Abstract
IgG antibodies can suppress more than 99% of the antibody response against the antigen to which they bind. This is used clinically to prevent rhesus-negative (Rh−) women from becoming immunized against Rh+ erythrocytes from their fetuses. The suppressive mechanism is poorly understood, but it has been proposed that IgG/erythrocyte complexes bind to the inhibitory Fc receptor for IgG (FcγRIIB) on the B cell surface, thereby triggering negative signals that turn off the B cell. We show that IgG induces the same degree of suppression of the response to sheep erythrocytes in animals lacking the known IgG-binding receptors FcγRIIB, FcγRI + III, FcγRI + IIB + III, and FcRn (the neonatal Fc receptor) as in wild-type animals. Reinvestigation of the ability of F(ab′)2 fragments to suppress antibody responses demonstrated that they were nearly as efficient as intact IgG. In addition, monoclonal IgE also was shown to be suppressive. These findings suggest that IgG inhibits antibody responses through Fc-independent mechanisms, most likely by masking of antigenic epitopes, thereby preventing B cells from binding and responding to antigen. In agreement with this, we show that T cell priming is not abolished by passively administered IgG. The results have implications for the understanding of in vivo regulation of antibody responses and Rh prophylaxis.
The ability of antibodies to inhibit induction of immunity has been known for almost a century. It was shown in 1909 that an excess of antitoxin inhibited development of immunity to diphtheria toxin in guinea pigs (1). In a system studying the antibody response in mice after immunization with sheep erythrocytes (SRBC), Henry and Jerne demonstrated that the molecules responsible for feedback inhibition of antibody responses were IgG antibodies (2). The ability of passively administered IgG to suppress immune responses since has been studied intensively. Microgram amounts of IgG can suppress more than 99% of a primary antibody response against SRBC (2, 3), whereas the suppressive effect on induction of immunological memory and a secondary antibody response is less pronounced (4–7). Suppression is induced by all murine IgG subclasses and is strictly antigen-specific, i.e., only the response to an antigen to which IgG can bind is affected (3, 8). The ability of IgG to suppress immune responses has been applied clinically in the so-called rhesus (Rh) prophylaxis. Rh− women, lacking the Rh antigen on their erythrocytes, may develop IgG antibodies against Rh+ erythrocytes acquired transplacentally from their Rh+ fetuses. Because IgG antibodies are transported actively via the placenta from mother to young, such antibodies can damage fetal erythrocytes (reviewed in ref. 9). To prevent this, IgG anti-Rh is administered routinely to Rh− women during pregnancy or immediately after delivery of an Rh+ baby. This treatment inhibits the production of maternal anti-Rh antibodies and has brought the incidence of hemolytic disease of the newborn down dramatically since it was first introduced in the 1960s (10).
Several models explaining antibody feedback suppression have been suggested. One is that passively administered IgG antibodies mask antigenic epitopes, thus preventing B cells from recognizing and responding to the antigen. Other models postulate the involvement of receptors for the Fc part of IgG (FcγRs). IgG/antigen complexes may be more rapidly eliminated by FcγR+ phagocytes than antigen alone. Alternatively, FcγRIIB, which is the only IgG receptor expressed on B cells, may be involved. FcγRIIB contains a cytoplasmic inhibitory motif (immune-receptor tyrosine-based inhibition motif or ITIM), which, when brought in proximity to receptors containing a specific activation motif (immune-receptor tyrosine-based activation motif or ITAM), inhibits cell activation through the latter (reviewed in ref. 11). ITAMs are present in the B cell receptor (BCR), and it has been shown in vitro that co-cross-linking of FcγRIIB and BCR inhibits B cell activation (12–16). An attractive hypothesis explaining negative feedback regulation of in vivo antibody responses is ITIM-mediated inhibition of B cells, resulting from co-cross-linking of FcγRIIB and BCR by the IgG/antigen complexes.
To understand the mechanism behind feedback suppression it is therefore important to determine whether or not suppression is dependent on the Fc part of the IgG molecule. Although a simple question, it has proven a difficult one to answer unequivocally in experimental systems. The most straightforward way of analyzing this is to compare the suppressive ability of intact IgG with that of F(ab′)2 fragments (where the Fc part has been proteolytically cleaved off). Such studies performed in vivo have given discrepant results, some claiming that F(ab′)2 fragments are less suppressive (4, 8, 17, 18) and others claiming that they are equally suppressive as intact IgG (19, 20). An indirect way of assessing Fc dependence has been to study whether or not suppression is epitope-specific. Suppression of the response only to the epitope recognized by IgG (21, 22) has been interpreted as evidence for the epitope-masking hypothesis whereas suppression of the response to all epitopes on the antigen (3, 8, 18, 23) was considered to indicate Fc dependence. The unresolved question of Fc dependence of IgG-mediated suppression is analyzed here in a novel system, using FcγR-deficient (FcγR−/−) mice. Our results strongly suggest that IgG is able to efficiently suppress antibody responses independently of the Fc part. A way of interpreting available experimental data that can explain many of the discrepancies in the literature is presented.
MATERIALS AND METHODS
Antigens.
