Abstract
Using Dynamic Causal Modeling (DCM) and functional magnetic resonance imaging (fMRI), we examined effective connectivity between three left hemisphere brain regions (inferior frontal gyrus, inferior parietal lobule, fusiform gyrus) and bilateral medial frontal gyrus in 12 children with reading difficulties (M age = 12.4, range: 8.11–14.10) and 12 control children (M age = 12.3, range: 8.9–14.11) during rhyming judgments to visually presented words. More difficult conflicting trials either had similar orthography but different phonology (e.g. pint-mint) or similar phonology but different orthography (e.g. jazz-has). Easier non-conflicting trials had similar orthography and phonology (e.g. dime-lime) or different orthography and phonology (e.g. staff-gain). The modulatory effect from left fusiform gyrus to left inferior parietal lobule was stronger in controls than in children with reading difficulties only for conflicting trials. Modulatory effects from left fusiform gyrus and left inferior parietal lobule to left inferior frontal gyrus were stronger for conflicting trials than for non-conflicting trials only in control children but not in children with reading difficulties. Modulatory effects from left inferior frontal gyrus to inferior parietal lobule, from medial frontal gyrus to left inferior parietal lobule, and from left inferior parietal lobule to medial frontal gyrus were positively correlated with reading skill only in control children. These findings suggest that children with reading difficulties have deficits in integrating orthography and phonology utilizing left inferior parietal lobule, and in engaging phonological rehearsal/segmentation utilizing left inferior frontal gyrus possibly through the indirect pathway connecting posterior to anterior language processing regions, especially when the orthographic and phonological information is conflicting.
Keywords: Functional magnetic resonance imaging (fMRI), Dynamic Causal Modeling (DCM), Reading difficulties, Orthography, Phonology
1. Introduction
Converging behavioral evidence suggests that a central problem in children with reading difficulties is a deficit in phonological processing, especially in identifying and manipulating the sound structure of a word (Bruck, 1992; Stanovich & Siegel 1994). Neuroimaging studies show that children with reading difficulties exhibit abnormal activation in left temporo-parietal regions and in left inferior frontal gyrus during reading tasks (Shaywitz et al., 2002). Abnormal activation in left superior temporal gyrus and inferior frontal gyrus could be the underlying neural basis of the deficits that children with reading difficulties have in phonological processing and abnormal activation in left inferior parietal cortex (including inferior parietal lobule and angular gyrus) could be the underlying neural basis of deficits that children with reading difficulties have in mapping between orthographic and phonological representations (Booth et al., 2002).
Most functional neuroimaging studies aim to identify network components that are selectively engaged by cognitive tasks. However, a network could shift from one behavioral goal to another not because of differences in the distribution of activations, but because of differences in the interactions among its components (Damasio, 1989; McIntosh, 2000; Mesulam, 1981, 1998). Analyses of effective connectivity (the modulatory influence that one brain region exerts upon another), and its non-directional counterpart known as functional connectivity (based on correlation of brain activation between regions), have, in fact, shown that network components can display task-dependent alterations in their interactions that are independent of amount of activation (Chaminade & Fonlupt, 2003; Homae, Yahata, & Sakai, 2003; Horwitz, Rumsey, & Donohue, 1998; McIntosh et al., 1994; Pugh et al., 2000). Components of distributed networks serve multiple roles including the integration of convergent inputs, the binding of distributed information, the relay of information from one region to another, and the control of neural activity within other network components (Mesulam, 1998).
Although most neuroimaging studies have sought to identify particular brain areas within which activation patterns discriminate controls from those with reading difficulties, a deeper understanding of the neurobiology of reading difficulties may emerge from examining connectivity among multiple brain regions that function cooperatively to process information during reading. Two studies have found that functional connectivity with left angular gyrus is dysfunctional in adults with reading difficulties. Adults with reading difficulties (18–40 years) did not show a correlation of left angular gyrus with left inferior frontal gyrus or with left fusiform gyrus as controls did during single word naming (Horwitz et al., 1998). Another study found that functional connectivity of left angular gyrus with occipital and temporal sites was disrupted during non-word rhyming in dyslexics (16–54 years) (Pugh et al., 2000). The findings of less intense activation and weaker functional connectivity of left inferior parietal cortex in adults with reading difficulties are consistent with reported group differences in brain morphology. Voxel-based morphology studies have found less white matter in left temporo-parietal cortex and less gray matter in left inferior parietal cortex in adults (18–33 years) and children (10–12 years) with reading difficulties (Eckert et al., 2005; Silani et al., 2005). Difusion tensor imaging studies also found that fractional anisotropy of left temporo-parietal cortex was significantly correlated with reading ability in adults (26–36 years) and children (7–13 years) with good to poor reading, indicating that a reduction of density, myelination and directional coherence may underlie reading problems in adults and children with reading difficulties (Deutsch et al., 2005; Klingberg et al., 2000).
In a previous study, Cao, Bitan, Chou, Burman and Booth (2006) reported that controls showed greater intensity of activation than children with reading difficulties in left inferior frontal gyrus, fusiform gyrus, and inferior parietal lobule during rhyming judgments to conflicting word pairs (e.g. pint-mint, jazz-has) presented in the visual modality, but there were no group differences in intensity of activation for non-conflicting word pairs (e.g. dime-lime, staff-gain) despite group differences in accuracy (Cao et al., 2006). This finding is consistent with behavioral research on children with reading disorder that shows a larger conflict effect in visual and auditory rhyming tasks as compared to controls (McPherson, Ackerman, & Dykman, 1997; Rack, 1985). Rhyming judgment to visually presented words is a relatively complex task that involves decoding orthographic stimuli, phonological rehearsal, phonological segmentation, and making an explicit determination of whether words rhyme. Cao et al. (2006) interpreted abnormal activation in left fusiform gyrus as reflecting an orthographic processing deficit, abnormal activation in left inferior parietal lobule as reflecting a deficit in mapping between orthographic and phonological representations, and abnormal activation in left inferior frontal gyrus as reflecting a deficit in phonological rehearsal/segmentation and/or top-down modulation of posterior processes (Cao et al., 2006). The current study examined whether differences in conflicting as well as non-conflicting trials are associated differences in effective connectivity. We used Dynamic Causal Modeling (DCM) to examine the directional influence that one brain region has on another (Friston, Harrison, & Penny, 2003). DCM is distinguished from alternative approaches by accommodating non-linear and dynamic aspects of neuronal interactions, and by framing the estimation in terms of perturbations that accommodate to experimentally designed inputs (Friston et al., 2003). We chose to use a rhyming task because several previous studies using this task have consistently implicated left inferior frontal gyrus and left temporo-parietal regions in phonological processing (Crosson et al., 1999; Kareken, Lowe, Chen, Lurito, & Mathews, 2000; Lurito, Kareken, Lowe, Chen, & Mathews, 2000; Paulesu et al., 1996; Pugh et al., 1996; Rumsey et al., 1992; Xu et al., 2001). Based on previous neuroimaging work on the rhyming task, our regions of interest (ROIs) included left inferior frontal gyrus, left inferior parietal lobule, left fusiform gyrus and bilateral medial frontal gyrus for the DCM analysis (Bitan et al., 2005; Bitan et al., 2006; Bitan, Burman, et al., 2007). However, based on functional connectivity studies (Horwitz et al., 1998; Pugh et al., 2000), our a priori connections of interest were modulatory effects into and out of left inferior parietal lobule. We expected children with reading difficulties to have disrupted effective connectivity especially for conflicting word pairs.
