Abstract
The study of ion channels has relied heavily on the use of pharmacological blocking agents. However, many of these agents have multiple effects, which may compromise interpretation of results when the affected mechanisms/pathways mediate similar functions. Volume regulated anion channels (VRAC) and connexin hemichannels can both mediate the release of glutamate and taurine, although these channels have distinct activation stimuli and hemichannels, but not VRAC, are permeable to Lucifer yellow (LY). It has been reported that some anion channel blockers may inhibit connexin hemichannels. We further examined the effects of classic gap junction/hemichannel blockers and anion channel blockers on these channels. The typical VRAC blockers NPPB, IAA-94 and tamoxifen blocked low divalent cation-induced glutamate and taurine release and LY loading, presumed due to hemichannel opening. The blocking action of these compounds on hemichannels was concentration dependent and fell within the same range where the drugs classically block VRACs. Conversely, carbenoxolone (CBX), the most widely used gap junction/hemichannel blocker, was an effective blocker of VRAC mediated glutamate and taurine release, and blocked these channels at similar concentrations at which it blocked hemichannels. The CBX effect on VRACs was verified using astrocytes from connexin 43 knock out (Cx43 KO) animals. In these cells, the hypotonic induced amino acid flux was retained while the low divalent cation solution flux was lost. These results extend our knowledge about ‘cross-inhibition’ of VRACs and gap junctions/hemichannels by certain pharmacological agents. Given the overlap in function of these two types of channels, great care must be exerted in using pharmacological blockers to identify one channel from the other.
Keywords: Astrocyte, glutamate, taurine, carbenoxolone, divalent cation, tamoxifen, NPPB, IAA-94
Introduction
Astrocytes are intimately involved in regulating extracellular glutamate levels (Danbolt, 2001). They are responsible for removing the majority of synaptically released glutamate via a group of high affinity Na+-dependent glutamate transporters (Szatkowski et al., 1990; Zerangue and Kavanaugh, 1996). However, astrocytes contain millimolar levels of glutamate (Ottersen, 1989; Levi and Patrizio, 1992; Ye et al., 2001) and can also release glutamate to the extracellular space. Presently, five release mechanisms have been identified: (1) Reverse operation of Na+-dependent glutamate transport (Nicholls and Attwell, 1990; Longuemare et al., 1999); (2) Release through VRACs activated by swelling (Kimelberg et al., 1990; Kimelberg et al., 1995); (3) Release mediated by increases in [Ca2+]i (Parpura et al., 1994; Bezzi et al., 1998; Parpura and Haydon, 2000), at least some of which may be vesicular release; (4) Glutamate release through the P2X7 receptor channel pore (Duan et al., 2003) and (5) Release through open hemichannels (Ye et al., 2003). Unfortunately, these mechanisms of release are sometimes difficult to distinguish from one another. For example, increase in [Ca2+]i can lead to vesicular release but may also promote release by opening hemichannels (De Vuyst et al., 2006). Another example is that P2X7 and hemichannel mediated glutamate release may both be activated by divalent cation removal (Parpura et al., 2004; Spray et al., 2006; Suadicani et al., 2006). This study, however, concerns the ambiguity that can arise due to lack of specificity of certain drugs presumed to have selective blocking actions; specifically, the ‘cross-inhibition’ between drugs that block hemichannels or swelling-activated anion channels.
Connexin hemichannels have long been thought to only exist as half of a gap junction. Recently, however, evidence suggests that they can also function as stand alone channels (Bennett et al., 2003; Ye et al., 2003; Ransom and Ye, 2005; Spray et al., 2006). Unlike gap junctions, hemichannels are exposed to extracellular Ca2+ and are primarily gated closed by physiological [Ca2+]o (Gomez-Hernandez et al., 2003). Connexin hemichannels can be blocked by most pharmacological compounds that block gap junctions, including AGA, octanol, heptanol, and carbenoxolone (Ransom and Ye, 2005).
Volume (or swelling) activated anion channels (VRAC) are permeable to chloride and anionic osmolytes. They are also called volume–sensitive organic anion channels (VSOACs) (Jackson et al., 1994), volume-sensitive chloride channels (ICl,vol) (Nilius et al., 1994), or swelling activated Cl− channel (ICl,swell) (Ackerman et al., 1994). Astrocytes likely express multiple types of VRAC that are closed under resting conditions (Parkerson and Sontheimer, 2004). These channels are activated by cell swelling as seen in brain edema or when astrocytes are exposed to high levels of K+. In order to restore osmotic gradients, astrocytes utilize VRAC to export osmolytically active molecules, mainly chloride and organic osmolytes, in a process called regulatory volume decrease (RVD). Many of the organic osmolytes released by astrocytes are also neuroactive, which include glutamate, aspartate and taurine (Kimelberg et al., 1990; Pasantes-Morales et al., 1990). Activated by hypotonic solution or by high [K+]o, astrocyte VRAC have also been implicated in spreading depression-mediated glutamate release, based on reducing glutamate release by the anion channel blocker NPPB (Basarsky et al., 1999). Similarly, glutamate release in the ischemic cortical penumbra has been attributed to VRAC based on blockade by tamoxifen, another highly effective blocker of these channels (Feustel et al., 2004).
Some VRAC blockers, however, have been shown to block Cx46 and Cx50 hemichannels expressed in oocytes. NPPB and flufenamic acid (FFA) blocked both, while niflumic acid only blocked Cx50 hemichannels (Eskandari et al., 2002). Other commonly used VRAC blockers were without effects on these hemichannels, including 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS), 4-acetamido-4′-isothiocyanostilbene-2,2′-disulfonic acid (SITS), tamoxifen and IAA-94. Channels formed by connexins vary in their characteristics (Harris, 2001), and the effects of these blockers on channels formed by Cx43, the connexin primarily expressed in astrocytes, has not been studied (except for FFA, see (Srinivas and Spray, 2003). Moreover, it seems possible that classical hemichannel blockers might have effects on VRAC. This has not been systematically studied, although we reported in passing that heptanol and octanol did not have significant effects on hypotonic solution-induced glutamate release (Ye et al., 2003).
In this study, we tested anion channel blockers on divalent cation removal-induced hemichannel opening detected by measuring amino acid release and lucifer yellow (LY) loading. In addition, we tested hemichannel blockers on VRAC activated by hypotonic solution. We identified several anion channel blockers, including NPPB, IAA-94 and tamoxifen that blocked Cx43 hemichannels. Conversely, CBX was found to be a very effective anion channel blocker, an effect that was verified using an astrocyte specific Cx43 KO mouse. These results document cross–inhibition between pharmacological agents previously thought to be either specific for VRAC or hemichannels. This study implies that one must be cautious in concluding the identity of a channel mediating amino acid release from astrocytes based solely on pharmacological findings.
Methods
Materials
Cell culture medium (Earle’s minimum essential media) was obtained from Gibco Invitrogen (Carlsbad, CA). Fetal bovine serum was purchased from Hyclone (Logan, UT). All other chemicals unless specifically mentioned were from Sigma-Aldrich Chemical Co. (St. Louis, MO). AGA, BGA, NPPB and IAA-94 were dissolved in dimethyl sulfoxide (DMSO) to make stock solution and diluted 1000 times in the corresponding bath solution. CBX was dissolved in ddH2O to 50 mM and diluted to reach the desired final concentrations.
Cell Culture
Hippocampal astrocytes were cultured from neonatal rat pups (Charles River, Wilmington, MA) as previously described (Ye et al., 2001). Cultures reached confluence within two weeks and > 90 % of cells stained positive for the astrocyte marker glial fibrillary acidic protein (GFAP, Sigma, St. Louis, MO). Cultures were typically used for experiments after 2–4 weeks in culture and they were essentially free of neurons.
Genetically modified B6129 mice in which Cx43 was selectively knocked out in astrocytes (developed and provided by Ken McCarthy) were used to establish cultures of Cx43 KO astrocytes (Wiencken-Barger et al., 2007). These cultures were prepared from individual mouse pups at P1-2 and the tails were clipped for genotyping. Wild type littermates were used as controls. Breeding pairs of B6129 mice with the P2X7R knocked out were purchased from Jackson Lab (Bar Harbor, Maine) and new born pups were used for culturing P2X7R KO astrocytes. To get sufficient cells for experiments using KO mice, astrocyte cultures were prepared using cortex.