SRBC and horse erythrocytes (HRBC) were purchased from The National Veterinary Institute (Uppsala, Sweden) and stored in sterile Alsevers solution at 4°C. For 2,4,6-trinitrophenyl (TNP) conjugation, a mixture of 1 ml packed SRBC, 1.5 ml dH2O, 1.75 ml of 0.56 M cacodylate buffer (cacodylic acid sodium salt; Sigma), pH 6.9, and 2 ml of a 12.5-mg/ml solution of TNP (picrylsulfonic acid hydrate; Sigma) was incubated at room temperature for 1 hr. The cells then were washed once in PBS, once in glycyl-glycine (1 mg/ml in PBS) (Merck), and three times in PBS before use. TNP and 5-iodo-4-hydroxyl-3-nitrophenacetyl (NIP) (NIP-CAP-Osu; Cambridge Research Biochemicals) were conjugated to BSA (Sigma) and SRBC as described (24, 25). Soluble antigens were dialyzed against PBS, sterile-filtered, and stored at 4°C.
Antibodies and F(ab′)2 Fragments.
Polyclonal IgG anti-SRBC were prepared from hyperimmune mouse sera. mAbs were derived from culture supernatants of B cell hybridomas producing IgG1 anti-TNP (B8401H5, H5), IgG2a anti-TNP (C4007B4, 7B4), IgG2b anti-TNP (C1901B4, 1B4) (26), or IgE anti-TNP (IGELb1, IGELb4, and IGELa2) (27). IgG from serum or supernatants was purified on a protein A-Sepharose column (Pharmacia) (28) and IgE was purified on a Sepharose column coupled with monoclonal rat anti-mouse κ (24). F(ab′)2 fragments were prepared by digesting purified IgG2a (7B4) with pepsin (Sigma) at an enzyme/antibody ratio of 1:100, pH 3.5, for 12 hr at 37°C. The digested material then again was passed over a protein A-Sepharose column to remove intact IgG. The nonbound fraction was tested for remaining undigested IgG2a in ELISA (24) on plates coated with BSA-TNP detecting bound antibody with a biotinylated anti-IgG2a antibody (Southern Biotechnology Associates) (Fig. 3A). The hemagglutination units are defined as the reciprocal of the highest antibody dilution able to agglutinate a 0.125% suspension of SRBC-TNP after 1-hr incubation at 37°C. Antibody concentrations were determined by absorbance at 280 nm (OD of 1.5 was assumed to equal 1 mg/ml of antibody). All antibodies used were dialyzed against PBS, sterile-filtered, and stored at −20°C.
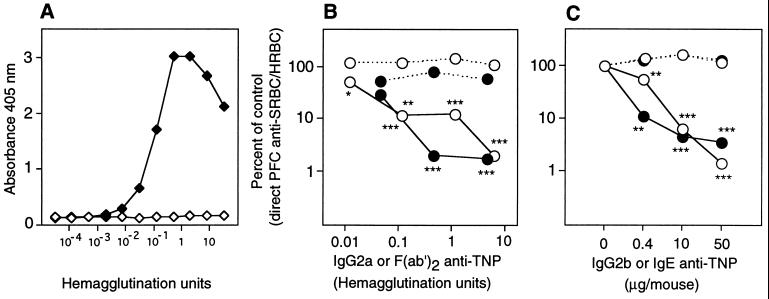
Figure 3.
(A) Binding of intact TNP-specific IgG2a (7B4) (solid symbols) and F(ab′)2 prepared from 7B4 (open symbols) to BSA-TNP in ELISA. Twofold serial dilutions were started with antibody preparations containing 32 hemagglutination units (HU)/ml [corresponding to 68 μg/ml 7B4 and 272 μg/ml of F(ab′)2]. (B) Groups of four to five (C57BL/6 × DBA/2)F1 mice were immunized with 1 × 106 SRBC-TNP and 4 × 105 HRBC preincubated for 1 hr at 37°C with the indicated amounts of intact TNP-specific IgG2a (7B4) (solid symbols), F(ab′)2 of IgG2a (7B4) (open symbols), or PBS. The antibody preparations were the same as those tested in A. Mice were given 54 (containing 6.4 HU), 10, 1, or 0.1 μg F(ab′)2 and 10 (containing 4.7 HU), 1, or 0.1 μg intact 7B4. Five days later the direct SRBC-specific (solid line) and HRBC-specific (broken line) PFC/spleen were assayed. PFC/spleen in the respective control groups (receiving antigen alone) were: 22,368 SRBC; 26,701 HRBC. This experiment was repeated twice: once with a single dose in (C57BL/6 × DBA/2)F1 mice and once as a titration in C57BL/6, with both experiments giving similar results. (C) Groups of three to five (C57BL/6 × DBA/2)F1 mice were immunized with 4 × 106 SRBC-TNP and 8 × 105 HRBC preincubated with 0–50 μg of IgE anti-TNP (IGELb4) (solid circles) or IgG2b anti-TNP (1B4) (open circles). Five days later the direct SRBC-specific (solid line) and HRBC-specific (broken line) PFC/spleen were assayed. PFC/spleen in the respective control groups (receiving antigen alone) were: 41,400 SRBC; 32,289 HRBC. In another experiment, mice were immunized with 50 μg IGELb4 or two other monoclonal TNP-specific IgE antibodies (IGELa2, IGELb1) and SRBC-TNP. All three IgE antibodies induced more than 90% suppression (not shown).
Mice.