2. Materials and methods
2.1. Participants
Twelve children with reading difficulties (M age = 12.4, range: 8.11–14.10; 10 males) and 12 age-matched children (M age = 12.3, range: 8.9–14.11; 8 males) participated in this study. One child with reading difficulties and one control were African–American. The other participants were Caucasian. The number of participants in this study is generally accepted as the minimum for a meaningful fMRI study (Desmond & Glover 2002) and previous studies using Dynamic Causal Modeling have employed similar numbers of participants (Bitan et al., 2005, 2006).
Children with reading difficulties had a past diagnosis of reading difficulties and met the following inclusionary criteria: (1) performance IQ (Wechsler, 1999) above 90, and (2) mean on word and non-word reading accuracy (Woodcock, McGrew, & Mather, 2001) and word and non-word reading speed (Torgeson, Wagner, & Rashotte, 1999) below 90 (see Table 1). All of the 12 children with reading difficulties were lower than 85 on at least one of the four standardized tests. All of the 12 children with reading difficulties had a discrepancy of at least 10 points between performance IQ and the lowest score on a standardized reading test, and 11 of them had a discrepancy of at least 10 points between performance IQ and the average of the four standardized reading tests. The age-matched control children met the following criteria: (1) difference of age with matched children with reading difficulties less than four months, (2) performance IQ (Wechsler, 1999) above 90, and (3) mean on word and non-word reading accuracy and speed tests (Torgeson et al., 1999; Woodcock et al., 2001) above 90. Table 1 presents the means and standard deviations of scaled scores of the standardized tests. We calculated a 2 group (children with reading difficulties and controls) × 2 test (performance IQ and average of reading tests), and we found a significant interaction between group and test (F(1, 22) = 13.993, p < .01). Follow-up t-test showed that although children with reading difficulties had a lower score on both performance IQ (t(22) = 4.140, p < .001) and the average of reading tests (t(22) = 8.839, p < .001) as compared to control children, the group difference was greater on the average of reading tests. However, group differences in brain activation revealed in the current study could still in part be due to IQ differences. Parents of all children were given an informal interview to insure that the children met the following inclusionary criteria: (1) native English speaker, (2) right-handed, and (3) free of neurological or psychiatric disorders. The Institutional Review Board at Northwestern University and Evanston Northwestern Healthcare Research institute approved the informed consent procedures.
Table 1.
Means (standard deviations) of the scaled scores on standardized tests for children with reading difficulties (RD) and controls
| RD | Controls | |
|---|---|---|
| Verbal IQ (WASI)** | 97 (12) | 110 (8) |
| Performance IQ (WASI)*** | 98 (7) | 109 (7) |
| Full scale IQ (WASI)*** | 97 (9) | 111 (7) |
| Word identification (WJ-III)*** | 84 (8) | 110 (7) |
| Word attack (WJ-III)*** | 83 (8) | 106 (9) |
| SWE (TOWRE)*** | 83 (8) | 105 (9) |
| PDE (TOWRE)*** | 76 (9) | 102 (12) |
| Average of four reading tests*** | 81 (6) | 106 (7) |
p < .001
p < .01 in independent-sample t-tests.
WASI: Wechsler Abbreviated Intelligence Scale; WJ-III, Woodcock Johnson III Tests of Achievement; TOWRE, Test of Word Reading Efficiency; SWE, Sight Word Efficiency; PDE, Phonetic Decoding Efficiency.
2.2. Functional activation task
2.2.1. Rhyming task
Two words were visually presented in sequential order and the participant had to determine whether the two words rhymed. If the word pair rhymed, the participant pressed a button with the right index finger; if the word pair did not rhyme, the participant pressed another button with the right middle finger. Each word was presented for 800 ms followed by a 200 ms blank interval. A red fixation-cross appeared on the screen after the second word, indicating the need to make a response during the subsequent 2600 ms interval. If no response was made during this time, it was considered an incorrect response.
Half of the word pairs rhymed and half did not. Half of the word pairs had similar orthography and half did not. The combination of these factors resulted in four types of trials (see Fig. 1): similar orthography similar phonology (O+P+), similar orthography different phonology (O+P−), different orthography similar phonology (O−P+) and different orthography different phonology (O−P−). O+P− and O−P+ are considered as conflicting trials because the word pairs have conflicting orthographic and phonological information, while O+P+ and O−P− are considered as non-conflicting trials, because the word pairs have non-conflicting orthographic and phonological information. There were 24 word pairs for each trial type. The four trial types were matched for their written word frequency based on child and adult norms (The Educator’s Word Frequency Guide, 1996). All words and symbols (see below) were presented in lower case, at the center of the screen, with a 0.5 letter offset of position between the first and second stimulus.
Fig. 1.
Schematic of experimental design. Two visual words were presented sequentially in the visual modality in one of 4 conditions involving a conflict between orthography and phonology (O+P−, O−P+) and a non-conflict between orthography and phonology (O+P+, O−P−). A red fixation cross was presented in the response interval.
2.2.2. Control trials
Two perceptual control trial types were used in which two symbol strings were presented visually in sequential order and the participant had to determine whether the strings matched. In the ‘Simple’ trials, the symbol string consisted of a single symbol, while in the ‘Complex’ trials the symbol string consisted of three different symbols. Timing parameters were the same as for the lexical trials. Twenty-four items were presented in each perceptual trial type, with half of them matching. In addition to the perceptual control trials, 72 fixation trials were included as null events. In the null trials, a black fixation-cross was presented for the same duration as the stimuli in the lexical and perceptual trials and participants were instructed to press a button when the black fixation-cross turned red. We used null trials as the baseline for our fMRI analysis because the difference in behavioral performance between groups was the smallest for these trials.
The order of lexical, perceptual and fixation trials was optimized for event-related design using OptSeq (http://surfer.nmr.mgh.harvard.edu/optseq) (Burock, Buckner, Woldor., Rosen, & Dale, 1998). The order of stimuli within task was fixed for all subjects.