Immunocytochemistry
Cells cultured on glass coverslips were stained for Cx43 and GFAP, as previously described (Ye et al., 2003) with slight modification. Antibodies were used at the following concentrations: 1 µg/ml affinity-purified rabbit anti-connexin 43 (Zymed, South San Francisco, CA) followed with anti-rabbit secondary antibody (Jackson ImmunoResearch, West Grove, PA) and 1:2,000 monoclonal Cy3-conjugated GFAP (Sigma). All coverslips were counterstained with sytox (Invitrogen/Molecular Probe) to stain nuclei. Stainings were examined using a Fluoview 1000 confocal microscope (Olympus, Japan).
Western blot
The expression of Cx43 and Cx30 in brain tissue and cultured cells were also assessed by western blot as previously described with small modification (Ye et al., 1999). Cortex and hippocampus were removed from 2 month old Cx43 KO mice and control WT littermates euthanized by CO2. Cultured astrocytes (2–4 weeks in culture) were mechanically scrapped from culture flasks and centrifuged in PBS. Harvested tissues and cell pellets were immediately sonicated in 5–7 fold volume of PBS supplemented with 10% protease inhibitor cocktail (Sigma). The sonicated samples were then centrifuged and sonicated again, and this was repeated 2–3 times. Aliquotes from the fully sonicated samples were taken for protein content determination and for boiling in Laemmli’s buffer. Boiled sample (contained 30µg protein) was loaded into each lane and separated by pre-casted gradient SDS-PAGE (Bio-Rad, Hercules, CA). Proteins were then transferred to polyvinylidene fluoride membrane (Immobilon-P; Millipore, Bedford, MA) and blocked overnight at 4°C in TBS-T (0.1% Tween-20 in tris-buffer solution) with 5% non fat dry milk, 1% bovine serum albumin and 1% FBS. After two brief washes with TBS-T, the membranes were probed with mouse anti-Cx43 antibody (Sigma, 1:1000) or mouse anti-Cx30 (Zymed, 1:200) in TBS-T with 1% FBS and 1% non-fat dry milk. For protein loading control, parallel blots loaded and run under identical conditions were probed with mouse anti-actin (Sigma, 1:1000). After 2h incubation with primary antibodies, blots were washed 3 times in TBS-T and incubated for 1h with peroxidase-conjugated goat anti-mouse antibodies (Sigma, 1:1000). After being washed in TBS-T (5 × 5 min), blots were visualized with ECL reagent (Santa Cruz, CA) and exposed on hypersensitive ECL film.
Amino acid release
As previously reported, hemichannel opening was evoked by omitting all divalent cations or Ca2+ alone (Ye et al., 2003). HEPES solution used in these experiments contained (in mM): 126.5 NaCl, 3.0 KCl, 1.25 NaH2PO4, 25 HEPES acid, 10 glucose, 2.0 MgSO4, and 2.0 CaCl2, with pH adjusted to 7.40 by NaOH. MgSO4 and CaCl2 were deleted in nominal divalent cation free solution (DCFS). Release experiments were performed in 24-well plates (500 µl/well) at 37 °C, where confluent hippocampal astrocytes were first rinsed twice with HEPES solution to wash out culture media. In experiments testing hemichannel and VRAC blockers, tested compounds were first applied in the presence of regular Ca2+ and Mg2+ (2.0 mM each) for 10 min, then rinsed once with the final solution (e.g. DCFS or hypotonic solution + blocker) and incubated for 5–20 minutes before collection of extracellular solutions for measurement of amino acid release. Collected samples were stored at −20°C for HPLC analysis. It is important to note that reported amino acid “release” is actually net release- i.e., release minus uptake.
To ensure that DCFS exposure did not lead to non-specific membrane permeability, we monitored LDH release after 20 or 60 min incubation with DCFS. No LDH release was detected and the cells were viable 24 hours later (data not illustrated).
Hypotonic solution was made by omitting 75 mM NaCl from the HEPES solution (i.e., 50% hypotonic solution). To differentiate the effects of hypo-osmolarity from the effects of lowering [Na+]o, a solution was made in which 75 mM NaCl was substituted with 75 mM choline-Cl (i.e., low Na+-isotonic solution).
Measure of cell volume change
To monitor volume changes induced by hypotonic solution, astrocytes were cultured on 25mm round coverslips and loaded with 1.0 µM calcein-AM (Invitrogen/Molecular Probe) for 30 min before being placed into a RC-40HP perfusion chamber (Harvard Apparatus, MA). Time serial confocal images were obtained with a Fluoview 1000 equipped with a 20x XLUMPlanPI dipping fluorescence objective (Olympus, Japan). The cells were perfused at 4 ml/min with solutions equilibrated to room temperature to avoid changes related to temperature shifts. Calcein was excited with a 488 nm Argon laser at minimal strength and scanned at 5s intervals. No significant bleaching of fluorescence was observed in the experiment paradigm. After the experiment, individual astrocytes in the field were selected and fluorescence intensity quantified.
Dye uptake and scrape loading
Dye uptake experiments were carried out using parallel procedures to those described above for amino acid release. Astrocytes were incubated with 0.5 mM LY for 10 minutes at 37 °C under test conditions and then rinsed five times with regular HEPES solution. For quantification of dye uptake, cells were fully lysed in 500 µl distilled water followed with bath sonication or pipette trituation, then the amount of LY was determined using a fluorescence plate reader (BMG Labtechnologies, Durham, NC) with a black quartz 96 well plate (Sunergia Group, Herndon, VA), and using an excitation filter of 420 ± 10 nm and an emission filter > 475 nm. Background readings were obtained from cells not exposed to LY and subtracted for accuracy. Intracellular LY was then normalized against cell protein levels to compensate for small variation among cell densities.
The scrape loading technique was used to evaluate the extent of gap junction communication (el-Fouly et al., 1987). Astrocytes were cultured to confluence on 22 mm square coverslips and bathed in DCFS (see above) containing 0.5 mM LY. The astrocyte layer was scraped by a razor blade and 2 minutes after the scraping, coverslips were removed from the bath and rinsed 5 times with HEPES solution and then fixed in 4% paraformaldehyde and examined with the Fluoview 1000 confocal microscope. Z-serial scanning was obtained with 1 µm intervals and stacked for the final image. The distribution of LY was quantified with Fluoview software by averaging linear intensity profiles vertical to the scrape and with the edge of the scrape as the staring point.
Measurement of Amino Acids
Amino acids were separated and measured as previously described (Ye et al., 2001) using a Breeze HPLC system from Waters (Milford, MA) with some minor modifications. Briefly, samples were centrifuged at 13,000g for 3 min and supernatants were pre-column derivatized with o-phthalialdehyde (OPA, Sigma, St. Louis, MO) and injected by a 717plus autosampler, followed by separation through a C18 reverse-phase Adsorbosphere OPA-HR column (Alltech, Deerfield, IL). Gradient elution was powered by Waters 1525 binary pumps with mobile phase A (pH 5.90) consisting of 25 mM sodium acetate, dioxane and isopropanol in a mixture of (v/v) 95.6/0.4/4.0 and mobile phase B a mixture of methanol, dioxane and isopropanol (v/v, 97/1.5/1.5). Fluorescent signals were detected by a 474 scanning fluorescent detector excited at 338 nm with emission detected at 450 nm. Chromatography was analyzed with Breeze software with external standards.
The amount of amino acid release was calculated from the volume of the solution and normalized to the protein content of the cultures. To measure protein contents and cytoplasmic amino acid contents, cultured cells were dissolved in 0.3 M NaOH, bath sonicated and neutralized with HCl. Aliquots were used to measure protein concentration using the Bio-Rad protein assay kit or to measure amino acid concentration by HPLC analysis.
Statistics
All data were expressed as mean ± standard error of the mean with each group consisting of 6 or 8 samples. To facilitate comparisons between groups, most data were normalized to corresponding control levels. Statistical differences were calculated by one-way ANOVA with Dunnett’s post hoc test. The following symbols were used throughout to indicate level of statistical significance: #, P<0.05; ##, P<0.01; ###, P<0.001 for comparison of control vs. DCFS or hypotonic, and *, P<0.05; **, P<0.01; ***, P<0.001 for comparisons of DCFS vs. DCFS + tested compounds or hypotonic vs. hypotonic + tested compounds.
Results
Hemichannels and volume activated anion channels (VRAC) have different permeabilities
Astrocytes abundantly express Na+-dependent glutamate transporters that generally keep extracellular glutamate concentration low (Danbolt, 2001). However, astrocytes can also release glutamate through several pathways, and the equilibrium between release and uptake determines extracellular glutamate concentration ([Glu]o).
