Abstract
Electrospinning is an enabling technology that can architecturally (in terms of geometry, morphology or topography) and biochemically fabricate engineered cellular scaffolds that mimic the native extracellular matrix (ECM). This is especially important and forms one of the essential paradigms in the area of tissue engineering. While biomimesis of the physical dimensions of native ECM’s major constituents (eg, collagen) is no longer a fabrication-related challenge in tissue engineering research, conveying bioactivity to electrospun nanofibrous structures will determine the efficiency of utilizing electrospun nanofibers for regenerating biologically functional tissues. This can certainly be achieved through developing composite nanofibers. This article gives a brief overview on the current development and application status of employing electrospun composite nanofibers for constructing biomimetic and bioactive tissue scaffolds. Considering that composites consist of at least two material components and phases, this review details three different configurations of nanofibrous composite structures by using hybridizing basic binary material systems as example. These are components blended composite nanofiber, core-shell structured composite nanofiber, and nanofibrous mingled structure.
Keywords: electrospinning, composites, nanofiber, tissue scaffolds, biomimetic, bioactive
Introduction
Electrospinning and tissue engineering scaffolds
Electrospinning, which is an ultrafine fiber manufacturing technology, was coined in the 1990’s from the earlier used term of “electrostatic spinning” (Formhals 1934) by Reneker and co-workers (Doshi and Reneker 1995; Reneker and Chun 1996). It has now attracted increasingly worldwide attention in both the academic community and industrial world (Reneker and Chun 1996; Huang, Zhang et al 2003; Li and Xia 2004). Electrospinning is capable of fabricating fibers with nanometer scale diameters that yield very high specific surface area – up to one to two orders of magnitude higher than micrometer scale fibers produced by conventional melting and dry/wet spinning methods. Electrospun nanofibers are therefore very useful for developing a variety of products or structures whose functional efficiency is surface area dependent. Among those potential applications proposed (Huang, Zhang et al 2003; Li and Xia 2004; Zhang, Lim et al 2005), construction of biomimetic1 cellular scaffold will represent one of the most promising applications for the electrospun nanofibers. Using ‘Electrospinning’ as the keyword for literature searching through the ISI Web of Science®, it was found that, of the top 10 most cited articles2 out of more than 1000 relevant papers, 3 of them pertain to the subject of nanofibrous tissue scaffolding applications with the other 7 articles being either reviews or related to the process of electrospinning. The underlying rationale of using nanofibers for constructing cellular scaffolds is based on the biomimesis principle that electrospun nanofibers can mimic the physical structure of the major constructive elements in the native ECM as biologically, almost all of the tissues and organs such as bone, skin, tendon and cartilage, are synthesized and hierarchically organized into fibrous form (structure) with fiber dimensions down to nanometer scale (Nishida, Yasumoto et al 1988; Kadler, Holmes et al 1996). Nanofibrous scaffold could therefore provide environmental or physical cues to the cells and promote cell growth and function well towards the synthesis of genuine extracellular matrices over time (Laurencin, Ambrosio et al 1999). Unlike other types of architectural scaffolds, using electrospun nanofiber for scaffolding implies that while the nanofibrous scaffold is responsible for the overall mechanical properties of the tissue or cell-scaffold complex, the nanolevel structures (nanofibers) can provide nanomechanical and biodegradation properties for cells to proactively interplay with the provisional matrix and functionalize and remodel it, similar to that of the native cellular remodeling process within the ECM.
Thus, electrospinning has recently established the reputation for its capability to make ECM-mimicking scaffolds, and is counted as a new addition to the conventional scaffold fabrication techniques (eg, solvent-casting and particulateleaching, gas foaming, fiber bonding, freeze drying, etc). However, despite the increasing interest in electrospinning for the past decade, making use of electrospun nanofibers for tissue engineering has only a mere short history of about 5–7 years (Fertala, Han et al 2001; Stitzel, Pawlowski et al 2001; Li, Laurencin et al 2002). Both the design, fabrication of the nanofibrous scaffolds and molecular level understanding of the interactions in vitro between the nanofibrous scaffolds and mammalian cells as well as in vivo tests and applications are still in the early stage of development. With respect to the materials used in electrospinning in the very first few years since 2001, traditional synthetic biodegradable aliphatic polyesters such as PLA, PLGA, and PCL are still the preferred and prevailing choices of materials for constructing nanofibrous scaffolds due to their well-known good processability and mechanical performance. Obviously, in the context of biomimicking nanoscale fibers, these electrospun synthetic polymers have replicated the physical dimensions and morphology of the major component collagen in the native ECM. Yet, two persistent problems can restrain the synthetic polymeric nanofibers from being effective during application. Firstly, unlike natural biopolymers, the pristine synthetic polymers lack cell recognition sites on the scaffold surfaces and that means poor cell affinity (Hubbell 1995; Cai, Yang et al 2002; Rosso, Marino et al 2005). Secondly, the aggravated hydrophobicity arising from their inherent hydrophobic attribute (Chen, Ushida et al 2000; Cai, Wan et al 2003) and nanoscale effect (Feng, Li et al 2002; Neimark, Kornev et al 2003) will affect cell seeding on the nanofibrous scaffolds and subsequent cellular activities. In addition, their acidic degradation products have detrimental effects to the cells. Hence, despite the scaffold being porous and possessing higher surface area, poor hydrophilicity will cause a majority of the pores to remain empty, potentially resulting in the underutilization of the 3-D scaffolds. These are certainly the immediate problems to be addressed prior to effective use.
Why composite nanofibers?
The above noted problems demand for the development of bioactive3 and functional electrospun nanofibers. Essentially, it is related to the biochemical attributes of the used materials. The most ideal candidate materials should be the native biomaterials such as collagen. However, one of the shortcomings for collagen is its inadequate mechanical properties after being processed from its native form. Thus, an alternative solution will be to make appropriate modification to the synthetic polymers. Whilst traditional surface chemical modification approaches used on the bulk synthetic polymers can be applied to ameliorate the synthetic nanofibers, simple physical hybridizing synthetic polymers with bioactive natural biopolymers and then converting the hybrids into nanofibers will offer a more facile and cost-effective route for modifying and tailoring the material properties. By definition, composite materials or composites are made from two or more components. As natural and synthetic polymers constitute the largest fraction of biomaterials for tissue scaffolding, here we will define a composite fiber as one whose materials are compounded from one synthetic sourced polymer and one from natural sourced polymer or inorganic nanoparticles. Unlike traditional engineering composites where inorganic components such as carbon and glass fibers are used to reinforce the matrix material, the natural biopolymers used are to impart bioactivity to the biologically passive synthetic polymers. With the versatile electrospinning, such composite nanofibers can be designed and fabricated in the form of either basically random blending or ordered structure (eg, core-sheath) from the available synthetic and natural polymers. A number of merits are conceivable with such composite nanofibers. Physically, the new composite nanofibers could provide better hydrophilicity (wettability) and improved mechanical properties, etc. Biologically, the incorporation of bioactive macromolecules (eg, collagenous proteins or growth factors) into the synthetic components could promote cell-surface recognition and also promote or control many aspects of cell physiology such as adhesion, spreading, activation, migration, proliferation and differentiation (Drumheller and Hubbell 2000). Due to the size of the nanofibers, such effects are being augmented or made more effective because of the high surface area for cells to access. Additionally, as controlled and sustained delivery of growth factors are deemed necessary for successful tissue engineering (Baldwin and Mark Saltzman 1998; Ikada and Tabata 2002), the biomimetic composite nanofibers, in particular, core-sheath structure could perform controlled and effective delivery of bioactive molecules purely from the nanofibrous scaffolds without using extra delivery devices.
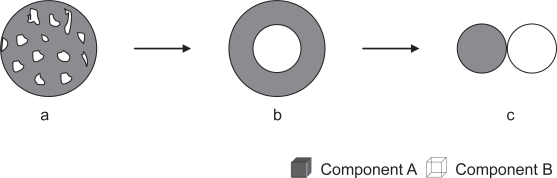
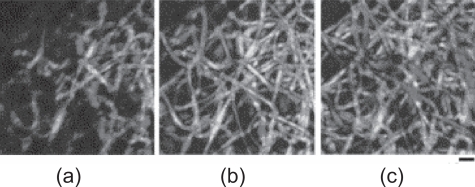
Here, we will focus on composite nanofibrous scaffolds primarily made from biodegradable synthetic and natural materials. Using a binary hybridizing system as an illustration, composite nanofibers in the forms of randomly blended structure, core-shell structure, and mingled nanofibers (Figure 1) will be the major three types of composite nanofibers discussed. Since composites involve different phases, the illustrations in Figure 1 also reflect the typical different phase separation or existence states in a biphasic structured composites or hybrid nanofibers.
Figure 1.
Schematic cross-sectional views of different structures of composite nanofibers from components of A and B. (a) randomly blended; (b) core-shell structured; and (c) nanofibers-mingled (from concurrent electrospinning).
Components blended composite nanofibrous scaffolds
The components blended composite nanofibers can be divided into two categories, ie, organic-organic blends and organic-inorganic blends. Both will be discussed in the following two sub-sections.