Founders for the FcγRIIB−/− (29) and the Fc receptor γ-chain (FcRγ)−/− mice (30) were a kind gift from J. V. Ravetch (29, 30). β2-Microglobulin (β2m)−/− mice (31) were purchased from The Jackson Laboratory. Double “knock-out” mice, lacking FcRγ as well as FcγRIIB (FcRγ−/− × FcγRIIB−/−), were purchased from Taconic Farms. C57BL/6, CBA, (C57BL/6 × 129/Sv)F1, and (C57BL/6J × DBA/2)F1 mice were from Bommice, Ry, Denmark. FcγRIIB−/−, FcRγ−/−, and (FcRγ−/− × FcγRIIB−/−) mice are on a mixed C57BL/6 and 129/Sv background, and, therefore, (C57BL/6 × 129/Sv)F1 or C57BL/6 mice were used as wild-type controls. The β2m−/− mice are on a homogenous C57BL/6 background, and controls were C57BL/6 mice. All animals were maintained and bred at the Department of Genetics and Pathology, Biomedicum, or at the Department of Animal Development and Genetics, Uppsala University. Mice were age- and sex-matched within each experiment.
Immunizations.
Mice were immunized in their tail veins with 0.2 ml antigen/antibody complexes formed by incubating antigen and antibodies (or antigen and PBS for controls) for 1 hr at 37°C immediately before injection. In a few experiments, as indicated, antigen and antibody were administered separately: 0.1 ml antibody followed within 1 hr by 0.1 ml antigen in PBS i.v.
Adoptive Transfers.
Adoptive transfers were conducted 5 months after primary immunization. Spleen cells (4 × 107) from donors primed with IgG and SRBC, primed with SRBC alone, or left unimmunized were transferred i.v. in 0.2 ml PBS to syngeneic, irradiated recipients (600 rad, 24 hr earlier). Thirty minutes later, 4 × 107 SRBC-NIP in 0.1 ml PBS was administered i.v.
Plaque-Forming Cell (PFC) and Enzyme-Linked Immunospot (ELISPOT) Assays.
A modified version of the Jerne hemolytic PFC assay (32) was used. One hundred microliters of a spleen cell suspension from the mouse to be tested, 25 μl of a 10% SRBC or HRBC suspension, and 25 μl of guinea pig serum (as a source of complement, diluted 1:4) (The National Veterinary Institute, Uppsala, Sweden) were added to 300 μl of 0.5% agarose [50% Seaplaque GTG (low melting point, Amersham) and 50% agarose (United States Biochemical)] kept at 45°C. The mixture was spread quickly on a microscope slide and incubated in a humid chamber for 3 hr at 37°C. All dilutions were made in Hanks’ balanced salt solution. Duplicate samples were counted “blindly” under a magnifying glass. The ELISPOT assays for measuring SRBC and NIP responses have been described in detail (25, 33). Briefly, ELISA plates were coated with SRBC or BSA-NIP. Spleen cells suspended in cell culture medium were applied and incubated at 37°C for 3 hr. Antibodies produced by the cells were visualized as spots after the addition of sheep anti-mouse IgG-alkaline phosphatase (Jackson ImmunoResearch) and the precipitating substrate BCIP (5-bromo-4-chloro-3-indolyl phosphate; Sigma). Statistical analyses were performed with Student’s t test, and P values are presented as not significant (ns), P > 0.05; ∗, P < 0.05; ∗∗, P < 0.01; or ∗∗∗, P < 0.001.
RESULTS
IgG-Mediated Suppression in Mice Lacking FcγRIIB.
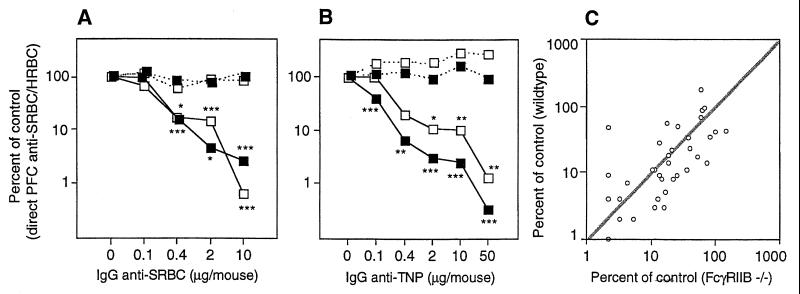
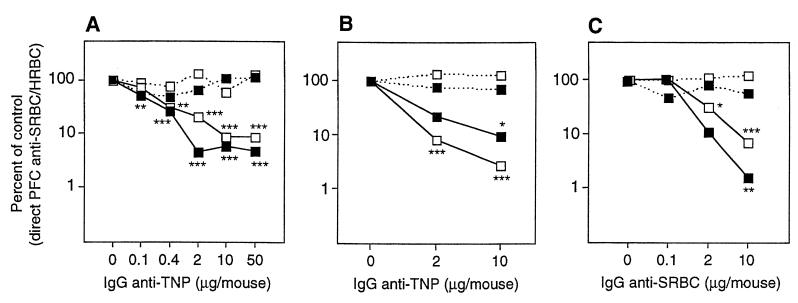
FcγRIIB binds IgG1, IgG2a, and IgG2b (but not IgG3) and is the only FcγR on B cells (reviewed in ref. 34). This receptor is involved in endocytosis and phagocytosis and can also negatively regulate cell activation, as described in the Introduction. To test the involvement of FcγRIIB in IgG-mediated suppression, we immunized FcγRIIB−/− and wild-type mice with polyclonal IgG anti-SRBC + SRBC + HRBC. Controls received SRBC + HRBC alone. The number of single cells producing SRBC- or HRBC-specific IgM was assayed 5 days later in a PFC assay. IgG was able to induce equally efficient suppression of the SRBC response in both strains (Fig. 1A). Suppression was antigen-specific, because the response to the non-cross-reacting antigen, HRBC, was normal (Fig. 1A). To test non-epitope-specific suppression also, mice were immunized with monoclonal IgG anti-TNP + SRBC-TNP + HRBC. Again, the response to SRBC, but not to HRBC, was suppressed efficiently both in FcγRIIB−/− and wild-type mice (Fig. 1B). Comparison of the suppressive effect of IgG in the two strains was done altogether in 14 experiments (46 matched pairs). Plotting the responses in wild-type versus FcγRIIB−/− mice show a distribution around a line with the slope 1, which is to be expected if suppression were equally efficient in both strains (Fig. 1C). Thus, both epitope-specific and non-epitope-specific suppression is highly efficient in the absence of FcγRIIB at all doses of IgG tested, and no significant difference in the degree of suppression could be seen.