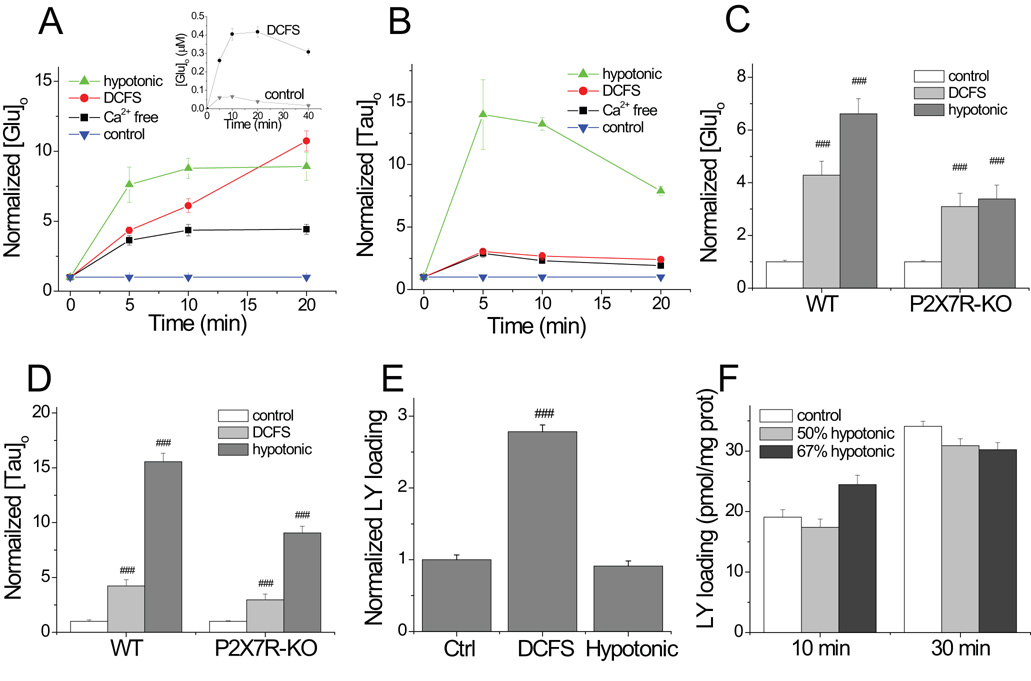
We have previously reported that both extracellular Ca2+ and Mg2+ can influence Cx43 hemichannel opening, and that Ca2+ is more potent than Mg2+ (Ye et al., 2003). As expected, removal of extracellular Ca2+ or Ca2+ and Mg2+ (i.e., divalent cation free solution or DCFS) led to glutamate release (Fig. 1A; [Glu]o levels were normalized to levels seen in HEPES solution containing 2.0 mM Ca2+ and Mg2+). Glutamate release appears not to reach a plateau level in DCFS but the inset shows that the absolute glutamate released did plateau, as expected; the matched “control” glutamate release, however, had a profile that ‘distorted’ the normalized data. After 10 minutes incubation, normalized [Glu]o is at 6.11 ± 0.49 in DCFS and 4.36 ± 0.41 in Ca2+ free solution.
Fig.1.
Comparison of hemichannel and VRAC mediated glutamate and taurine release and dye loading. (A) Both divalent cation removal (Ca2+ or both Ca2+ and Mg2+ (i.e., DCFS)) and hypotonic solution increased [Glu]o in astrocyte cultures. Hypotonic solution or Ca2+ removal induced glutamate release (normalized to release in control solution) that reached equilibrium after about 5 min. Normalized [Glu]o appears to increase continuously in DCFS, but absolute glutamate release does plateau (see insert graph; see text). (B) Hypotonic solution induced more taurine release than divalent cation removal. (C and D) Hypotonic solution (50% hypotonic for 10 min) and DCFS (10 min) caused significant glutamate and taurine release from astrocytes from P2X7 receptor KO mice, although somewhat less release than seen in WT astrocytes. (E) DCFS, but not hypotonic solution, caused LY loading, indicating that hypotonic solution did not evoke hemichannel opening. (F) Prolonged incubation with hypotonic solution (either 50% or 67% hypotonic; see text) failed to induce LY loading into astrocytes. (n=6–8 each, ### =P<0.001, DCFS vs. Ctrl).
Astrocytes swell in hypotonic solution and release glutamate and taurine (Fig. 1 A and B; (Kimelberg et al., 1990)). Hypotonic solution caused slightly greater glutamate release than did DCFS), but it produced much greater release of taurine (Fig 1A–C; after 10 min incubation, taurine increased to 13.24 ± 0.49 (n=6) in 50% hypotonic solution vs. 2.67 ± 0.32 (n=6) in DCFS (P<0.001)). This suggested that swelling activated anion channels were more abundant than hemichannels. If not, the bigger pore diameter of the hemichannel, would likely have mediated a higher flux rate per channel and greater taurine release than a similar number of anion channels. In addition, it was previously reported that swelling activated channels are more permeable to taurine than to glutamate (i.e., compare Fig 1A to 1B; (Jackson et al., 1994).
Control experiments indicated that the majority of DCFS-induced amino acid release was not mediated by P2X7 receptors, because DCFS induced similar amounts of glutamate and taurine release from astrocytes from WT and P2X7R KO animals (Fig 1C and 1D). Interestingly, while hypotonic-induced amino acid release remained robust in P2X7R KO astrocytes, it was significantly reduced compared to WT cells (P<0.05; see discussion).
The channels opened by DCFS, presumably hemichannels, were permeability to LY. In contrast, the channels opened by hypotonic solution did not admit LY (Fig. 1E). Even prolonged exposure to a greater hypotonic stimulus (i.e., 67% hypotonic for 30 min) did not increase LY loading beyond control levels (Fig. 1F). LY permeability has been commonly used as an indicator of the formation of channels with big pores (Spray et al., 2006). These results indicate that hypotonic solution opened a channel whose pore was smaller than that of a hemichannel.
Inhibition of Cx43 hemichannels by VRAC blockers
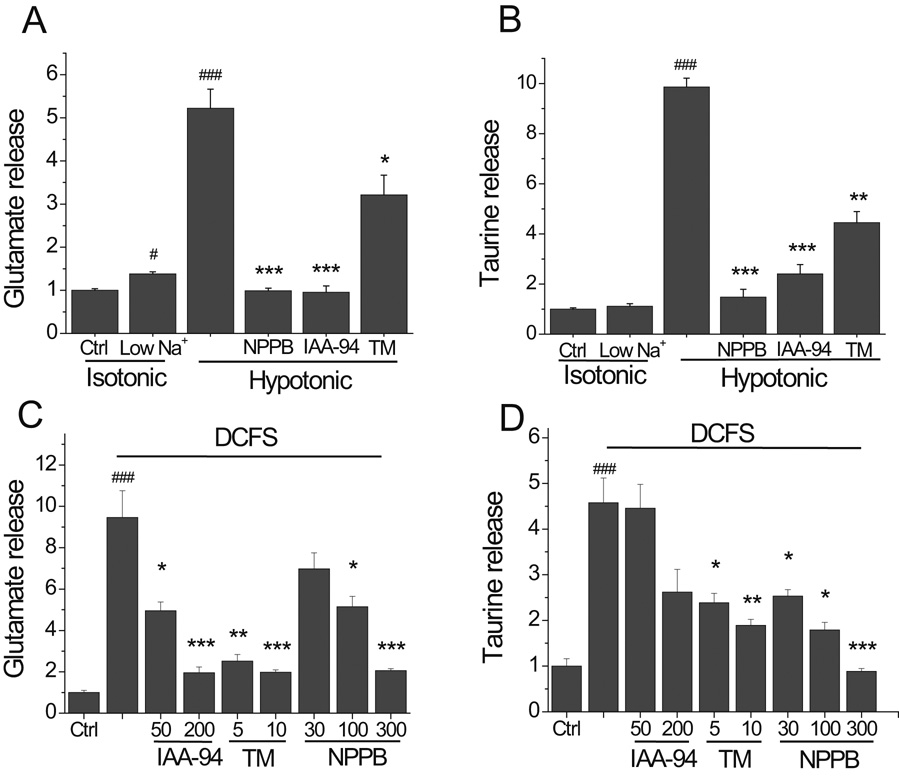
Hypotonic solution was prepared by reducing the amount of NaCl (i.e., 147.5 to 75mM for a 50% hypotonic solution). Lowering extracellular [Na+] by itself, without altering solution osmolality (“isotonic” condition), had minimal effects on amino acid release (Fig 2 A, B). The small increase in [Glu]o seen in isotonic low Na+ solution (i.e., 1.38 ± 0.05 vs. 1.00 ± 0.04 in control, P<0.05) was most likely due to reduced Na+ dependent glutamate uptake. Note that no such effect was seen for taurine release (Fig 2B), presumably because Na+ dependent transport contributes less significantly to setting the transmembrane taurine gradient in this time frame (i.e., 10 min).
