Abstract
The potential to induce therapeutic angiogenesis through gene transfer has engendered much excitement as a possible treatment for tissue ischemia. After 10 years of clinical experimentation, however, it now appears clear that several crucial issues are still to be resolved prior to achieving clinical success. These include the understanding of whether functional blood vessels might arise as a result of the delivery of a single angiogenic factor or require more complex cytokine combinations, the identification of the proper timing of therapeutic gene expression and, most notably, the development of more efficacious gene delivery tools. Viral vectors based on the adeno-associated virus (AAV) appear particularly suitable to address the last requirement, since they display a specific tropism for skeletal muscle cells and cardiomyocytes, and drive expression of the therapeutic genes in these cells for indefinite periods of time. In this review, I discuss the current applications of gene therapy for cardiovascular disorders, with particular attention to the possible improvements in the technologies involved in virus-mediated gene transfer.
Keywords: therapeutic angiogenesis, ischemia, adenovirus, cardiomyocytes
Despite the remarkable progresses made over the last several years in early diagnosis and prevention, cardiovascular disorders still represent the leading cause of morbidity and mortality in the industrialized world and a rising concern in most developing countries (Srinath Reddy et al 2005). In Europe alone, over 600,000 deaths per year are registered due to myocardial infarction or arise as a consequence of ischemic cardiomyopathy, with an incidence of 1:6 in men and 1:17 in women (http://www.heartstats.org). The current approaches of medical (trombolysis) or surgical (angioplasty and aorto-coronary bypass) revascularization have significantly modified the natural history of ischemic cardiomyopathy. However, the limitations of these interventions and their relatively poor efficacy in the long term render the progression toward heart failure inevitable in several patients. Therefore, novel approaches for therapeutic intervention are clearly needed. Similar considerations also apply to critical limb ischemia, which is estimated to develop in 500–1,000 individuals per million per year.
Over the last several years, the possibility of exploiting gene delivery for the production of therapeutic factors as a new strategy to treat cardiovascular disease has been supported by the success in pre-clinical experimentation in small and large animal models of human disease. As opposed to other gene therapy applications, at least three considerations support the relative feasibility of the gene therapy approach in the cardiovascular system. First, both the heart and selected vascular districts are easily accessible by using the same devices and techniques that are commonly used by interventional cardiology. Gene delivery is thus local and, in most instances, specific cell targeting is not required. Second, basic research on the molecular and cellular pathways of cardiac and vascular metabolism has provided an impressive list of genes encoding factors that support cardiac function or trigger new blood vessel formation; for several of these genes, preclinical proof of the efficacy of their overexpression has already been obtained. Last, most gene therapy applications in the cardiovascular field will be successful even if achieving relatively modest results when compared to the more demanding goals that are required, for example, by gene therapy for hereditary disorders. Even a positive modulation of cardiac function, a significant decrease in arterial restenosis, or a slowing down of progression toward heart failure would represent excellent clinical endpoints from which to build more ambitious applications.
This review discusses the current applications of gene therapy for the induction of new blood vessel formation in ischemic tissues. I will pay particular attention to the technologies involved in virus-mediated gene transfer to the myocardium and to the arterial wall, given the pivotal role played by issues concerning delivery in determining the outcome of any gene therapy procedure.
Vectors for cardiovascular gene transfer
The success of a gene therapy application depends on three major parameters, namely the choice of an appropriate therapeutic gene, the delivery of this gene to a sufficient number of cells and the achievement of appropriate levels of gene expression. One of the major hurdles that have so far hampered a wider range of gene therapy applications has been the lack of an ideal vector, capable of fulfilling the above requirements and, at the same time, of providing an acceptable safety profile. Table 1 reports the gene delivery procedures that are currently available for gene therapy in the cardiovascular arena.
Table 1.
Gene transfer procedures for cardiovascular gene therapy applications
| Strategy | Method | Advantages | Disadvantages |
|---|---|---|---|
| Naked DNA (plasmids and oligonucleotides) | Direct injection | Easiness of production and use | Modest efficiency
Transitory effect |
| Lipofection | Liposomes
Cationic lipids Complexes liposomes/proteins |
Easiness of production and use | Modest efficiency
Transitory effects |
| Viral vectors | Adenoviral vectors | High multiplicity of infection
High levels of transgene expression Highly concentrated vector preparations High cloning capacity Infection of both quiescent and proliferating cells Broad cell tropism |
Transient transduction
Activation of a powerful antiviral inflammatory and immune response |
| AAV vectors | Non pathogenic
Specific tropism for skeletal and cardiac myocytes and artery smooth muscle cells Highly concentrated vector preparations High multiplicity of infection Transduction of quiescent cells Not inflammatory and not immunogenic Trangene expression persisting indefinitely in vivo |
Relatively limited cloning capacity (<5 kb)
Absence of packaging cell lines |
|
| Physical methods | Electroporation | Relatively easy of set up (for the skeletal muscle) | Low efficiency |
| Physical methods | Ultrasounds | Exploits established clinical procedures | Still not optimized |
The plasma membrane of mammalian cells is a highly selective barrier that normally precludes the entry of polypeptides and other macromolecules, including highly charged nucleic acids. In contrast to this general rule, for reasons that are still unclear, skeletal muscle cells and cardiomyocytes are indeed capable of spontaneously uptaking small quantities of naked DNA. This property has been at the basis of diverse gene therapy experimentation based on plasmid DNA injection to promote therapeutic angiogenesis, which will be discussed later. However, the very low efficiency at which DNA internalization occurs (at least 2 orders of magnitude lower than virus-mediated gene delivery) and the limited period of time during which the transgene is expressed (usually less than 10–14 days) still represent major obstacles that have so far prevented the broad success of this approach.
A potentially interesting and novel way to improve the delivery of plasmid DNA into both endothelial cells and – after disruption of endothelial cell junctions – the underlying muscle cells is the utilization of ultrasounds, either alone or in combination with ecographic contrast media based on gas microbubbles (Lawrie et al 1999; Amabile et al 2001; Chen et al 2003). When the latter are used, their rupture induced by ultrasounds determines an acoustic cavitation event that creates physical pores in the cell plasma membrane. Through these pores, naked DNA can penetrate into the cell’s cytoplasm. Although promising (Ay et al 2001; Danialou et al 2002; Miura et al 2002; Taniyama et al 2002; Huber et al 2003), the outcome of this procedure still appears quite variable and further optimization is required before it reaches the clinic.
Currently, viral vectors based on recombinant adenovirus (Ad) or adeno-associated virus (AAV) are the most successful and extensively used tools to deliver genes in the cardiovascular system (Guzman et al 1993; Fisher et al 1997). These two vectors share a few similar features, such as the ability to transduce a variety of quiescent cell types after in vivo injection, including skeletal muscle cells and cardiomyotyes, and amenability to easy laboratory manipulation.
Adenoviral vectors
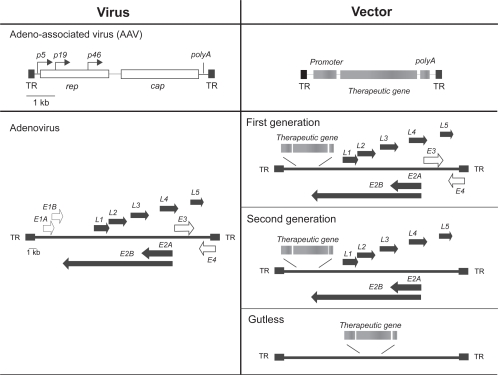
Ad vectors based on serotype 5 have been by far the most commonly used vectors for delivery to the cardiovascular system. These vectors can accommodate large inserts, mediate transient but high levels of protein expression, and can be easily produced at high titers, all remarkable properties in the gene therapy scenario (St George 2003). A schematic representation of the genetic organization of wild type adenovirus and of the vectors derived from this virus is shown in Figure 1. A first generation of vectors contained the therapeutic genes cloned in order to substitute the early E1A and E1B genes, the E1 region being the only portion of the adenoviral genome absolutely required for viral replication. These defective adenoviruses have been used extensively in a number of gene therapy clinical trials, including experimentation in the cardiovascular field (see below). Although very efficient in terms of transduction and transgene expression in the infected cells, the in vivo application of these vectors is notably limited by their strong immunogenicity and by the stimulation of a potent inflammatory response (Yang et al 1996). Major safety concerns about a wider use of these vectors have been raised by the death of a young patient recruited in a gene therapy trial for the treatment of a rare metabolic disorder (OCT deficiency). The lethal event was most probably due to a systemic inflammatory response to the vector triggered by the expression of several wild type adenoviral genes by the transduced cells (Lehrman 1999).