Figure 1.
(A) Groups of six to nine FcγRIIB−/− (open symbols) or five to seven (C57BL/6 × 129/Sv)F1 (solid symbols) mice were immunized with 0–10 μg of polyclonal IgG anti-SRBC followed within 1 hr by 1 × 107 SRBC and 4 × 105 HRBC. Five days later the direct SRBC-specific (solid line) and HRBC-specific (broken line) PFC/spleen were assayed. The response is shown as the percentage of the response in control groups. PFC/spleen in the respective control groups (receiving antigen alone) were: FcγRIIB−/−, 106,615 SRBC, 21,183 HRBC; (C57BL/6 × 129/Sv)F1, 120,666 SRBC, 37,583 HRBC. (B) Groups of five FcγRIIB−/− or four to five C57BL/6 mice were immunized with 4 × 106 SRBC-TNP and 8 × 105 HRBC preincubated with 0–50 μg of a mixture of equal amounts of monoclonal IgG1 (H5), IgG2a (7B4), and IgG2b (1B4) anti-TNP. Symbols and assay are as in A. PFC/spleen in the respective control groups (receiving antigen alone) were: FcγRIIB−/−, 127,231 SRBC, 83,472 HRBC; C57BL/6, 35,080 SRBC, 34,260 HRBC. (C) Summary of the 14 experiments performed in which FcγRIIB−/− and wild-type control mice were immunized with polyclonal IgG anti-SRBC, monoclonal IgG1, IgG2a, or IgG2b anti-TNP, or mixtures of the mAbs. The suppression of direct PFC anti-SRBC (expressed as percentage of control response + 1) in FcγRIIB−/− mice is plotted vs. the suppression in corresponding wild-type mice (46 matched pairs; overlapping points are shown only as one symbol). A line with slope 1:1 is added to the figure for comparison.
We have chosen to analyze the number of B cells producing IgM anti-SRBC 5 days after immunization. This decision was based on studies showing that the optimal IgM response in FcγRIIB−/− mice immunized with 4 × 106 SRBC occurred at this time point and that the IgG response was too low to allow reliable analysis (not shown).
IgG-Mediated Suppression in Mice Lacking FcγRI + III (FcRγ−/−), FcRn (β2m−/−), or FcγRI + II + III (FcRγ−/− × FcγRIIB−/−).
Apart from FcγRIIB, known murine receptors for IgG are FcγRI, FcγRIII, and the neonatal FcR (FcRn) (reviewed in refs. 34 and 35). FcγRI binds IgG2a and IgG3 (36) and is the only high-affinity receptor for IgG. FcγRIII binds IgG1, IgG2a, and IgG2b. Mice lacking FcRγ, which is a constituent of both FcγRI and FcγRIII, do not express either receptor (30). The data here show that the anti-SRBC response was profoundly suppressed by IgG in animals lacking FcγRI + III (Fig. 2A). FcRn transports IgG from mother to young (37) and protects IgG from catabolism in adult mice (38). It is a heterodimer of a class I-like α-chain and β2m, which also is needed for its expression (39). FcRn is the only receptor reported to bind all subclasses of IgG (40) and was an interesting candidate because all IgG subclasses can induce suppression. However, we found no evidence for less efficient IgG-mediated suppression in β2m−/− mice compared with C57BL/6 controls (Fig. 2B). Finally, double “knock-out” mice lacking both FcRγ and FcγRIIB were tested for suppression. We found that IgG also induced efficient suppression in these animals (Fig. 2C).
Figure 2.
(A) Groups of four to five FcRγ−/− (lacking FcγRI + III) (open symbols) and C57BL/6 mice (solid symbols) were immunized with 4 × 106 SRBC-TNP and 8 × 105 HRBC preincubated with 0–50 μg of a mixture of equal amounts of monoclonal IgG1 (H5), IgG2a (7B4), and IgG2b (1B4) anti-TNP. Five days later the direct SRBC-specific (solid line) and HRBC-specific (broken line) PFC/spleen were assayed. The response is shown as the percentage of the response in control groups. PFC/spleen in the respective control groups (receiving antigen alone) were: FcRγ−/−, 178,969 SRBC, 16,133 HRBC; C57BL/6, 73,409 SRBC, 6,932 HRBC. This experiment was repeated twice using polyclonal IgG anti-SRBC without evidence of less efficient suppression in FcRγ−/− mice (not shown). (B) Groups of five β2m−/− (lacking FcRn) and C57BL/6 mice were immunized with 4 × 106 SRBC-TNP and 8 × 105 HRBC preincubated with 0, 2, and 10 μg of a mixture of equal amounts of monoclonal IgG1 (H5), IgG2a (7B4), and IgG2b (1B4) anti-TNP. Symbols and assay are as in A. PFC/spleen in the respective control groups (receiving antigen alone) were: β2m−/−, 77,854 SRBC, 49,238 HRBC; C57BL/6, 66,251 SRBC, 65,938 HRBC. This experiment was repeated once using 50 μg of polyclonal IgG anti-SRBC and SRBC, showing more than 99% suppression in both strains (not shown). (C) Groups of four (FcRγ−/− × FcγRIIB−/−) and C57BL/6 mice were immunized with 4 × 106 SRBC and 8 × 105 HRBC preincubated with 0–10 μg of polyclonal IgG anti-SRBC. Symbols and assay are as in A. PFC/spleen in the respective control groups (receiving antigen alone) were: (FcRγ−/− × FcγRIIB−/−), 54,055 SRBC, 104,172 HRBC; C57BL/6, 15,072 SRBC, 62,202 HRBC.
