Yang et al., in review).
5. Future research topics
With the broadening use of aquarium fishes as research models, new mutants, transgenic individuals, strains, and lines are being continually created. To protect and maintain this large number of valuable individuals, strains and lines, it is essential to develop a germplasm repository program with gamete and embryo cryopreservation. Sperm cryopreservation will be the main focus based on current technologies and approaches until the development of protocols for egg and embryo crypreservation in fish. At present, protocols for sperm cryopreservation are available for use with zebrafish, medaka, and Xiphophorus fishes, and live offspring have been produced with cryopreserved sperm. Future research needs include the following topics.
5.1 Standardization of the procedures for sperm cryopreservation
To enable application of protocols developed for sperm cryopreservation across different laboratories, procedure standardization is necessary, especially for determination and choice of sperm concentration, sample packaging, and labeling. Standardization is also necessary for terminology (e.g. strict definition of terms such as fertilization and motility) and in defining the essential parameters necessary for complete reporting of results. Sperm concentration is an extremely important factor in sample preparation for cryopreservation, and can directly influence results (Dong et al., 2007a). Because most studies of aquatic species cryopreservation do not control or report sperm concentrations, it is likely that this is the single largest uncontrolled variable in this research area (Tiersch et al., 2007). Also, due to the limited sperm availability in aquarium fishes, maximized use of cryopreserved samples for strain or line recovery requires control of sperm concentration and determination of suitable sample loading in each container (e.g., French straw). Hemocytometer counts and spectrophotometry are commonly used methods to measure sperm concentration. For aquarium fishes, specialized spectrophotometers with a 2-μl sample size are desirable to avoid sample waste.
5.2. Evaluation of gamete quality
Considerable variation in characteristics of post-thaw sperm is generally the rule in most species studied (Holt, 2000; Thurston et al., 2002; Mazur et al., 2008) such as that reported for boar (Holt et al., 2005), rhesus monkey Macaca mulatta (Leibo et al., 2007), sea bream Sparus aurata (Cabrita et al., 2005), Pacific oyster Crassostrea gigas (Dong et al., 2005), eastern oyster Crassostrea virginica (Paniagua-Chavez and Tiersch, 2001), zebrafish Danio rerio (Yang et al., 2007b), and X. couchianus (Yang et al., in review). The reasons for this variability, for example a range in post-thaw sperm fertility of from 5 to 81% in samples with the same initial motility in zebrafish (Yang et al., 2007b), have not been identified. A more comprehensive understanding of sperm quality needs to be obtained for assessing within-species variation in the susceptibility of spermatozoa to damage during cryopreservation, including factors such as membrane integrity and acrosome-like structure disruption. In zebrafish, body condition factor has been found to positively relate with the motility and fertility of thawed sperm, and has been suggested as an indicator for evaluating sperm quality for cryopreservation (Yang et al., 2007a).
A genetic basis for variation in post-thaw semen viability has been suggested (Thurston et al., 2002), and it has been proposed that certain molecular markers could be identified to link with the genes influencing this variation. Currently, estimation of sperm quality before cryopreservation provides an opportunity to predict the outcome of sperm cryopreservation, but it will be necessary to develop a functional and molecular understanding of the factors that influence sperm cryopreservation. Sperm quality estimation could include: analysis of the relationship of sperm performance with body condition and nutrition, the use of flow cytometry for measuring membrane integrity and mitochondrial integrity, the use of computer assisted sperm analysis (Cosson, 2004) for quantifying motility characteristics, and detection of changes in protein profiles in sperm after cryopreservation for predictive biomarkers.