Fig.2.
Anion channel blockers reduced hemichannel mediated amino acid release. (A and B) Glutamate and taurine release in hypotonic solution was primarily due to cell swelling. Reduced Na+ solution, made isotonic by replacement with choline, caused minimal amino acid release (i.e., Isotonic, low Na+). NPPB (300 µM), IAA-94 (200 µM) and tamoxifen (TM, 10 µM) blocked/reduced hypotonic-induced glutamate and taurine release. (C and D) DCFS-induced glutamate and taurine release, presumably hemichannel mediated, were blocked in a dose-dependent fashion by IAA-94, tamoxifen and NPPB. (n=6 for all experiments;# = P<0.05, ### = P<0.001 for Low Na+ isotonic, hypotonic or DCFS vs. Ctrl; * = P<0.05, ** = P<0.01, *** = P<0.001 for hypotonic vs. hypotonic + tested compounds or DCFS vs DCFS + tested compound).
In line with previous reports, the anion channel blockers NPPB (300µM), IAA-94 (200 µM) and tamoxifen (10 µM) all significantly, if not completely, blocked glutamate release in hypotonic solution (Fig 2A). Likewise, hypotonic solution induced taurine release was significantly blocked by NPPB, IAA-4 and tamoxifen (Fig. 2B).
A variety of anion channel blockers also blocked hemichannel-mediated amino acid release from astrocytes in a concentration-dependent fashion (Fig. 2C, D). Interestingly, in contrast to their lack of effects on Cx46 and Cx50 hemichannels (Eskandari et al., 2002), IAA-94 and tamoxifen also blocked Cx43 hemichannels as judged by reduced DCFS-induced glutamate and taurine release (Fig. 2C and 2D).
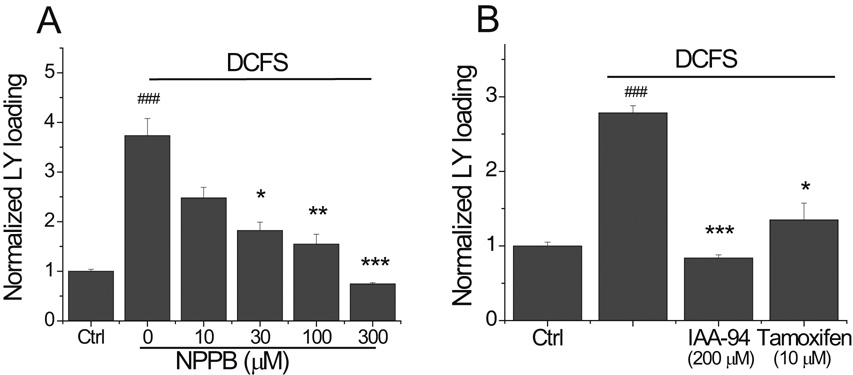
It was hypothetically possible that DCFS itself opened anion channels, in addition to hemichannels, offering another explanation for why DCFS-induced amino acid release was blocked by VRAC blockers. We tested this possibility by looking at the effect of VRAC blockers on DCFS-induced LY loading (recall that VRAC are not permeable to LY; Fig 1E). As shown in Fig. 3A, DCFS-induced LY loading was blocked in a concentration dependent manner by NPPB, and its efficiency on this primarily Cx43 hemichannel mediated event was similar to its reported effects on Cx46 and Cx50 hemichannels (Eskandari et al., 2002). In addition, tamoxifen and IAA-94 also significantly blocked DCFS-induced Lucifer yellow loading (Fig 3B). These results strongly support the idea that a range of anion channel blocking agents also blocked Cx43 hemichannels.
Fig.3.
Anion channel blockers reduced hemichannel mediated LY loading. (A) The anion channel blocker NPPB reduced DCFS induced LY loading in a concentration-dependent manner. (B) Two other anion channel blockers, IAA-94 and tamoxifen, blocked LY loading into astrocytes in DCFS. (n=8 for all experiments, ### = P<0.001 for DCFS vs. Ctrl; * = P<0.05, ** = P<0.01, *** = P<0.001, for DCFS vs. DCFS + tested compounds).
Carbenoxolone and 18β-glycyrrhetinic acid effectively blocked VRAC mediated amino acid release
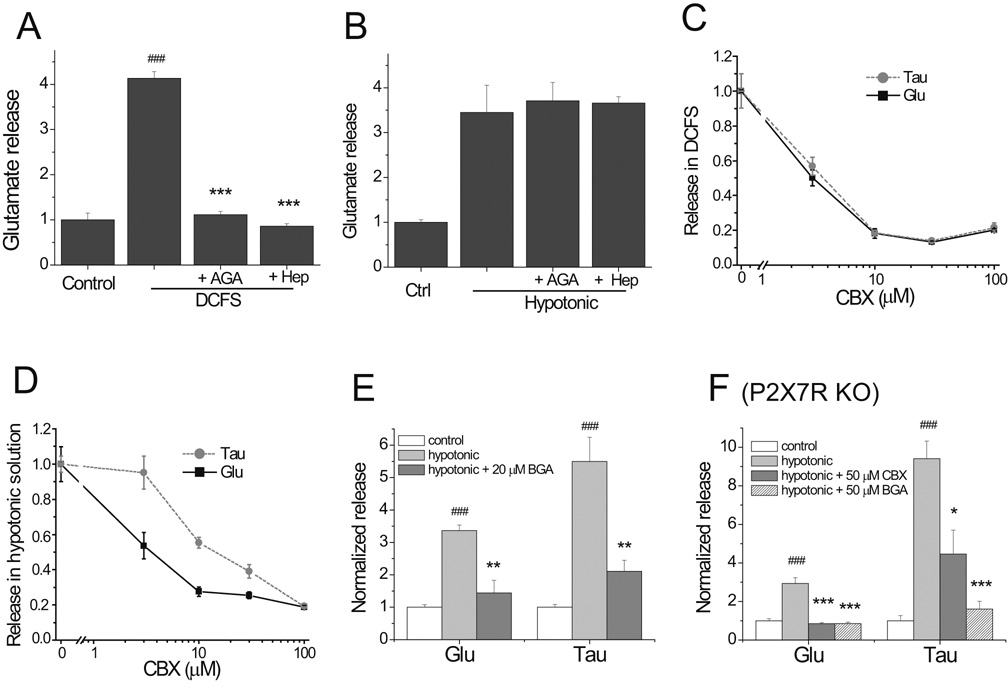
Little is known about the effects of commonly used hemichannel blockers on VRAC. As previously reported (Ye et al., 2003), glutamate release in DCFS was blocked by 40 µM AGA and 2 mM heptanol (Fig 4A). At the same concentrations, AGA and heptanol had no significant effects on glutamate release in hypotonic solution implying no blocking activity on VRAC (Fig 4B). Likewise, hypotonic taurine release was not affected by these drugs (not illustrated).
Fig.4.
Effects of gap junction/hemichannel blockers on swelling activated anion channels. (A) AGA (40 µM) and heptanol (2mM) blocked DCFS-induced glutamate release from astrocytes. (B) AGA (40 µM) and heptanol (2 mM) had no significant effects on hypotonic solution-induced glutamate release. (C and D) Carbenoxolone (CBX) blocked, in a concentration-dependent manner, hemichannel mediated (C) and anion channel (D) mediated glutamate and taurine release. Anion channel mediated taurine release was less sensitive to CBX than glutamate release. (E) In WT astrocytes BGA reduced hypotonic-induced glutamate and taurine release. (F) In P2X7R-KO astrocytes CBX and BGA blocked hypotonic induced glutamate and taurine release. (all n=6, ## = P<0.01, ### = P<0.001 for Ctrl vs. DCFS or Hypotonic; ** = P<0.01, *** = P<0.001 for DCFS vs. DCFS + tested compound or hypotonic vs. hypotonic + tested compound).
Carbenoxolone (CBX), the most commonly used gap junction and hemichannel blocker (in part because of its high aqueous solubility), potently blocked glutamate and taurine release in DCFS with an EC50 around 3 µM (Fig 4C). Unlike AGA and heptanol, however, CBX was also very effective at blocking glutamate and taurine release in hypotonic solution (Fig 4D). Interestingly, CBX blockade of VRAC also had an EC50 around 3 µM for glutamate release (around 10 µM for taurine release). The slightly lower efficiency of CBX on blocking taurine release compared to glutamate release might be related to the fact that VRAC have a higher permeability for taurine than glutamate (Jackson et al., 1994).