Suppression by F(ab′)2 Fragments of IgG and by IgE.
Because no involvement of FcγRs in IgG-mediated suppression could be demonstrated, and because the other major Fc-mediated function of IgG, complement activation, is not required for suppression (41), the ability of F(ab′)2 fragments to suppress was reinvestigated. F(ab′)2 fragments were prepared from purified monoclonal TNP-specific IgG2a (clone 7B4). Intact IgG was not detected in the F(ab′)2 preparation (Fig. 3A), and, based on the sensitivity of the ELISA, contamination with more than 17 ng/ml (0.006% of total protein) of intact IgG could be excluded. F(ab′)2 was a very efficient suppressor and inhibited almost 90% of the response at low concentrations (0.12 hemagglutination units, corresponding to 1 μg/mouse) (Fig. 3B). This demonstrated that the Fc part of IgG is not mandatory for suppression and that F(ab′)2 fragments specific for one epitope can induce suppression of the response to other epitopes. Therefore, non-epitope-specific suppression cannot be used as an argument for involvement of the Fc part. Moreover, the results suggested that antibody classes other than IgG may also induce suppression. Indeed, in mice immunized with monoclonal IgE anti-TNP + SRBC-TNP the magnitude of suppression was similar to that induced by a monoclonal IgG2b anti-TNP (Fig. 3C). Thus, 90–99% suppression of primary SRBC responses can be mediated by F(ab′)2 fragments of IgG as well as by intact IgE.
IgG Antibodies Causing Complete Suppression of the Antibody Response Do Not Abolish Priming of T Helper Cells.
The findings described above suggest that masking of B cell epitopes by IgG explains IgG-mediated suppression. This would not prevent IgG/antigen complexes from being captured, internalized, processed, and presented to T cells by FcγR+ antigen-presenting cells. To test whether T cell priming in vivo is inhibited by IgG, we used a hapten-carrier system (25, 42) (Table 1). Donor mice were injected with polyclonal SRBC-specific IgG and SRBC, with SRBC alone, or they were left unimmunized. As expected, IgG completely suppressed the primary response (not shown). Five months later, cells from these three groups were adoptively transferred to irradiated syngeneic recipients that subsequently were immunized with SRBC-NIP. The IgG anti-NIP response in the recipients reflects the number of SRBC (carrier)-specific T helper cells induced in the donor mice during primary immunization. A memory response was induced in the recipients because mice given SRBC-primed cells and challenged with SRBC-NIP had a significantly higher IgG response than mice receiving unprimed cells [288,606 vs. 1,655 anti-SRBC-SFC (spot-forming cells) and 56,245 vs. 5,605 anti-NIP-SFC]. Interestingly, mice receiving cells from groups primed with IgG + SRBC exhibited a secondary type of IgG anti-NIP response (34,445 SFC) although it was slightly lower than in recipients of SRBC-primed cells (56,245 SFC). Therefore, although the primary antibody response was suppressed completely by IgG, the induction of T helper cells was not abolished.
Table 1.
IgG does not abolish T helper cell induction
| Donor* | Recipient* | 2° IgG anti-NIP† | P vs. c‡ | 2° IgG anti-SRBC† | P vs. c‡ |
|---|---|---|---|---|---|
| Nil | SRBC-NIP | 3.75 ± 0.09 (5,605) | c | 3.22 ± 0.17 (1.655) | c |
| SRBC | SRBC-NIP | 4.75 ± 0.08 (56,245) | 0.001 vs. c | 5.46 ± 0.05 (288,606) | 0.001 vs. c |
| IgG + SRBC | SRBC-NIP | 4.54 ± 0.06 (34,445) | 0.001 vs. 0.005 | 4.42 ± 0.05 (26,104) | 0.001 vs. 0.001 |
CBA donor mice were primed with 50 μg polyclonal SRBC-specific IgG and 4 × 107 SRBC, or 4 × 107 SRBC alone, or were left unprimed. Five months later, groups of eight irradiated, syngeneic recipients received 6 × 107 spleen cells from the different groups of donors and were secondary-immunized with 4 × 107 SRBC-NIP immediately after transfer. Five days after adoptive transfers, the antibody response in recipient mice was tested in an ELISPOT assay. This experiment was repeated once with similar results (not shown).
Mean of the log10 value of IgG anti-NIP/SRBC (SFC/spleen) ± SD (geometrical mean) in recipients 5 days after transfer/secondary immunization.
P value vs. control value (c).