5.3. Establishment of a working repository system for cryopreserved sperm
After cryopreservation, frozen samples stored in liquid nitrogen require permanent and clear identification. The use of plastic straws for packaging of sperm samples offers the advantage of permanent labeling by direct printing and the use of an automated barcode reader. A labeling and coding system for each cryopreserved sample needs to be developed, including information on biology, genetics, intellectual property rights, source and ownership, sample collection, and handling. Integrated databases need to be developed with the existing genetic databases for these fishes, inventory procedures need be established to allow easy access to cryopreserved samples from a specific species and strain, and biosecurity procedures need be established to minimize or prevent transfer of pathogenic contaminants with cryopreserved samples (Jenkins 2000a, b; Tiersch and Jenkins, 2003).
5.4. Evaluation of the potential for high-throughput processing
To establish a working repository, high-throughput processing will be necessary for large numbers of samples. This high throughput will include the use of automated or semi-automated sample handling systems for straw labeling, filling, sealing, and freezing. Recently the National Center for Research Resources of the National Institutes of Health (2007) held a workshop to address the achievement of high-throughput repositories for biomedical germplasm preservation including aquarium fishes. This workshop assessed the current status of germplasm cryopreservation for animal models, discussed current problems and challenges, especially the availability of germplasm preservation technologies, identified gaps in current scientific knowledge such as the mechanisms of injury during cryopreservation, and evaluated the potential for application of novel technologies for high-throughput germplasm preservation capabilities. All of these topics are worthy of future research to achieve high-throughput processing. Indeed, development of the capability for large-scale processing is probably the greatest current need for aquarium fish cryopreservation. Consider if we have 20,000 lines of aquarium fish that require preservation in germplasm repositories, and we choose to freeze only ten samples per line. If we process 100 samples per day (5 days per week), it would require 8 years to simply address the current backlog of samples. These are conservative estimates and serve to illustrate the need for rapid, standardized, automated, and effective protocols and technologies.
Acknowledgments
We thank R. Walter and L. Hazlewood of the Xiphophorus Genetic Stock Center, Z. Varga and C. Carmichael of the Zebrafish International Resource Center, and R. Winn and M. Norris of the Aquatic Biotechnology and Environmental Laboratory of the University of Georgia for providing fish, technical help and valuable discussion. This review was supported by funding from USPHS grant P-40-RR17072 from the NIH National Center for Research Resources, the U.S. Department of Agriculture, and the National, Louisiana Sea Grant College Programs, and the ACRES-LSU system collaborative program. This manuscript has been approved for publication by the Director of the Louisiana Agricultural Experiment Station as number 08-xx-xxxx.
Appendix
Extender formulations
-
BSMIS (Morris et al., 2003):
75 mM NaCl, 70 mM KCl, 2mM CaCl2, 1mM MgSO4 and 20 mM Tris, pH 8.0, store at 4 °C
-
BSS (Balanced salt solution) (Iwamatsu, 1983):
Solution A: 0.111 M NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.81 mM MgSO4-7H2O
Solution B: 0.6 M NaHCO3
Working solution: Adjust the solution A with solution B to bring the pH to 7.4
-
Ginsberg buffer (Ginsburg, 1963):
0.111 M NaCl, 3.4 mM KCl, 2.7 mM CaCl2-2H2O, 2.4 mM NaHCO3. Note: the order of addition is important to prevent precipitation.
Freezing medium (www.zfin.org): 9 ml Ginsburg buffer, 1ml methanol, and 1.5 g powered skim milk (Carnation brand). Note: the order of ingredients is important to prevent precipitation of the milk, and this medium needs to be used within 3 hr.