Figure 1.
Schematic representation of the genetic organization of AAV and adenovirus (left side) and of the respective vectors. The location at which the therapeutic gene cassette (promoter+gene+polyadenylation site) is inserted is indicated. TR, Terminal repeat sequence.
In order to limit these highly undesirable effects, a second generation adenoviral vector was developed, in which other viral genes (in particular, E3 and E4) had been deleted. These second generation vectors are better tolerated in terms of immune response and persist longer in the injected animals, at least in some specific tissues. However, for reasons that are still to be understood, the levels of transgene expression from these vectors appears quite unsatisfactory (Qian et al 2001).
Finally, a third generation adenoviral vector consists of the so called “gutless” or helper-dependent vectors, which contain the therapeutic gene and the two repeated regulatory regions at the 3′ and 5′ of the wild type genome, which also include the signals required for packaging the genome into viral particles. The production of these gutless viruses requires co-infection of the producing cells by a first generation adenoviral vector carrying a modification in the 5′ repeated region. Through this, the Cre recombinase produced by the infected cells determines the deletion of the packaging signal of the helper construct, which is flanked by two LoxP sites (Mitani et al 1995; Parks et al 1996). These vectors are efficient and very well tolerated in vivo; in addition, they permit cloning of large DNA inserts, thus opening the way to the possibility of transferring large cDNAs or even entire genomic regions including their normal regulatory genetic elements. However, their production is quite cumbersome and the efficiency of purification of the vector from the helper virus particles is rather inefficient, thus still preventing broad clinical application.
Adeno-associated virus (AAV) vectors
A constantly growing number of pre-clinical gene therapy studies exploit vectors based on AAV, a small non-enveloped, single-stranded (ss) DNA virus with a diameter of 18–25 nm, which belongs to the Parvoviridae family and is classified in the Dependovirus genus. Members of this genus are widely diffuse in nature: well over 100 AAV variants have so far been isolated from primate sources, and new serotypes are continuously discovered. All serotypes share a similar structure, genome size and genetic organization. Over the last few years, viral vectors based on AAV have gained increasing popularity due to several favorable characteristics, including the high efficiency of transduction of post-mitotic tissues such as muscle, heart, brain and retina and the long-term persistence of transgene expression in the absence of inflammation or immune response (Favre et al 2001). The wild type, 4.7 kb long AAV genome contains two open reading frames, corresponding to the rep and cap genes encoding for the replicative and capsid proteins of the virus, respectively (Figure 1). Through the use of two different promoters and the alternative inclusion of one exon, the rep gene gives rise to four protein isoforms (rep78, 68, 52 and 40). Three different products (VP1, VP2, VP3) are also generated from the cap gene after the alternative usage of three different translation start sites and of a common polyadenylation signal. The coding region of AAV is flanked by two 145 bp interted terminal repeats (ITRs), which are complementary within the first 125 bp and form a T-shaped hairpin at both ends of the genome. This palindromic sequence is the only cis-acting element required for all the major functions of AAV (viral DNA replication, assembly of the viral particles, integration/excision from the host genome) and is the only sequence of viral origin present in the vector DNA.
The life cycle of wild type AAV strictly depends on the presence or absence of host cell coinfection with a helper virus. Under non-permissive conditions (ie, without a helper virus), the AAV genome mainly integrates into a specific region (AAVS1) of human chromosome 19q13.3 (Kotin et al 1992; Linden et al 1996; Dutheil et al 2000), where it establishes a latent infection for indefinite periods of time. This represents the only characterized instance of site-specific integration of a virus in a mammalian cell; the exploitation of this event would permit the safe insertion of exogenous genes into the human genome, a highly desirable goal in the gene therapy field. In this respect, however, it needs to be pointed out that site-specific integration is entirely dependent on the function of the AAV Rep proteins. Since the rep gene is removed from the vector DNA – mainly because its unregulated expression is toxic to the infected cells (see Marcello et al (2000) and citations therein) – the current generation of AAV vectors persists inside non-dividing cells mainly as extrachromosomal, concatemerized DNA (Schnepp et al 2005); if the vector genome integrates, it does so in a random manner (Miller et al 2005).
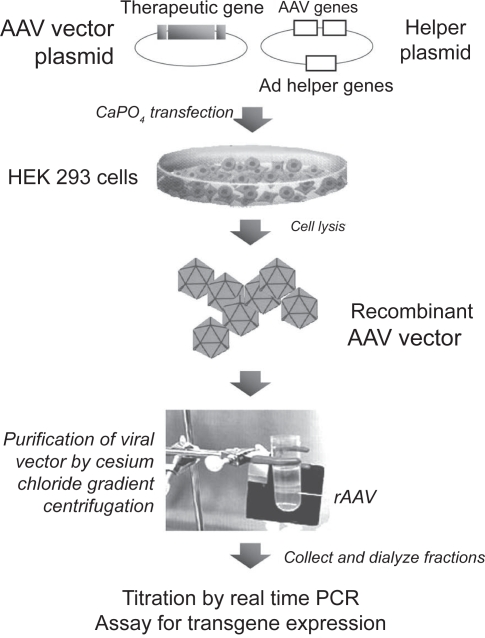
The characteristics of the AAV life cycle, including its defectiveness and ability to persist in infected cells as a latent viral genome, suggested early on that this virus could be an excellent tool for in vivo gene transfer (Tratschin et al 1984). Since the AAV genome cloned into a plasmid is still infectious and able to produce viral particles, any exogenous gene (less than 4.5 kb in length) can, in theory, be placed within the two 145 bp ITRs to obtain a circular backbone suitable for vector production. Unlike other delivery systems that have evolved over several generations, the original composition of the AAV vector plasmid (a transgene expression cassette flanked by the two ITRs) is essentially the same as in the current version. The traditional method for rAAV production was based on co-transfection of the vector plasmid together with a second plasmid, supplementing the Rep and Cap gene functions, into Ad helper-infected cells (usually HeLa or HEK293 cells); more recently, adenovirus co-infection has been substituted by the co-transfection of a plasmid also expressing a few adenoviral genes, which provide the helper functions (Grimm et al 1998) (Figure 2).
Figure 2.
Production of AAV vectors. A plasmid containing the therapeutic gene cassette (promoter+gene+polyadenylation site) cloned between the AAV terminal repeats is co-transfected into epithelial HEK293 cells together with a plasmid containing the rep and cap AAV genes and some genes from adenovirus that provide helper function. After 48 hours, cells are lysed and recombinant vector particles are purified by cesium chloride gradient centrifugation. Fractions are collected from the gradient and the number of particles containing viral genomes are quantified by real-time PCR.
Therapeutic angiogenesis
The possibility to exploit viral vectors to deliver genes to the myocardium and the skeletal muscle at high efficiency permits the development of novel therapeutic strategies for the treatment of cardiac and limb ischemia. Ischemia refers to a lack of oxygen due to inadequate perfusion, which results from an imbalance between oxygen supply and demand. The most common cause of myocardial ischemia is the atherosclerosis of epicardial coronary arteries. Slowly developing, high-grade coronary artery stenosis does not usually precipitate acute infarction, because of the progressive development of a rich collateral network. Myocardial infarction generally occurs when there is an abrupt decrease in coronary blood flow, following the thrombotic occlusion of a coronary artery, previously narrowed by atherosclerosis. This event is associated with the almost instantaneous failure of normal muscle contraction and relaxation. The events following myocardial infarction often have a devastating impact on the patient’s conditions, principally because of the irreversible death of the cardiomyocytes and their substitution over time by a scar of akinetic fibrous tissue. The extent of the infarction depends on the duration and the severity of the perfusion defect. However, the entity of the myocardial injury is also modulated by a number of factors, including development of a functional collateral circulation, medical therapy and ischemic preconditioning. Beyond scar contraction, progressive ventricular remodelling can further reduce cardiac function in the weeks following the initial event (Pfeffer et al 1991).
Most of the currently available therapies – for instance angioplasty and thrombolysis – can significantly relieve the cause of the infarction, notably improving the prognosis of patients. Conversely, after the occurrence of the irreversible injury, no medication or procedure has so far shown efficacy in restoring adequate blood supply and replacing the fibrous tissue with new contractile fibres. Consequently, most of the patients experiencing a myocardial infarction inexorably progress toward heart failure.