2.3. MRI data acquisition and analysis
Images were acquired using a 1.5 Tesla General Electric (GE) scanner. The Blood oxygen level dependent (BOLD) functional images were acquired using the echo planar imaging (EPI) method (time of echo (TE) = 35 ms, flip angle = 90°, matrix size = 64 × 64, field of view = 24 cm, slice thickness = 5 mm, number of slices = 24; time of repetition (TR) = 2000 ms). Two 108 trial runs (8 min), with 240 repetitions each, were administered. Structural T1 weighted 3D images were also acquired (TR = 21 ms, TE = 8 ms, flip angle = 20°, matrix size = 256 × 256, field of view = 22 cm, slice thickness = 1 mm, number of slices = 124).
Data analysis was performed using SPM2 (Statistical Parametric Mapping) (http://www.fil.ion.ucl.ac.uk/spm). Images were spatially realigned to the first volume to correct for head movements. No individual runs had more than 3 mm maximum displacement in either X, Y, or Z translation (M = 1.41, SD = 0.92 for children with reading difficulties; M = 0.87, SD = 0.45 for controls). Functional images were co-registered with the anatomical image, and normalized to the standard T1 Montreal Neurological Institute (MNI) template volume. Data were then smoothed with a 10 mm isotropic Gaussian kernel. Statistical analyses at the first level were calculated using an event-related design, with 4 lexical trial types, 2 perceptual trial types, and null trial types as trials of interest. A high pass filter with a cutoff period of 128 s was applied. Trials were modeled using a canonical hemodynamic response function (HRF). Global normalization scaled the mean of each scan to a common value. Both correct and incorrect trials were used in the statistical analysis, because we assumed that children were engaged in the cognitive task for the incorrect trials, since there appeared to be no speed-accuracy trade off for children with low accuracy. In addition, a previous conventional analysis showed similar group differences between controls and children with reading difficulties for both correct and incorrect trials as well as for correct trials only (Cao et al., 2006). Parameter estimates from contrasts in single subject models were entered into random-effect analysis using one-sample t-tests across all participants in each group. Direct group comparisons were executed using two-sample t-tests. All reported areas of activation were significant using p < 0.001 uncorrected with a cluster size greater than 10 voxels.
Three left hemisphere ROIs (fusiform gyrus, inferior frontal gyrus, and inferior parietal lobule) were chosen that were previously identified as involved in the rhyming task (Bitan et al., 2005, 2006). Bilateral medial frontal gyrus was chosen as a fourth ROI because this region is implicated in conflict monitoring (Bitan, Burman, et al., 2007). Group maxima for each group in all lexical trials versus null trials were identified in each of the four ROIs using anatomical masks of those regions in SPM2 (left inferior frontal gyrus: −45, 9, 27 for controls, −60, 6, 30 for children with reading difficulties; left fusiform gyrus: −42, −48, −18 for controls, −39, −51, −15 for children with reading difficulties; left inferior parietal lobule: −36, −42, 39 for both groups; medial frontal gyrus: −9, 6, 57 for both groups). The distance between the two group maxima was less than 25 mm for left inferior frontal gyrus and fusiform gyrus. For left inferior parietal lobule, children with reading difficulties did not show activation in this region; therefore, we used the group maximum in the control children to define this ROI for both children with reading difficulties and the control children. All ROIs were 6 mm radius spheres centered on the most significant voxel within 25 mm of the group maximum with the constraint that each individual’s peak was within the following anatomical masks in SPM2 (left inferior frontal gyrus: within left inferior frontal gyrus; left fusiform gyrus within left fusiform gyrus or inferior temporal gyrus; left inferior parietal lobule within left inferior parietal lobule, superior parietal lobule, precuneus gyrus, angular gyrus, or supramarginal gyrus; bilateral medial frontal gyrus within bilateral medial frontal gyrus, anterior cingulate or superior frontal gyrus). A 6 mm sphere was used so as not to include many inactive voxels and this volume is consistent with several previous effective connectivity studies (Brazdil, Mikl, Marecek, Krupa, & Rektor, 2007; Ethofer et al., 2006; Sonty et al., 2007). A weaker peak was chosen in individuals where the maximum peak was not in an appropriate Brodmann area (left inferior frontal gyrus within BA 44, 45, or 9; left fusiform gyrus within BA 19 or 37; left inferior parietal lobule within BA 40 or 7; bilateral medial frontal gyrus within BA 6 or 8).
Effective connectivity analysis was performed using the DCM tool in Statistical Parametric Mapping (SPM2) (Friston et al., 2003; Penny, Stephan, Mechelli, & Friston, 2004). DCM is a non-linear systems identification procedure that uses Bayesian estimation of parameters to make inferences about effective connectivity between brain regions and how this connectivity is affected by experimental conditions. In DCM, three sets of parameters are estimated: the direct influence of stimuli on regional activity; the intrinsic or latent connections between regions (i.e. the interregional influences in the absence of modulating experimental effects); and the changes in the intrinsic connectivity between regions induced by the experimental design (modulatory effects) (Mechelli, Price, Noppeney, & Friston, 2003). The modulatory effects are in arbitrary units in DCM. Since ‘connectivity’ in DCM is measured through the coupling of changes in imaging signals, rather than anatomically, a significant unidirectional modulatory influence of one brain region upon another does not necessarily reflect the presence of a direct and unidirectional anatomical connection. Instead, the connectivity revealed by DCM reflects the inferred direction of neural influences that are specific to the experimental context and that may be mediated through inter-neurons or other brain regions not explicitly included in the model.