HBSS (Hanks' balanced salt solution):
0.137 M NaCl, 5.4 mM KCl, 1.3 mM CaCl2, 1.0 mM MgSO4, 0.25 mM Na2HPO4, 0.44 mM KH2PO4, 4.2 mM NaHCO3, and 5.55 mM glucose, pH = 7.2
Footnotes
Footnote for cover page: This paper is derived from a presentation given at the 4th Aquatic Animal Models of Human Disease Conference: hosted by Duke University's Nicholas School of the Environment and Earth Sciences, and Duke's Comprehensive Cancer Center, Durham, NC, USA, January 31 - February 3, 2008.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amemiya I, Murayama S. Some remarks on the existence of developing embryos in the body of an oviparous cyptinodont, Oryzias latipes (Temminck et Schlegel) Proc Imperial Acad (Tokyo) 1931;7:176–178. [Google Scholar]
- Aoki K, Okamoto M, Tatsumi K, Ishikawa Y. Cryopreservation of medaka spermatozoa. Zool Sci. 1997;14:641–644. [Google Scholar]
- Atz JW. Fundulus heteroclitus in the laboratory - a history. Am Zool. 1986;26:111–120. [Google Scholar]
- Billard R. Effects of celomic and seminal fluids and various saline diluents on the fertilizing ability of spermatozoa in the rainbow trout, Salmo gairdneri. J Reprod Fertil. 1983;68:77–84. doi: 10.1530/jrf.0.0680077. [DOI] [PubMed] [Google Scholar]
- Blaxter JHS. Sperm storage and cross-fertilization of spring and autumn spawning herring. Nature. 1953;172:1189–1190. [Google Scholar]
- Blesbios E, Labbe C. Main improvement in semen and embryos cryopreservation for fish and fowl. In: Planchenault D, editor. Workshop on Cryopreservation of Animal Genetic Resources in Europe; Paris, France. 2003. pp. 55–66. [Google Scholar]
- Burnett KG, Bain LJ, Baldwin WS, Callard GV, Cohen S, DiGiulio RT, Evans DH, Gomez-Chiarri M, Hahn ME, Hoover CA, Karchner SI, Katoh F, MacLatchy DL, Marshall WS, Meyer JN, Nacci DE, Oleksiak MF, Rees BB, Singer TD, Stegeman JJ, Towle DW, Van Veld PA, Vogelbein WK, Whitehead A, Winn RN, Crawford DL. Fundulus as the premier teleost model in environmental biology: Opportunities for new insights using genomics. Comp Biochem Physiol D. 2007;2:257–286. doi: 10.1016/j.cbd.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrita E, Robles V, Cunado S, Wallace JC, Sarasquete C, Herraez MP. Evaluation of gilthead sea bream, Sparus aurata, sperm quality after cryopreservation in 5ml macrotubes. Cryobiology. 2005;50:273–284. doi: 10.1016/j.cryobiol.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Cosson J. The ionic and osmotic factors controlling motility of fish spermatozoa. Aquacult Int. 2004;12:69–85. [Google Scholar]
- DeMarais A, Oldis D. Matrotrophic transfer of fluorescent microspheres in Peociliid fishes. Copeia. 2005;3:632–636. [Google Scholar]
- Devireddy RV, Raha D, Bischof JC. Measurement of water transport during freezing in cell suspensions using a differential scanning calorimeter. Cryobiology. 1998;36:124–155. doi: 10.1006/cryo.1997.2071. [DOI] [PubMed] [Google Scholar]
- Dong Q, Huang C, Eudeline B, Tiersch TR. Systematic factor optimization for cryopreservation of shipped sperm samples of diploid Pacific Oysters, Crassostrea gigas. Cryobiology. 2005;51:176–197. doi: 10.1016/j.cryobiol.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Dong Q, Huang C, Tiersch TR. Control of sperm concentration is necessary for standardization of sperm cryopreservation in aquatic species: Evidence from sperm agglutination in oysters. Cryobiology. 2007a;54:87–98. doi: 10.1016/j.cryobiol.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Dong Q, Huang C, Tiersch TR. Post-thaw amendment of cryopreserved sperm for use in artificial insemination of a viviparous fish, the green swordtail Xiphophorus helleri. Aquaculture. 