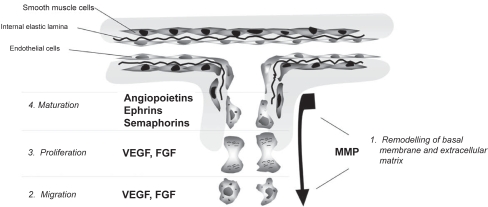
The possibility to promote the neovascularization of ischemic tissues is a highly ambitious objective, rendered possible by the identification, over the last 15 years, of several cytokines and growth factors able to physiologically activate an angiogeneic process. Traditionally, new blood vessel formation in the adult life occurs through capillary sprouting from pre-existing vessels (Figure 3). The process is initiated by the metabolic activation, proliferation and migration of endothelial cells, concomitant with vast remodeling of the extracellular matrix (reviewed in Risau 1997). In physiological conditions, the main regulator of angiogenesis is hypoxia. In conditions of low oxygen tension, the cellular transcription factor HIF-1 (Hypoxia Inducible Factor–1) is post-translationally stabilized and activates the expression of a vast series of genes, among which the vascular endothelial growth factor (VEGF) and its receptors, which are required for all phases of angiogenesis (Liu et al 1995). The VEGF family is composed of at least six members (VEGF-A, B, C, D, E and PlGF – Placental Growth Factor), with different isoforms arising from alternative splicing (Ferrara 1999). The best characterized family member is VEGF-A, the action of which is mediated by its receptors VEGFR-1/Flt-1 and VEGFR-2/KDR/Flk-1 present in endothelial cells (Carmeliet and Collen 1999; Neufeld et al 1999). It has become increasingly apparent that these main receptors act in concert with several other co-receptors, belonging to the integrin, cadherin, neuropilin and ephrin receptor families; the expression of these co-receptors on endothelial cells potentiates or modulates the proliferative and chemotactic effect of VEGF (Soker et al 1998; Gale and Yancopoulos 1999; Bussolino et al 2001). In addition, different members of the ephrin and semaphorin (neuropilin ligands) families, originally identified as guides of axonal growth, have recently been shown to exert similar roles in nascent vasculature, where they act in concert with VEGF (Gale et al 2001).
Figure 3.
Sprouting angiogenesis. In adult organisms, angiogenesis is initiated upon the metabolic activation, proliferation and mobilization of endothelial cells lining pre-existing vessels. This process is triggered by various angiogenic cytokines, including VEGF and FGF2 and is accompanied by the destabilization of the extracellular matrix by matrix metalloproteases (MMP). At later time points, the newly formed capillary matures by the acquisition of pericytes and smooth muscle cells to form a new medial layer. The maturation process requires the action of several other cytokines, among which the angiopoietins, ephrins and semaphorins play a major role.
Besides endothelial cell sprouting, functional new blood vessel formation also requires other factors that act at later time points to promote vessel maturation. Among these, an essential role is played by the angiopoietins, and, in particular, by angiopoietin-1 (Ang1), which, by interacting with the Tie2 receptor on the endothelial surface, reduces the permeability of the newly formed vasculature (Asahara et al 1998; Davis and Yancopoulos 1999; Thurston et al 2000; Arsic et al 2003). Different molecular mechanisms support the favorable effects of Ang1 on vessel maturation and function, including the recruitment of pericytes (Dumont et al 1994; Suri et al 1996) or mesenchymal precursors (Metheny-Barlow et al 2004), the strengthening of intercellular junctions between adjacent endothelial cells (Gamble et al 2000) and the direct interference with the intracellular signaling-pathways responsible for VEGF-triggered vascular leakiness (Jho et al 2005).
In addition to VEGF, several other growth factors are able to activate endothelial cells and promote the angiogenic response. Among these factors, much interest has been engendered by some members of the fibroblast growth factor family (FGF) (Battler et al 1993). By interacting with different ubiquitous receptors, these factors are able to stimulate, on one side, endothelial cell proliferation, while, on the other side, the secretion of proteases that are essential for extracellular matrix degradation and cellular migration in the initial phases of the angiogenic process (Giordano et al 1996). It is estimated that there are 23 structurally similar forms of FGF. The specific role of these different FGF family members during normal angiogenesis in vivo and their relevance in different physiological and pathological conditions still needs to be thoroughly addressed. FGF1, 2, 4 and 5 have been used in angiogenic studies and no significant differences in efficacy have been detected (Yla-Herttuala and Alitalo 2003).
Gene therapy clinical trials to induce therapeutic angiogenesis
After the identification of VEGF and FGF as powerful inducers of angiogenesis, various clinical trials were conducted with the purpose of evaluating the effect of these factors when delivered as recombinant proteins (in particular, TRAFFIC and FIRST, which assessed the effect of FGF2 on peripheral (Lederman et al 2002) and coronary artery (Simons et al 2002) disease respectively, and VIVA, which evaluated rhVEGF in patients with coronary heart disease (Henry et al 2003). Quite unexpectedly, and in contrast to the brilliant results obtained by the same formulations in different animal models of acute or progressive ischemia (Sato et al 2000), the administration of these recombinant proteins produced only modest or no result at all in the treated patients. The negative outcome of this experimentation might be attributed to a number of different reasons, among which the very short half-life of these cytokines in vivo and the de-sensitization of chronic ischemic tissues to growth factor treatment.
Injection of naked plasmid DNA
One applicable way to overcome the poor success of recombinant protein administration is the delivery of the cDNAs encoding for these factors, which, in principle, should ensure the prolonged expression of the desired cytokine by the ischemic tissues in vivo. Indeed, this approach has initially engendered great enthusiasm after the vectors expressing the VEGF and FGF2 cDNAs have been successfully used in various pre-clinical models of myocardial and limb ischemia in small and large animals (Faries et al 2000; Khan et al 2002). Clinical experimentation in patients began in the mid '90s, by identifying patients with critical limb ischemia who had exhausted all conventional options for revascularization (Isner et al 1996). The treatment consisted in the injection of a plasmid coding for the 165 amino acid isoform of VEGF-A. The success of this treatment was evaluated by angiography and nuclear magnetic resonance, revealing the formation of new collateral vessels and a significant perfusion improvement in the VEGF-treated group (Baumgartner et al 1998). The only reported major side effect was the occurrence of a remarkable edema of the leg, probably due to the increase in vessel permeability caused by VEGF (Baumgartner et al 2000). A series of over 20 clinical trials followed, which entailed the injection of naked plasmids encoding VEGF to the myocardium through a mini left anterior thoracotomy (Losordo et al 1998; Symes et al 1999; Vale et al 2000; Fortuin et al 2003; Reilly et al 2005), or by a transendocardial approach using a NOGA catheter (Losordo et al 2002). Most of these procedures were well tolerated, with few major adverse cardiac events and without complications directly related to gene expression. The results of these early studies performed by naked DNA injection should be interpreted with caution, considering their uncontrolled, open-label design and the significant sham or placebo effect observed by any intervention in patients with coronary artery disease. Indeed, a double-blinded, placebo-controlled experimentation performed in Europe a few years later (the Euroinject One trial) failed to show any significant difference between injection of a plasmid encoding VEGF165 and placebo in patients (Gyongyosi et al 2005; Kastrup et al 2005).
Other currently ongoing clinical trials, also based on naked plasmid DNA gene transfer, take advantage of a plasmid encoding FGF1 (aFGF) fused to a heterologous secretion signal under the control of a constitutive strong promoter (NV1FGF). A phase I, multicenter, open label experimentation has already been conducted in patients with severe peripheral vascular disease by intramuscular injection of increasing and repeated doses to patients with unreconstructible, end-stage peripheral arterial occlusive disease (Comerota et al 2002). Following these results, a Phase IIb, double-blind, randomized, placebo-controlled clinical trial has been recently conducted in 6 European countries in patients with critical limb ischemia at high risk of amputation. Despite the fact that no improvement in wound healing was seen, the results showed that this new treatment significantly reduced the risk of amputation and might thus lead to lower mortality rates in patients. This treatment will be further evaluated in a larger, Phase III experimentation, which is scheduled to begin in the second quarter of 2006.