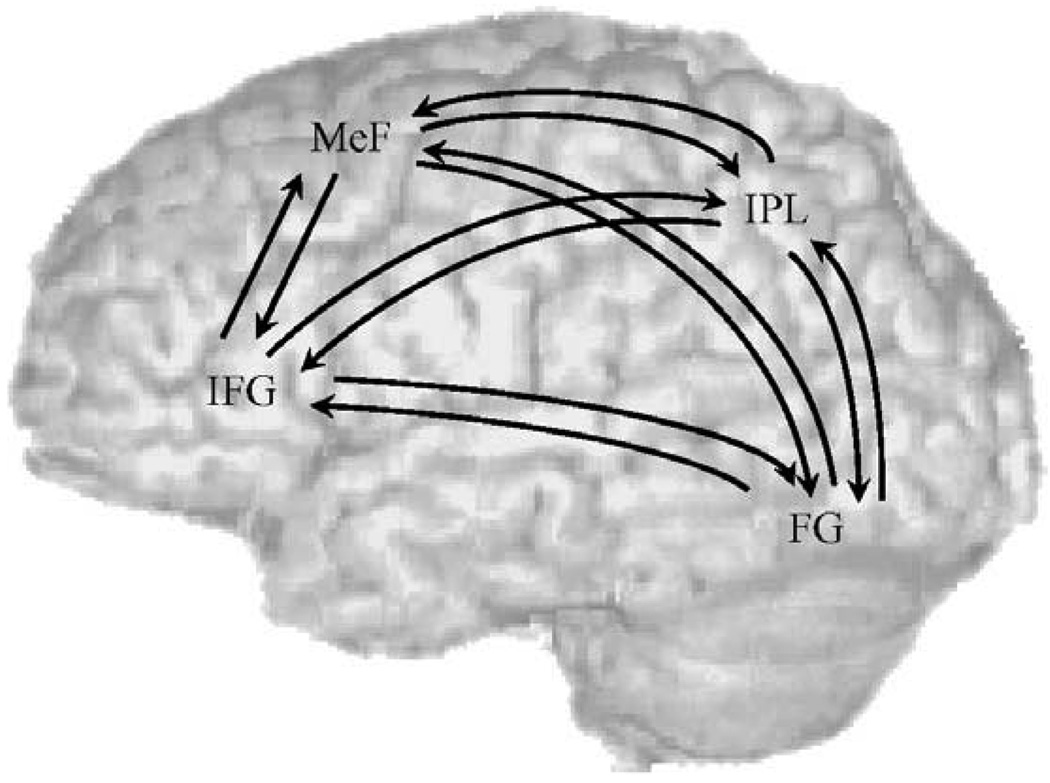
For the DCM analysis, the modulatory effects from the subject-specific, first level models were taken to a second, between-subject random-effect level (Bitan et al., 2006). Subject-specific DCMs were fully and reciprocally connected (resulting in 24 connections), with modulatory effects of conflicting or non-conflicting trials specified on the coupling among all regions. Fig. 2 presents the complete DCM model. We chose not to calculate comparisons between separate models with different numbers of connections because nothing is known about the pattern of effective connectivity in people with reading difficulties. Direct input was specified on left fusiform gyrus and included conflicting, non-conflicting and perceptual trials. Group differences in modulatory effects for the conflicting or non-conflicting trials were evaluated through three-way analyses of variance (ANOVAs): 2 groups (control, children with reading difficulties) × 2 conditions (conflicting, non-conflicting) × 3 coupled regions separately for input to and output from each region of interest. This resulted in 8 ANOVA models. Based on previous functional connectivity studies (Horwitz et al., 1998; Pugh et al., 2000), our a priori connections of interest were input to and output from inferior parietal lobule, so we corrected for two comparisons (p < .05/2 = p < .025). All other models were corrected for 6 comparisons (p < .05/6 = p < .008). We also calculated exploratory analyses of the correlation between behavioral performance and modulatory effects within controls and children with reading difficulties.
Fig. 2.
The connectivity path model tested separately for the conflicting and non-conflicting condition separately in controls and children with reading difficulties
3. Results
Table 2 presents accuracy and reaction time for controls and children with reading difficulties on the visual rhyming task and the baseline task. We calculated a 2 group (controls, children with reading difficulties) × 3 condition (conflicting, non-conflicting, and null) ANOVA separately for accuracy and reaction time on the lexical task. Overall, children with reading difficulties were less accurate (F(1, 22) = 38.945, p < .001) and slower (F(1, 22) = 22.963, p < .01) than controls. There was also a significant main effect of condition for accuracy (F(1, 22) = 106.753, p < .001) but not for reaction time, indicating that conflicting trials were more difficult than non-conflicting trials (t(23) = 7.438, p < .001), and non-conflicting trials were more difficult than null trials (t(23) = 4.201, p < .001). There was a significant interaction between group and condition for accuracy (F(2, 44) = 23.528, p < .001) but not for reaction time. Follow-up t-tests showed that although both lexical trial types resulted in lower percent correct (conflicting: t(22) = 6.999, p < .001; non-conflicting: t(22) = 4.170, p < .001) for children with reading difficulties compared to controls, there was no group difference for null trials. The group difference was significantly greater on conflicting trials than on non-conflicting trials, because the interaction between group and lexical trial types was significant (F(1, 22) = 12.285, p < .01). Although children with reading difficulties were near chance on the conflicting trials, the fact that they had slower reaction times than for the non-conflicting trials suggests that there was not a speed-accuracy trade off.
Table 2.
Means (and standard deviations) for accuracy (%) and reaction time (ms) for controls and children with reading difficulties (RD) in the two conflicting (O+P−, O−P+), two non-conflicting (O+P+. O−P−) and null trials
| O+P+ | O+P− | O−P+ | O−P− | Null | |
|---|---|---|---|---|---|
| Accuracy (%) | |||||
| Controls | 94 (6) | 73 (19) | 86 (10) | 94 (9) | 96 (8) |
| RD | 74 (18) | 42 (23) | 51 (16) | 87 (9) | 95 (6) |
| Reaction time (ms) | |||||
| Controls | 1107 (293) | 1220 (268) | 1120 (265) | 1054 (260) | 1124 (277) |
| RD | 1506 (302) | 1637 (335) | 1542 (245) | 1496 (286) | 1469 (159) |
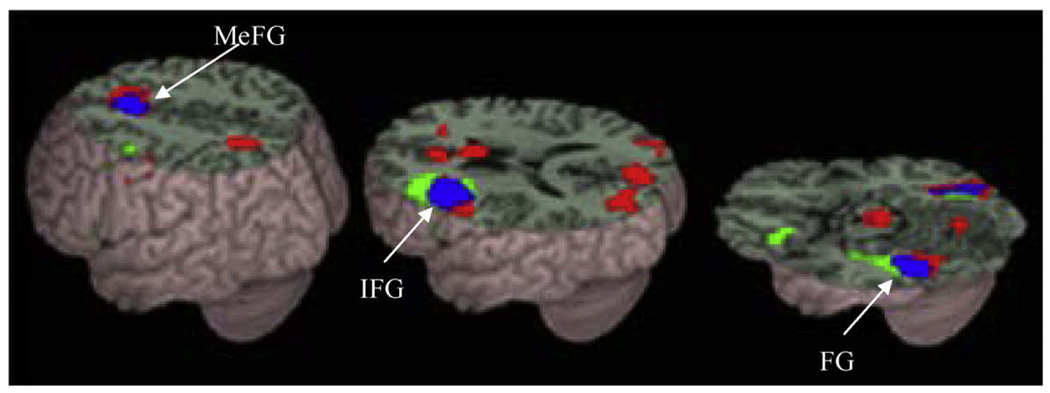
Table 3 and Fig. 3 present regions that were active for all lexical trials compared to null trials in controls and children with reading difficulties. Both groups showed activation including left fusiform gyrus, left inferior frontal gyrus, and bilateral medial frontal gyrus. Table 4 presents group differences between control children and children with reading difficulties. Consistent with previous studies, control children showed greater activation than children with reading difficulties in left inferior frontal gyrus, left inferior parietal lobule and left middle temporal gyrus. Children with reading difficulties showed greater activation than control children in right posterior cingulate gyrus
Table 3.