Although AGA had no significant effects on VRAC at the tested concentration (Fig 4B), surprisingly, 18β-glycyrrhetinic acid (BGA) at concentration ≥20 µM effectively blocked glutamate and taurine release in hypotonic solution (Fig 4E).
It has been reported that CBX is an effective blocker of P2X7R-mediated pore formation, perhaps by blocking the receptor itself (Suadicani et al., 2006) or by blocking pannexin-1 which can be associated with P2X7R (Locovei et al., 2007). Our results indicated that P2X7R-KO astrocytes had robust hypotonic amino acid release (Fig. 1C and 1D), making it unlikely that CBX blocked hypotonic release by an effect on P2X7R or pannexin-1. To test this hypothesis, we applied CBX or BGA to P2X7R-KO astrocytes. Indeed, hypotonic-induced release was blocked by CBX or BGA in the absence of P2X7R. Notably, BGA was more effective at blocking VRAC mediated taurine release than CBX (Fig 4F).
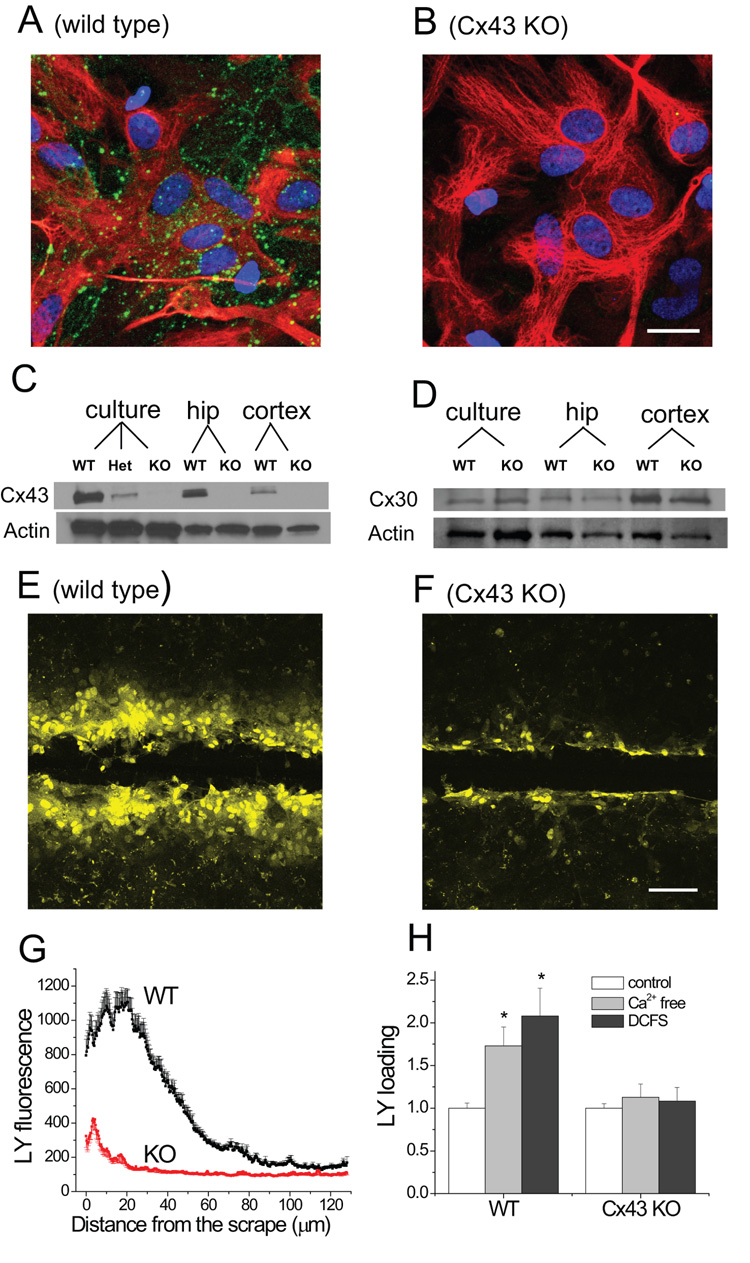
To prove that CBX effects on VRAC did not involve hemichannels, we took advantage of Cx43 KO astrocytes (Fig. 5). Astrocytes cultured from Cx43 KO mice have no detectable expression of Cx43 by immunohistochemistry (Fig 5A, B), or by western blot (Fig. 5C). Knocking out Cx43 can cause up regulation of Cx30 in some mouse lines (Wallraff et al., 2006), but no significant upregulation of Cx30 was detected in our Cx43 KO astrocytes either in culture or in hippocampal and cortical tissues (Fig. 5D).
Fig.5.
Cx43 KO astrocytes had reduced gap junction and hemichannel functions. (A and B) Cx43 staining in WT and Cx43 KO astrocytes (Green: Cx43, Red: GFAP. Blue: nuclei). (C and D) Western blots of Cx43 (C) and Cx30 (D) expression in culture astrocytes, and in adult brain (hippocampus (hip) and cortex; WT = wild type, KO = knockout, Het = heterozygote). (E and F). Scrape-loading of LY into cultured astrocytes; only WT astrocytes showed significant LY loading beyond the edge of the scrape. (G) LY fluorescence (in arbitrary intensity units) in astrocytes after scrape-loading as a function of distance from the scrape edge. (H) LY loading into WT and KO astrocytes in the presence of Ca2+ free solution or DCFS. (scale bar = 10 µm in A and B, 50 µm in E and F)
The abundance of gap junctions was assessed functionally using the LY scrape loading technique (see methods). In WT cultured astrocytes this technique indicated robust gap junction coupling between astrocytes (Fig 5E). In Cx43 KO astrocytes, however, gap junctions were dramatically decreased based on minimal scrape loading beyond the cells immediately adjacent to the scrape (Fig. 5F). These results were quantified by plotting LY fluorescence intensity as a function of distance from the scrape (Fig 5G). Scrape-loaded LY not only diffused further in WT astrocytes, but also the absolute amplitude of LY loading near the scrape was much greater in WT compared to Cx43 KO astrocytes. This may indicate that the initial loading of LY activated by mechanical scraping was primarily mediated by Cx43 hemichannels, as opposed to non-specific uptake due to membrane damage. Furthermore, at distances > 100 µm from the scrape, LY fluorescence intensity reached a low constant value suggesting that this LY loading was unlikely due to diffusion from the loading zone, but rather was a consequence of a low level of direct loading through hemichannels and other non-specific mechanisms. Note that WT astrocytes have a slightly higher LY intensity at these distant areas (Fig 5G) consistent with the idea that Cx43 hemichannels participated in this loading away from the scrape zone. Overall, these data supported the notion that Cx43 is the major connexin expressed by astrocytes. Not surprisingly, Cx43 KO astrocytes also had no significant loading of LY in Ca2+ free solution or DCFS (Fig 5H).
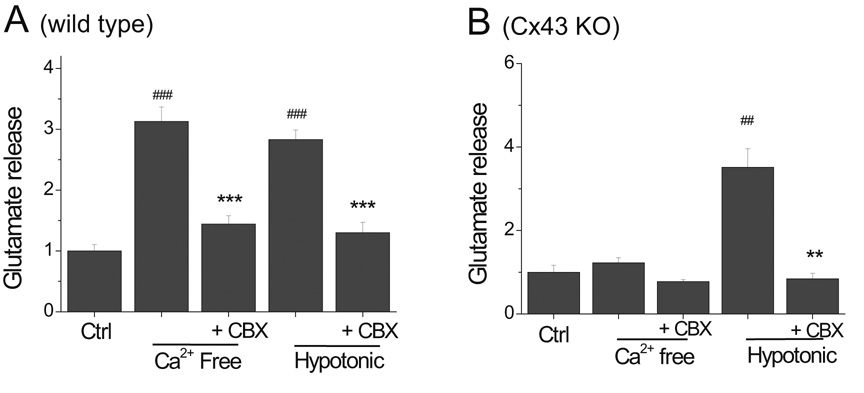
While removing Ca2+ no longer caused significant glutamate release in Cx43 KO astrocytes, hypotonic solution released glutamate in amounts equal to those seen in WT astrocytes (Fig 6A, B). Importantly, glutamate release induced by hypotonic solution in Cx43-KO astrocytes was still blocked by CBX (Fig. 6B). Similar results were found for taurine release (not illustrated). These findings confirm that CBX blocks VRAC independently of hemichannels. The small release of glutamate seen in Cx43-KO astrocytes in the absence of Ca2+ was likely due to activation of remaining hemichannels composed of other connexins expressed in small amounts by astrocytes, such as Cx30 (Nagy and Rash, 2000; Frisch et al., 2003), Cx40 and/or Cx45 (Dermietzel et al., 2000).