Finally, two additional ongoing trials exploit naked DNA gene transfer in patients with peripheral artery disease. The first is aimed at delivering the hepatocyte growth factor (HGF) cDNA, a cytokine that, besides mitogenic potential on liver cells, also plays an important, multifaceted role in angiogenesis, organogenesis in embryonic development, as well as in the regeneration of tissues and organs damaged by a various forms of injury (Morishita et al 2004). The second experimentation is based on the administration of a plasmid encoding the angiomatrix protein Del-1 (developmentally regulated endothelial locus 1), a unique αvβ3 integrin ligand that is normally produced by endothelial cells and mediates cell attachment, migration, and activation of cytoplasmic signaling molecules in focal contacts (Rajagopalan et al 2004). The latter experimentation is carried out by mixing the Del-1 plasmid with Poloxamer-188, a surfactant polymer with antithrombotic and hemorheologic properties, which is frequently used in the pharmaceutical industry as an emulsifier or solubilizer.
Adenoviral vectors
Despite the relative ease of production and administration of naked plasmid DNA, the clinical success of this approach has been, so far, generally poor, and the therapeutic benefit that has to be expected from the ongoing experimentation is thus limited. This is mainly due to the very low efficiency of tissue transfection currently attainable by simple naked DNA injection and the relatively short period of transgene expression. Given these limitations, other clinical trials have exploited first generation Ad vectors as a mean to deliver various therapeutic genes.
The angiogenic gene therapy (AGENT) studies have addresses the safety and efficacy of the non-surgical, intracoronary injection of an Ad vector expressing FGF4 (Ad5FGF4) in patients with stable angina. In particular, AGENT has compared the effects of 5 ascending Ad5FGF4 doses (Grines et al 2002), while AGENT2 has been the first phase I/IIa, randomized, double-blind, placebo-controlled trial of therapeutic angiogenesis, comparing the effect of a single intracoronary injection of 1 × 1010 Ad5FGF4 viral particles (Grines et al 2003). The AGENT trials established that intracoronary administration of Ad5FGF4 could be performed with reasonable safety in patients with coronary artery disease, and that a one-time dose could provide an anti-ischemic effect up to 12 weeks of evaluation. Further appraisal of the efficacy and safety of Ad5FGF-4 has then been planned in two simultaneous phase IIb/III multicenter, randomized, double-blind, placebo-controlled pivotal trials in the United States and the European Union, with the designed enrollment of approximately 1,000 treated subjects. Unfortunately, however, an interim analysis of the preliminary data from one of these trials has clearly indicated that the trials, as designed, would provide insufficient evidence of efficacy. Therefore, further recruitment was stopped at the beginning of 2004.
Another series of clinical experimentation was based on the delivery of a defective Ad expressing the VEGF121 cDNA under the control of the CMV enhancer/promoter (Ad(GV)VEGF121.10). The RAVE trial was a phase II, double-blind, placebo-controlled study designed to test the efficacy and safety of intramuscular delivery of this vector to the lower extremities of 105 subjects with unilateral peripheral artery obstructive disease. This study showed that a single unilateral intramuscular administration of AdVEGF121 was not associated with improved exercise performance or quality of life, and did not support further experimentation (Rajagopalan, Mohler et al 2003). The same vector has also been tested in two different phase I trials in patients with coronary artery disease entailing direct intramyocardial injection during coronary bypass artery grafting (CABG) (Rosengart et al 1999). Sixteen patients, who could not be completely revascularized by CABG, received the vector as intramyocardial injections in conjunction with CABG. Another 15, who were not candidates for traditional mechanical revascularization and who had severe angina despite being on maximal medical treatment, received the adenovirial vector as 10–30 intramyocardial injections administered via a mini-thoracotomy. Similar to the peripheral vascular disease population, vector administration was well tolerated without any consistent drug-related side effects. There were indications towards improvement in angina symptoms, quality of life and treadmill times as well as in myocardial perfusion scans. However, as with the peripheral vascular disease population, these results are difficult to interpret in the context of an open-labeled, non-controlled trial. Nevertheless, the suggested therapeutic activity along with the safety profile, justified activation of a phase II randomized prospective, proof-of-concept' trial in ‘no-option’ patients. This phase II trial, called REVASC, involved 20 sites in North America; 71 patients with severe coronary artery disease were enrolled. The preliminary results showed significant improvements in both cardiac function and quality of life (Stewart 2002). Currently, a multicenter, randomized, double-blind placebo-controlled study is ongoing in Denmark, Israel and United Kingdom to evaluate the efficacy of Ad-VEGF121 in patients with advanced coronary artery disease and moderate to severe angina despite optimal medical treatment, and who are not amenable to percutaneous coronary revascularization or bypass grafting. The end-point of this study will include functional measures (exercise capability), physiological measures (SPECT) and symptomatic/quality of life measures.
Two trials have assessed the efficiency of an adenovirus expressing the 165 amino acid isoform of VEGF (VEGF-Adv) or of a plasmid expressing the same gene delivered in a liposome formulation (VEGF-P/L) injected intra-arterially after percutaneous transluminal angioplasty in patients with peripheral (Makinen et al 2002) and coronary artery disease (Hedman et al 2003) – the KAT trial. Both the adenovirus and plasmid formulations were reported to improve the vascularity of the treated limbs 3 months after therapy and to enhance myocardial perfusion in the coronary heart disease patients 6 months after therapy.
Finally, a phase I clinical trial evaluating an adenovirus vector expressing HIF-1α/VP16 (Ad2HIF-1α/VP16) for the treatment of peripheral arterial disease has recently been completed. Although the final results of this initial trial have not been yet published, preliminary data on the 28 participants appear promising (Rajagopalan, Deitcher et al 2003). The hypoxia-inducible factor (HIF-1) is a trimeric master transcription factor controlling the expression of several genes involved in a variety of processes in endothelial cells, including glucose metabolism, erythro-poiesis, regulation of vascular tone, and cell proliferation and survival (Wenger and Gassmann 1997). Among the genes that are activated, several exert powerful angiogenic activity, including VEGF; as neovascularization results from the complex interplay of a variety of factors, an upstream regulatory protein such as HIF-1 could potentially be more effective as a therapeutic agent than any single pro-angiogenic factor. Intracellular oxygen concentration regulates HIF-1 activity by influencing both stability and transcriptional activity of the HIF-1α subunit of HIF-1 (Poellinger and Johnson 2004); under normoxic conditions, HIF-1α is targeted for ubiquitination and proteosomal degradation. A constitutively-active version of HIF-1α was generated by replacing the C-terminus of the protein, including its oxygen-dependent and endogenous transac-tivation domains, with the strong transactivation domain from the herpes virus VP16 protein. Administration of plasmid DNA encoding HIF-1α/VP16 has been shown to increase blood flow in animal models of both cardiac and peripheral ischemic disease (Vincent et al 2000; Shyu et al 2002). In a similar approach, constitutively-active, modified versions of the native HIF-1α protein have been reported to promote angiogenesis in the hindlimbs of mice and rabbits (Pajusola et al 2005; Patel et al 2005). After the successful demonstration of the safety of the Ad2HIF-1α/VP16 delivery, a phase II trial has recently been started in patients with peripheral artery disease. This is a placebo-controlled study that will enroll up to 200 patients at approximately 35 medical centers in the United States and Europe.
Table 2 reports a summary of the above described clinical experimentation.
Table 2.
Major gene therapy clinical trials
| Vector | Gene | Disorder | Route | Reference |
|---|---|---|---|---|
| Naked DNA plasmid | VEGF-A 165 aa | PAOD
CAD |
Intramuscular
Intramyocardial through minithoracotomy |
Baumgartner et al (1998) Losordo et al (1998); Symes et al (1999); Vale et al (2000); Fortuin et al (2003); Reilly et al (2005) |
| Transendocardial catheter-based |
Losordo et al (2002) Gyongyosi et al (2005); Kastrup et al (2005) (EUROINJECT 1) |
|||
| FGF1 (NV1FGF) | PAOD | Intramuscular | Comerota et al (2002) | |
| HGF | PAOD | Intramuscular | Morishita et al (2004) | |
| Del-1 | PAOD | Intramuscular | Rajagopalan et al (2004) | |
| Plasmid DNA/liposome | VEGF-A 165 aa | PAOD
CAD |
Intraarterial after PTA
Intraarterial after PTA |
Makinen et al (2002) Hedman et al (2003) (KAT) |
| Adenoviral vectors | FGF4 | CAD | Intracoronary |
Grines et al (2002); Grines et al (2003) (AGENT) |
| VEGF-A 121 aa | PAOD
CAD |
Intramuscular
Intramyocardial during CABG or via minithoracotomy |
Rajagopalan, Mohler et al (2003) (RAVE)
Rosengart et al (1999); Stewart (2002) (REVASC) |
|
| VEGF-A 165 aa | PAOD
CAD |
Intraarterial after PTA
Intraarterial after PTA |
Makinen et al (2002) Hedman et al (2003) (KAT) |
|
| HIF1α/VP16 | PAOD | Intramuscular | Rajagopalan, Deitcher et al (2003) |
Abbreviations: CAD, coronary artery disease; PAOD, peripheral artery occlusive disease; PTA, percutaneous transluminal angioplasty; CABG, coronary artery bypass grafting.