Activations in controls and children with reading difficulties (RD) in lexical versus null trials
| Region | H | BA | z-Test | voxels | x | y | z | |
|---|---|---|---|---|---|---|---|---|
| Control | Inferior/middle frontal gyrus | L | 45/47/44/46/9 | 4.21 | 526 | −48 | 30 | 6 |
| L | 9 | 4.09 | −45 | 9 | 27 | |||
| Fusiform gyrus | L | 37/19 | 4.63 | 191 | −39 | −45 | −21 | |
| L | 37 | 3.68 | −42 | −48 | −18 | |||
| Superior temporal gyrus | L | 22 | 3.83 | 24 | −60 | −39 | 6 | |
| Middle/inferior occipital gyrus, inferior temporal gyrus | R | 19/18/20 | 3.71 | 93 | 48 | −75 | −9 | |
| Fusiform/interior temporal gyrus | R | 37/20 | 3.65 | 32 | 36 | −51 | −15 | |
| Cingulate gyrus/medial frontal gyrus | L | 32/8 | 3.35 | 13 | −9 | 12 | 45 | |
| L | 6 | 3.03 | −9 | 6 | 57 | |||
| RD | Middle/inferior occipital/fusiform gyrus | R | 18/19/17/37 | 4.26 | 61 | 27 | −90 | −3 |
| Middle/inferior occipital gyrus/fusiform gyrus | L | 18/19/37 | 3.49 | 246 | −42 | −78 | −6 | |
| L | 37 | 3.46 | −39 | −51 | −15 | |||
| Cuneus | R/L | 18/17 | 3.28 | 357 | 9 | −72 | 12 | |
| Superior frontal gyrus | L | 6 | 2.92 | 32 | −3 | 6 | 60 | |
| Cingulate gyrus/medial frontal gyrus | L/R | 32/8 | 2.80 | 43 | 6 | 24 | 39 | |
| L | 6 | 2.03 | −9 | 6 | 57 | |||
| Inferior frontal gyrus | L | 45/46/9 | 2.92 | 53 | −33 | 27 | 3 | |
| L | 9 | 2.50 | −60 | 6 | 30 |
Note. Peaks of activations are listed in bold for areas spanning different regions; H, hemisphere; L, left; R, right; BA, Brodmann Area. p < .001 uncorrected, greater than 10 voxels.
Fig. 3.
Brain activations for the lexical minus ‘null’ trials in controls (green) and in children with reading difficulties (red). The overlap between groups is represented in blue. Both controls and children with reading difficulties showed activation in left inferior frontal gyrus (IFG), left fusiform gyrus (FG) and medial frontal gyrus (MeFG). p < .001 uncorrected, 10 or greater voxels. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 4.
Direct comparisons between controls and children with reading difficulties (RD) for lexical versus null trials
| Region | H | BA | z-Test | voxels | x | y | z | |
|---|---|---|---|---|---|---|---|---|
| Control > RD | Middle/inferior frontal gyrus | L | 11, 47 | 3.44 | 113 | −24 | 42 | −9 |
| Inferior frontal gyrus | L | 46 | 3.20 | 12 | −39 | 33 | 12 | |
| Precentral gyrus | L | 4 | 3.17 | 27 | −42 | 6 | 15 | |
| Inferior parietal lobule | L | 40 | 3.11 | 15 | −51 | −42 | 48 | |
| Middle temporal gyrus | L | 21 | 3.03 | 16 | −54 | −57 | 3 | |
| RD > Control | Posterior cingulate | R | 29 | 3.16 | 56 | 6 | −51 | 12 |
Note. Peaks of activations are listed in bold for areas spanning different regions; H, hemisphere; L, left; R, right; BA, Brodmann Area. p < .001 uncorrected, greater than 10 voxels.
Fig. 4 shows the strength of modulatory effects for conflicting and non-conflicting trials in controls and children with reading difficulties (significant modulatory effects within each group are represented with an asterisk, p < .05). For conflicting trials, controls had significant modulatory effects for all connections except for left inferior frontal gyrus to left inferior parietal lobule and medial frontal gyrus to left inferior parietal lobule, whereas children with reading difficulties only had significant modulatory effects from left fusiform gyrus to left inferior frontal gyrus and left fusiform gyrus to medial frontal gyrus. For non-conflicting trials, controls had no significant modulatory effects, whereas children with reading difficulties had significant modulatory effects for all connections except from left inferior frontal gyrus to left fusiform gyrus, left inferior frontal gyrus to left fusiform gyrus, left fusiform gyrus to left inferior parietal lobule, and medial frontal gyrus to left fusiform gyrus.
Fig. 4.
Modulatory effects in controls (white bars) and in children with reading difficulties (gray bars) in conflicting (left) and non-conflicting (right) trials for modulatory effects going in and out of left inferior frontal gyrus (IFG), left inferior parietal lobule (IPL), left fusiform gyrus (FG), and medial frontal gyrus (MeFG). *, p < .05 in one-sample t-tests.
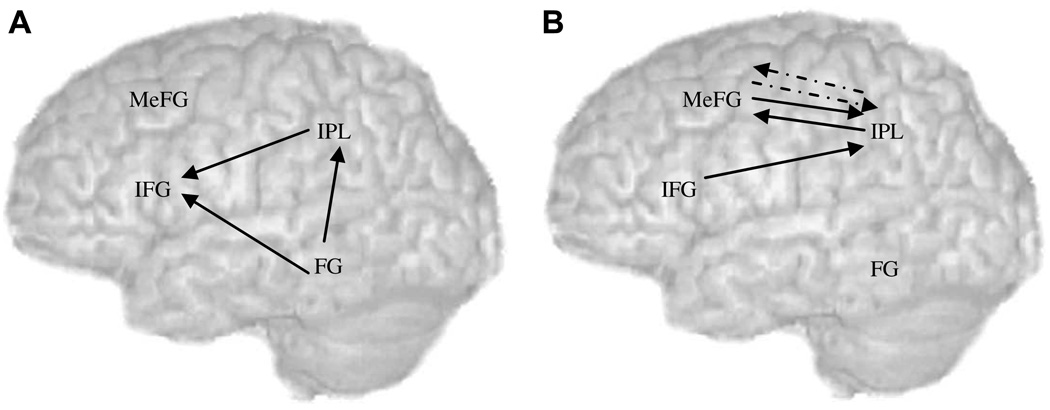
In order to evaluate the group differences, ANOVAs were performed on modulatory effects separately for 8 different models (4 regions (left inferior frontal gyrus, left inferior parietal lobule, left fusiform gyrus, and bilateral medial frontal gyrus) in 2 directions (diverging and converging)). Each model consisted of 2 groups (controls, children with reading difficulties) × 2 conditions (conflicting, non-conflicting) × 3 coupled regions. We established a prior hypothesis of the group differences in converging to and diverging from left inferior parietal lobule based on previous studies (Horwitz et al., 1998; Pugh et al., 2000), therefore, for the two models of left inferior parietal lobule, we used p < .025 as the corrected level for multiple comparisons. For the other six models, we used p < .008 as the corrected level for multiple comparisons. For the sake of brevity, we will only report main effects or interactions involving group. There was not a significant main effect of group nor a significant two-way interaction including group. There were two significant three-way interactions including group (Fs(2, 44) = 6.6 and10.6 for converging to left inferior parietal lobule, p < .01, and converging to left inferior frontal gyrus, p < .001, respectively). Follow-up tests found that for the converging effect to left inferior parietal lobule, there was a significant two way interaction of group and coupled regions only for conflicting trials (F(2,44) = 4.17, p < .05), but not for non-conflicting trials (F(2,44) = 0.30, p = .74). The modulatory effect from left fusiform gyrus to left inferior parietal lobule was significantly stronger in controls than in children with reading difficulties for conflicting trials (t(22) = 2.15, p < .05) (see in Fig. 5A), while the modulatory effects from left inferior frontal gyrus and medial frontal gyrus to left inferior parietal lobule were not significantly different between groups (ps = .1, and .08, respectively). For the converging effect to left inferior frontal gyrus, we broke up the three-way interaction by group and found that there was a significant two-way interaction of condition and coupled region only for control children (F(2, 22) = 13.95, p < .001), but not for children with reading difficulties (F(2, 22) = 0.05, p = .95). As it shows in Fig. 5A, the modulatory effects from left fusiform gyrus to left inferior frontal gyrus and from left inferior parietal lobule to left inferior frontal gyrus were significantly greater for conflicting than for non-conflicting trials in controls (t(11) = 3.89, p < .01; t(11) = 2.23, p < .05, respectively), while the modulatory effect from medial frontal gyrus to left inferior frontal gyrus was not significantly different between the two conditions (p = .07).