2007b;259:403–414. doi: 10.1016/j.aquaculture.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper BW, McCallum CM, Scout JL, Slade AJ, Moens CB. A high-throughput method for identifying N-Ethyl-N-Nitrosourea (ENU)-induced point mutations in zebrafish. In: Detrich HW IIII, Westerfield M, Zon LI, editors. The zebrafish genomics, and informatics, Methods in Cell Biology. Vol. 77. Elsevier Press; San Diego: 2004. pp. 91–112. [DOI] [PubMed] [Google Scholar]
- Driever W, Stemple D, Schier A, Solnicakrezel L. Zebrafish - genetic tools for studying vertebrate development. Trends Genet. 1994;10:152–159. doi: 10.1016/0168-9525(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Earley RL. Xiphophorus: carving a niche towards a broader understanding of aggression and dominance. Zebrafish. 2006;3:287–298. doi: 10.1089/zeb.2006.3.287. [DOI] [PubMed] [Google Scholar]
- Elgar G, Sandford R, Aparicio S, Macrae A, Venkatesh B, Brenner S. Small is beautiful: Comparative genomics with the pufferfish (Fugu rubripes) Trends Genet. 1996;12:145–150. doi: 10.1016/0168-9525(96)10018-4. [DOI] [PubMed] [Google Scholar]
- Engeszer RE, Patterson LB, Rao AA, Parichy DM. Zebrafish in the wild: a review of natural history and new notes from the field. Zebrafish. 2007;4:21–40. doi: 10.1089/zeb.2006.9997. [DOI] [PubMed] [Google Scholar]
- Full B, Lane N, Benson E. Life in the Frozen State. CRC Press; New York: 2004. [Google Scholar]
- Ginsburg AS. Sperm-egg association and its relationship to activation of egg in Salmonid fishes. J Embryol Exp Morphol. 1963;11:13–33. [PubMed] [Google Scholar]
- Grier HJ. Sperm development in teleost Oryzias latipes. Cell Tissue Res. 1976;168:419–431. doi: 10.1007/BF00215993. [DOI] [PubMed] [Google Scholar]
- Grier HJ, Linton JR, Leatherland JF, Devlaming VL. Structural evidence for two different testicular types in teleost fishes. Am J Anat. 1980;159:331–345. doi: 10.1002/aja.1001590307. [DOI] [PubMed] [Google Scholar]
- Hagedorn M, Kleinhans FW, Artemov D, Pilatus U. Characterization of a major permeability barrier in the zebrafish embryo. Biol Reprod. 1998;59:1240–1250. doi: 10.1095/biolreprod59.5.1240. [DOI] [PubMed] [Google Scholar]
- Harrison RAP, Dott HM, Foster GC. Effect of ionic-strength, serum-albumin and other macromolecules on maintenance of motility and surface of mammalian spermatozoa in a simple medium. J Reprod Fertil. 1978;52:65–73. doi: 10.1530/jrf.0.0520065. [DOI] [PubMed] [Google Scholar]
- Harvey B, Norman KR, Ashwood-Smith MJ. Cryopreservation of zebrafish spermatozoa using methanol. Can J Zool. 1982;60:1867–1870. [Google Scholar]
- Hawkins WE, Clark MS, Shima A, Walter RB, Winn RN, Westerfield M. Four resource centers for fishes: specifies, stocks, and services. Mar Biotechnol. 2001;3:S239–S248. doi: 10.1007/s10126-001-0046-x. [DOI] [PubMed] [Google Scholar]
- Holt WV. Fundamental aspects of sperm cryobiology: the importance of species and individual differences. Theriogenology. 2000;53:47–58. doi: 10.1016/s0093-691x(99)00239-3. [DOI] [PubMed] [Google Scholar]