Organic-organic blends
As mentioned above, the organic-organic blends is meant to be made from synthetic and natural sourced polymers with improving bioactivity and functions as the chief concern. Table 1 gives a summary of organic-organic blend nanofibrous scaffolds which have been explored by different researchers. As one of the earliest groups of applying the composite concept for developing biomimetic and bioactive nanofibrous scaffolds, we have demonstrated the efficacy of using a combination of the natural collagen-derived biopolymer gelatin (Gt) with the synthetic poly(ɛ-caprolactone) (PCL) to acquire desired physical, chemical and biological properties of nanofibrous scaffolds (Zhang, Ouyang et al 2005). Our results showed that composite nanofibrous scaffold Gt/PCL had very good wettability and/or hydrophilicity and balanced mechanical properties compared to its constituents. In vitro cell culture experiments manifested very significant cell proliferation and infiltration compared to the biologically inert synthetic PCL alone scaffolds. Cellular infiltration into the Gt/PCL composite nanofibrous scaffolds up to 110 μm was, for the first time, quantitatively measured through a laser scanning microscopy. The favorable cellular responses were attributed to the materials hybridization effect. Introduction of the bioactive biopolymer of Gt into the PCL had remarkably improved the wettability and cell affinity of the fibrous scaffolds. Although electrospun nanofibrous scaffolds are deemed porous with interstices formed by fiber interlacing, the ‘pores’ formed would be much smaller than the normal cell size of a few to tens of microns which could inhibit cell migration to the interior of the electrospun nanofibrous structure. Nevertheless, we speculate that three factors could be responsible for the observed cellular infiltration phenomenon. Firstly, the introduction of natural biopolymer of Gt into the PCL confers good hydrophilicity/wettability and biological recognition signals, which will consequently facilitate nutrients/oxygen transfer and removal of metabolic products and encourage pioneering cells to migrate deeper into the scaffold. Such a favorable local microenvironment as a result of material constituents can definitely modulate the cellular responsive behaviour (Chen, Ushida et al 2002; Coombes, Verderio et al 2002; Telemeco, Ayres et al 2005). Secondly, Gt/PCL composites had lower tensile strength, but very good elongation and deformation properties. These favorable mechanical properties can provide easier opening of spaces for cell penetration to deeper levels of the scaffold. Matched nanomechanical properties will be one of the important factors to account for cell penetration. The resilience and deformability of scaffolds at nano-, meso-, and macro-scale do influence in vitro migration and morphology of cells (Carnegie and Cabaca 1993). Lastly, the gelatin component in the Gt/PCL scaffold is gradually dissolved during cell culture resulting in the emergence of porous fibers. This will in situ make extra space for cell migration and easy transportations of nutrients and waste. The formation of 3-D porous fibers was demonstrated in our later study (Zhang, Feng et al 2006) by leaching the gelatin component out of the composite fibers as shown in Figure 2. The 3-D porous fiber morphology also suggests that the phase separation of gelatin and PCL in the composite nanofibers is in a randomly blended fashion. Further, BET surface area measurement indicated that the 3-D porous fibers possessed a surface area of about 2.4 times that of the pristine Gt/PCL fibers. With these encouraging results, very recently we have electrospun Gt/PCL composite nanofibers onto a polyurethane dressing (Tegaderm™, 3M Medical) for potential dermal wound healing application (Chong, Phan et al 2007). Significant cell adhesion, growth and proliferation on the Tegaderm-nanofiber construct were achieved, providing great potential and feasibility in the treatment of wounds through layered dermal reconstitution. In another study using a similar strategy, Li et al (Li, Mondrinos et al 2006) also fabricated gelatin-containing composite nanofibrous scaffolds of PLGA/gelatin/elastin for potential soft tissue engineering applications. The cultured H9c2 rat cardiac myoblasts and rat bone marrow stromal cells (BMSCs) were found to grow well and cell penetration into the scaffolds were also observed through histological characterization. These studies also indicate that as the bioactive component of gelatin is a hydrogel dissolvable in water, blending gelatin with a structural stable synthetic polymer to form composites circumvents the chemical cross-linking related cytotoxicity problem of gelatin scaffolds (Zhang, Venugopal et al 2006).
Table 1.
Organic–organic blend composite nanofibrous scaffolds
| Scaffold materials | Solvents used | Diameters of electrospun fibers | Cells cultured | Potential uses for tissue engineering | References |
|---|---|---|---|---|---|
| DNA/PLGA or PLA– PEG block copolymer | DMF/Tris–EDTA buffer | 250–875 nm, 375 nm–1.1μm | A pre–osteoblastic cell line, MC3T3E1 | Bone | (Luu, Kim et al 2003) |
| Gelatin/PCL | TFE | 500–900 nm | BMSCs, Fibroblasts | Skin | (Zhang, Ouyang et al 2005; Chong, Phan et al 2007) |
| Collagen/PEUU | HFIP | 100–900 nm | smooth muscle cells | Soft tissues | (Stankus, Guan et al 2004) |
| Collagen/Elastin/PEO | Aqueous HCl | 220–600 nm | SMCs | Blood vessel | (Buttafoco, Kolkman et al 2006) |
| PLCL/Collagen (or Heparin) | HFIP | 120–520 nm | HUVEC | Vascular graft | (Kwon and Matsuda 2005) |
| NGF–BSA/PCLEEP | DCM/PBS | 0.5~3.0μm | PC12 cells | Nerve | (Chew, Wen et al 2005) |
| Collagen/GAG(eg, CS) | TFE/Water | 260 nm | RCFs | / | (Zhong, Teo et al 2005) |
| Collagen/Elastin/PLGA (blend ratio 45:15:40) | HFIP | 720 ± 350 nm | Bovine endothelial and smooth muscle cells | Vascular substitute | (Stitzel, Liu et al 2006) |
| Gelatin/PANi | HFIP | 60–800 nm | H9c2 rat cardiac myoblast | Cardiac/nerve | (Li, Guo et al 2006) |
| Gelatin/Elastin/PLGA | HFIP | 380 ± 80 nm | H9c2 rat cardiac myoblast, BSCs | Heart/blood vessel | (Li, Mondrinos et al 2006) |
| Collagen/P(LLA–CL) | HFIP | 100–300 nm | HCAECs | Blood vessel | (He, Yong et al 2005) |
| PGA/Chitin | HFIP | 50–350 nm | Fibroblasts | / | (Park, Kang et al 2006) |
| PHBV/Collagen | HFIP | 300–600 nm | NIH3T3 | / | (Meng, Kim et al 2007) |
| PDO/Elastin | HFIP | 400–800 nm | Human dermal fibroblasts | Vascular graft | (Sell, McClure et al 2006) |
| GDNF/PCLEEP | DCM/PBS | 3.96 ± 0.14μm | In vivo test | Nerve | (Chew, Mi et al 2007) |
| Collagen/PCL | HFIP | ~275 nm | HDFs | Skin | (Venugopal, Zhang et al 2006) |
| Collagen/PCL (75:25) | HFIP | 541 ± 164 nm | Schwann cells, fibroblasts, olfactory ensheathing cells | Nerve | (Schnell, Klinkhammer et al 2007) |
Abbreviations: BMSC: bone marrow stromal cell; BSA, bovine serum albumin; CS, chondroitin sulfate; DCM, dichloromethane; DMF, dimethylformamide; GAG, glycos-aminoglycan; GDNF, human glial cell–derived neurotrophic factor; HCAEC, human coronary artery endothelial cell; HCl, hydrochloric acid; HDF, human dermal fibroblast; hESF, human embryo skin fibroblast; HUVEC, human umbilical vein endothelial cell; HFIP, hexafluoroisopropanol; NGF, nerve growth factor; P(LLA–CL), poly(L–lactic acid)–co–poly(ɛ–caprolactone); PANi, polyaniline; PBS, phosphate buffered saline; PCL, poly(ɛ–caprolactone); PCLEEP, polymer(ɛ–caprolactone–co–ethylethylene phosphate); PDO, polydioxanone; PEG, poly(ethylene glycol); PEO, poly(ethylene oxide); PEUU, poly(ester urethane)urea; PGA, poly(glycolic acid); PHBV, poly(3–hydroxybutyrate–co–3–hydroxyvalerate); PLA, polylactide; PLCL, poly(L–lactide–co–ɛ–caprolactone); PLGA, poly (D,L–lactide–co–glycolide); PlnDI, perlecan domain I; PVA, poly(vinyl alcohol); RCF, rabbit conjunctiva fibroblast; SMC, Smooth muscle cell; TFE, trifluoroethanol.
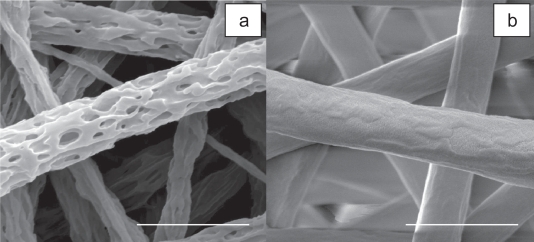
Figure 2.
SEM images of 3-D porous fibers (a) after gelatin was leached out of the electrospun Gt/PCL composite fibers (b) (Zhang, Feng et al 2006). Scale bar 2 μm.
It is also noted that many investigations are based on the collagen/synthetics blends to produce biomimetic and bioactive scaffolds. Bioactivity and/or biofunctions have been remarkably achieved in the nanofibrous form. These studies have similarly demonstrated that compared to the synthetic nanofibrous counterparts, collagen-containing composite nanofibrous scaffolds facilitated cell adhesion (Stankus, Guan et al 2004; Kwon and Matsuda 2005; Li, Mondrinos et al 2006; Park, Kang et al 2006; Venugopal, Zhang et al 2006; Meng, Kim et al 2007; Schnell, Klinkhammer et al 2007), spreading (He, Yong et al 2005; Kwon and Matsuda 2005; Venugopal, Zhang et al 2006), viability (He, Yong et al 2005; Schnell, Klinkhammer et al 2007), migration (Stankus, Guan et al 2004; Sell, McClure et al 2006; Schnell, Klinkhammer et al 2007), proliferation (Stankus, Guan et al 2004; Kwon and Matsuda 2005; Venugopal, Zhang et al 2006; Meng, Kim et al 2007; Schnell, Klinkhammer et al 2007), phenotypic morphological preservation and differentiation (He, Yong et al 2005; Schnell, Klinkhammer et al 2007), and possible collagenase degradation function (Stankus, Guan et al 2004). In addition, introduction of collagen in processing can facilitate the generation of even finer electrospun fibers (Kwon and Matsuda 2005; Li, Guo et al 2006; Li, Mondrinos et al 2006; Park, Kang et al 2006; Venugopal, Zhang et al 2006). Apart from these gelatin/collagen/elastin-containing composite nanofibrous scaffolds, in a different strategy, Li et al fabricated polyaniline (PANi)-contained gelatin composite nanofibrous scaffolds by doping gelatin with a small amount of conductive polymer PANi and demonstrated biocompatibility of such conductive nanofibrous scaffolds (Li, Guo et al 2006). This groundwork will prompt future probing of the electroactive effect of such scaffolds for engineering cardiac or neuronal tissues. In another study, for even better mimicking of the natural ECM which is mainly composed of collagen and glycosaminoglycans (GAGs), Zhong et al prepared collagen/condroitin sulfates composite nanofibrous scaffolds and demonstrated their excellent biocompatibility through conducting in vitro culturing of rabbit conjunctive fibroblasts on the developed scaffolds (Zhong, Teo et al 2005).