Fig.6.
Hypotonic, but not Ca2+-free, solution caused amino acid release in Cx43 KO astrocytes. (A) In Cx43 WT astrocytes, CBX blocked glutamate release induced by Ca2+ free or by hypotonic solution. (B) In Cx43 KO astrocytes, Ca2+-free solution did not induce significant glutamate release. Hypotonic solution, however, induced glutamate release that remained sensitive to blockade by CBX.
Hypotonic solution causes rapid and reversible swelling in WT and Cx43 KO astrocytes
The mechanism of hypotonic solution-induced amino acid release is presumed to be activation of VRAC by cell swelling. Cell volume can be monitored in real time by measuring fluorescence of a preloaded impermeable dye molecule such as calcein.
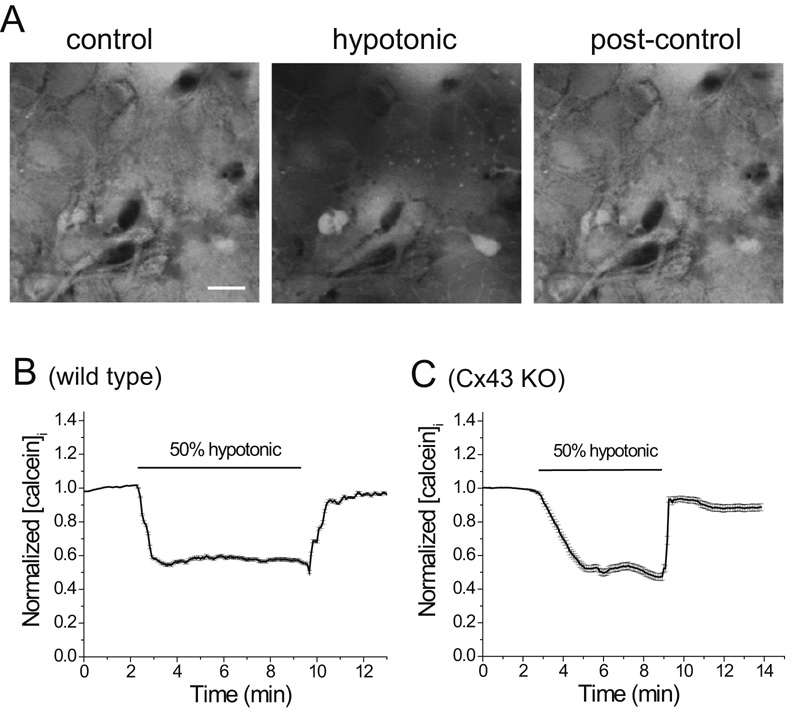
Astrocytes were loaded with calcein AM, a dye that can’t pass through most ion channels because of its large size. Application of 50% hypotonic solution caused a rapid and large reduction in fluorescence implying dye dilution because of osmotic water uptake and cell swelling (Fig 7A). The fluorescence rapidly recovered to baseline level when the hypotonic solution was removed. This was quantified as normalized calcein fluorescence (Fig 7B). Interestingly, hypotonic solution induced similar levels of swelling in WT and Cx43KO astrocytes (Fig 7B,C). That calcein concentration returned to baseline level after hypotonic solution exposure indicated that no dye leaked from the cell, a result expected based on the observation that LY did not permeate astrocytes exposed to hypotonic solution (Fig 1E)
Fig.7.
Hypotonic solution caused rapid swelling in both WT and Cx43-deficient astrocytes monitored by intracellular dye dilution. (A) Confocal images of calcein loaded WT astrocytes before, during and 2 minutes after exposure to hypotonic solution, note decrease in calcein fluorescence in the presence of hypotonic solution, indicating cell swelling. (B and C) Hypotonic solution produced similar decreases in calcein fluorescence in normal and Cx43 KO astrocytes during brief exposure to hypotonic solution. scale bar = 10 µm.
Discussion
Pharmacological agents are very important tools for studying biological mechanisms. If truly selective in their mode of action, for example tetrodotoxin that blocks some types of Na+ channels, they can be strategically employed to detect the involvement of particular channels or receptors in a biological event. Unfortunately, only a few drugs offer such selectivity. Most drugs have multiple actions that can compromise, sometimes seriously, their utility for solving biological problems. If the multiple actions of a compound are not fully understood, opportunity arises for experimental results to be misinterpreted. Of course, this is most likely to be a problem when an unexpected drug action produces an effect that seems consistent with the traditional or best known action of the drug.
We undertook these studies to further evaluate the extent of promiscuous action of agents that ‘primarily’ affect anion channels, particularly those activated by cell swelling or VRAC, or gap junctions/hemichannels. The practical motivation was that both VRAC and hemichannels can mediate amino acid release from cells, and it was obvious that non-specificity of drug action could lead to mistaken identification of the channels involved. It is known, for example, that some hemichannels can be blocked by anion channel blockers (Eskandari et al., 2002).
VRAC and Cx43 hemichannels
Both hemichannels and VRAC release amino acids when open. We took pains to insure proper identification of these channels in the astrocytes studied. Amino acid release from hemichannels activated by DCFS or zero [Ca2+]o solution depended on Cx43 expression. In astrocytes from Cx43 KO animals, these stimuli failed to elicit amino acid release. This is strong confirmation that Cx43 forms hemichannels in these cells that are opened by diminishing [Ca2+]o and release glutamate and taurine. On the other hand, hypotonic solution was just as effective in eliciting cell swelling and amino acid release in astrocytes from Cx43 KO compared to WT animals.
Hemichannels and VRAC also had distinct permeabilities. Whereas the Cx43 channel was permeable to Lucifer Yellow, the VRAC was not. To our knowledge, this detail about the permeability of VRAC and hemichannels has not been previously reported. VRAC, but not Cx43 hemichannels, were relatively more permeable to taurine than glutamate.
It was important to verify that the VRAC operated in the absence of Cx43 expression because confusing functional linkages can exist between proteins. For example, it is now suggested that P2X7 receptors can ‘link’ with pannexins such that activation of the P2X7 receptor leads to opening of a pannexin hemichannel (Locovei et al., 2007). As a precaution, we verified that DCFS-induced and hypotonic-induced amino acid release persisted in P2X7R KO animals (Fig 1C, D). Interestingly, however, hypotonic-induced amino acid release was significantly reduced in the P2X7R KO compared to WT astrocytes. The explanation for this finding is not clear and was not pursued. Of course, it raises the possibility that the expression of VRAC might have been depressed in the absence of P2X7R expression, but certainly other explanations are also possible.
A genetically modified mouse lacking Cx43 expression selectively in astrocytes was used in some of our experiments. Astrocytes also express Cx30, but less abundantly than Cx43. Astrocytes in the CX43 KO animal used here showed no significant change in Cx30 expression, as has been reported in other Cx43 KO mice where Cx30 was up regulated (Wallraff et al., 2006). Our results indicate that gap junction and hemichannel functions were greatly depressed in Cx43 KO astrocytes implying that Cx43 is the dominate protein for the formation of these channels in astrocytes. By inference, Cx30 and other connexins weakly expressed in astrocytes have a minor functional role in gap junctions and hemichannels under our culture conditions. It is important to note, however, that connexin expression can be influenced by environmental factors such as the close physical presence of neurons (Koulakoff et al., 2008). Likewise, differing genetic backgrounds in mouse strains might account for variable phenotypes when knocking out the same gene. For example, this could conceivably explain how expression levels of other connexins or behavioral phenotype might vary between different mouse strains in which Cx43 has been selectively knocked out of astrocytes (Wiencken-Barger et al., 2007).
Some compounds blocked both VRAC and connexin hemichannels
Our primary findings were that volume regulated anion channels, or VRAC, and gap junction/hemichannels were blocked by some of the same drugs. The VRAC blockers tamoxifen, IAA-94 and NPPB were effective blockers of Cx43 hemichannels at roughly the same concentrations that blocked anion channels. While NPPB was previously reported to block Cx50 hemichannels, that report indicated that tamoxifen and IAA-94 were without effects on hemichannels composed of Cx50/57 (Eskandari et al., 2002). This makes the important point that drug action appears to depend on the specific connexin protein under study, or less likely, depends on the cell type expressing a given connexin protein. Conversely, CBX was very effective at blocking VRAC, and did so at similar concentrations that are effective in blocking gap junction/hemichannels. This was not a universal property of gap junction/hemichannel blockers, however. At concentrations sufficient to block hemichannels, heptanol and AGA had no effect on VRAC-mediated amino acid release.