- Holt WV, Medrano A, Thurston LM, Watson PE. The significance of cooling rates and animal variability for boar sperm cryopreservation: insights from the cryomicroscope. Theriogenology. 2005;63:370–382. doi: 10.1016/j.theriogenology.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Huang C, Dong Q, Tiersch TR. Sperm cryopreservation of a live-bearing fish, the platyfish Xiphophorus couchianus. Theriogenology. 2004a;62:971–989. doi: 10.1016/j.theriogenology.2003.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Dong Q, Walter RB, Tiersch TR. Initial studies on sperm cryopreservation of a live-bearing fish, the green swordtail Xiphophorus helleri. Theriogenology. 2004b;62:179–194. doi: 10.1016/j.theriogenology.2003.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Dong Q, Walter RB, Tiersch TR. Sperm cryopreservation of green swordtail Xiphophorus helleri, a fish with internal fertilization. Cryobiology. 2004c;48:295–308. doi: 10.1016/j.cryobiol.2004.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Takei Y. Diverse adaptability in Oryzias species to high environmental salinity. Zool Sci. 2002;19:727–734. doi: 10.2108/zsj.19.727. [DOI] [PubMed] [Google Scholar]
- Iwamatsu T. A new technique for dechorionation and observations on the development of the naked egg in Oryzias latipes. J Exp Zool. 1983;228:83–89. [Google Scholar]
- Jamieson BGM. Fish Evolution and Systematics: Evidence from Spermatozoa. Cambridge University Press; Cambridge, UK: 1991. [Google Scholar]
- Jenkins JA. Infectious disease and quality assurance considerations for the transfer of cryopreserved fish gametes. In: Tiersch TR, Mazik PM, editors. Cryopreservation in Aquatic Species. World Aquaculture Society; Baton Rouge, LA: 2000a. pp. 343–363. [Google Scholar]
- Jenkins JA. Regulatory considerations for the global transfer of cryopreserved fish gametes. In: Tiersch TR, Mazik PM, editors. Cryopreservation in Aquatic Species. World Aquaculture Society; Baton Rouge, LA: 2000b. pp. 364–379. [Google Scholar]
- Kallman KD. How the Xiphophorus problem arrived in San Marcos, Texas. Mar Biotechnol. 2001;3:S6–S16. doi: 10.1007/s10126-001-0022-5. [DOI] [PubMed] [Google Scholar]
- Kari G, Rodeck U, Dicker AP. Zebrafish: An emerging model system for human disease and drug discovery. Clin Pharmacol Ther. 2007;82:70–80. doi: 10.1038/sj.clpt.6100223. [DOI] [PubMed] [Google Scholar]
- Krone A, Wittbrodt J. A simple and reliable protocol for cryopreservation of medaka Oryzias latipes spermatozoa. Fish Biol J Medaka. 1997;9:47–48. [Google Scholar]
- Laale HW. Biology and use of zebrafish, Brachydanio rerio in fisheries research - literature review. J Fish Biol. 1977;10:121–173. [Google Scholar]
- Leibo SP. Sources of variation in cryopreservation. In: Tiersch TR, Mazik PM, editors. Cryopreservation In Aquatic Species. World Aquaculture Society; Baton Rouge, Louisiana: 2000. pp. 75–83. [Google Scholar]
- Leibo SP, Kubisch HM, Schramm RD, Harrison RM, VandeVoort CA. Male-to-male differences in post-thaw motility of rhesus spermatozoa after cryopreservation of replicate ejaculates. J Med Primatol. 2007;36:151–163. doi: 10.1111/j.1600-0684.2006.00194.x. [DOI] [PubMed] [Google Scholar]
- Leung LKP. Principles of biological cryopreservation. In: Jamieson BGM, editor. Fish Evolution and Systematics: Evidence from Spermatozoa. Cambridge University Press; Cambridge, UK: 1991. pp. 231–244. [Google Scholar]
- Long JA, Trinajstic K, Young GC, Senden T. Live birth in the Devonian period. Nature. 2008;453:650–652. doi: 10.1038/nature06966. [DOI] [PubMed] [Google Scholar]
- Maack G, Segner H. Morphological development of the gonads in zebrafish. J Fish Biol. 2003;62:895–906. [Google Scholar]
- Mazur P, Leibo SP, Seidel GE. Cryopreservation of the germplasm of animals used in biological and medical research: Importance, impact, status, and future directions. Biol Reprod. 2008;78:2–12. doi: 10.1095/biolreprod.107.064113. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Machida T, Kawashima S. Influence of environmental salinity on the development of chloride cells of freshwater and brackish water Medaka Oryzias latipes. Zool Sci. 1986;3:859–865. [Google Scholar]