Improved strategies to induce efficient therapeutic angiogenesis
What are the main lessons learned from these clinical trials? Despite over 10 years of clinical experimentation, it is very clear that gene therapy for ischemic heart and peripheral artery disease is still in its infancy. An initial series of at least seven cohort studies, in which patients have been followed up to 2 years post-injection, reported highly positive results (Losordo et al 1998; Rosengart et al 1999; Symes et al 1999; Vale et al 2000; Fortuin et al 2003). These studies, however, essentially suffered from a lack of control groups. In contrast, the overall outcome of the randomized trials have been much more disappointing (Yla-Herttuala and Alitalo 2003). While the definitive results of ongoing experimentation has to be carefully evaluated before a definitive conclusion is drawn, it nevertheless appears that novel vectors and improved delivery methods are needed before definitive clinical success might be met.
Improvement of gene delivery
Irrespective of the therapeutic gene involved, it appears that the efficiency of gene delivery currently represents one of the major limitations that still hamper clinical success. Plasmid DNA delivery is simple and fraught with major safety concerns; however, the efficiency of uptake of naked DNA by muscle and cardiac cells, even if surprisingly higher than with most other cell types, is still very poor. Despite preparations of plasmids injected in quantities to the order of hundred micrograms or milligrams, the levels of the DNA that is internalized by the cells remain orders of magnitude lower than those obtained using viral vectors. In addition, most preclinical investigations have revealed that measurable levels of gene expression are maintained only for the first couple of weeks after injection. This condition might not be sufficient to exert an angiogenic stimulus able to generate a stable neovasculature.
Other clinical trials have been conducted using first generation adenoviral vectors, which carry deletions in the E1 and, in a few instances, in the E3 genes. These vectors are excellent tools for gene transfer given the efficacy at which they both infect cells and express genes. However, these vectors are fraught with several problems, mainly due to the strong inflammatory and immune response they elicit. This essentially reduces the vector dose that can be injected, prevents the possibility of vector re-injection, and limits the expression of transgene to 1–2 weeks after transduction, after which the host’s immune system eliminates the transduced cells (Gilgenkrantz et al 1995). A completely new generation of adenoviral vectors devoid of viral genes, which are still infectious but less immunogenic, would definitely be desirable. However, after almost 10 years of development of gutless adenoviral vectors (Mitani et al 1995; Parks et al 1996), the problems intrinsic to the development of these vectors are still not solved.
Given the current concerns on safety and performance of lentiviral vectors, the only viral vector system that, at present, appears suitable for gene therapy of cardiovascular disorders is the one based on AAV. As outlined above, these vectors display a number of appealing features for gene transfer in the heart and in the skeletal muscle. Among these features are the lack of relevant immunogenicity, the absence of an inflammatory response at the site of injection, the possibility of obtaining relatively pure vector preparations at high titres, the capacity of transducing cells at high multiplicity of infection – which allows mixing of different preparations and thus therapy with gene cocktails –. In addition, even more relevant characteristics are the specific – and still unexplained – tropism for muscle cells and cardiomyocytes, and the ability of these vectors to drive expression of the therapeutic gene they carry for indefinite periods of time in these post-mitotic tissues. Given these features, it might be reasonably expected that these vectors will play a central role in the clinical cardiovascular gene therapy experimentation in the near future.
Cell targeting
Strictly related to the issue of gene delivery for cardiovascular disorders is the problem of conferring upon vectors the property of targeting specific cells. In general, the currently available methods, including non-viral systems and both adenovirus and AAV, have a propensity to transduce non-vascular tissues with greater ease than vascular cells, thereby limiting their application in cardiovascular disease. Two strategies are currently envisaged to improve targeting of gene transfer, namely the modification of the properties of the surface proteins of viral vectors (Ad and AAV) and the improvement of the currently available non-viral delivery methods by the inclusion of proteins conferring targeting properties.
Ad serotype 5 virions primarily bind, via the knob domain of the virus fiber protein, to the coxsackie and adenovirus receptor (CAR) (Bergelson et al 1997; Tomko et al 1997). Upon CAR binding, a secondary interaction between αvβ3/αvβ5 integrins and the penton base of the Ad capsid (containing a RGD motif) occurs that mediates internalization of the virion (Wickham et al 1993). In addition, the fiber protein shaft region makes contact with the cell surface heparan sulphate proteoglycans (HSPGs) of the target cell. Thus, modifications of the amino acid sequence of the Ad knob domain (CAR binding), of the penton base (integrin binding) and of the intervening fiber shaft (HSPG binding) have been conceived as ways to modify Ad serotype 5 tropism in vitro and in vivo. These modifications include serotype switching by fiber swapping among the over 50 natural Ad serotypes and insertion of targeting peptides into CAR-ablated vectors (reviewed in Baker 2004). The majority of these targeting peptides, which mediate attachment of the virus to target cells, have been isolated by phage display technology. This allows high throughput screening of random peptide libraries expressed on the coat protein of filamentous phage for their ability to target selected cell populations, either in vitro or in vivo (reviewed Hajitou et al 2006). Over the last few years, this technology has generated a wealth of peptides that exploit receptor diversity on different cell types, including different endothelial cell variants. Although the studies so far performed with these targeted Ad vectors are encouraging, there is little data to suggests that retargeting will be successful in vivo in terms of efficiency of gene transfer.
An alternative, promising strategy to target Ad vectors to specific cells is the utilization of a dual-specific antibody, which recognizes and inactivates the vector CAR binding domain on one side, while on the other side binds a specific cell receptor of interest. In the cardiovascular system, cell-specific gene delivery has so far been achieved using antibodies against a variety of possible targets, which include E-selectin, FGF2 receptor, the extracellular domain of the angiotensin converting enzyme (ACE) and the Flt-1 tyrosine kinase receptor (reviewed in Baker 2004).
Compared to Ad, the AAV serotype 2 vector capsid is less tolerant to modifications, with the exception of a few sites in which short amino acid targeting peptides can be safely inserted without determining a significant loss of infectivity (reviewed in Flotte and Berns 2005). In the cardiovascular system, targeted AAV2 vectors might overcome one of the most notable limitations of wild type AAV2, namely its inability to transduce vascular endothelial cells. Capsid modeling studies for targeting peptide insertions have originally been carried out based on the structure of the homologous canine parvovirus (Girod et al 1999) and have subsequently taken advantage of the solution of the crystal structure of AAV2 (Xie et al 2002). Based on this information, different laboratories have recently obtained AAV vectors targeted to specific cell types (reviewed in Ding et al. 2005). For example, the incorporation of a venous endothelial cell targeting peptide into AAV2 has recently allowed transduction of venous (but not arterial) endothelial cells both in vitro and invivo (White et al 2004). In this respect, however, it must be noted that transduction of endothelial cells by AAV, similar to other cell types, is limited not only at the level of virus entry into the cells, but also, most importantly, at the levels of uncoating (Thomas et al 2004), of transport of the viral genome to the nucleus (Ding et al 2005) and of conversion of the single-stranded viral genome to double-stranded DNA (Ferrari et al 1996).
Over the last few years, more than 100 AAV variants have been isolated from primate sources, and new sero-types are continuously being discovered (Gao et al 2005). Each of these variants possesses peculiar characteristics in terms of efficiency of transduction and tropism for specific cell populations in vivo. The primary receptors required for transduction for some of these AAV serotypes have been identified (such as sialic acid for AAV4 and AAV5 (Kaludov et al 2001; Walters et al 2001), including the sialo-glycoprotein PDGF-R (Di Pasquale et al 2003)) and can thus be associated with the transduction pattern for the relevant AAV. In most instances, however, the molecular determinants of tropism of these alternate AAV serotypes (whether at the level of cell surface receptor or at later steps after cell entry) is still unknown. Some of these serotypes appear very promising for gene therapy applications in the cardiovascular system, among which AAV1 and AAV6 for local muscle gene delivery by direct intramuscular injection and AAV8 and AAV9 for systemic gene transfer to the muscle and the heart (Wang et al 2005; Inagaki et al 2006).