Although the above attempts of introducing natural materials have resulted in improved biological properties, there appeared some biophysical and mechanical inadequacies with these systems. One of the noted problems is the dissolving solvent which implicates modification to the natural biopolymer structure. As seen in Table 1, except work by Buttafoco et al (2006) where aqueous acidic solutions were used for electrospinning collagen/elastin/PEO blends, almost all the other composite nanofibers produced employed the strong polarity organic solvent of fluorinated alcohols, in particular the HFIP as the dissolving solvent. The reason is that to have the blend of collagen/synthetics successfully electrospun, selecting an organic solvent which is capable of dissolving both the collagen and the used synthetic polymer is a prerequisite. In this regard, the specialty organic solvents such as HFIP, TFE which can dissolve a wide range of polymers including those tough polymers such as polyglycolide, polyamides, polypeptides, could be the only choice as collagen is insoluble in the ordinary organic solvents. Huang et al (Huang, Nagapudi et al 2001) once attempted electrospinning collagen dissolved in a traditional weak acid solution. However, very high fiber-forming aiding agent PEO with a ratio of more than 50% was used. Later, with HFIP as the dissolving solvent, Matthews et al (Matthews, Wnek et al 2002) successfully electrospun pure collagens into nanofibers and demonstrated the collagen’s banding characteristic remains. In addition to its high polarity strong dissolving capability to various polymers, its other physical properties such as being volatile, miscible with water and many organic solvents, and low surface tension also favor it to be an ideal solvent for electrospinning. But, HFIP is a rather costly organic solvent. And there are also reports that using HFIP could modify the collagen native structure. For example, Stankus et al (Stankus, Guan et al 2004) used circular dichroism spectroscopy to evaluate the preservation of collagen secondary structure in the electrospun PEUU/collagen blends and found signs of some structural modification, in particular to those blends with collagen contents less than 50%. Previously, Doillon et al also investigated the negative influence of HFIP on the secondary structure of collagen (Doillon, Drouin et al 1997). Although using the high polar HFIP is still disputable, the relatively less polar TFE could be an alternative choice of candidate solvent because TFE could facilitate reconstruction of the helical configuration of collagen (Buck 1998). In another of our work on cross-linking the electrospun gelatin nanofibers, we found that the ‘crystallinity’ which reflects the triple-helix content was increased by about 20% (Zhang, Venugopal et al 2006). Despite gelatin being a denatured substance from collagen which involves rupture of the triple-helix structure by breaking the hydrogen bonds and rearranging the triple helix into a random configuration, under proper conditions, the chains are able to undergo a conformational disorder-order transition to recover the triple-helix structure (Pezron, Djabourov et al 1991; Ross-Murphy 1992). Another issue is it has been commonly found that the random blending system gave rise to a decrease in certain mechanical properties, eg, tensile strength, especially for the blending ratio of natural components up to 50% in the blending system (Stankus, Guan et al 2004; He, Yong et al 2005; Kwon and Matsuda 2005; Zhang, Ouyang et al 2005; Sell, McClure et al 2006). Severe phase separation and weak physical interactions between the binary blend system are probably responsible for the weakening mechanical performance (Zhang, Ouyang et al 2005; Park, Kang et al 2006; Zhang, Feng et al 2006). Mechanical properties are of crucial important in scaffold design for engineering load-bearing tissues. Electrospun nanofibers are able to emulate the nanoscale collagen in the ECM, which means matched nanomechanical properties to the cells. However, the macroscopic mechanical properties of their assembled form (eg, fibrous membranes) did not seem comparable to other types of scaffolds fabricated from the same materials. In this regard, besides optimizing the constituent ratio to minimize the decrease in mechanical properties of composite nanofibers, combination of nanofibers with other types of substrate such as microfibers and films could be a better solution for load-bearing tissue regeneration (Tuzlakoglu, Bolgen et al 2005; Sahoo, Ouyang et al 2006; Chong, Phan et al 2007; In Jeong, Kim et al 2007).
As mentioned before, introduction of structural proteins such as collagen (gelatin) and elastin is one of the approaches to improve the physicochemical and biological properties of the nanofibrous scaffolds. However, bioactivity of electrospun nanofibrous scaffolds can also be achieved through incorporating very tiny amount of function-regulating biomolecules such as DNA and a variety of growth factors into the scaffolds. Thereafter, they can then be released out of the scaffolds in a controlled manner to the cell microenvironment to modulate cell behavior. In such a case, the scaffold works additionally as a drug delivery functional device. For example, Luu et al demonstrated the first successful incorporation of DNA into the electrospun PLGA random copolymer and PLA-PEG block copolymer nanofibrous scaffolds for gene delivery (Luu, Kim et al 2003). The loaded DNA was claimed to be able to be sustainably released over a period of 20 days with the scaffold still structurally intact and capable of cell transfection and bioactivity. In another study, Chew et al investigated the feasibility of encapsulating human β-nerve growth factor (NGF) in an electrospun scaffold of ɛ-caprolactone and ethyl ethylene phosphate (PCLEEP) copolymer (Chew, Wen et al 2005). PC12 neurite outgrowth assay suggested a partial retaining of the bioactivity. Furthermore in another study, human glial cell-derived neurotrophic factor (GDNF, 0.13 wt%) was encapsulated in the PCLEEP for in vivo testing the efficacy of electrospun aligned protein/polymer composite fibers through a rat model for peripheral nerve-injury treatment (Chew, Mi et al 2007). Definitely, drug-loaded composite nanofibrous scaffolds have great potential in locally controlling the cellular process. However, retention of bioactivity and realization of controlled delivery of the loaded bioactive molecules remain to be the major research interests of utilizing nanofibers. More improvements and exploration are clearly needed in this context.
Organic-inorganic blends
For organic-inorganic blends, inorganic nanoparticles have often been incorporated into polymer matrix to add functionalities and/or to improve mechanical properties for bone tissue engineering as summarized in Table 2. Generally, inorganic phase such as bioactive nanoparticles nano-hydroxyapatite (nHA) (Kim, Song et al 2005; Kim, Lee et al 2006; Li, Vepari et al 2006; Thomas, Jagani et al 2006; Wutticharoenmongkol, Sanchavanakit et al 2006; Venugopal, Vadgama et al 2007), carbon nanotubes (CNT) (Saeed, Park et al 2006; Jose, Steinert et al 2007), nanoclays (Ji, Li et al 2006) and whiskers (Junkasem, Rujiravanit et al 2006) have been reported for preparing nanofibrous tissue engineering scaffolds. Bone is a natural composite material which is composed of an organic matrix (mostly type I collagen) with an array of inorganic apatite nanocrystals. To mimic the bone structure, hydroxyapatite and other calcium phosphate in combination with biodegradable and biocompatible polymers are natural choices for bone tissue engineering application. Fujihara et al (2005) reported polycaprolactone PCL/CaCO3 composite nanofibers with two different PCL to calcium carbonate (CaCO3) ratios (PCL: CaCO3 75:25 wt% and 25:75 wt%). Good cell attachment was observed for the studied composition range, which indicated a potential to utilize PCL/CaCO3 composite nanofibers to guide bone regeneration (GBR) membranes. Similar results were reported for composite nanofibres of hydroxyapatite nanoparticles incorporated in other polymer systems such as synthetic poly(lactic acid) (Kim, Lee et al 2006) and natural polymers (eg, gelatin (Kim, Song et al 2005) and silk (Li, Vepari et al 2006)). Incorporating cell-signaling molecules such as RGD peptides and growth factors have been proven to further improve the cellular behaviour of the tissue engineering scaffolds. Venugopal, Vadgama et al (2007) reported a significant increased mineralization (55%) in PCL/nHA/Collagen biocomposite nanofibrous scaffolds after 10 days of cell culture using human fetal osteoblast cells (hFOB). They concluded that such a unique combination of nanostructures and bioactivity in nanofibrous scaffolds had inherent surface functionality for hFOB adhesion, migration, proliferation and mineralization to form a bone tissue. Li et al (Li, Vepari et al 2006) reported electrospun silk fibroin nanofibrous scaffolds containing bone morphogenetic protein 2 (BMP-2) and/or nanoparticles of hydroxyapatite for in vitro bone formation from human bone marrow-derived mesenchymal stem cells (hMSCs). They found that the co-existence of BMP-2 and nHA in the electrospun silk fibroin nanofibers resulted in the highest calcium deposition and upregulation of BMP-2 transcript levels when compared with other systems.
Table 2.
Organic – inorganic blend composite nanofibrous scaffolds
| Scaffold materials | Solvents | Diameters of electrospun fibers | Cells cultured | Potential uses for tissue engineering | References |
|---|---|---|---|---|---|
| HA/Gelatin | HFIP | 200–400 nm | human osteoblastic cells MG63 | bone | (Kim, Song et al 2005) |
| PCL/CaCO3 | Chloroform/Methanol | 760 ± 190 nm | human osteoblast hFOB1.19 | bone | (Fujihara, Kotaki et al 2005) |
| PHBV/HAp | TFE | 100–2,000 nm | COS– 7 cells from the monkey kidney | / | (Ito, Hasuda et al 2005) |
| HA/PLA | Chloroform | 1~2 μm | MG63 cells | bone | (Kim, Lee et al 2006) |
| Silk/PEO/nHAP/BMP– 2 | water | 520 ± 55 nm | hMSCs | bone | (Li, Vepari et al 2006) |
| PLLA/HA | DCM/1,4-dioxane | <500 nm | human osteosarcoma MG– 63 | bone | (Deng, Sui et al 2007) |
| PCL/HA/Collagen | HFIP | 373 ± 191 nm | hFOB | bone | (Venugopal, Vadgama et al 2007) |
| PLLA/MWCNT/HA | DCM | 250–950 nm | DPSCs | dental | (Deng, Xu et al 2007) |
Abbreviations: BMP– 2, bone morphogenetic protein 2; CaCO3, calcium carbonate; DCM, dichloromethane; DPSC, dental pulp stem cell; HA/nHAP/HAp, hydroxyapatite; HFIP, hexafluoroisopropanol; hFOB, human fetal osteoblasts; hMSC, human bone marrow– derived mesenchymal stem cell; MWCNT, multi– wall carbon nanotube; PHBV, poly(3– hydroxybutyrate– co– 3– hydroxyvalerate); PLLA, poly(L– lactic acid); PCL, poly(ɛ– caprolactone); PEO, poly(ethylene oxide); PLA, polylactide; TFE, trifluoroethanol.