Tamoxifen, by blocking estrogen receptors, has been the most successful chemotherapy for treating breast cancer in the last 30 years (Ponzone et al., 2006). Tamoxifen also has many other effects including blockade of Na+ and K+ channels (Smitherman and Sontheimer, 2001), anion channels (Feustel et al., 2004; Kimelberg, 2005; Abdullaev et al., 2006) and Na+-dependent glutamate transport (Marcaggi et al., 2005). Tamoxifen reduction of glutamate release during ischemia, which reduced neuronal death in the penumbra area (Feustel et al., 2004), has been credited to a blockade of anion channels (Feustel et al., 2004; Kimelberg, 2005; Abdullaev et al., 2006). However, tamoxifen has been shown to block Cx43 gap junction in cultured cardiac myocytes (Verrecchia and Herve, 1997). Our finding that tamoxifen is also a very effective blocker of connexin hemichannels raises the possibility that at least part of the beneficial effects of this drug may be contributed by blocking hemichannel mediated glutamate release. This possibility is further supported by the fact that the ischemic penumbra provides an ideal environment for hemichannel opening: dramatically decreased [Ca2+]o, reduced energy supply and free radical generation (Contreras et al., 2002). Interestingly, it has been shown in some systems, that glutamate release in the penumbra area is not mediated by glutamate transporters (Feustel et al., 2004), an indication of channel mediated release (but see (Tekkok et al., 2007)). Moreover, direct effects of tamoxifen on glutamate transporters should be insignificant at the concentrations used (i.e., 10 µM); tamoxifen blocks reverse glutamate transport with an Ec50 > 100 µM (Marcaggi et al., 2005).
The mechanism of tamoxifen blockage of Cx43 hemichannels remains to be elucidated. Interestingly, tamoxifen’s effects on hemichannel mediated glutamate release appeared to be time dependent. We observed maximal inhibitory effects of tamoxifen on Cx43 hemichannels with 10 min preincubation, while prolonged incubation (i.e., >30 min) reduced and then reversed its effects (Ye et al, unpublished data). In fact, prolonged pre-exposure to tamoxifen facilitates hemichannel opening (Lin et al., 2008).
Carbenoxolone has been commonly used as a gap junction and connexin hemichannel blocker. Recently, CBX has been reported to have other actions independent of it is blockade of connexin channels, such as being extremely effective at blocking P2X7R pore formation, as tested on exogenously expressed P2X7 receptors in cell lines (Suadicani et al., 2006). In this study, we showed that CBX can effectively block VRAC mediated glutamate and taurine release. The definitive proof of this was the ability of CBX to block hypotonic amino acid release in cells not expressing Cx43. In addition, the report by Suadicani et al. (2006) raised the possibility that the effects of CBX on VRAC may work through P2X7R. However, P2X7R KO astrocytes retained VRAC mediated amino acid release, suggesting that CBX effects on VRAC are not related to P2X7R nor Cx43. Finally, preliminary studies on double KO astrocytes lacking both P2X7R and Cx43, confirm that VRAC mediated amino acid release is retained (Ye and Ransom, unpublished results).
Blocking gap junctions by CBX had been implicated as protective against ischemic brain injury (Frantseva et al., 2002; de Pina-Benabou et al., 2005). Employing the same logic as above, one must now consider that the CBX benefit on ischemia brain injury could be mediated by blocking VRAC. The lack of specificity of CBX is especially unfortunate because the drug has desirable chemical stability and water solubility making it the most widely used gap junction/hemichannel blocker for in vitro and in vivo experiments.
Mechanism of CBX and BGA mediated VRAC blockade
Currently, we don’t know the mechanisms underlying hemichannel blocker antagonism of VRAC or anion channel blocker inhibition of hemichannels. It is believed that CBX, among many similar compounds, closes gap junctions and connexin hemichannels by inserting into plasma membranes (lipid bilayers) and changing the membrane properties in a manner that affects connexin hemichannel structure and function (Rozental et al., 2001). Given this rather non-specific mechanism of action, it is perhaps not surprising that the function of other protein channels such as VRAC might also be altered. If CBX intercalates into membranes to cause channel blockade or occlusion, it offers a possible explanation for the observation that this drug more strongly reduced the permeation of glutamate than taurine through VRAC. Glutamate is slightly larger than taurine and would be more impeded in passing a channel made smaller by CBX’s steric effect. Interestingly, CBX has a similar Ec50 at blocking Cx43 hemichannels and VRAC.
Astrocytes swell immediately in hypotonic solution independent of Cx43 expression. Preliminary observations indicate that CBX does not block swelling at concentrations that block amino acid release, suggesting that the target of CBX block of hypotonic mediated release is downstream of the physical swelling. This makes it more likely that CBX directly targets VRAC.
Most of the other gap junction blockers, including AGA and heptanol, do not block VRAC at effective concentrations for hemichannels (Fig 4E). We found that BGA, structurally related to AGA, did block VRAC, but only at concentrations that are ~20 times higher than those needed to block hemichannel (Ye and Ransom, unpublished data).
Acknowledgement
The authors thank Ken McCarthy for conditional Cx43 KO mice, Thomas Moeller for comments on the manuscripts and Nathan Y.C. Lam for HPLC analysis. This study was supported by NIH grant NS 15589 (BRR).
Abbreviation
- AGA
18α-glycyrrhetinic acid
- BGA
18β-glycyrrhetinic acid
- CBX
carbenoxolone
- DCFS
divalent cation free solution
- IAA-94
indanyloxyacetic acid 94
- LY
Lucifer Yellow
- NPPB
5-Nitro-2-(3-phenylpropyl-amino)-benzoic acid
References
- Abdullaev IF, Rudkouskaya A, Schools GP, Kimelberg HK, Mongin AA. Pharmacological comparison of swelling-activated excitatory amino acid release and Cl- currents in rat cultured astrocytes. J Physiol. 2006 doi: 10.1113/jphysiol.2005.103820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman MJ, Wickman KD, Clapham DE. Hypotonicity activates a native chloride current in Xenopus oocytes. J Gen Physiol. 1994;103:153–179. doi: 10.1085/jgp.103.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basarsky TA, Feighan D, MacVicar BA. Glutamate release through volume-activated channels during spreading depression. J Neurosci. 1999;19:6439–6445. doi: 10.1523/JNEUROSCI.19-15-06439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MV, Contreras JE, Bukauskas FF, Saez JC. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci. 2003;26:610–617. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Contreras JE, Sanchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MV, Saez JC. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci U S A. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- de Pina-Benabou MH, Szostak V, Kyrozis A, Rempe D, Uziel D, Urban-Maldonado M, Benabou S, Spray DC, Federoff HJ, Stanton PK, Rozental R. Blockade of gap junctions in vivo provides neuroprotection after perinatal global ischemia. Stroke. 2005;36:2232–2237. doi: 10.1161/01.STR.0000182239.75969.d8. [DOI] [PubMed] [Google Scholar]
- De Vuyst E, Decrock E, Cabooter L, Dubyak GR, Naus CC, Evans WH, Leybaert L. Intracellular calcium changes trigger connexin 32 hemichannel opening. Embo J. 2006;25:34–44. doi: 10.1038/sj.emboj.7600908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel R, Gao Y, Scemes E, Vieira D, Urban M, Kremer M, Bennett MV, Spray DC. Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Res Brain Res Rev. 2000;32:45–56. doi: 10.1016/s0165-0173(99)00067-3. [DOI] [PubMed] [Google Scholar]
- Duan S, Anderson CM, Keung EC, Chen Y, Swanson RA. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci. 2003;23:1320–1328. doi: 10.1523/JNEUROSCI.23-04-01320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Fouly MH, Trosko JE, Chang CC. Scrape-loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res. 1987;168:422–430. doi: 10.1016/0014-4827(87)90014-0. [DOI] [PubMed] [Google Scholar]