Fig. 5.
(A) Differences between controls and children with reading difficulties in modulatory effects. Modulatory effect from left fusiform gyrus (FG) to left inferior parietal lobule (IPL) was significantly stronger in control children than in children with reading difficulties only for conflicting trials. Modulatory effects from left FG and left IPL to left inferior frontal gyrus (IFG) was significantly stronger for conflicting trials than for non-conflicting trials only in control children. (B) Correlations with reading skills in control children. The modulatory effects between left IPL and bilateral medial frontal gyrus (MeFG) were positively correlated with the standard scores on Word Attack for non-conflicting (solid) and conflicting (dotted) trials. The modulatory effect from IFG to IPL was also positively correlated with the standard scores on Word Attack for non-conflicting trials.
We calculated correlations of accuracy of the rhyming task with modulatory effects separately for each condition (conflicting, non-conflicting) and separately for each group (controls, children with reading difficulties), but none of them were significant. We also calculated the correlation of scores on standardized reading tests with modulatory effects. Fig. 5B presents these correlations for conflicting trials, controls showed positive correlations of standard scores on Word Attack with modulatory effects from medial frontal gyrus to left inferior parietal lobule (r = 0.55, p < .1) and left inferior parietal lobule to medial frontal gyrus (r = 0.57, p < .1). For non-conflicting trials, controls showed positive correlations of standard scores on Word Attack with modulatory effects from medial frontal gyrus to left inferior parietal lobule (r = 0.71, p < .01), left inferior parietal lobule to medial frontal gyrus (r = 0.72, p < .01), and left inferior frontal gyrus to left inferior parietal lobule (r = 0.55, p < .1). Children with reading difficulties showed no brain-behavior correlations.
Taken together, group analyses revealed that control children showed a stronger modulatory effect from left fusiform gyrus to left inferior parietal lobule as compared to children with reading difficulties, but only for the conflicting trials. Control children, but not children with reading difficulties, showed stronger modulatory effects from left fusiform gyrus and left inferior parietal lobule to left inferior frontal gyrus for conflicting trials as compared to non-conflicting trials. Correlation analyses showed that control children with higher reading skill showed stronger modulatory effects from bilateral medial frontal gyrus to left inferior parietal lobule, left inferior frontal gyrus to inferior parietal lobule and left inferior parietal lobule to medial frontal gyrus.
4. Discussion
The present study examined effective connectivity between controls and children with reading difficulties on a reading task that required them to determine whether two visually presented words rhymed. Some of the trials contained conflicting pairs (e.g. pint-mint, jazz-has) and other trials contained non-conflicting pairs (e.g. dime-lime, staff-gain). This is the first study to use effective connectivity to examine differences between controls and people with reading difficulties in the directional influence that one brain region has on another. Our first main finding is that the modulatory effect from left fusiform gyrus to left inferior parietal lobule was weaker in children with reading difficulties compared to controls for conflicting trials. This is consistent with a functional connectivity study showing that adults with reading difficulties had weaker connection between left fusiform gyrus and left angular gyrus during word reading (Horwitz et al., 1998) and with another functional connectivity study showing that adults with reading difficulties had a weaker connection between left lateral extrastriate cortex and left angular gyrus during word and non-word reading (Pugh et al., 2000). However, these functional connectivity studies are not directionally specific, so the current study additionally implicates the deficit in feed-forward connections from left fusiform gyrus to left inferior parietal lobule. Left inferior parietal lobule has been implicated in integrating orthography and phonology (Booth et al., 2002, 2003). Our results suggest that children with reading difficulties have deficits in computations that link orthographic codes involving the left fusiform gyrus to brain regions involved in mapping orthographic to phonological representations. This process is especially compromised when the orthographic and phonological information are in conflict.
Our second main finding is that the modulatory effect from left fusiform gyrus to inferior frontal gyrus was significantly greater for conflicting trials than for non-conflicting trials in control children, but not in children with reading difficulties. This is consistent with a previous study that found the modulatory effect from left fusiform gyrus to left inferior frontal gyrus was significantly greater for conflicting trials than for non-conflicting trials in typical children (Bitan, Cheon, Lu, Burman, & Booth, 2007). Left fusiform gyrus has been implicated in processing orthographic representations (Booth et al., 2002), while left inferior frontal gyrus has been implicated in sub-vocal phonological rehearsal (Pugh et al., 1996) and phonological segmentation (Fiez & Petersen 1998). When word pairs have similar orthography but different phonology or different orthography but similar phonology, there are greater demands on phonological rehearsal/segmentation. Children with reading difficulties may not have shown increasing connectivity of left fusiform gyrus with left inferior frontal gyrus for conflicting trials because they cannot effectively recruit these task-selective regions. Previous studies using the general linear model examining functional connectivity focused on the left angular gyrus (Horwitz et al., 1998; Pugh et al., 2000). They did not examine connectivity between other regions, so our study is the first to report a weaker connection from left fusiform gyrus to left inferior frontal gyrus in children with reading difficulties.