Apart from the compositions of composite nanofibers, the nano-/micro-structures and fiber morphology have also been reported to have significant effects on biological responses, which ultimately are dependent on fabrication processing. So far, most of the nanocomposites were fabricated by mixing nanoparticles with polymers using simple stirring and ultrasonification for dispersion. The particle size of nHA ranged from 10 nm to 150 nm. One of the processing related problems was the agglomeration of nanoparticles due to their large surface areas and surface interactions. The reported micro-/nano-structures of composite nanofibers had neither uniform distribution of nHA within polymer matrix nor controlled orientation and alignment of non-spheric nanoparticles such as HA nano-plates or CNTs. This not only compromises the mechanical properties but may also take a longer time to remodel into bone tissue during regeneration for such composite nanofibers in contrast to the native ECM. To overcome this problem, the interfacial forces between nanoparticles and polymers have to be carefully manipulated. Kim et al (2006) reported the use of a surfactant hydroxysteric acid (HSA) to control the interaction between the hydrophilic nHA powders and the hydrophobic chloroform-dissolved PLA. They found improved dispersability of nHA powders and resulted uniformality of composite nanofibers. However, the fiber diameters were still relatively large (1–2 μm), a common feature for electrospun fibers made from filled nanoparticles. To mimic the structures and compositions of human tissues, a biomimetic approach has to be adopted (Chan, Kumar et al 2006).
Significant progress in understanding of hierarchical structure of bone in the past decades has prompted research into how to build a scaffold that mimics the bone structure. Bone is a hierarchically structured material with remarkable mechanical properties. It is regarded as a nanocomposite material which is made up of hydroxyapatite nanocrystals and collagen over several length scales. The current approaches by utilizing nHA particles with particle sizes of tens or hundreds nanometers are far from ideal to mimic the natural bone structures where the nHA is typically platelike with a dimension of 50 × 25 × 3 nm (Landis, Song et al 1993). Various attempts have been carried out since the late 1990s to perform biomimetic synthesis of nHA/collagen nanocomposites and composite scaffolds (Bradt, Mertig et al 1999; Du, Cui et al 2000). But thus far, it has failed to produce any electrospun composite nanofibers because of the processing difficulty in electrospinning of aqueous mineralised collagen system. A recent study on gelatin/HA biomimetic nanofibers was attempted to produce nanofibers for guided tissue regeneration. Kim et al (Kim, Song et al 2005) used a co-precipitation method to produce biomimetic gelatin/HA nanocomposite from both Ca- and P-containing gelatin solutions under alkaline condition at 40 °C. After washing and freeze drying, the co-precipitated nanocomposite was re-dissolved in a highly polar solvent, 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP). Such fluorinated alcohols have been widely used in electrospinning of natural biopolymers such as collagen (Matthews, Wnek et al 2002) and gelatin (Zhang, Ouyang et al 2005). TEM micrographs revealed improved homogeneity over nanoparticles filled nanofibers. More importantly, the selected area electron diffraction (SAED) revealed a diffusion ring pattern on elongated HA crystals, which was characteristic of the typical apatite structure when grown in a biomimetic process where the mediation of the amino acid structure in the collagen-based organic matrix induced a preferential apatite growth along its c-axis direction (Kikuchi, Ikoma et al 2004). The disadvantages of fluorinated alcohols such as HFIP are their costs, possible toxicity and environmental concerns. The future directions in electrospinning of biomimetic nanocomposite fibers should focus on the use of more eco-friendly aqueous system which mimics more the in vivo cellular growth conditions of tissues.
Core-shell structured composite nanofibrous scaffolds
Another category of composite nanofibers is in the form of core-shell or core-sheath structure. Conventionally, a core-sheath larger sized fiber consists of a core of one type of polymer and a shell of a different polymer. The mechanical properties of the fiber are chiefly dictated by the core material, whereas the shell polymer offers external functions or properties (eg, adhesion, friction, softness). With electrospinning, core-shell structured nanofibers can be produced as well.
Coaxial electrospinning
Feasibility of fabricating core-shell nanofibers through a technique called coaxial electrospinning have been recently demonstrated by several research groups (Loscertales, Barrero et al 2002; Sun, Zussman et al 2003; Li and Xia 2004; Yu, Fridrikh et al 2004; Zhang, Huang et al 2004). Essentially, coaxial electrospinning is a modification or extension to the ordinary electrospinning process. The major difference is that coaxial electrospinning employs a compound spinneret which consists of one (or more) inner capillary housed by an outer tube from which different fluids are separately fed into their respective channels and integrated into a core-sheath structured composite fiber as they are charged and emitted from the compound spinneret.
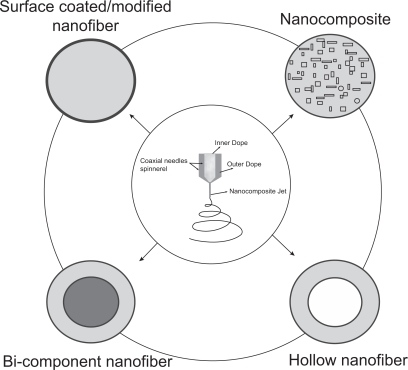
With coaxial electrospinning, at least four types of functional nanofibers (Figure 3) can be envisioned and actually have been demonstrated workable in the past few years. The basic fiber form is generally of concentric bi-component in morphology or surface-coating like form dependent on control of processing parameters while coaxial electrospinning two homogeneous solutions (Figure 4a). If nanoparticles-containing fluid was used as core dope, nanoparticles-loaded composite nanofibers can be prepared (Figure 4b). Li et al and Loscertales et al have creatively demonstrated the feasibility of directly performing one-step fabrication of hollow nanofibers (Figure 4c) via combining the coaxial electrospinning and sol-gel chemistry (Li and Xia 2004; Loscertales, Barrero et al 2004). Furthermore, very recently Zhao et al developed multichannel microtubes (Figure 4d) by extending the two-channel coaxial electrospinning approach (Zhao, Cao et al 2007) to multi-channels. Obviously, coaxial electrospinning provides a novel route to design and fabricate a variety of functional nanofiber structures.
Figure 3.
Illustrated cross-sectional views of a variety of novel and functional polymeric nanofibers from coaxial electrospinning, including basic bi-component nanofiber, surface coated/modified nanofiber through tuning the sheath thickness, nanoparticles encapsulated nanocomposite nanofiber, and hollow nanofibers where the core component is removed.
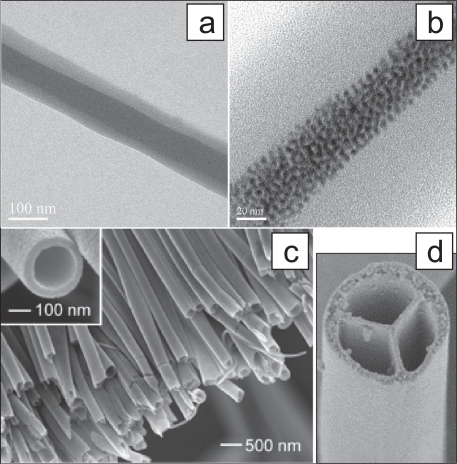
Figure 4.
Coaxial electrospinning were employed to develop core-shell nanofibers (a) (Zhang, Huang et al 2004), self-assembled FePt magnetic nanoparticles (ca. 4 nm) encapsulated nanofibers (b) (Song, Zhang et al 2005), hollow nanofibers (c) (Li and Xia 2004), and multichannel tubes (d) (Zhao, Cao et al 2007).
The prospect of core-shell structured nanofibers from coaxial electrospinning looks very attractive to numerous industrial applications. However, current investigation on this technique is still quite limited and some issues such as the mechanism of forming core-shell structure and processing control on core-sheath configuration remain to be thoroughly investigated. With respect to the formation mechanism of core component, some researchers suggested that the rapid stretching of the sheath causes strong viscous stress, which will be passed onto the core fluid. The shear stress would stretch the core component and elongate it along with the sheath solution via mechanism such as viscous dragging and/or contact friction (Li and Xia 2004; Zussman, Yarin et al 2006). Another issue is under what kind of conditions can high yield of core-shell structured nanofibers be produced, and how those commonly appreciated processing variables such as applied electric field strength, solution viscosity and/or concentrations, and flow rate would affect the control of sheath-thickness as well as the resultant fiber dimensions. Presently, our work indicated that by altering the inner polymer solution concentrations and flow rates, both the inner and overall diameter of coaxially electrospun bi-component nanofibers can be consequently changed (Zhang, Huang et al 2004; Zhang, Wang et al 2006). Li et al investigated the influences of varying flow rate and electrical strength. They found increasing the feeding rates led to larger inner diameter, and both the inner and outer diameters of the core-shell fibers decreased as the electrical field was enhanced.
As bioactive tissue scaffolds
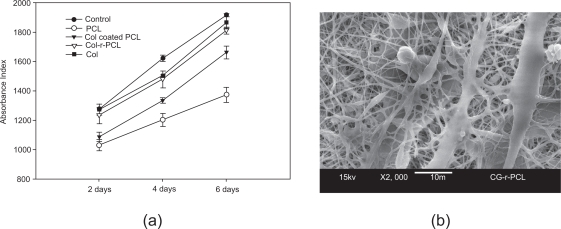
Coaxial electrospun core-shell structured composite nanofibers can be used for constructing bioactive cellular scaffolds by using electrospinnable bioactive macromolecules such as collagen as the shell (to impart bioactivity) and synthetic polymer as core (to retain mechanical and structural advantage). This concept and efficacy have been demonstrated in our group (Zhang, Venugopal et al 2005). In this work, we examined the cell proliferation and morphological differences by culturing human dermal fibroblasts (HDFs) on the collagen-r-PCL (representing collagen and PCL being the shell and core, respectively) scaffolds, and other substrates for comparison including electrospun nanofibrous scaffolds of PCL and collagen, tissue culture plate (TCP) control, and collagen-coated electrospun PCL prepared by immersing the electrospun PCL into a collagen solution overnight. After 6 days of culture, it was found that nanofibers with coatings either achieved by coaxial electrospinning or by simple immersion-coating were definitely favorable for cell proliferation. But, the efficiency is dependent on coating approaches used. Compared to pure nanofibrous PCL, the HDFs density on the core-shell nanofibrous scaffolds increased linearly by 19.5% (2 days), 22.9% (4 days), and 31.8% (6 days). In contrast, the simple immersion collagen-coated electrospun PCL increased only by 5.5% (2 days), 11.0% (4 days), and 21.0% (6 days) (Figure 5a). In addition, for the PCL involved nanofibers, we also found that the HDFs could penetrate beneath the collagen-r-PCL composite nanofibers (Figure 5b). However, there is no such finding either in the pristine PCL or the simple immersion collagen-coated PCL nanofibrous scaffold. This study suggests that current core-shell composite nanofibers tend to resemble the natural ECM architectural constituent of collagen, which makes cells have a propensity to interact well with them. Core-sheath nanofibers would also be a possible solution for the components-incompatible-induced limited improvement in the mechanical properties as discussed in section 2.1.