- Eskandari S, Zampighi GA, Leung DW, Wright EM, Loo DD. Inhibition of gap junction hemichannels by chloride channel blockers. J Membr Biol. 2002;185:93–102. doi: 10.1007/s00232-001-0115-0. [DOI] [PubMed] [Google Scholar]
- Feustel PJ, Jin Y, Kimelberg HK. Volume-regulated anion channels are the predominant contributors to release of excitatory amino acids in the ischemic cortical penumbra. Stroke. 2004;35:1164–1168. doi: 10.1161/01.STR.0000124127.57946.a1. [DOI] [PubMed] [Google Scholar]
- Frantseva MV, Kokarovtseva L, Perez Velazquez JL. Ischemia-induced brain damage depends on specific gap-junctional coupling. J Cereb Blood Flow Metab. 2002;22:453–462. doi: 10.1097/00004647-200204000-00009. [DOI] [PubMed] [Google Scholar]
- Frisch C, Theis M, De Souza Silva MA, Dere E, Sohl G, Teubner B, Namestkova K, Willecke K, Huston JP. Mice with astrocyte-directed inactivation of connexin43 exhibit increased exploratory behaviour, impaired motor capacities, and changes in brain acetylcholine levels. Eur J Neurosci. 2003;18:2313–2318. doi: 10.1046/j.1460-9568.2003.02971.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Hernandez JM, de Miguel M, Larrosa B, Gonzalez D, Barrio LC. Molecular basis of calcium regulation in connexin-32 hemichannels. Proc Natl Acad Sci U S A. 2003;100:16030–16035. doi: 10.1073/pnas.2530348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- Jackson PS, Morrison R, Strange K. The volume-sensitive organic osmolyte-anion channel VSOAC is regulated by nonhydrolytic ATP binding. Am J Physiol. 1994;267:C1203–C1209. doi: 10.1152/ajpcell.1994.267.5.C1203. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Astrocytic swelling in cerebral ischemia as a possible cause of injury and target for therapy. Glia. 2005;50:389–397. doi: 10.1002/glia.20174. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Rutledge E, Goderie S, Charniga C. Astrocytic swelling due to hypotonic or high K+ medium causes inhibition of glutamate and aspartate uptake and increases their release. J Cereb Blood Flow Metab. 1995;15:409–416. doi: 10.1038/jcbfm.1995.51. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci. 1990;10:1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulakoff A, Ezan P, Giaume C. Neurons control the expression of connexin 30 and connexin 43 in mouse cortical astrocytes. Glia. 2008 doi: 10.1002/glia.20698. [DOI] [PubMed] [Google Scholar]
- Levi G, Patrizio M. Astrocyte heterogeneity: endogenous amino acid levels and release evoked by non-N-methyl-D-aspartate receptor agonists and by potassium-induced swelling in type-1 and type-2 astrocytes. J Neurochem. 1992;58:1943–1952. doi: 10.1111/j.1471-4159.1992.tb10073.x. [DOI] [PubMed] [Google Scholar]
- Lin JH, Lou N, Kang N, Takano T, Hu F, Han X, Xu Q, Lovatt D, Torres A, Willecke K, Yang J, Kang J, Nedergaard M. A central role of connexin 43 in hypoxic preconditioning. J Neurosci. 2008;28:681–695. doi: 10.1523/JNEUROSCI.3827-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. FEBS Lett. 2007;581:483–488. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longuemare MC, Rose CR, Farrell K, Ransom BR, Waxman SG, Swanson RA. K(+)-induced reversal of astrocyte glutamate uptake is limited by compensatory changes in intracellular Na+ Neuroscience. 1999;93:285–292. doi: 10.1016/s0306-4522(99)00152-9. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Hirji N, Attwell D. Release of L-aspartate by reversal of glutamate transporters. Neuropharmacology. 2005;49:843–849. doi: 10.1016/j.neuropharm.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Rash JE. Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain Res Brain Res Rev. 2000;32:29–44. doi: 10.1016/s0165-0173(99)00066-1. [DOI] [PubMed] [Google Scholar]
- Nicholls D, Attwell D. The release and uptake of excitatory amino acids. Trends Pharmacol Sci. 1990;11:462–468. doi: 10.1016/0165-6147(90)90129-v. [DOI] [PubMed] [Google Scholar]
- Nilius B, Oike M, Zahradnik I, Droogmans G. Activation of a Cl- current by hypotonic volume increase in human endothelial cells. J Gen Physiol. 1994;103:787–805. doi: 10.1085/jgp.103.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen OP. Quantitative electron microscopic immunocytochemistry of neuroactive amino acids. Anat Embryol (Berl) 1989;180:1–15. doi: 10.1007/BF00321895. [DOI] [PubMed] [Google Scholar]
- Parkerson KA, Sontheimer H. Biophysical and pharmacological characterization of hypotonically activated chloride currents in cortical astrocytes. Glia. 2004;46:419–436. doi: 10.1002/glia.10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Haydon PG. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci U S A. 2000;97:8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Scemes E, Spray DC. Mechanisms of glutamate release from astrocytes: gap junction "hemichannels", purinergic receptors and exocytotic release. Neurochem Int. 2004;45:259–264. doi: 10.1016/j.neuint.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Moran J, Schousboe A. Volume-sensitive release of taurine from cultured astrocytes: properties and mechanism. Glia. 1990;3:427–432. doi: 10.1002/glia.440030514. [DOI] [PubMed] [Google Scholar]
- Ponzone R, Biglia N, Jacomuzzi ME, Mariani L, Dominguez A, Sismondi P. Antihormones in prevention and treatment of breast cancer. Ann N Y Acad Sci. 2006;1089:143–158. doi: 10.1196/annals.1386.037. [DOI] [PubMed] [Google Scholar]
- Ransom BR, Ye ZC. Gap junctions and hemichannels. In: Kettenmann H, Ransom BR, editors. Neuroglia. New York: Oxford University Press; 2005. pp. 177–189. [Google Scholar]
- Rozental R, Srinivas M, Spray DC. How to close a gap junction channel. Efficacies and potencies of uncoupling agents. Methods Mol Biol. 2001;154:447–476. doi: 10.1385/1-59259-043-8:447. [DOI] [PubMed] [Google Scholar]
- Smitherman KA, Sontheimer H. Inhibition of glial Na+ and K+ currents by tamoxifen. J Membr Biol. 2001;181:125–135. doi: 10.1007/s00232-001-0016-2. [DOI] [PubMed] [Google Scholar]
- Spray DC, Ye ZC, Ransom BR. Functional connexin "hemichannels": a critical appraisal. Glia. 2006;54:758–773. doi: 10.1002/glia.20429. [DOI] [PubMed] [Google Scholar]
- Srinivas M, Spray DC. Closure of gap junction channels by arylaminobenzoates. Mol Pharmacol. 2003;63:1389–1397. doi: 10.1124/mol.63.6.1389. [DOI] [PubMed] [Google Scholar]
- Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatkowski M, Barbour B, Attwell D. Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature. 1990;348:443–446. doi: 10.1038/348443a0. [DOI] [PubMed] [Google Scholar]
- Tekkok SB, Ye Z, Ransom BR. Excitotoxic mechanisms of ischemic injury in myelinated white matter. J Cereb Blood Flow Metab. 2007;27:1540–1552. doi: 10.1038/sj.jcbfm.9600455. [DOI] [PubMed] [Google Scholar]
- Verrecchia F, Herve J. Reversible inhibition of gap junctional communication by tamoxifen in cultured cardiac myocytes. Pflugers Arch. 1997;434:113–116. doi: 10.1007/s004240050370. [DOI] [PubMed] [Google Scholar]
- Wallraff A, Kohling R, Heinemann U, Theis M, Willecke K, Steinhauser C. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci. 2006;26:5438–5447. doi: 10.1523/JNEUROSCI.0037-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiencken-Barger AE, Djukic B, Casper KB, McCarthy KD. A role for Connexin43 during neurodevelopment. Glia. 2007;55:675–686. doi: 10.1002/glia.20484. [DOI] [PubMed] [Google Scholar]
- Ye ZC, Rothstein JD, Sontheimer H. Compromised glutamate transport in human glioma cells: reduction-mislocalization of sodium-dependent glutamate transporters and enhanced activity of cystine-glutamate exchange. J Neurosci. 1999;19:10767–10777. doi: 10.1523/JNEUROSCI.19-24-10767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZC, Ransom BR, Sontheimer H. (1R,3S)-1-Aminocyclopentane-1,3-dicarboxylic acid (RS-ACPD) reduces intracellular glutamate levels in astrocytes. J Neurochem. 2001;79:756–766. doi: 10.1046/j.1471-4159.2001.00581.x. [DOI] [PubMed] [Google Scholar]
- Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerangue N, Kavanaugh MP. Flux coupling in a neuronal glutamate transporter. Nature. 1996;383:634–637. doi: 10.1038/383634a0. [DOI] [PubMed] [Google Scholar]