Our third major finding is that the modulatory effect from left inferior parietal lobule to left inferior frontal gyrus was significantly greater for conflicting trials than for non-conflicting trials in control children, but not in children with reading difficulties. This is consistent with a functional connectivity study showing that adults with reading difficulties had weaker connection between left angular gyrus and left inferior frontal gyrus during word reading (Horwitz et al., 1998). However, functional connectivity studies are not directionally specific, so our study additionally implicates feed-forward connections from left inferior parietal lobule to left inferior frontal gyrus as the locus of deficit in children with reading difficulties. A recent diffusion tensor imaging study found that there is both a direct and an indirect pathway from left superior temporal gyrus to left inferior frontal gyrus (Catani, Jones, & ffytche, 2005). The indirect pathway goes from left superior temporal gyrus to left inferior parietal lobule and then to left inferior frontal gyrus. The pathway from left inferior parietal lobule to left inferior frontal gyrus has been implicated in spontaneous speech. No anatomical studies have reported an abnormality in the indirect pathway in people with reading difficulties. However, our finding of a lack of conflict effect in modulatory effects from left inferior parietal lobule to left inferior frontal gyrus in children with reading difficulties suggests that the indirect pathway may be dysfunctional. This could indicate that children with reading difficulties are less able to recruit left inferior frontal gyrus for phonological rehearsal/segmentation, especially for trials in which there is conflicting orthographic and phonological information.
The last main finding of the current study is that the modulatory effect from left inferior frontal gyrus to left inferior parietal lobule, and bidirectional modulatory effects between left inferior parietal lobule and medial frontal gyrus were positively correlated with reading skills only in control children. Although left inferior frontal gyrus has been implicated in phonological rehearsal/segmentation, recent studies have demonstrated its important role in modulation on the posterior language regions. Bitan et al. (2005, 2006) examined developmental differences in effective connectivity in spelling and rhyming tasks in the visual modality (Bitan et al., 2005, 2006). They showed that the modulation of left inferior frontal gyrus on left intraparietal sulcus for the spelling task and on left superior temporal sulcus for the rhyming task was stronger for adults compared to children. This result suggests that greater modulatory effects for left inferior frontal gyrus in adults reflect their relatively effective top-down modulation of posterior task-selective regions. This finding is also consistent with learning studies in adults. Learning an artificial grammar is associated with increasing connectivity of left inferior frontal gyrus with left parietal lobe and right inferior frontal gyrus (Fletcher, Buechel, Josephs, Friston, & Dolan, 1999). Our finding of a positive correlation between reading skill and the modulatory effect from left inferior frontal gyrus to left inferior parietal lobule in control children may indicate children with higher reading skill are more effective at the top-down modulation of posterior language processing regions. Anterior cingulate/medial frontal gyrus has been implicated in conflict detection, selective attention, and error monitoring (Kiehl, Liddle, & Hopfinger, 2000; Weissman, Giesbrecht, Song, Mangun, & Woldor., 2003). The positive correlation of reading skill with modulatory effects between left inferior parietal lobule and medial frontal gyrus in control children suggests that higher skill is associated with more effective detection and resolution of conflicts between orthography and phonology.
In conclusion, this study found that children with reading difficulties showed weaker modulatory effect from left fusiform gyrus to left inferior parietal lobule compared to controls for conflicting trials suggesting a deficit in integrating orthography and phonology. Modulatory effects from left fusiform gyrus and inferior parietal lobule to inferior frontal gyrus were stronger for conflicting trials than for non-conflicting trials only in control children indicating children with reading difficulties have a deficit in utilizing and phonological rehearsal/segmentation system in left inferior frontal gyrus. In addition, the weaker conflict effect in children with reading difficulties from left inferior parietal lobule to left inferior frontal gyrus implicates a deficit in the indirect pathway from posterior to anterior language processing regions. In addition, control children had a positive correlation of reading skill with modulatory effect from left inferior frontal gyrus to left inferior parietal lobule suggesting children with higher skill are more effective in top-down modulation of orthographic and phonological information integration, and with modulatory effect between medial frontal gyrus and left inferior parietal lobule suggesting children with higher skill are more effective in conflict detection and resolution.
Acknowledgments
This research was supported by grants from the National Institute of Child Health and Human Development (HD042049) and by the National Institute of Deafness and Other Communication Disorders (DC06149) to J.R.B.
References
- Bitan T, Booth JR, Choy J, Burman DD, Gitelman DR, Mesulam MM. Shifts of effective connectivity within a language network during rhyming and spelling. Journal of Neuroscience. 2005;25:5397–5403. doi: 10.1523/JNEUROSCI.0864-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Burman DD, Lu D, Cone N, Gitelman DR, Mesulam MM, et al. Weaker top-down modulation from left inferior frontal gyrus area in children. NeuroImage. 2006;33:991–998. doi: 10.1016/j.neuroimage.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Burman DD, Chou TL, Dong L, Cone NE, Cao F, et al. The interaction of orthographic and phonological information in children: An fMRI study. Human Brain Mapping. 2007;28:880–891. doi: 10.1002/hbm.20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Cheon J, Lu D, Burman DD, Booth JR. Age-dependent top-down control vs age-dependent bottom-up processing in children reconciling orthographic and phonological information: An effective connectivity, fMRI study. NeuroImage. 2007;36:S75. [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TR, Mesulam MM. Functional anatomy of intra- and cross-modal lexical tasks. NeuroImage. 2002;16:7–22. doi: 10.1006/nimg.2002.1081. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TR, Mesulam MM. The relation between brain activation and lexical performance. Human Brain Mapping. 2003;19:155–169. doi: 10.1002/hbm.10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazdil M, Mikl M, Marecek R, Krupa P, Rektor I. Effective connectivity in target stimulus processing: A dynamic causal modeling study of the visual oddball task. NeuroImage. 2007;35:827–835. doi: 10.1016/j.neuroimage.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Bruck M. Persistence of dyslexic’s phonological awareness deficits. Developmental Psychology. 1992;28:874–886. [Google Scholar]