DISCUSSION
We show here that IgG can efficiently inhibit antibody responses in mice lacking the known Fc receptors for IgG. The lack of any demonstrable role of FcγRIIB for IgG-mediated suppression was an unexpected finding. However, previous reports that IgG3 (which is unable to bind to FcγRIIB) can induce efficient suppression in vivo (3, 8) also argue against an exclusive role for this receptor. The antibody production in FcγRIIB−/− mice after immunization with thymus-dependent and thymus-independent antigens (without IgG) is markedly enhanced, suggesting that the receptor plays an important negative regulatory role in vivo in some experimental systems (29). We and others have found indications of Fc-receptor involvement in negative feedback regulation by IgG in vitro. Deglycosylated monoclonal IgG, unable to activate complement and to bind to FcγRs, was less suppressive than glycosylated IgG (43), whereas IgG that was deficient only in classical complement activation was able to suppress (41). Suppression could not be induced in spleen cell cultures depleted of FcγR-positive B cells (44) and was partially inhibited by an mAb that blocks FcγRIIB and III (45). The considerable differences between in vitro and in vivo systems for studying antibody responses probably are responsible for these discrepancies. Obvious differences are the lack of lymphoid organ structure in vitro and the fact that B cells studied in vitro are memory cells derived from spleens primed with high doses of antigen in vivo.
In addition to in vitro studies, the view that IgG-mediated suppression is Fc-dependent was suggested by two types of experimental in vivo findings, both of which are controversial: the inability of F(ab′)2 fragments to induce suppression (4, 8, 17, 18) and the ability of IgG to induce non-epitope-specific suppression (3, 8, 18, 23). There are many difficulties in working with F(ab′)2 fragments, and, in retrospect, it is hard to explain the reason for the conflicting results discussed earlier. A small contamination by intact IgG may be sufficient to induce suppression by “F(ab′)2 preparations.” Alternatively, inefficient suppression by F(ab′)2 could be a consequence of elimination of such fragments more rapidly than that of intact IgG because of the lack of protection by FcRn (38). In this sense, the ability of IgG to induce suppression in vivo can be said to be Fc-dependent although the actual mechanism of action may be independent of FcγRs. In the present study we have excluded contamination exceeding 1.7 ng/mouse, a dose that does not induce suppression. The immunization schedule (preincubating antibody and antigen) minimizes the difference in elimination rate between intact IgG and F(ab′)2 (46). Here, F(ab′)2 also induces significant suppression at concentrations as low as 1 μg/mouse. The slightly less efficient suppression by F(ab′)2 than by intact IgG is probably caused by a more rapid elimination of F(ab′)2 fragments in spite of the immunization schedule. One of the most crucial findings in the present report is that TNP-specific F(ab′)2 fragments can suppress the response to SRBC determinants when injected together with SRBC-TNP. This shows that, contrary to the dogma, the existence of non-epitope-specific suppression cannot be used as evidence for involvement of the Fc portions of IgG. In many experimental systems IgG must recognize an epitope present at high density on the erythrocyte surface to induce suppression (3, 23, 47). TNP-specific monoclonal IgG, for example, can inhibit the SRBC response when given together with SRBC-TNP with a high (but not with a low) TNP density (23). Because TNP is a small hapten (329 Da) that spontaneously couples to the ɛ-amino groups of lysine, we find it likely that it is conjugated to SRBC in a sufficiently even and dense pattern so that IgG, binding to TNP-epitopes, also will sterically hinder recognition of SRBC epitopes. On the other hand, when IgG binds to an epitope that is not so abundant, epitope-specific suppression would be expected. This interpretation leads to the conclusion that, depending on the epitope density, suppression will be epitope- or non-epitope-specific and may explain many of the discrepancies in the literature.
We demonstrate that IgE antibodies can act as negative regulators of antibody responses, adding strong support to the existence of suppression independent of the Fc-part of IgG. Involvement of the Fc part of IgE is unlikely: although IgE binds to FcɛRI and FcɛRII as well as to FcγRIIB and FcγRIII (49), no decrease in IgE-mediated suppression of the response to SRBC-TNP was found in mice lacking these receptors (FcɛRII−/−, FcγRIIB−/−, FcRγ−/−) (data not shown). IgM is an isotype known to enhance antibody responses (2), but at least one monoclonal IgM has been reported to suppress (8). There is a correlation between affinity and suppressive ability among monoclonal IgG antibodies (8). These findings imply that all high-affinity antibodies, when present in molar excess of antigen, have the capacity to inhibit antibody responses to erythrocytes. Both IgG (23) and IgE (48) have dual immunoregulatory roles and can enhance the antibody response to soluble protein antigens. This probably takes place via increased uptake of antigen by FcɛRII or FcγRs on antigen-presenting cells, followed by efficient presentation of peptides to T cells. In addition to increased T cell help, the molar excess of antigen present in such experimental systems would allow the triggering of B cells through their BCR, explaining why the net result is increased antibody production.