Figure 5.
Core-shell structured collagen-r-PCL nanofibers favored HDFs proliferation (a) and cellular infiltration (b) (Zhang, Venugopal et al 2005).
Except for coaxial electrospun core-shell nanofibers, other means such as previously used immersion coating (He, Ma et al 2005; Zhang, Venugopal et al 2005) and chemical conjunctions (Chua, Lim et al 2005; Ma, He et al 2005; Kim and Park 2006; Park, Kim et al 2006; Casper, Yang et al 2007; Zhu, Leong et al 2007) have also been attempted to make bioactive molecules coated nanofibers as summarized in Table 3. However, it should be noted that simple immersion coating could make the coating happened only on the shallow layer of the whole nanofibrous structure rather than on each individual fiber because of the hydrophobicity of aliphatic polyesters (eg, PLA and PCL) and nanofibrous structure contributed hydrophobic effect (Feng, Li et al 2002; Neimark, Kornev et al 2003). For the chemical surface modification method, to have desired biomolecules conjugated on the nanofiber surface, the inert electrospun nanofibers are usually subjected to pretreatment via technique like argon plasma or UV irradiation to generate reactive species such as carboxylic or hydroxyl. This severe pretreatment would likely affect the mechanical properties of the delicate nanofibers. Furthermore, as the plasma effect only happens to a depth of several hundred angstroms, a deeper surface modification of the nanofibrous scaffold structure may be difficult to attain as well.
Table 3.
Core-shell structured nanofiber scaffolds
| Scaffold materials | Solvents used | Diameters of electrospun fibers | Cells cultured | Potential uses for tissue engineering | References |
|---|---|---|---|---|---|
| Collagen-r-PCL | TFE | 385 ± 82 nm | Fibroblasts | Skin | (Zhang, Venugopal et al 2005) |
| Collagen-P(LLA-CL) | Aqueous HCl, DCM/DMF | 470 ± 130 nm | HCAECs | Vascular graft | (He, Ma et al 2005) |
| Gelatin-[PMAA]-PET | TFA | 200–600 nm | Endothelial cells | Blood vessel | (Ma, Kotaki et al 2005) |
| Galactose -[PAAc]- PCLEEP | Acetone | 760 nm | Hepatocytes | Liver | (Chua, Lim et al 2005) |
| BMP-2-[SMCC]-Chitosan | HFIP | / | Osteoblastic MC3T3 cell | Bone | (Park, Kim et al 2006) |
| Gelatin-[EDAC]-PCL | Chloroform/DMF (70:30) | 200–1000 nm | Endothelial cells | Blood vessel | (Ma, He et al 2005) |
| Fibronectin-PLLC | HFIP | 100–500 nm | Porcine esophageal epithelial cells | esophagus | (Zhu, Leong et al 2007) |
| RGD-(PLGA-b-PEG-NH2)/PLGA | DMF/THF (1:1) | 449 ± 150 nm | NIH3T3 fibroblasts | / | (Kim and Park 2006) |
| PlnDI-collagen (or gelatin) | HFIP | 2–6 μm | MG63 osteoblastic cells | Bone | (Casper, Yang et al 2007) |
Abbreviations: BMP-2, bone morphogenetic protein-2; DCM, dichloromethane; DMF, N,N-dimethyl formamide; EDAC, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride; HCAEC, human coronary artery endothelial cell; HCl, Hydrochloric acid; PAAc, poly(acrylic acid); PCL, poly(ɛ-caprolactone); PCLEEP, poly(e-caprolactone-co-ethyl ethylene phosphate); PET, poly(ethylene terephthalate); PlnDI, perlecan domain I; PLGA, poly(D,L-lactic-co-glycolic acid); PLLC, poly(L-lactide-co-caprolactone); PMAA, poly(methacrylic acid); SMCC, succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate; RGD, Arg-Gly-Asp; TFA, trifluoroacetic acid; THF, tetrahydrofuran.
Delivery of bioactive molecules
If drugs or bioactive agents are encapsulated by a shell polymer, core-shell electrospun nanofibers can be used for functional drug delivery. In this regard, coaxial electrospinning might be particularly suitable for making biomimetic scaffolds with drug delivery capability. The advantage is that it does not require the drug to be electrospinnable or for it to have good physicochemical interaction with the carrier polymer. In contrast, for the cases of drugs loaded by blend electrospinning, poor interaction between the drug and polymer (Luu, Kim et al 2003; Zeng, Xu et al 2003; Kim, Luu et al 2004; Zeng, Y ang et al 2005), and drug non-electrospinnability (Zhang, Wang et al 2006) both tremendously affect the drug distribution in the polymer matrix and consequently the release behavior. The benefits of using core-shell nanofibers for such a purpose are quite obvious. Firstly, it will be able to preserve those labile biological agents such as DNA and growth factors from being deactivated or denatured even when the applying environment is aggressive. In fact, such protection begins as early as during the fabrication stage because, unlike blend electrospinning, the aqueous solution containing bioactive agents and the shell polymer solution are separately prepared and pumped through different spinning channels. This would greatly reduce the possible influence of being exposed to organic solvents. Secondly, core-shell nanofibers belong to reservoir type drug release device; therefore it will be possible to address the burst release problem noted in those electrospun fibers where drugs were usually incorporated through electrospinning a blend of the drug and polymer carrier (Kenawy, Bowlin et al 2002; Zong, Kwangsok et al 2002; Luu, Kim et al 2003; Kim, Luu et al 2004). Furthermore, by manipulating the core-shell nano-/micro-structure, desired and controlled releasing kinetics could be achieved.
Sustainable release of proteins or drugs with core-shell nanofibers have recently been demonstrated by us and others (Jiang, Hu et al 2005; Huang, He et al 2006; Zhang, Wang et al 2006). Jiang et al (Jiang, Hu et al 2005) encapsulated BSA and lysozyme in PCL nanofibers and found the released lysozyme maintained its structure and bioactivity. Huang et al coaxial electrospun Resyeratrol (RT, an antioxidant) and Gentamycin Sulfate (GS, an antibiotic) loaded nanofibers for controlled release application. In our recent work, we demonstrated the burst-release suppressing ability of core-shell nanofibers by entrapping a fluorescein-conjugated BSA in the PCL shell. These results will provide a basis for further design and optimization of processing conditions to control the core-sheath nanostructure so as to achieve highly sustainable, controllable, and effective bioactive factor releases. In the context of tissue engineering applications, as delivery of growth factors is indispensable in the course of tissue regeneration, it is believed that coaxial electrospinning and the produced core-shell nanofibers will have great potential to locally regulate cellular process for a prolonged time through controlled release of these appropriate growth factors directly into the cell living microenvironment.
Nanofibers mingled structure
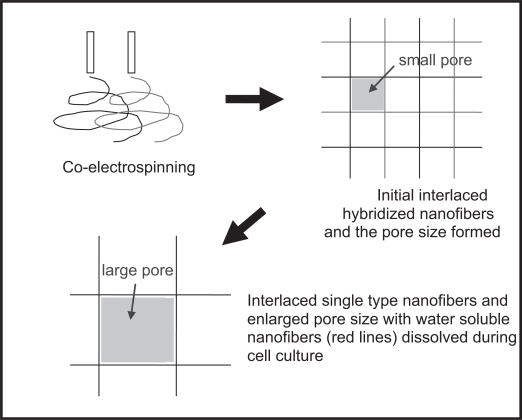
Mingled nanofibers refer to two (or more) different nanofibers which are concurrently electrospun to attain random and homogenous hybridization of them at individual fiber level. Besides envisioning achievable advantages in physical and mechanical properties, one of the most attractive points for the nanofibers mingled structure is that it could offer a solution to cell penetration problem associated with the electrospun nanofibrous scaffolds (Zhang 2004). The working principle as shown in Figure 6 is by simultaneously electros-pinning two kinds of biodegradable and biocompatible polymers (one of them being water soluble) to form nanofibers randomly mingled structure. From here, larger pores can then be formed in situ through leaching out of the water soluble nanofibers during cell culture. Formation of larger space can thus encourage cellular infiltration. This concept was also previously proposed by Kidoaki et al (Kidoaki, Kwon et al 2005) and implemented by co-electrospinning segmented polyurethane (SPU) with poly(ethylene oxide) (PEO) to form mixed fiber mesh (Figure 7). But experimental in vitro cell culture work to provide evidence of cell infiltration has not been attempted by anyone yet. Very recently, Duan et al (2007) simultaneously electrospun PLGA and blend of chitosan/PVA to generated mingled PLGA-chitosan/PVA composite nanofibrous scaffolds. They found such a manner of introducing chitosan/PVA component had changed the hydrophilic/hydrophobic balance, and consequently influenced degradation and mechanical properties as well as cell attachment, proliferation and migration with the nanofibrous scaffolds.
Figure 6.
Schematic illustration showing the formation of larger pores by electrospinning of mingled nanofibers and in situ leaching out of the water soluble nanofibers (red lines) during cell cultivation (Zhang 2004).
Figure 7.
Confocal laser scanning micrographs of electrospun mingled fibrous structure of SPU/PEO. (a) Bottom region of the mixed fiber mesh. SPU and PEO were stained with rhodamine and FITC, respectively. (b) Middle region of the mesh observed at the 4 μm-upper region than (a). (c) Top region of the mesh observed at the 4 μm-upper region than (b) (Kidoaki, Kwon et al 2005). Scale bar 10 μm
Indeed, although electrospun nanofibers can resemble the physical dimensions of the native ECM constituents, the small pores/interstices formed from nanofiber lacing of each others will be too small for cells to pass through (Eichhorn and Sampson 2005; Kidoaki, Kwon et al 2005; Badami, Kreke et al 2006; Pham, Sharma et al 2006; Stankus, Guan et al 2006). Despite the fact that numerous research works have revealed favorable cell adhesion, proliferation and phenotype preservation and functions on the electrospun nanofibrous scaffolds, supporting cellular ingrowth to form cell-scaffold integrated 3-D complex is a critical issue that needs to be resolved. After all, formation of merely a monolayer of cells on the electrospun nanofibrous scaffolds has limited application in tissue engineering. To overcome the cell infiltration problem and achieve a highly cellularized tissue engineered construct in addition to the fiber leaching methods of creating micropores or microvoids in situ (Kidoaki, Kwon et al 2005; Zhang, Ouyang et al 2005; Zhang, Feng et al 2006), different approaches and strategies have been adopted by researchers. For instance, simultaneous electrospinning nanofibers and living cells to achieve a uniform distribution of cells through the scaffold thickness had been proposed (Stankus, Guan et al 2006). Alternatively, using coaxial electrospinning to directly produce cells-encapsulated nanofiber scaffolds is also possible to generate three dimensional distribution of cells within the electrospun scaffolds (Townsend-Nicholson and Jayasinghe 2006). Pham et al (Pham, Sharma et al 2006) electrospun PCL scaffolds consisting of alternating layers of relatively larger microfibers (2~10 μm) and nanofibers to investigate cell infiltration.