- Morisawa M, Suzuki K. Osmolality and potassium ion - their roles in initiation of sperm motility in teleosts. Science. 1980;210:1145–1147. doi: 10.1126/science.7444445. [DOI] [PubMed] [Google Scholar]
- Morris GJ. The origin, ultrastructure, and microbiology of the sediment accumulation in liquid nitrogen storage vessels. Cryobiology. 2005;50:231–238. doi: 10.1016/j.cryobiol.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Morris JP, Berghmans S, Zahrieh D, Neuberg DS, Kanki JP, Look AT. Zebrafish sperm cryopreservation with N,N-dimethylacetamide. Biotechniques. 2003;35:956–968. doi: 10.2144/03355st03. [DOI] [PubMed] [Google Scholar]
- Naruse K. Classification and phylogeny of fishes of the genus Oryzias and its relatives. Fish Biol J Medaka. 1996;8:1–9. [Google Scholar]
- Naruse K, Shima A, Matsuda M, Sakaizumi M, Iwamatsu T, Soeroto B, Uwa H. Distribution and phylogeny of rice fish and their relatives belonging to the suborder Adrianichthyoidei in Sulawesi, Indonesia. Fish Biol J Medaka. 1993;5:11–15. [Google Scholar]
- National Center for Research Resources of National Institute of Health. Natcher Conference Center, National Institute of Health; Bethesda, MD: 2007. Workshop report for “Achieving high-throughput repositories for biomedical germplasm preservation”. downloaded from website: www.esi-bethesda.com/ncrrworkshops/Biomedical/index.aspx. [Google Scholar]
- Paniagua-Chavez CG, Buchanan JT, Tiersch TR. Effect of extender solutions and dilution on motility and fertilizing ability of Eastern oyster sperm. J Shellfish Res. 1998;17:231–237. [Google Scholar]
- Paniagua-Chavez CG, Tiersch TR. Laboratory studies of cry preservation f sperm and trochophore larvae of the Eastern oyster. Cryobiology. 2001;43:211–213. doi: 10.1006/cryo.2001.2346. [DOI] [PubMed] [Google Scholar]
- Parenti LR, Grier HJ. Evolution and phylogeny of gonad morphology in bony fishes. Integr Comp Biol. 2004;44:333–348. doi: 10.1093/icb/44.5.333. [DOI] [PubMed] [Google Scholar]
- Pinisetty D, Huang C, Dong Q, Tiersch TR, Devireddy RV. Subzero water permeability parameters and optimal freezing rates for sperm cells of the southern platyfish, Xiphophorus maculatus. Cryobiology. 2005;50:250–263. doi: 10.1016/j.cryobiol.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana KJ. Cryopreservation of fish spermatozoa. In: Day JG, McCellan MR, editors. Methods in Molecular Biology: Cryopreseration and Freeze-drying Protocols. 1995. pp. 151–165. [DOI] [PubMed] [Google Scholar]
- Ryan MJ. Sexual selection, receiver biases, and the evolution of sex differences. Science. 1998;281:1999–2002. doi: 10.1126/science.281.5385.1999. [DOI] [PubMed] [Google Scholar]
- Scott AP, Baynes SM. A review of the biology, handling and storage of salmonid spermatozoa. J Fish Biol. 1980;17:707–739. [Google Scholar]
- Stoss J, Holtz W. Cryopreservation of rainbow-trout Salmo gairdneri sperm. 2 Effect of pH and presence of a buffer in the diluents. Aquaculture. 1981;25:217–222. [Google Scholar]
- Tavolga WN. Embryonic development of the platyfish (Platypoecilus), the swordtail (Xiphophorus) and their hybrids. B Am Mus Nat Hist. 1949;94:161–230. [Google Scholar]
- The office of NBRP Medaka. Medaka Book: a guide for the laboratory use of medaka Oryzias latipes. 2007 http://shigen.lab.nig.ac.jp/medaka/medakabook/index.php.