In conclusion, we have presented several novel lines of experimental evidence that suggest that negative feedback regulation of primary antibody responses by passively administered IgG in vivo is Fc-independent and probably caused by epitope masking. This model, which we are “reintroducing,” may seem to be at variance with many previous reports. However, if we limit the discussion to in vivo studies and accept that it is possible to get “false” lack of suppression using F(ab′)2 fragments and that, depending on the epitope density on the antigen, both epitope and non-epitope-specific suppression can be induced, the epitope-masking model is, in fact, compatible with the majority of previously published data. The greater efficiency of high-affinity antibodies and the ability of antibody classes other than IgG to suppress fit easily into the model. The relative difficulty in inhibiting induction of memory and secondary responses can be explained by unperturbed T helper cell priming. In addition, memory B cells express high-affinity receptors for antigen that probably compete more effectively with passively administered IgG than naive B cells. Finally, the stoichiometry of the process is compatible with the epitope-masking model. Ten micrograms of IgG corresponds to 4 × 1013 molecules. In mice given 10 μg IgG + 4 × 106 SRBC-TNP, the molar ratio of IgG/SRBC-TNP is 107:1. Assuming that a F(ab′)2 fragment has a diameter of 12.2 × 10−9 m (50), 107 IgG molecules would cover approximately 11.7 × 10−10 m2. An erythrocyte (diameter 7 × 10−6 m) has a surface area of approximately 1.54 × 10−10 m2. Therefore, it is plausible that the doses of IgG shown to cause suppression can do so through steric inhibition. Immunization with preformed complexes of IgG/erythrocytes, employed in most of our experiments, hypothetically biases the experimental system toward epitope masking. However, no evidence of FcR involvement was seen when IgG was injected 1 hr before the antigen (Fig. 1A), making it unlikely that different immunization routes significantly alter the mechanism of suppression.
Allogeneic Rh+ erythrocytes presumably would be less immunogenic in humans than xenogeneic SRBC would be in mice. Therefore, the antibody response against the Rh factor can be expected to be easier to suppress than the response to SRBC, possibly explaining why doses as low as 100–300 μg are sufficient to inhibit Rh immunization in clinical practice (9). Today, Rh− women are treated with polyclonal IgG anti-Rh preparations derived from large pools of human immune sera. Such pools are a potential risk for infections, and the immunization of volunteers, from whom immune sera is derived, is not without complications. The presented findings may aid in the ongoing search for suppressive, monoclonal Rh-specific antibodies, where perhaps emphasis should be put on efficient antigen binding rather than on Fc-mediated properties.
Acknowledgments
We thank Ms. I. Brogren for skillful technical assistance, Dr. J. V. Ravetch for the gift of FcγRIIB−/− and FcRγ−/− founder animals, Dr. M. Wabl for IgE and Dr. P. Coulie for IgG hybridomas, and Drs. S. Applequist and O. Sjöberg for critical review of the manuscript. This work was supported by Agnes and Mac Rudberg’s Foundation; Ankarstrand’s Foundation; Ellen, Walter, and Lennart Hesselman’s Foundation; Hans von Kantzow’s Foundation; King Gustaf V’s 80-Year Foundation; Lilly and Ragnar Åkerham’s Foundation; The Swedish Foundation for Health Care Sciences and Allergy Research; The Swedish Medical Research Council; and The Swedish Council for Work Life Research.
ABBREVIATIONS
- SRBC
sheep erythrocytes
- Rh
rhesus
- FcγR
Fc receptor for IgG
- BCR
B cell receptor
- HRBC
horse erythrocytes
- TNP
2,4,6-trinitrophenyl
- NIP
5-iodo-4-hydroxyl-3-nitrophenacetyl
- FcRγ
Fc receptor γ-chain
- β2m
β2-microglobulin
- PFC
plaque-forming cell(s)
- FcRn
neonatal Fc receptor
- SFC
spot-forming cells
References
- 1.Smith T. J Exp Med. 1909;11:241–260. doi: 10.1084/jem.11.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henry C, Jerne N. J Exp Med. 1968;128:133–152. doi: 10.1084/jem.128.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heyman B, Wigzell H. J Immunol. 1984;132:1136–1143. [PubMed] [Google Scholar]
- 4.Sinclair N R S C. J Exp Med. 1969;129:1183–1201. doi: 10.1084/jem.129.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safford J J W, Tokuda S. J Immunol. 1971;107:1213–1225. [PubMed] [Google Scholar]
- 6.Axelrad M A. J Exp Med. 1971;133:857–863. doi: 10.1084/jem.133.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heyman B, Wigzell H. Scand J Immunol. 1985;21:255–266. doi: 10.1111/j.1365-3083.1985.tb01428.x. [DOI] [PubMed] [Google Scholar]
- 8.Brüggemann M, Rajewsky K. Cell Immunol. 1982;71:365–373. doi: 10.1016/0008-8749(82)90270-2. [DOI] [PubMed] [Google Scholar]
- 9.Bowman J M. Transfus Med Rev. 1988;2:129–150. doi: 10.1016/s0887-7963(88)70039-5. [DOI] [PubMed] [Google Scholar]