So far, there has been work reporting cellular ingrowth to some extent (Matthews, Wnek et al 2002; Bhattarai, Bhattarai et al 2004; Stankus, Guan et al 2004; Telemeco, Ayres et al 2005; Zhang, Ouyang et al 2005; Li, Mondrinos et al 2006). Cellular ingrowth phenomenon was explained as a result of bioactivity effect due to incorporation of bioactive components. In addition to this, appropriate nanomechanical properties of the scaffold nanofibers also allow cells to enter into the matrix through amoeboid movement to push the surrounding fibers aside to make necessary spaces. In spite of these experimental results, whether the electrospun nanofibrous scaffolds support cell infiltration is still open to debate. Systematic investigation from materials selection, manipulated geometry and physical properties of nanofibrous scaffolds, to the cell types, culture methods and conditions need to be performed. We believe while endowing nanofibers with appropriate wettability and biochemical signals would be workable for facilitating and encouraging cell migration into the scaffold interior as reported in our work and others (Stankus, Guan et al 2004; Telemeco, Ayres et al 2005; Zhang, Ouyang et al 2005; Badami, Kreke et al 2006), physical characteristics such as pore size, pore structure, pore distribution and the overall porosity of the nanofibrous scaffolds would equally play important role. Both will have direct influence on supply of the oxygen and nutrients to the cells and removal of waste products – which are the determinant factors for cellular infiltration (Sachlos and Czernuszka 2003).
Concluding remarks
It has been widely acknowledged in the tissue engineering research community that nanofibers produced from electrospinning technique are able to emulate the architecture of the native extracellular matrix, which is a complex fibrous network of proteins and glycosaminoglycans with hierarchical dimensions down to nanometer scale. Here, we discussed the potential of using electrospun composite nanofibers, in the form of components blended, core-shell structured, and nanofibrous mingled structures for developing biomimetic and bioactive cellular scaffolds, as well as the limitations and issues to be resolved.
In comparison to those commonly used biodegradable and biocompatible synthetic polymers, the strategy of introducing natural bioactive components into biologically inert but mechanically meritorious synthetics and converting such combinations into nanofiber form offers a facile approach to bioactivate and functionalize nanofibrous scaffolds. Because of the versatility of electrospinning, with currently established knowledge and understanding about the structure, constituents, and functions of ECM, it is conceivable that more elaborate biological recognition and signaling functions of the extracellular milieu can be integrated into the nanofibrous scaffolds for even precise recapitulation and spatiotemporal control in vitro and in vivo of the cell living environment. On the other hand, as interplays between cells and artificial scaffolds are crucial for modulating cellular functions in vitro and in vivo, the bioactive composite nanofibrous scaffolds might be an ideal biomimic platform for systematic research to enhance our understanding on cell-matrix interactions from which future design and fabrication of biomimetic nanofibrous scaffolds can be achieved and implemented in an accurate and rational manner.
It is believed endowing electrospun nanofibers bioactivity and biological functions will represent the mainstream trend in future nanofibrous scaffold related research activities. In this sense, with continual advances in electrospinning technology and biological evaluation of such scaffolds, biomimetic and bioactive composite nanofibers will be the right candidate materials in fulfilling the successful application of nanofibers in tissue engineering and regenerative medicine.
Footnotes
Refers to an artificial material or structure that mimics a biological material/structure/function.
As of May 7, 2007, the top 10 most cited articles from ISI Web of Science® are: 1) Reneker DH, Chun I, 1996. Nanotechnology, 7(3):216–23. (448 times). 2) Doshi J, Renker DH, 1995. J Electrost, 35 (2–3):151–60. (362 times). 3) Reneker DH, Yarin AL, Fong H, et al. 2000. J Appl Phys, 87 (9):4531–47. (302 times). 4) Huang ZM, Zhang YZ, Kotaki M, et al. 2003. Compos Sci Technol, 63 (15):2223–53. (284 times). 5) Fong H, Chun I, Reneker DH. 1999. Polymer, 40 (16):4585–92. (279 times). 6) Deitzel JM, Kleinmeyer J, Harris D, et al. 2001. Polymer, 42 (1):261–72. (259 times). 7) Li WJ, Laurencin CT, Caterson EJ, et al. 2002. J Biomed Mater Res, 60 (4):613–21. (227 times). 8) Matthews JA, Wnek GE, Simpson DG, et al. 2002. Biomacromolecules, 3 (2):232–8. (218 times). 9) Yoshimoto H, Shin YM, Terai H, et al. 2003. Biomaterials, 24 (12):2077–82. (192 times). 10) Li D, Xia YN. 2004. Adv Mater, 16 (14):1151–70. (186 times). Articles 7–9 are pertaining to tissue scaffolding applications.
The term ‘bioactive’ usually refers to a material or structure that would have positive effect on the living cells in vitro and/or in vivo, due to it containing certain bioactive substances such as proteins (eg, peptides, collagens). The bioactive substances can be physically (eg, via blending) or chemically (eg, by covalently immobilization) incorporated into the material. In this paper, we define a nanofiber being bioactive if it promotes cell-scaffold interaction in terms of cellular adhesion, proliferation, migration, maintaining normal cell morphology and functions, etc.
References
- Badami AS, Kreke MR, et al. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials. 2006;27:596–606. doi: 10.1016/j.biomaterials.2005.05.084. [DOI] [PubMed] [Google Scholar]
- Baldwin SP, Mark Saltzman W. Materials for protein delivery in tissue engineering. Advanced Drug Delivery Reviews. 1998;33:71–86. doi: 10.1016/s0169-409x(98)00021-0. [DOI] [PubMed] [Google Scholar]
- Bhattarai SR, Bhattarai N, et al. Novel biodegradable electrospun membrane: scaffold for tissue engineering. Biomaterials. 2004;25:2595–602. doi: 10.1016/j.biomaterials.2003.09.043. [DOI] [PubMed] [Google Scholar]
- Bradt J-H, Mertig M, et al. Biomimetic mineralization of collagen by combined fibril assembly and calcium phosphate formation. Chem Mater. 1999;11:2694–701. [Google Scholar]
- Buck M. Trifluoroethanol and colleagues: cosolvents come of age. Recent studies with peptides and proteins. Quarterly Reviews of Biophysics. 1998;31:297–355. doi: 10.1017/s003358359800345x. [DOI] [PubMed] [Google Scholar]
- Buttafoco L, Kolkman NG, et al. Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials. 2006;27:724–34. doi: 10.1016/j.biomaterials.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Cai Q, Wan Y, et al. Synthesis and characterization of biodegradable polylactide-grafted dextran and its application as compatilizer. Biomaterials. 2003;24:3555–62. doi: 10.1016/s0142-9612(03)00199-6. [DOI] [PubMed] [Google Scholar]
- Cai Q, Yang J, et al. A novel porous cells scaffold made of polylactide-dextran blend by combining phase-separation and particle-leaching techniques. Biomaterials. 2002;23:4483–92. doi: 10.1016/s0142-9612(02)00168-0. [DOI] [PubMed] [Google Scholar]
- Carnegie JA, Cabaca O. Extracellular matrix composition and resilience: two parameters that influence the in vitro migration and morphology of rat inner cell mass-derived cells. Biology of Reproduction. 1993;48:287–99. doi: 10.1095/biolreprod48.2.287. [DOI] [PubMed] [Google Scholar]
- Casper CL, Yang W, et al. Coating electrospun collagen and gelatin fibers with perlecan domain I for increased growth factor binding. Biomacromolecules. 2007;8:1116–23. doi: 10.1021/bm061003s. [DOI] [PubMed] [Google Scholar]
- Chan CK, Kumar TS, et al. Biomimetic nanocomposites for bone graft applications. Nanomedicine. 2006;1:177–88. doi: 10.2217/17435889.1.2.177. [DOI] [PubMed] [Google Scholar]
- Chen G, Ushida T, et al. Hybrid biomaterials for tissue engineering: a preparative method for PLA or PLGA-collagen hybrid sponges. Advanced Materials. 2000;12:455–7. [Google Scholar]
- Chen G, Ushida T, et al. Scaffold design for tissue engineering. Macromolecular Bioscience. 2002;2:67–77. [Google Scholar]
- Chew SY, Mi R, et al. Aligned protein-polymer composite fibers enhance nerve regeneration: a potential tissue-engineering platform. Advanced Functional Materials. 2007;17:1288–96. doi: 10.1002/adfm.200600441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew SY, Wen J, et al. Sustained release of proteins from electrospun biodegradable fibers. Biomacromolecules. 2005;6:2017–24. doi: 10.1021/bm0501149. [DOI] [PubMed] [Google Scholar]
- Chong EJ, Phan TT, et al. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomaterialia. 2007;3:321–30. doi: 10.1016/j.actbio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Chua K-N, Lim W-S, et al. Stable immobilization of rat hepatocyte spheroids on galactosylated nanofiber scaffold. Biomaterials. 2005;26:2537–47. doi: 10.1016/j.biomaterials.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Coombes AGA, Verderio E, et al. Biocomposites of non-crosslinked natural and synthetic polymers. Biomaterials. 2002;23:2113–18. doi: 10.1016/s0142-9612(01)00341-6. [DOI] [PubMed] [Google Scholar]
- Deng XL, Sui G, et al. Poly(L-lactic acid)/hydroxyapatite hybrid nanofibrous scaffolds prepared by electrospinning. Journal of Biomaterials Science-Polymer Edition. 2007;18:117–30. doi: 10.1163/156856207779146123. [DOI] [PubMed] [Google Scholar]
- Deng XL, Xu MM, et al. Electrospun PLLA/MWNTs/HA hybrid nanofiber scaffolds and their potential in dental tissue engineering. Key Engineering Materials. 2007:330–2. 393–6. [Google Scholar]
- Doillon CJ, Drouin R, et al. Chemical inactivators as sterilization agents for bovine collagen materials. Journal of Biomedical Materials Research. 1997;37:212–21. doi: 10.1002/(sici)1097-4636(199711)37:2<212::aid-jbm10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Doshi J, Reneker DH. Electrospinning process and applications of electrospun fibers. Journal of Electrostatics. 1995;35:151–60. [Google Scholar]
- Drumheller P, Hubbell J. The biomedical engineering handbook. CRC Press LLC; Boca Raton, Florida: 2000. [Google Scholar]
- Du C, Cui FZ, et al. Formation of calcium phosphate/collagen composites through mineralization of collagen matrix. Journal of Biomedical Materials Research. 2000;50:518–27. doi: 10.1002/(sici)1097-4636(20000615)50:4<518::aid-jbm7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Duan B, Wu L, et al. Degradation of electrospun PLGA-chitosan/PVA membranes and their cytocompatibility in vitro. Journal of Biomaterials Science, Polymer Edition. 2007;18:95–115. doi: 10.1163/156856207779146105. [DOI] [PubMed] [Google Scholar]
- Eichhorn S, Sampson W. Statistical geometry of pores and statistics of porous nanofibrous assemblies. Journal of The Royal Society Interface. 2005;2:309–18. doi: 10.1098/rsif.2005.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Li S, et al. 2002Super-hydrophobic surface of aligned polyacrylonitrile nanofibers Angewandte Chemie(International ed. In English),4171221–3. [DOI] [PubMed] [Google Scholar]
- Fertala A, Han WB, et al. Mapping critical sites in collagen II for rational design of gene-engineered proteins for cell-supporting materials. Journal of Biomedical Materials Research. 2001;57:48–58. doi: 10.1002/1097-4636(200110)57:1<48::aid-jbm1140>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Formhals A.1934. US patent 1,975,504.