Final remarks
It is worth emphasizing that the gene transfer technologies that are being developed should be considered not only for their eventual therapeutic applications, but also for their utility in basic research. In particular, the peculiar feature of AAV vectors to express genes for prolonged periods of time in the skeletal muscle and heart in the absence of inflammation offers a simple and straightforward way to examine gene function in adult organisms, thus representing an alternative tool to the use of transgenic animals. Moreover, since these vectors infect cells at high multiplicity, they also allow the simultaneous delivery of several combinations of genes to the same tissue. This possibility appears to be of great importance, since it will eventually permit the definition of the cytokine cocktail that has the greatest efficacy in the induction of a new functional vascular network, definitely an essential requirement for the induction of really “therapeutic” angiogenesis.
Acknowledgments
This work was supported by grants from the FIRB program of the “Ministero dell’Istruzione, Universita’ e Ricerca”, Italy; from the “Fondazione Cassa di Risparmio” of Trieste, Italy and from the Telethon Foundation, Italy. The author wishes to express his gratitude to Suzanne Kerbavcic for her precious and outstanding assistance.
References
- Amabile PG, Waugh JM, Lewis TN, et al. High-efficiency endo-vascular gene delivery via therapeutic ultrasound. J Am Coll Cardiol. 2001;37:1975–80. doi: 10.1016/s0735-1097(01)01253-0. [DOI] [PubMed] [Google Scholar]
- Arsic N, Zentilin L, Zacchigna S, et al. Induction of functional neo-vascularization by combined VEGF and angiopoietin-1 gene transfer using AAV vectors. Mol Ther. 2003;7:450–9. doi: 10.1016/s1525-0016(03)00034-0. [DOI] [PubMed] [Google Scholar]
- Asahara T, Chen D, Takahashi T, et al. Tie2 receptor ligands, angio-poietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998;83:233–40. doi: 10.1161/01.res.83.3.233. [DOI] [PubMed] [Google Scholar]
- Athanasopoulos T, Fabb S, Dickson G. Gene therapy vectors based on adeno-associated virus: characteristics and applications to acquired and inherited diseases (review) Int J Mol Med. 2000;6:363–75. doi: 10.3892/ijmm.6.4.363. [DOI] [PubMed] [Google Scholar]
- Ay T, Havaux X, Van Camp G, et al. Destruction of contrast micro-bubbles by ultrasound: effects on myocardial function, coronary perfusion pressure, and microvascular integrity. Circulation. 2001;104:461–6. doi: 10.1161/hc3001.092038. [DOI] [PubMed] [Google Scholar]
- Baker AH. Designing gene delivery vectors for cardiovascular gene therapy. Prog Biophys Mol Biol. 2004;84:279–99. doi: 10.1016/j.pbiomolbio.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Barr E, Carroll J, Kalynych AM, et al. Efficient catheter-mediated gene transfer into the heart using replication-defective adenovirus. Gene Ther. 1994;1:51–8. [PubMed] [Google Scholar]
- Battler A, Scheinowitz M, Bor A, et al. Intracoronary injection of basic fibroblast growth factor enhances angiogenesis in infarcted swine myocardium. J Am Coll Cardiol. 1993;22:2001–6. doi: 10.1016/0735-1097(93)90790-8. [DOI] [PubMed] [Google Scholar]
- Baumgartner I, Pieczek A, Manor O, et al. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998;97:1114–23. doi: 10.1161/01.cir.97.12.1114. [DOI] [PubMed] [Google Scholar]
- Baumgartner I, Rauh G, Pieczek A, et al. Lower-extremity edema associated with gene transfer of naked DNA encoding vascular endo-thelial growth factor. Ann Intern Med. 2000;132:880–4. doi: 10.7326/0003-4819-132-11-200006060-00005. [DOI] [PubMed] [Google Scholar]
- Bergelson JM, Cunningham JA, Droguett G, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–3. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- Bussolino F, Serini G, Mitola S, et al. Dynamic modules and heterogeneity of function: a lesson from tyrosine kinase receptors in endothelial cells. EMBO Rep. 2001;2:763–7. doi: 10.1093/embo-reports/kve181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Collen D. Role of vascular endothelial growth factor and vascular endothelial growth factor receptors in vascular development. Curr Top Microbiol Immunol. 1999;237:133–58. doi: 10.1007/978-3-642-59953-8_7. [DOI] [PubMed] [Google Scholar]
- Chen S, Shohet RV, Bekeredjian R, et al. Optimization of ultrasound parameters for cardiac gene delivery of adenoviral or plasmid deoxyribonucleic acid by ultrasound-targeted microbubble destruction. J Am Coll Cardiol. 2003;42:301–8. doi: 10.1016/s0735-1097(03)00627-2. [DOI] [PubMed] [Google Scholar]
- Comerota AJ, Throm RC, Miller KA, et al. Naked plasmid DNA encoding fibroblast growth factor type 1 for the treatment of end-stage unreconstructible lower extremity ischemia: preliminary results of a phase I trial. J Vasc Surg. 2002;35:930–6. doi: 10.1067/mva.2002.123677. [DOI] [PubMed] [Google Scholar]
- Danialou G, Comtois AS, Dudley RW, et al. Ultrasound increases plasmid-mediated gene transfer to dystrophic muscles without collateral damage. Mol Ther. 2002;6:687–93. [PubMed] [Google Scholar]
- Davis S, Yancopoulos GD. The angiopoietins: Yin and Yang in angio-genesis. Curr Top Microbiol Immunol. 1999;237:173–85. doi: 10.1007/978-3-642-59953-8_9. [DOI] [PubMed] [Google Scholar]
- Di Pasquale G, Davidson BL, Stein CS, et al. Identification of PDGFR as a receptor for AAV-5 transduction. Nat Med. 2003;9:1306–12. doi: 10.1038/nm929. [DOI] [PubMed] [Google Scholar]
- Ding W, Zhang L, Yan Z, et al. Intracellular trafficking of adeno-associated viral vectors. Gene Ther. 2005;12:873–80. doi: 10.1038/sj.gt.3302527. [DOI] [PubMed] [Google Scholar]
- Dumont DJ, Gradwohl G, Fong GH, et al. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- Dutheil N, Shi F, Dupressoir T, et al. Adeno-associated virus site-specifically integrates into a muscle-specific DNA region. Proc Natl Acad Sci USA. 2000;97:4862–6. doi: 10.1073/pnas.080079397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faries PL, Pomposelli FB, Jr, Quist WC, et al. Assessing the role of gene therapy in the treatment of vascular disease. Ann Vasc Surg. 2000;14:181–8. doi: 10.1007/s100169910033. [DOI] [PubMed] [Google Scholar]
- Favre D, Provost N, Blouin V, et al. Immediate and long-term safety of recombinant adeno-associated virus injection into the nonhuman primate muscle. Mol Ther. 2001;4:559–66. doi: 10.1006/mthe.2001.0494. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol. 1999;237:1–30. doi: 10.1007/978-3-642-59953-8_1. [DOI] [PubMed] [Google Scholar]
- Ferrari FK, Samulski T, Shenk T, et al. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–34. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher KJ, Jooss K, Alston J, et al. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–12. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- Flotte TR, Berns KI. Adeno-associated virus: a ubiquitous commensal of mammals. Hum Gene Ther. 2005;16:401–7. doi: 10.1089/hum.2005.16.401. [DOI] [PubMed] [Google Scholar]
- Fortuin FD, Vale P, Losordo DW, et al. One-year follow-up of direct myocardial gene transfer of vascular endothelial growth factor-2 using naked plasmid deoxyribonucleic acid by way of thoracotomy in no-option patients. Am J Cardiol. 2003;92:436–9. doi: 10.1016/s0002-9149(03)00661-1. [DOI] [PubMed] [Google Scholar]
- Gale NW, Baluk P, Pan L, et al. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev Biol. 2001;230:151–60. doi: 10.1006/dbio.2000.0112. [DOI] [PubMed] [Google Scholar]
- Gale NW, Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 1999;13:1055–66. doi: 10.1101/gad.13.9.1055. [DOI] [PubMed] [Google Scholar]
- Gamble JR, Drew J, Trezise L, et al. Angiopoietin-1 is an antiperme-ability and anti-inflammatory agent in vitro and targets cell junctions. Circ Res. 2000;87:603–7. doi: 10.1161/01.res.87.7.603. [DOI] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5:285–97. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- Gilgenkrantz H, Duboc D, Juillard V, et al. Transient expression of genes transferred in vivo into heart using first-generation adenoviral vectors: role of the immune response. Hum Gene Ther. 1995;6:1265–74. doi: 10.1089/hum.1995.6.10-1265. [DOI] [PubMed] [Google Scholar]
