Abstract
During the last decade, the application of nanotechnologies for anticancer drug delivery has been extensively explored, hoping to improve the efficacy and to reduce side effects of chemotherapy. The present review is dedicated to a certain kind of anticancer drug nanovectors developed to target tumors with the help of an external magnetic field. More particularly, this work treats anticancer drug nanoformulations based on superparamagnetic iron oxide nanoparticles coated with biocompatible polymers. The major purpose is to focus on the specific requirements and technological difficulties related to controlled delivery of antitumoral agents. We attempt to state the problem and its possible perspectives by considering the three major constituents of the magnetic therapeutic vectors: iron oxide nanoparticles, polymeric coating and anticancer drug.
Keywords: magnetic drug targeting, iron oxide nanoparticles, anticancer agent
Introduction
Nanosciences and nanotechnologies form a growing strategic sector with enormous economic potential: “small is big business” (Maliksi 2006). Nanostructures and nanosystems developed include macromolecules as for example DNA, colloid particles, macromolecular assemblies, or even viruses (Emerich and Thanos 2005). Nanosystems are governed by surface effects such as hydrophobic interactions, hydrogen bonding, ionic bonding or repulsion, covalent bonding.
There is an enormous potential for nanotechnology applied to drug delivery (Kreuter 2004; Whelan and Mayenne 2006). The vehicle might be a functionalized nanoparticle which contains the therapeutic agent and is capable of targeting specific diseased cells or organs. Efficient drug targeting is mainly explored in the field of cancer treatment. As cancer cells tend to develop multidrug resistance (Baird and Kaye 2003), and the serious side effects encountered limit the efficacy of chemotherapy (Alexiou et al 2005), the search for alternative treatments is currently one of the most active areas of cancer research (Sachdeva 1998).
A number of different targeting strategies for cancer therapy have been comprehensively reviewed in many publications (Sachdeva 1998; Begent 1999). One rational approach involves conjugating cancer chemotherapeutics with ligands specific to the cancer cell surface (antibodies or small molecules) in the hope of promoting their localization in tumors (Sachdeva 1998; Huang and Oliff 2001). Another approach is magnetic drug targeting: magnetoliposomes (De Cuyper and Joniau 1988) or magnetic polymer particles (Gupta and Gupta 2005) carrying active molecules represent an attractive tool since they allow one to concentrate the drug at a defined target site by local application of an external magnetic field. Nanoparticles of iron oxides, in particular magnetite and maghemite, have been shown to offer potential applications because of their unique magnetic properties and low toxicity (Pulfer and Gallo 2000; Lübbe et al 2001, 2003).
The purpose of our article is to focus on the specific requirements and the most recent developments related to the use of iron oxide nanoparticles for magnetically controlled delivery of anticancer drugs. The optimization of critical parameters makes the development of magnetic vectors of anticancer drugs a multidisciplinary challenge. Indeed, the success of this optimization depends not only on novel protocols of particle preparation but also on appropriate methods of physicochemical characterization and biological evaluation in vitro and in vivo. We attempt to illustrate the state of the problem and possible perspectives by considering the three major constituents of the nanoparticular magnetic vectors: iron oxide nanoparticles, polymeric coating and anticancer drugs.
Principle, challenges and limits of magnetic drug targeting
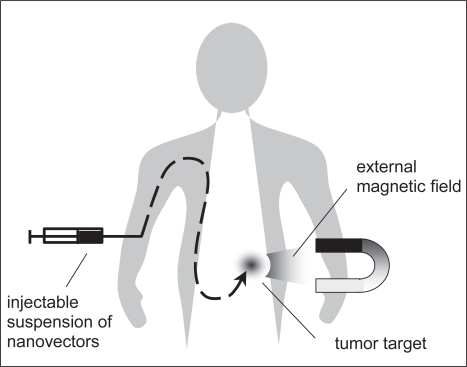
The principle of magnetic drug targeting is relatively simple: after intravascular injection (Figure 1), magnetic nanoparticles can be transported by the blood circulation and concentrated at the tumor with the aid of a magnetic field applied at the affected zone (Lübbe et al 2003).
Figure 1.
Principle of magnetic drug targeting.
Iron oxide particles with diameters below ~30–40 nm are of particular interest because they exhibit superparamagnetic behavior (Bean and Livingstone 1959). This means that once the magnetic field is removed, they do not retain any magnetization (no hysteresis). Superparamagnetic iron oxide nanoparticles are already used as contrast media in magnetic resonance imaging, and their low toxicity has been demonstrated for a long time (Neuberger et al 2005).