- Fujihara K, Kotaki M, et al. Guided bone regeneration membrane made of polycaprolactone/calcium carbonate composite nanofibers. Biomaterials. 2005;26:4139–47. doi: 10.1016/j.biomaterials.2004.09.014. [DOI] [PubMed] [Google Scholar]
- He W, Ma Z, et al. Fabrication of collagen-coated biodegradable polymer nanofiber mesh and its potential for endothelial cells growth. Biomaterials. 2005;26:7606–15. doi: 10.1016/j.biomaterials.2005.05.049. [DOI] [PubMed] [Google Scholar]
- He W, Yong T, et al. Fabrication and endothelialization of collagen-blended biodegradable polymer nanofibers: potential vascular graft for blood vessel tissue engineering. Tissue Engineering. 2005;11:1574–88. doi: 10.1089/ten.2005.11.1574. [DOI] [PubMed] [Google Scholar]
- Huang L, Nagapudi K, et al. Engineered collagen-PEO nanofibers and fabrics. J. Biomater Sci Polymer Edn. 2001;12:979–93. doi: 10.1163/156856201753252516. [DOI] [PubMed] [Google Scholar]
- Huang ZM, He CL, et al. Encapsulating drugs in biodegradable ultrafine fibers through co-axial electrospinning. Journal of Biomedical Materials Research, Part A. 2006;77A:169–79. doi: 10.1002/jbm.a.30564. [DOI] [PubMed] [Google Scholar]
- Huang Z-M, Zhang Y-Z, et al. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Composites Science and Technology. 2003;63:2223–53. [Google Scholar]
- Hubbell JA. Biomaterials in tissue engineering. Bio/Technology. 1995;13:565–76. doi: 10.1038/nbt0695-565. [DOI] [PubMed] [Google Scholar]
- Ikada Y, Tabata Y. Significance of drug delivery in tissue engineering. In: Lewandrowski K-U, et al., editors. Tissue engineering and biodegradable equivalents: scientific and clinical applications. Marcel Dekker, Inc; 2002. pp. 145–63. [Google Scholar]
- In Jeong S, Kim SY, et al. Tissue-engineered vascular grafts composed of marine collagen and PLGA fibers using pulsatile perfusion bioreactors. Biomaterials. 2007;28:1115–22. doi: 10.1016/j.biomaterials.2006.10.025. [DOI] [PubMed] [Google Scholar]
- Ito Y, Hasuda H, et al. A composite of hydroxyapatite with electrospun biodegradable nanofibers as a tissue engineering material. Journal of Bioscience and Bioengineering. 2005;100:43–9. doi: 10.1263/jbb.100.43. [DOI] [PubMed] [Google Scholar]
- Ji Y, Li B, et al. Structure and Nanomechanical Characterization of Electrospun PS/Clay Nanocomposite Fibers. Langmuir. 2006;22:1321–8. doi: 10.1021/la0525022. [DOI] [PubMed] [Google Scholar]
- Jiang H, Hu Y, et al. A facile technique to prepare biodegradable coaxial electrospun nanofibers for controlled release of bioactive agents. Journal of Controlled Release. 2005;108:237–43. doi: 10.1016/j.jconrel.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Jose MV, Steinert BW, et al. Morphology and mechanical properties of Nylon 6/MWNT nanofibers. Polymer. 2007;48:1096–104. [Google Scholar]
- Junkasem J, Rujiravanit R, et al. Fabrication of alpha-chitin whisker-reinforced poly(vinyl alcohol) nanocomposite nanofibres by electrospinning. Nanotechnology. 2006;17:4519–28. [Google Scholar]
- Kadler KE, Holmes DF, et al. Collagen fibril formation. Biochemical Journal. 1996;316:1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenawy E-R, Bowlin GL, et al. Release of tetracycline hydrochloride from electrospun poly(ethylene-co-vinylacetate), poly(lactic acid), and a blend. Journal of Controlled Release: Official Journal Of The Controlled Release Society. 2002;81:57–64. doi: 10.1016/s0168-3659(02)00041-x. [DOI] [PubMed] [Google Scholar]
- Kidoaki S, Kwon IK, et al. Mesoscopic spatial designs of nano- and microfiber meshes for tissue-engineering matrix and scaffold based on newly devised multilayering and mixing electrospinning techniques. Biomaterials. 2005;26:37–46. doi: 10.1016/j.biomaterials.2004.01.063. [DOI] [PubMed] [Google Scholar]
- Kikuchi M, Ikoma T, et al. Biomimetic synthesis of bone-like nanocomposites using the self-organization mechanism of hydroxyapatite and collagen. Composites Science and Technology. 2004;64:819–25. [Google Scholar]
- Kim H-W, Lee H-H, et al. Electrospinning biomedical nanocomposite fibers of hydroxyapatite/poly(lactic acid) for bone regeneration. Journal of Biomedical Materials Research Part A. 2006;79A:643–9. doi: 10.1002/jbm.a.30866. [DOI] [PubMed] [Google Scholar]
- Kim H-W, Song J-H, et al. Nanofiber generation of gelatin-hydroxyapatite biomimetics for guided tissue regeneration. Advanced Functional Materials. 2005;15:1988–94. [Google Scholar]
- Kim K, Luu YK, et al. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. Journal of Controlled Release. 2004;98:47–56. doi: 10.1016/j.jconrel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Kim TG, Park TG. Biomimicking extracellular matrix: cell adhesive RGD peptide modified electrospun poly(D,L-lactic-co-glycolic acid) nanofiber mesh. Tissue Engineering. 2006;12:221–33. doi: 10.1089/ten.2006.12.221. [DOI] [PubMed] [Google Scholar]
- Kwon IK, Matsuda T. Co-electrospun nanofiber fabrics of poly(L-lactide-co-E-caprolactone) with type I collagen or heparin with type I collagen or heparin. Biomacromolecules. 2005;6:2096–105. doi: 10.1021/bm050086u. [DOI] [PubMed] [Google Scholar]
- Landis WJ, Song MJ, et al. Mineral and organic matrix interaction in normally calcifying tendon visualized in three dimensions by high-voltage electron microscopic tomography and graphic image reconstruction. Journal of Structural Biology. 1993;110:39–54. doi: 10.1006/jsbi.1993.1003. [DOI] [PubMed] [Google Scholar]
- Laurencin C, Ambrosio A, et al. Tissue engineering: orthopedic applications. Annual Review of Biomedical Engineering. 1999;1:19–46. doi: 10.1146/annurev.bioeng.1.1.19. [DOI] [PubMed] [Google Scholar]
- Li C, Vepari C, et al. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27:3115–24. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Li D, Xia Y. Direct fabrication of composite and ceramic hollow nanofibers by electrospinning. Nano Letters. 2004;4:933–8. [Google Scholar]
- Li D, Xia Y. Electrospinning of nanofibers: reinventing the wheel? Advanced Materials. 2004;16:1151–70. [Google Scholar]
- Li M, Guo Y, et al. Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials. 2006;27:2705–15. doi: 10.1016/j.biomaterials.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Li M, Mondrinos MJ, et al. Co-electrospun poly(lactide-co-glycolide), gelatin, and elastin blends for tissue engineering scaffolds. Journal of Biomedical Materials Research Part A. 2006;79A:963–73. doi: 10.1002/jbm.a.30833. [DOI] [PubMed] [Google Scholar]
- Li W-J, Laurencin CT, et al. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. Journal of Biomedical Materials Research. 2002;60:613–21. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- Loscertales IG, Barrero A, et al. Micro/nano encapsulation via electrified coaxial liquid jets. Science. 2002;295:1695–8. doi: 10.1126/science.1067595. [DOI] [PubMed] [Google Scholar]
- Loscertales IG, Barrero A, et al. Electrically forced coaxial nanojets for one-step hollow nanofiber design. Journal of the American Chemical Society. 2004;126:5376–7. doi: 10.1021/ja049443j. [DOI] [PubMed] [Google Scholar]
- Luu YK, Kim K, et al. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA-PEG block copolymers. Journal of Controlled Release. 2003;89:341–53. doi: 10.1016/s0168-3659(03)00097-x. [DOI] [PubMed] [Google Scholar]
- Ma Z, He W, et al. Grafting of gelatin on electrospun poly(caprolactone) nanofibers to improve endothelial cell spreading and proliferation and to control cell orientation. Tissue Engineering. 2005;11:1149–58. doi: 10.1089/ten.2005.11.1149. [DOI] [PubMed] [Google Scholar]
- Ma Z, Kotaki M, et al. Surface engineering of electrospun polyethylene terephthalate (PET) nanofibers towards development of a new material for blood vessel engineering. Biomaterials. 2005;26:2527–36. doi: 10.1016/j.biomaterials.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Matthews JA, Wnek GE, et al. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3:232–8. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- Meng W, Kim S-Y, et al. Electrospun PHBV/collagen composite nanofibrous scaffolds for tissue engineering. Journal of Biomaterials Science, Polymer Edition. 2007;18:81–94. doi: 10.1163/156856207779146114. [DOI] [PubMed] [Google Scholar]
- Neimark A, Kornev K, et al. Wetting of nanofibers. Polymer Preprints. 2003;44:160. [Google Scholar]
- Nishida T, Yasumoto K, et al. The network structure of corneal fibroblasts in the rat as revealed by scanning electron microscopy. Investigative Ophthalmology and Visual Science. 1988;29:1887–90. [PubMed] [Google Scholar]