- Burock MA, Buckner RL, Woldor MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. NeuroReport. 1998;9:3735–3739. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- Cao F, Bitan T, Chou TL, Burman DD, Booth JR. Deficient orthographic and phonological representations in children with dyslexia revealed by brain activation patterns. Journal of Child Psychology and Psychiatry. 2006;47:1041–1050. doi: 10.1111/j.1469-7610.2006.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain [see comment] Annals of Neurology. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Chaminade T, Fonlupt P. Changes of effective connectivity between the lateral and medial parts of the prefrontal cortex during a visual task. European Journal of Neuroscience. 2003;18(3):675–679. doi: 10.1046/j.1460-9568.2003.02787.x. [DOI] [PubMed] [Google Scholar]
- Crosson B, Rao SM, Woodley SJ, Rosen AC, Bobholz JA, Mayer A, et al. Mapping of semantic, phonological, and orthographic verbal working memory in normal adults with functional magnetic resonance imaging. Neuropsychology. 1999;13(2):171–187. doi: 10.1037//0894-4105.13.2.171. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The brain binds entities and events by multiregional activation from convergence zones. Neural Computation. 1989;1:123–132. [Google Scholar]
- Desmond JE, Glover GH. Estimating sample size in functional MRI (fMRI) neuroimaging studies: Statistical power analyses. Journal of Neuroscience Methods. 2002;118(2):115–128. doi: 10.1016/s0165-0270(02)00121-8. [DOI] [PubMed] [Google Scholar]
- Deutsch GK, Dougherty RF, Bammer R, Siok WT, Gabrieli JD, Wandell B. Children’s reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex. 2005;41(3):354–363. doi: 10.1016/s0010-9452(08)70272-7. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Wilke M, Eckert M, Richards T, Richards A. Anatomical signatures of dyslexia in children: Unique information from manual and voxel based morphometry brain measures. Cortex. 2005;41(3):304–315. doi: 10.1016/s0010-9452(08)70268-5. [DOI] [PubMed] [Google Scholar]
- Ethofer T, Anders S, Erb M, Herbert C, Wietho S, Kissler J, et al. Cerebral pathways in processing of affective prosody: A dynamic causal modeling study. NeuroImage. 2006;30(2):580–587. doi: 10.1016/j.neuroimage.2005.09.059. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proceedings of the National Academy of Sciences. 1998;95:914–921. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P, Buechel C, Josephs O, Friston K, Dolan R. Learning-related neuronal responses in prefrontal cortex studied with functional neuroimaging. Cerebral Cortex. 1999;9(2):168–178. doi: 10.1093/cercor/9.2.168. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. NeuroImage. 2003;19(4):1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Homae F, Yahata N, Sakai KL. Selective enhancement of functional connectivity in the left prefrontal cortex during sentence processing. NeuroImage. 2003;20(1):578–586. doi: 10.1016/s1053-8119(03)00272-6. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proceedings of the National Academy of Sciences. 1998;95:8939–8944. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Lowe M, Chen SHA, Lurito J, Mathews V. Word rhyming as a probe of hemispheric language dominance with functional magnetic resonance imaging. Neuropsychiatry Neuropsychology & Behavioral Neurology. 2000;13(4):264–270. [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: An event-related fMRI study. Psychophysiology. 2000;37(2):216–223. [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, et al. Microstructure of temporo-parietal white matter as a basis for reading ability: Evidence from diffusion tensor magnetic resonance imaging [see comments] Neuron. 2000;25(2):493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Lurito JT, Kareken DA, Lowe MJ, Chen SHA, Mathews VP. Comparison of rhyming and word generation with FMRI. Human Brain Mapping. 2000;10(3):99–106. doi: 10.1002/1097-0193(200007)10:3<99::AID-HBM10>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR. Towards a network theory of cognition. Neural Networks. 2000;13(8–9):861–870. doi: 10.1016/s0893-6080(00)00059-9. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Grady CL, Ungerleider LG, Haxby JV, Rapoport SI, Horwitz B. Network analysis of cortical visual pathways mapped with PET. Journal of Neuroscience. 1994;14(2):655–666. doi: 10.1523/JNEUROSCI.14-02-00655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson BW, Ackerman PT, Dykman RA. Auditory and visual rhyme judgements reveal differences and similarities between normal and disabled adolescent readers. Dyslexia. 1997;3:63–77. [Google Scholar]
- Mechelli A, Price CJ, Noppeney U, Friston KJ. A dynamic causal modeling study on category effects: Bottom-up or topdown mediation? [see comment] Journal of Cognitive Neuroscience. 2003;15(7):925–934. doi: 10.1162/089892903770007317. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Annals of Neurology. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RSJ, et al. Is developmental dyslexia a disconnection syndrome? Brain. 1996;119:143–157. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, Friston KJ. Comparing dynamic causal models. NeuroImage. 2004;22(3):1157–1172. doi: 10.1016/j.neuroimage.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl E, Shaywitz BA, Shaywitz SE, Fulbright RK, Constable RT, et al. The angular gyrus in developmental dyslexia: Task specific differences in functional connectivity within posterior cortex. Psychological Science. 2000;11(1):51–56. doi: 10.1111/1467-9280.00214. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, et al. Cerebral organization of component processes in reading. Brain. 1996;119(4):1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Rack JP. Orthographic and phonetic coding in developmental dyslexia. British Journal of Psychology. 1985;76(3):325–340. doi: 10.1111/j.2044-8295.1985.tb01956.x. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Andreason P, Zametkin AJ, Aquino T, King AC, Hamburger SD, et al. Failure to activate the left tempoparietal cortex in dyslexia. Archives of Neurology. 1992;49:527–534. doi: 10.1001/archneur.1992.00530290115020. [DOI] [PubMed] [Google Scholar]
- Shaywitz B, Shaywitz S, Pugh K, Mencl E, Fulbright R, Skudlarski P, et al. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Silani G, Frith U, Demonet JF, Fazio F, Perani D, Price C, et al. Brain abnormalities underlying altered activation in dyslexia: A voxel based morphometry study. Brain. 2005;128(Pt 10):2453–2461. doi: 10.1093/brain/awh579. [DOI] [PubMed] [Google Scholar]
- Sonty SP, Mesulam MM, Weintraub S, Johnson NA, Parrish TB, Gitelman DR. Altered effective connectivity within the language network in primary progressive aphasia. Journal of Neuroscience. 2007;27(6):1334–1345. doi: 10.1523/JNEUROSCI.4127-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanovich KE, Siegel LS. Phenotypic performance profile of children with reading disabilities: A regression based approach to the phonological-core variable-difference model. Journal of Educational Psychology. 1994;86:24–53. [Google Scholar]
- The Educator’s Word Frequency Guide. Brewster, NY: Touchstone Applied Science Associates, Inc; 1996. [Google Scholar]
- Torgeson JK, Wagner RK, Rashotte CA. Test of Word Reading Efficiency. Austin. TX: PRO-ED, Inc; 1999. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation; a Harcourt Brace and Company; 1999. [Google Scholar]
- Weissman DH, Giesbrecht B, Song AW, Mangun GR, Woldor MG. Conflict monitoring in the human anterior cingulate cortex during selective attention to global and local object features. NeuroImage. 2003;19(4):1361–1368. doi: 10.1016/s1053-8119(03)00167-8. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Achievement. Itasca IL: The Riverside Publishing Company; 2001. [Google Scholar]
- Xu B, Grafman J, Gaillard WD, Ishii K, Vega-Bermudez F, Pietrini P, et al. Conjoint and extended neural networks for the computation of speech codes: The neural basis of selective impairment in reading words and pseudowords. Cerebral Cortex. 2001;11(3):267–277. doi: 10.1093/cercor/11.3.267. [DOI] [PubMed] [Google Scholar]