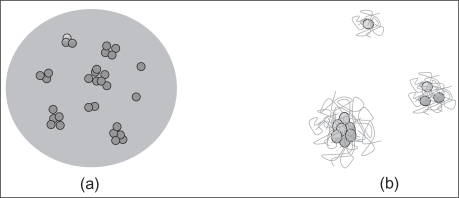
The iron oxide nanoparticles constitute the core of the final forms of therapeutic nanovectors. From a physicochemical point of view, the main objective in the preparation of magnetic nanoparticles consists in a strict control of particle size and colloid stability/dispersibility under physiological conditions. These properties can be modulated by coating the particles in two different ways: either the iron oxide nanoparticles are physically incorporated in a polymer matrix, or their surface is functionalized with polymer molecules (Figure 2). As for the drug, it can be dispersed in the polymer matrix, chemically bound to the polymer, or directly attached to the iron oxide surface.
Figure 2.
Schematic representation of the coating of iron oxide nanoparticles with polymers via encapsulation (a) or via surface treatment (b). In this latter case, a polymer layer may form around individual iron oxide particles or around aggregates.
After coating, the particle size is increased, but it still should remain in the sub-micron range. In this case, they will not block vessels and capillaries and thus avoid embolization (Alexiou et al 2005). Moreover, size and surface of resulting particles are determinant with respect to pharmacokinetics in vivo, where major limitations are quick blood clearance and non-specific uptake by macrophages. To maximize circulation times and targeting ability, the optimal size should be less than 100 nm in diameter and the surface should be hydrophilic (Bazile et al 1995; Storm et al 1995). Ideally, these properties should render the particles “furtive”, which means that they are not cleared by the reticulo-endothelial system. In addition to biocompatibility, the coating should regulate drug loading rates and release kinetics.
Once at the target site, the drug is released from the magnetic carrier creating a high local concentration in the tumor tissue while minimizing the amount of the drug throughout the rest of the body (Lübbe et al 2001). However, this implies that the magnetic field must be applied long enough for the drug delivery. The magnet must create a sufficiently strong magnetic field to retain nanoparticles at the desired site. The success of the method depends on the competitive forces between the external magnetic field and blood flow pressure in the arteries and capillaries. Another problem is the depth of the target site because the strength of the magnetic field decreases with distance. This implies that one can use this technique only in the case of solid tumors close to the surface of the body (Pulfer and Gallo 2000).
First clinical trials
Since 1996, Lübbe performed clinical trials for the treatment of breast cancer by magnetic carriers of epirubicin (Lübbe, Bergemann, Riess et al 1996). These trials followed pre-clinical studies (Lübbe, Bergemann, Huhnt et al 1996) that documented tolerance and efficacy. In the first trials, epirubicin was ionically bound to a modified carbohydrate layer on iron oxide nanoparticles. The authors clearly observed the accumulation of nanoparticles in the target area after exposure to the magnetic field. However, the drug release was rather dependent on variable physiological parameters (Lübbe et al 2001). Thus, technological improvements were necessary to make this treatment more effective. Nevertheless, the feasibility of the technique was demonstrated, and these results were encouraging for many researchers all over the world.
Synthesis of superparamagnetic iron oxide nanoparticles
Magnetite (Fe3O4) is a common magnetic oxide that has a cubic inverse spinel structure (Tron et al 2000). The most common method to prepare magnetite nanoparticles consists in mixing solutions of FeCl3 and FeCl2, followed by precipitation of magnetite by addition of a base (Massart 1981). Fe3O4 nanoparticles may then be superficially oxidized to the more stable maghemite (γ-Fe2O3) by addition of ferric nitrate to the colloidal suspension (Fauconnier et al 1999). Size, shape and composition of iron oxide nanoparticles depend on the operating conditions (pH, nature of the base, Fe2+ and Fe3+ ratio....). The content of magnetite and maghemite in these nanoparticles can be estimated by techniques such as Mössbauer spectroscopy (Muller et al 1999) and X-ray diffraction (Lottichi et al 1998). Recently, we have reported a possibility to deduce the magnetite/maghemite ratio in the solid phase of ferrofluids from Raman spectra (Chourpa et al 2005), using the ratio of their respective characteristic vibrational bands (671 cm−1/721 cm−1). It has also been observed that exposure to oxygen and elevated temperatures favored the formation in the ferrofluids of undesirable species like antiferromagnetic hematite.
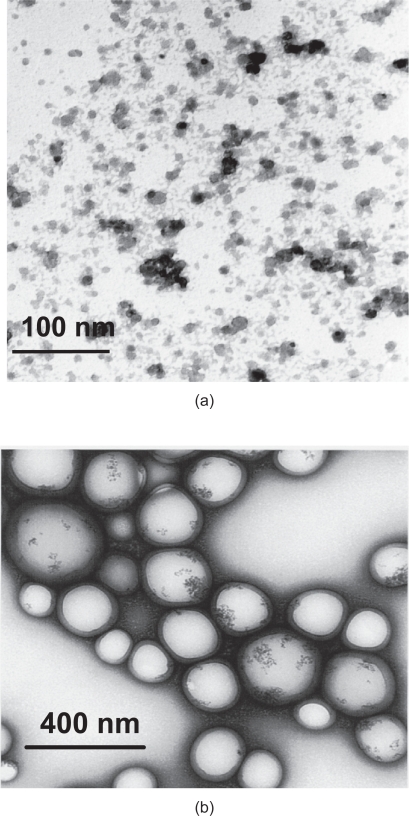
According to transmission electron microscopy (TEM) data (Figure 3a), the iron oxide nanocrystals are nearly spherical and their size is most often described in the literature to be within limits of 6–12 nm. As we mentioned above, the iron oxide nanoparticles constitute the nucleus of the final nanovector which will necessarily be much bigger.