- Park KE, Kang HK, et al. Biomimetic nanofibrous scaffolds: preparation and characterization of pga/chitin blend nanofibers. Biomacromolecules. 2006;7:635–43. doi: 10.1021/bm0509265. [DOI] [PubMed] [Google Scholar]
- Park YJ, Kim KH, et al. Immobilization of bone morphogenetic protein-2 on a nanofibrous chitosan membrane for enhanced guided bone regeneration. Biotechnology and Applied Biochemistry. 2006;43:17–24. doi: 10.1042/BA20050075. [DOI] [PubMed] [Google Scholar]
- Pezron I, Djabourov M, et al. Conformation of gelatin chains in aqueous solutions: 1. A light and small-angle neutron scattering study. Polymer. 1991;32:3201–10. [Google Scholar]
- Pham QP, Sharma U, et al. Electrospun poly(-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7:2796–805. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- Reneker D, Chun I. Nanometre diameter fibres of polymer – produced by electrospinning. Nanotechnology. 1996;7:216–23. [Google Scholar]
- Ross-Murphy SB. Structure and rheology of gelatin gels: recent progress. Polymer. 1992;33:2622–7. [Google Scholar]
- Rosso F, Marino G, et al. Smart materials as scaffolds for tissue engineering. Journal of Cellular Physiology. 2005;203:465–70. doi: 10.1002/jcp.20270. [DOI] [PubMed] [Google Scholar]
- Sachlos E, Czernuszka JT. Making tissue engineering scaffolds work. review on the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. European Cells and Materials. 2003;5:29–40. doi: 10.22203/ecm.v005a03. [DOI] [PubMed] [Google Scholar]
- Saeed K, Park S-Y, et al. Preparation of electrospun nanofibers of carbon nanotube/polycaprolactone nanocomposite. Polymer. 2006;47:8019–25. [Google Scholar]
- Sahoo S, Ouyang H, et al. Characterization of a novel polymeric scaffold for potential application in tendon/ligament tissue engineering. Tissue Engineering. 2006;12:91–9. doi: 10.1089/ten.2006.12.91. [DOI] [PubMed] [Google Scholar]
- Schnell E, Klinkhammer K, et al. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-[epsilon]-caprolactone and a collagen/poly-[epsilon]-caprolactone blend. Biomaterials. 2007;28:3012–25. doi: 10.1016/j.biomaterials.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Sell SA, McClure MJ, et al. Electrospun polydioxanone-elastin blends: potential for bioresorbable vascular grafts. Biomedical Materials. 2006;1:72–80. doi: 10.1088/1748-6041/1/2/004. [DOI] [PubMed] [Google Scholar]
- Song T, Zhang YZ, et al. Encapsulation of self-assembled FePt magnetic nanoparticles in PCL nanofibers by coaxial electrospinning. Chemical Physics Letters. 2005;415:317–22. [Google Scholar]
- Stankus JJ, Guan J, et al. Microintegrating smooth muscle cells into a biodegradable, elastomeric fiber matrix. Biomaterials. 2006;27:735–44. doi: 10.1016/j.biomaterials.2005.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankus JJ, Guan J, et al. Fabrication of biodegradable elastomeric scaffolds with sub-micron morphologies. Journal of Biomedical Materials Research Part A. 2004;70A:603–14. doi: 10.1002/jbm.a.30122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzel J, Liu J, et al. Controlled fabrication of a biological vascular substitute. Biomaterials. 2006;27:1088–94. doi: 10.1016/j.biomaterials.2005.07.048. [DOI] [PubMed] [Google Scholar]
- Stitzel JD, Pawlowski KJ, et al. 2001Arterial smooth muscle cell proliferation on a novel biomimicking, biodegradable vascular graft scaffold Journal of Biomaterials Applications 16(July):22–33. [DOI] [PubMed] [Google Scholar]
- Sun Z, Zussman E, et al. Compound core-shell polymer nanofibers by co-electrospinning. Advanced Materials. 2003;15:1929–32. [Google Scholar]
- Telemeco TA, Ayres C, et al. Regulation of cellular infiltration into tissue engineering scaffolds composed of submicron diameter fibrils produced by electrospinning. Acta Biomaterialia. 2005;1:377–85. doi: 10.1016/j.actbio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Thomas V, Jagani S, et al. Electrospun bioactive nanocomposite scaffolds of polycaprolactone and nanohydroxyapatite for bone tissue engineering. Journal of Nanoscience and Nanotechnology. 2006;6:487–93. doi: 10.1166/jnn.2006.097. [DOI] [PubMed] [Google Scholar]
- Townsend-Nicholson A, Jayasinghe SN. Cell electrospinning: a unique biotechnique for encapsulating living organisms for generating active biological microthreads/scaffolds. Biomacromolecules. 2006;7:3364–9. doi: 10.1021/bm060649h. [DOI] [PubMed] [Google Scholar]
- Tuzlakoglu K, Bolgen N, et al. Nano- and micro-fiber combined scaffolds: A new architecture for bone tissue engineering. Journal of Materials Science: Materials in Medicine. 2005;16:1099–104. doi: 10.1007/s10856-005-4713-8. [DOI] [PubMed] [Google Scholar]
- Venugopal J, Vadgama P, et al. Biocomposite nanofibres and osteoblasts for bone tissue engineering. Nanotechnology. 2007;18:1–8. [Google Scholar]
- Venugopal JR, Zhang YZ, et al. In vitro culture of human dermal fibroblasts on electrospun polycaprolactone collagen nanofibrous membrane. Artificial Organs. 2006;30:440–6. doi: 10.1111/j.1525-1594.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- Wutticharoenmongkol P, Sanchavanakit N, et al. Novel bone scaffolds of electrospun polycaprolactone fibers filled with nanoparticles. Journal of Nanoscience and Nanotechnology. 2006;6:514–22. doi: 10.1166/jnn.2006.090. [DOI] [PubMed] [Google Scholar]
- Yu JH, Fridrikh SV, et al. Production of submicrometer diameter fibers by two-fluid electrospinning. Advanced Materials. 2004;16:1562–6. [Google Scholar]
- Zeng J, Xu X, et al. Biodegradable electrospun fibers for drug delivery. Journal of Controlled Release: Official Journal of The Controlled Release Society. 2003;92:227–31. doi: 10.1016/s0168-3659(03)00372-9. [DOI] [PubMed] [Google Scholar]
- Zeng J, Yang L, et al. Influence of the drug compatibility with polymer solution on the release kinetics of electrospun fiber formulation. Journal of Controlled Release. 2005;105:43–51. doi: 10.1016/j.jconrel.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Zhang YZ.2004. Electrospinning of gelatin/PCL bioactive composite nanofibrous structures for tailored biomedical applications. National University of Singapore: (unpublished document).
- Zhang YZ, Feng Y, et al. Fabrication of porous electrospun nanofibers. Nanotechnology. 2006;17:901–8. [Google Scholar]
- Zhang YZ, Huang Z-M, et al. Preparation of core-shell structured PCL-r-gelatin bi-component nanofibers by coaxial electrospinning. Chem Mater. 2004;16:3406–9. [Google Scholar]
- Zhang YZ, Lim CT, et al. Recent development of polymer nanofibers for biomedical and biotechnological applications. Journal of Materials Science: Materials in Medicine. 2005;16:933–46. doi: 10.1007/s10856-005-4428-x. [DOI] [PubMed] [Google Scholar]
- Zhang YZ, Ouyang HW, et al. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. Journal of Biomedical Materials Research, Part B: Applied Biomaterials. 2005;72B:156–65. doi: 10.1002/jbm.b.30128. [DOI] [PubMed] [Google Scholar]
- Zhang YZ, Venugopal J, et al. Characterization of the surface biocompatibility of the electrospun PCL-collagen nanofibers using fibroblasts. Biomacromolecules. 2005;6:2583–9. doi: 10.1021/bm050314k. [DOI] [PubMed] [Google Scholar]
- Zhang YZ, Venugopal J, et al. Crosslinking of the electrospun gelatin nanofibers. Polymer. 2006;47:2911–17. [Google Scholar]
- Zhang YZ, Wang X, et al. Coaxial electrospinning of (fluorescein isothiocyanate-conjugated bovine serum albumin)-encapsulated poly(epslon-caprolactone) nanofibers for sustained release. Biomacromolecules. 2006;7:1049–57. doi: 10.1021/bm050743i. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Cao X, et al. Bio-mimic multichannel microtubes by a facile method. J Am Chem Soc. 2007;129:764–5. doi: 10.1021/ja068165g. [DOI] [PubMed] [Google Scholar]
- Zhong SP, Teo WE, et al. Formation of collagen-glycosaminoglycan blended nanofibrous scaffolds and their biological properties. Biomacromolecules. 2005;6:2998–3004. doi: 10.1021/bm050318p. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Leong MF, et al. Esophageal epithelium regeneration on fibronectin grafted poly(l-lactide-co-caprolactone) (PLLC) nanofiber scaffold. Biomaterials. 2007;28:861–8. doi: 10.1016/j.biomaterials.2006.09.051. [DOI] [PubMed] [Google Scholar]
- Zong X, Kwangsok K, et al. Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer. 2002;43:4403–12. [Google Scholar]
- Zussman E, Yarin AL, et al. Electrospun polyaniline/poly(methyl methacrylate)-derived turbostratic carbon micro-/nanotubes. Advanced Materials. 2006;18:348–53. [Google Scholar]