- 10.Clarke C A, Donohoe W T A, Woodrow J C, Finn R, Krevans J R, Kulke W, Lehane D, Sheppard P M. Br Med Journal. 1963;1:979–984. doi: 10.1136/bmj.1.5336.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vivier E, Daëron M. Immunol Today. 1997;18:286–291. doi: 10.1016/s0167-5699(97)80025-4. [DOI] [PubMed] [Google Scholar]
- 12.Sidman C L, Unanue E R. J Exp Med. 1976;144:882–896. doi: 10.1084/jem.144.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips N E, Parker D C. J Immunol. 1984;132:627–632. [PubMed] [Google Scholar]
- 14.Bijsterbosch M K, Klaus G G B. J Exp Med. 1985;162:1825–1836. doi: 10.1084/jem.162.6.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amigorena S, Bonnerot C, Drake J R, Choquet D, Hunziker W, Guillet J-G, Webster P, Sautes C, Mellman I, Fridman W H. Science. 1992;256:1808–1812. doi: 10.1126/science.1535455. [DOI] [PubMed] [Google Scholar]
- 16.Muta T, Kurosaki T, Misulovin Z, Sanchez M, Nussenzweig M C, Ravetch J V. Nature (London) 1994;368:70–73. doi: 10.1038/368070a0. [DOI] [PubMed] [Google Scholar]
- 17.Sinclair N R S, Lees R K, Elliott E V. Nature (London) 1968;220:1048–1049. doi: 10.1038/2201048a0. [DOI] [PubMed] [Google Scholar]
- 18.Enriques-Rincon F, Klaus G G B. Immunology. 1984;52:129–136. [PMC free article] [PubMed] [Google Scholar]
- 19.Tao T W, Uhr J W. Nature (London) 1966;212:208–209. doi: 10.1038/212208a0. [DOI] [PubMed] [Google Scholar]
- 20.Cerottini J C, McConahey P J, Dixon F J. J Immunol. 1969;102:1008–1015. [PubMed] [Google Scholar]
- 21.Brody N I, Walker J G, Siskind G W. J Exp Med. 1967;126:81–91. doi: 10.1084/jem.126.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Möller G. Eur J Immunol. 1985;15:409–412. doi: 10.1002/eji.1830150420. [DOI] [PubMed] [Google Scholar]
- 23.Wiersma E J, Coulie P G, Heyman B. Scand J Immunol. 1989;29:439–448. doi: 10.1111/j.1365-3083.1989.tb01143.x. [DOI] [PubMed] [Google Scholar]
- 24.Gustavsson S, Hjulström S, Liu T, Heyman B. J Immunol. 1994;152:4793–4800. [PubMed] [Google Scholar]
- 25.Gustavsson S, Kinoshita T, Heyman B. J Immunol. 1995;154:6524–6528. [PubMed] [Google Scholar]
- 26.Coulie P, Van Snick J. Eur J Immunol. 1985;15:793–798. doi: 10.1002/eji.1830150810. [DOI] [PubMed] [Google Scholar]
- 27.Rudolph A K, Burrows P D, Wabl M R. Eur J Immunol. 1981;11:527–529. doi: 10.1002/eji.1830110617. [DOI] [PubMed] [Google Scholar]
- 28.Ey P L, Prowse S J, Jenkin C R. Immunochemistry. 1978;15:429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- 29.Takai T, Ono M, Hikida M, Ohmori H, Ravetch J V. Nature (London) 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 30.Takai T, Li M, Sylvestre D, Clynes R, Ravetch J V. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 31.Koller B H, Marrack P, Kappler J W, Smithies O. Science. 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 32.Jerne N K, Nordin A A. Science. 1963;140:405. doi: 10.1126/science.140.3565.405. [DOI] [PubMed] [Google Scholar]
- 33.Heyman B, Brogren I, Klareskog L. J Immunol Methods. 1991;139:293–296. doi: 10.1016/0022-1759(91)90200-y. [DOI] [PubMed] [Google Scholar]
- 34.Hulett M D, Hogarth P M. Adv Immunol. 1994;57:1–127. doi: 10.1016/s0065-2776(08)60671-9. [DOI] [PubMed] [Google Scholar]
- 35.Ghetie V, Ward E S. Immunol Today. 1997;18:592–598. doi: 10.1016/s0167-5699(97)01172-9. [DOI] [PubMed] [Google Scholar]
- 36.Gavin A L, Barnes N, Dijstelbloem H M, Hogarth P M. J Immunol. 1998;160:20–23. [PubMed] [Google Scholar]
- 37.Brambell F W R. Lancet. 1966;ii:1087–1093. doi: 10.1016/s0140-6736(66)92190-8. [DOI] [PubMed] [Google Scholar]
- 38.Junghans R P, Anderson C L. Proc Natl Acad Sci USA. 1996;93:5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simister N E, Mostov K E. Nature (London) 1989;337:184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- 40.Guyer R L, Koshland M E, Knopf P. J Immunol. 1976;117:587–593. [PubMed] [Google Scholar]
- 41.Heyman B, Wiersma E, Nose M. Eur J Immunol. 1988;18:1739–1743. doi: 10.1002/eji.1830181113. [DOI] [PubMed] [Google Scholar]
- 42.Mitchison N A. Eur J Immunol. 1971;2:10–17. doi: 10.1002/eji.1830010103. [DOI] [PubMed] [Google Scholar]
- 43.Heyman B, Nose M, Weigle W O. J Immunol. 1985;134:4018–4023. [PubMed] [Google Scholar]
- 44.Stockinger B, Lemmel E-M. Cell Immunol. 1978;40:395–403. doi: 10.1016/0008-8749(78)90347-7. [DOI] [PubMed] [Google Scholar]
- 45.Heyman B. Scand J Immunol. 1989;29:121–126. doi: 10.1111/j.1365-3083.1989.tb01106.x. [DOI] [PubMed] [Google Scholar]
- 46.Spiegelberg H L, Weigle W O. J Exp Med. 1965;121:323–325. doi: 10.1084/jem.121.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quintana I Z, Silveira A V, Möller G. Eur J Immunol. 1987;17:1343–1349. doi: 10.1002/eji.1830170919. [DOI] [PubMed] [Google Scholar]
- 48.Heyman B, Liu T, Gustavsson S. Eur J Immunol. 1993;23:1739–1742. doi: 10.1002/eji.1830230754. [DOI] [PubMed] [Google Scholar]
- 49.Takizawa F, Adamczewski M, Kinet J-P. J Exp Med. 1992;176:469–476. doi: 10.1084/jem.176.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crothers D, Metzger H. Immunochemistry. 1972;9:341–357. doi: 10.1016/0019-2791(72)90097-3. [DOI] [PubMed] [Google Scholar]