- Giordano FJ, Ping P, McKirnan MD, et al. Intracoronary gene transfer of fibroblast growth factor-5 increases blood flow and contractile function in an ischemic region of the heart. Nat Med. 1996;2:534–9. doi: 10.1038/nm0596-534. [DOI] [PubMed] [Google Scholar]
- Girod A, Ried M, Wobus C, et al. Genetic capsid modifications allow efficient re-targeting of adeno-associated virus type 2. Nat Med. 1999;5:1052–6. doi: 10.1038/12491. [DOI] [PubMed] [Google Scholar]
- Grimm D, Kern A, Rittner K, et al. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum Gene Ther. 1998;9:2745–60. doi: 10.1089/hum.1998.9.18-2745. [DOI] [PubMed] [Google Scholar]
- Grines CL, Watkins MW, Helmer G, et al. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation. 2002;105:1291–7. doi: 10.1161/hc1102.105595. [DOI] [PubMed] [Google Scholar]
- Grines CL, Watkins MW, Mahmarian JJ, et al. A randomized, double-blind, placebo-controlled trial of Ad5FGF-4 gene therapy and its effect on myocardial perfusion in patients with stable angina. J Am Coll Cardiol. 2003;42:1339–47. doi: 10.1016/s0735-1097(03)00988-4. [DOI] [PubMed] [Google Scholar]
- Guzman RJ, Lemarchand P, Crystal RG, et al. Efficient gene transfer into myocardium by direct injection of adenovirus vectors. Circ Res. 1993;73:1202–7. doi: 10.1161/01.res.73.6.1202. [DOI] [PubMed] [Google Scholar]
- Gyongyosi M, Khorsand A, Zamini S, et al. NOGA-guided analysis of regional myocardial perfusion abnormalities treated with intramyocar-dial injections of plasmid encoding vascular endothelial growth factor A-165 in patients with chronic myocardial ischemia: subanalysis of the EUROINJECT-ONE multicenter double-blind randomized study. Circulation. 2005;112:I157–65. doi: 10.1161/01.CIRCULATIONAHA.105.525782. [DOI] [PubMed] [Google Scholar]
- Hajitou A, Pasqualini R, Arap W. Vascular targeting: recent advances and therapeutic perspectives. Trends Cardiovasc Med. 2006;16:80–8. doi: 10.1016/j.tcm.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedman M, Hartikainen J, Syvanne M, et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT) Circulation. 2003;107:2677–83. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]
- Henry TD, Annex BH, McKendall GR, et al. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003;107:1359–65. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- Huber PE, Mann MJ, Melo LG, et al. Focused ultrasound (HIFU) induces localized enhancement of reporter gene expression in rabbit carotid artery. Gene Ther. 2003;10:1600–7. doi: 10.1038/sj.gt.3302045. [DOI] [PubMed] [Google Scholar]
- Inagaki K, Fuess S, Storm TA, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isner JM, Pieczek A, Schainfeld R, et al. Clinical evidence of angio-genesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet. 1996;348:370–4. doi: 10.1016/s0140-6736(96)03361-2. [DOI] [PubMed] [Google Scholar]
- Jho D, Mehta D, Ahmmed G, et al. Angiopoietin-1 opposes VEGF-induced increase in endothelial permeability by inhibiting TRPC1-dependent Ca2 influx. Circ Res. 2005;96:1282–90. doi: 10.1161/01.RES.0000171894.03801.03. [DOI] [PubMed] [Google Scholar]
- Kaludov N, Brown KE, Walters RW, et al. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J Virol. 2001;75:6884–93. doi: 10.1128/JVI.75.15.6884-6893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastrup J, Jorgensen E, Ruck A, et al. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris A randomized double-blind placebo-controlled study: the Euroinject One trial. J Am Coll Cardiol. 2005;45:982–8. doi: 10.1016/j.jacc.2004.12.068. [DOI] [PubMed] [Google Scholar]
- Khan TA, Sellke FW, Laham RJ.2002. Therapeutic angiogenesis for coronary artery disease. 4:65–74. [DOI] [PubMed]
- Kotin RM, Linden RM, Berns KI. Characterization of a preferred site on human chromosome 19q for integration of adeno-associated virus DNA by non-homologous recombination. Embo J. 1992;11:5071–8. doi: 10.1002/j.1460-2075.1992.tb05614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen M, Makinen K, Manninen H, et al. Adenovirus-mediated gene transfer to lower limb artery of patients with chronic critical leg ischemia. Hum Gene Ther. 1998;9:1481–6. doi: 10.1089/hum.1998.9.10-1481. [DOI] [PubMed] [Google Scholar]
- Lawrie A, Brisken AF, Francis SE, et al. Ultrasound enhances reporter gene expression after transfection of vascular cells in vitro. Circulation. 1999;99:2617–20. doi: 10.1161/01.cir.99.20.2617. [DOI] [PubMed] [Google Scholar]
- Lederman RJ, Mendelsohn FO, Anderson RD, et al. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomised trial. Lancet. 2002;359:2053–8. doi: 10.1016/s0140-6736(02)08937-7. [DOI] [PubMed] [Google Scholar]
- Lehrman S. Virus treatment questioned after gene therapy death. Nature. 1999;401:517–18. doi: 10.1038/43977. [DOI] [PubMed] [Google Scholar]
- Linden RM, Ward P, Giraud C, et al. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA. 1996;93:11288–94. doi: 10.1073/pnas.93.21.11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Cox SR, Morita T, et al. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ Res. 1995;77:638–43. doi: 10.1161/01.res.77.3.638. [DOI] [PubMed] [Google Scholar]
- Losordo DW, Vale PR, Hendel RC, et al. Phase 1/2 placebo-controlled, double-blind, dose-escalating trial of myocardial vascular endothelial growth factor 2 gene transfer by catheter delivery in patients with chronic myocardial ischemia. Circulation. 2002;105:2012–18. doi: 10.1161/01.cir.0000015982.70785.b7. [DOI] [PubMed] [Google Scholar]
- Losordo DW, Vale PR, Symes JF, et al. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation. 1998;98:2800–4. doi: 10.1161/01.cir.98.25.2800. [DOI] [PubMed] [Google Scholar]
- Makinen K, Manninen H, Hedman M, et al. Increased vascularity detected by digital subtraction angiography after VEGF gene transfer to human lower limb artery: a randomized, placebo-controlled, double-blinded phase II study. Mol Ther. 2002;6:127–33. doi: 10.1006/mthe.2002.0638. [DOI] [PubMed] [Google Scholar]
- Marcello A, Massimi P, Banks L, et al. Adeno-associated virus type 2 Rep protein inhibits human papillomavirus type 16 E2 recruitment of the transcriptional coactivator p300. J Virol. 2000;74:9090–8. doi: 10.1128/jvi.74.19.9090-9098.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metheny-Barlow LJ, Tian S, Hayes AJ, et al. Direct chemotactic action of angiopoietin-1 on mesenchymal cells in the presence of VEGF. Microvasc Res. 2004;68:221–30. doi: 10.1016/j.mvr.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Miller DG, Trobridge GD, Petek LM, et al. Large-scale analysis of adeno-associated virus vector integration sites in normal human cells. J Virol. 2005;79:11434–42. doi: 10.1128/JVI.79.17.11434-11442.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani K, Graham FL, Caskey CT, et al. Rescue, propagation, and partial purification of a helper virus-dependent adenovirus vector. Proc Natl Acad Sci U S A. 1995;92:3854–8. doi: 10.1073/pnas.92.9.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S, Tachibana K, Okamoto T, et al. In vitro transfer of antisense oligodeoxynucleotides into coronary endothelial cells by ultrasound. Biochem Biophys Res Commun. 2002;298:587–90. doi: 10.1016/s0006-291x(02)02467-1. [DOI] [PubMed] [Google Scholar]
- Morishita R, Aoki M, Hashiya N, et al. Safety evaluation of clinical gene therapy using hepatocyte growth factor to treat peripheral arterial disease. Hypertension. 2004;44:203–9. doi: 10.1161/01.HYP.0000136394.08900.ed. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Gengrinovitch S, et al. Vascular endothelial growth factor (VEGF) and its receptors. Faseb J. 1999;13:9–22. [PubMed] [Google Scholar]
- Pajusola K, Kunnapuu J, Vuorikoski S, et al. Stabilized HIF-1alpha is superior to VEGF for angiogenesis in skeletal muscle via adeno-associated virus gene transfer. Faseb J. 2005;19:1365–7. doi: 10.1096/fj.05-3720fje. [DOI] [PubMed] [Google Scholar]