- Thirumala S, Huang C, Dong Q, Tiersch TR, Devireddy RV. A theoretically estimated optimal cooling rate for the cryopreservation of sperm cells from a live-bearing fish, the green swordtail Xiphophorus helleri. Theriogenology. 2005;63:2395–2415. doi: 10.1016/j.theriogenology.2004.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston LM, Watson PF, Holt WV. Semen cryopreservation: A genetic explanation for species and individual variation. Cryoletters. 2002;23:255–262. [PubMed] [Google Scholar]
- Tiersch TR. Introduction. In: Tiersch TR, Mazik PM, editors. Cryopreservation in Aquatic Species. World Aquacultrue Society; Baton Rouge, LA: 2000. pp. xix–xxvi. [Google Scholar]
- Tiersch TR. Cryopreservation in aquarium fishes. Mar Biotechnol. 2001;3:S212–S223. doi: 10.1007/s10126001-0044-z. [DOI] [PubMed] [Google Scholar]
- Tiersch TR, Mazik PM. Cryopreservation in Aquatic Species. World Aquaculture Society; Baton Rouge, LA: 2000. [Google Scholar]
- Tiersch TR, Jenkins JA. Biosecurity and regulatory considerations for transfer of cryopreserved sperm and early life stages of aquatic species. In: Lee CS, O'Bryen PJ, editors. Biosecurity in Aquaculture Production Systems: Exclusion of Pathogens and Other Undesirables. The World Aquaculture Society; Baton Rouge, LA: 2003. pp. 171–198. [Google Scholar]
- Tiersch TR, Yang H, Jenkins JA, Dong Q. Sperm cryopreservation in fish and shellfish. In: Roldan ERS, Gomendio M, editors. Spermatology (Society of Reproduction and Fertility supplement 65) Nottingham University Press; Nottingham, U.K.: 2007. pp. 493–508. [PubMed] [Google Scholar]
- Walter RB, Kazianis S. Xiphophorus interspecies hybrids as genetic models of induced neoplasia. J Ins Lab Anim Res. 2001;42:299–322. doi: 10.1093/ilar.42.4.299. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book, A Guide for the Laboatory Use of Zebrifsh Danio rerio. The Univeristy of Oregon Press; Eugene, OR: 1995. [Google Scholar]
- Wittbrodt J, Shima A, Schartl M. Medaka - A model organism from the Far East. Nature Rev Genet. 2002;3:53–64. doi: 10.1038/nrg704. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. Introductory Remarks on the Medaka. In: Yamamoto T, editor. Medaka, Biology and Strains. Yugakusya Publisher; 1975a. pp. 1–16. [Google Scholar]
- Yamamoto T. Medaka (killifish): Biology and Strains. Keigaku Publishing Co; Tokyo, Japan: 1975b. [Google Scholar]
- Yang H, Hazelwood L, Walter RB, Tiersch TR. Effect of osmotic immobilization on refrigerated storage and cryopreservation of sperm from a viviparous fish, the green swordtail Xiphophorus helleri. Cryobiology. 2006;52:209–218. doi: 10.1016/j.cryobiol.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Carmichael C, Varga ZM, Tiersch TR. Development of a simplified and standardized protocol with potential for high-throughput for sperm cryopreservation in zebrafish Danio rerio. Theriogenology. 2007a;68:128–136. doi: 10.1016/j.theriogenology.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Hazlewood L, Heater SJ, Guerrero PA, Walter RB, Tiersch TR. Production of F-1 interspecies hybrid offspring with cryopreserved sperm from a live-bearing fish, the swordtail Xiphophorus helleri. Biol Reprod. 2007b;76:401–406. doi: 10.1095/biolreprod.106.056549. [DOI] [PMC free article] [PubMed] [Google Scholar]