- Parks RJ, Chen L, Anton M, et al. A helper-dependent adeno-virus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci USA. 1996;93:13565–70. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel TH, Kimura H, Weiss CR, et al. Constitutively active HIF-1alpha improves perfusion and arterial remodeling in an endovascular model of limb ischemia. Cardiovasc Res. 2005;68:144–54. doi: 10.1016/j.cardiores.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Pfeffer JM, Pfeffer MA, Fletcher PJ, et al. Progressive ventricular remodeling in rat with myocardial infarction. Am J Physiol. 1991;260:H1406–14. doi: 10.1152/ajpheart.1991.260.5.H1406. [DOI] [PubMed] [Google Scholar]
- Poellinger L, Johnson RS. HIF-1 and hypoxic response: the plot thickens. Curr Opin Genet Dev. 2004;14:81–5. doi: 10.1016/j.gde.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Qian HS, Channon K, Neplioueva V, et al. Improved adenoviral vector for vascular gene therapy: beneficial effects on vascular function and inflammation. Circ Res. 2001;88:911–17. doi: 10.1161/hh0901.090926. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Deitcher S, Olin J, et al. 2003Harnessing the response to hypoxia: Use of a constitutively active hypoxia-inducible factor 1-alpha transgene in no-option critical limb ischemia patients Circulation 108Suppl IVIV, 442(abstract). [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Mohler ER, 3rd, Lederman RJ, et al. Regional angio-genesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adeno-viral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108:1933–8. doi: 10.1161/01.CIR.0000093398.16124.29. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Olin JW, Young S, et al. Design of the Del-1 for therapeutic angiogenesis trial (DELTA-1), a phase II multicenter, double-blind, placebo-controlled trial of VLTS-589 in subjects with intermittent claudication secondary to peripheral arterial disease. Hum Gene Ther. 2004;15:619–24. doi: 10.1089/104303404323142060. [DOI] [PubMed] [Google Scholar]
- Reilly JP, Grise MA, Fortuin FD, et al. Long-term (2-year) clinical events following transthoracic intramyocardial gene transfer of VEGF-2 in no-option patients. J Interv Cardiol. 2005;18:27–31. doi: 10.1111/j.1540-8183.2005.04026.x. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–4. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Rosengart TK, Lee LY, Patel SR, et al. Angiogenesis gene therapy: phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEGF121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation. 1999;100:468–74. doi: 10.1161/01.cir.100.5.468. [DOI] [PubMed] [Google Scholar]
- Sato K, Laham RJ, Pearlman JD, et al. Efficacy of intracoronary versus intravenous FGF-2 in a pig model of chronic myocardial ischemia. Ann Thorac Surg. 2000;70:2113–18. doi: 10.1016/s0003-4975(00)02018-x. [DOI] [PubMed] [Google Scholar]
- Schnepp BC, Jensen RL, Chen CL, et al. Characterization of adeno-associated virus genomes isolated from human tissues. J Virol. 2005;79:14793–803. doi: 10.1128/JVI.79.23.14793-14803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimpo M, Ikeda U, Maeda Y, et al. AAV-mediated VEGF gene transfer into skeletal muscle stimulates angiogenesis and improves blood flow in a rat hindlimb ischemia model. Cardiovasc Res. 2002;53:993–1001. doi: 10.1016/s0008-6363(01)00546-6. [DOI] [PubMed] [Google Scholar]
- Shyu KG, Wang MT, Wang BW, et al. Intramyocardial injection of naked DNA encoding HIF-1alpha/VP16 hybrid to enhance angiogenesis in an acute myocardial infarction model in the rat. Cardiovasc Res. 2002;54:576–83. doi: 10.1016/s0008-6363(02)00259-6. [DOI] [PubMed] [Google Scholar]
- Simons M, Annex BH, Laham RJ, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation. 2002;105:788–93. doi: 10.1161/hc0802.104407. [DOI] [PubMed] [Google Scholar]
- Soker S, Takashima S, Miao HQ, et al. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–45. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- Srinath Reddy K, Shah B, Varghese C, et al. Responding to the threat of chronic diseases in India. Lancet. 2005;366:1744–9. doi: 10.1016/S0140-6736(05)67343-6. [DOI] [PubMed] [Google Scholar]
- St George JA. Gene therapy progress and prospects: adenoviral vectors. Gene Ther. 2003;10:1135–41. doi: 10.1038/sj.gt.3302071. [DOI] [PubMed] [Google Scholar]
- Stewart DJ.2002A phase 2, randomized, multicenter, 26-week study to assess the efficacy and safety of BIOBYPASS (AdGVVEGF121) delivered through minimally invasive surgery versus maximum medical treatment in patients with severe angina, advanced coronary artery disease, and no options for revascularizations Circulation 1062986-a(abstract).12460884 [Google Scholar]
- Su H, Lu R, Kan YW. Adeno-associated viral vector-mediated vascular endothelial growth factor gene transfer induces neovascular formation in ischemic heart. Proc Natl Acad Sci USA. 2000;97:13801–6. doi: 10.1073/pnas.250488097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–80. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Symes JF, Losordo DW, Vale PR, et al. 1999Gene therapy with vascular endothelial growth factor for inoperable coronary artery disease Ann Thorac Surg 68830–6.discussion 836–7. [DOI] [PubMed] [Google Scholar]
- Taniyama Y, Tachibana K, Hiraoka K, et al. Development of safe and efficient novel nonviral gene transfer using ultrasound: enhancement of transfection efficiency of naked plasmid DNA in skeletal muscle. Gene Ther. 2002;9:372–80. doi: 10.1038/sj.gt.3301678. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Storm TA, Huang Z, et al. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J Virol. 2004;78:3110–22. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston G, Rudge JS, Ioffe E, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–3. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- Tomko RP, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–6. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tratschin JD, West MH, Sandbank T, et al. A human parvovirus, adeno-associated virus, as a eucaryotic vector: transient expression and encapsidation of the procaryotic gene for chloramphenicol acetyl-transferase. Mol Cell Biol. 1984;4:2072–81. doi: 10.1128/mcb.4.10.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale PR, Losordo DW, Milliken CE, et al. Left ventricular electromechanical mapping to assess efficacy of phVEGF(165) gene transfer for therapeutic angiogenesis in chronic myocardial ischemia. Circulation. 2000;102:965–74. doi: 10.1161/01.cir.102.9.965. [DOI] [PubMed] [Google Scholar]
- Vincent KA, Shyu KG, Luo Y, et al. Angiogenesis is induced in a rabbit model of hindlimb ischemia by naked DNA encoding an HIF-1alpha/VP16 hybrid transcription factor. Circulation. 2000;102:2255–61. doi: 10.1161/01.cir.102.18.2255. [DOI] [PubMed] [Google Scholar]
- Walters RW, Yi SM, Keshavjee S, et al. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J Biol Chem. 2001;276:20610–16. doi: 10.1074/jbc.M101559200. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhu T, Qiao C, et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005;23:321–8. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- Wenger RH, Gassmann M. Oxygen(es) and the hypoxia-inducible factor-1. Biol Chem. 1997;378:609–16. [PubMed] [Google Scholar]
- White SJ, Nicklin SA, Buning H, et al. Targeted gene delivery to vascular tissue in vivo by tropism-modified adeno-associated virus vectors. Circulation. 2004;109:513–19. doi: 10.1161/01.CIR.0000109697.68832.5D. [DOI] [PubMed] [Google Scholar]
- Wickham TJ, Mathias P, Cheresh DA, et al. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–19. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- Xie Q, Bu W, Bhatia S, et al. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc Natl Acad Sci USA. 2002;99:10405–10. doi: 10.1073/pnas.162250899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Haecker SE, Su Q, et al. Immunology of gene therapy with adenoviral vectors in mouse skeletal muscle. Hum Mol Genet. 1996;5:1703–12. doi: 10.1093/hmg/5.11.1703. [DOI] [PubMed] [Google Scholar]
- Yla-Herttuala S, Alitalo K. Gene transfer as a tool to induce therapeutic vascular growth. Nat Med. 2003;9:694–701. doi: 10.1038/nm0603-694. [DOI] [PubMed] [Google Scholar]