Figure 3.
TEM photomicrograph of cationic iron oxide nanoparticles (a), TEM photomicrograph of iron oxide nanoparticles encapsulated in PLGA polymer (b). Both samples were prepared in our laboratory according to the methods described previously (Chourpa et al 2005; Ngaboni et al 2005).
Surface properties of iron oxide nanoparticles in colloidal suspensions
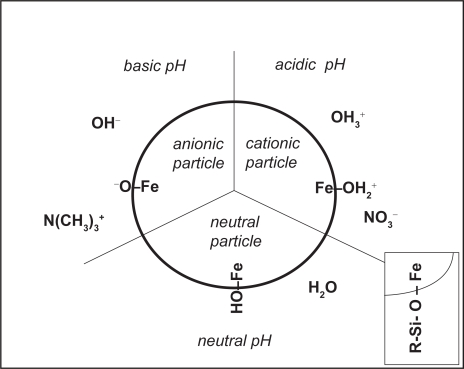
The iron oxide nanoparticles can be dispersed in suitable solvents to form homogenous suspensions called ferrofluids or magnetic fluids. They present a very interesting amphoteric character (Figure 4) due to the presence of hydroxyl groups at their surface (Fauconnier et al 1999). Because the stability of an aqueous ferrofluid is mainly due to electrostatic repulsion between charged particles, they flocculate around the point of zero charge (PZC, established around pH 7). Typically, the iron oxide nanoparticles are used as cationic ferrofluids, which means that the particle surface is positively charged and accompanied by anionic counterions (usually nitrates). A PZC close to physiological pH should not necessarily be considered an inconvenient, as the particles still have to be coated before injection. Thus, the iron oxide nanoparticle is just a precursor, and its surface properties need only be compatible with coating and drug loading.
Figure 4.
Diagram of surface properties of iron oxide nanoparticles. Insert: surface silanization.
As the nanoparticles have large surface area/volume ratios, they tend to adsorb plasma proteins and agglomerate in vivo. As a result, without coating the ferrite nanoparticles are rapidly cleared by macrophages in the reticulo-endothelial system.
As we noted above, one possibility of coating is to encapsulate the magnetic particles in a solid polymer matrix (Figures 2a and 3b). Alternatively, the hydroxyl groups at the surface of iron oxide nanoparticles can be used to directly anchor active molecules or polymers. To enrich the choice of possible reactions, one can use silanes (Figure 4 insert) as coupling intermediates : silanes form Fe-O-Si bonds with the particle surface and offer on their opposite end functional groups (amines, thiols) that can interact with therapeutical agents, biologically relevant ligands and polymers (Xu et al 1999).
Association of iron oxide nanoparticles with pharmaceutical polymers by encapsulation
In this case, the objective is to formulate multi-functional nanoparticular carrier systems with magnetic material in a solid polymer matrix (Häfeli 2004). An important challenge in the preparation of magnetic polymer particles, especially for drug targeting application, is that the magnetite content of the polymer particles should be large enough to permit magnetic guidance and delivery of these magnetic particles to the target site. Actually, it seems difficult to conclude about the minimum magnetic content required.
Among a wide variety of synthetic biodegradable polymers, only a few are biocompatible and commonly used to manufacture polymeric nanoparticles for medical applications. They include poly(alkylcyanoacrylates), poly(methylidene malonate), polyanhydrides, polyorthoesters and polyesters such as poly(lactic acid), poly(glycolic acid), poly(ε-caprolactone) and their copolymers (Barratt 2000).
Defined spherical magnetic microspheres were made for the first time at the end of the 1970s by Widder et al (1979). They engineered magnetic albumin microspheres containing doxorubicin at low doses and demonstrated their utilitity in an animal tumor model (Widder et al 1980, 1983). However, these albumin particles were generally not smaller than 1 μm which precludes intravenous administration. In addition, albumin has the disadvantage of possibly provoking an immune response, so interest has been progressively focused on magnetite particles covered with dextranes and/or synthetic polymers. Ibrahim described for the first time the preparation of polyalkylcyanoacrylate nanoparticles loaded with magnetite and an anticancer drug, dactinomycin (Ibrahim et al 1982). Magnetic polyalkylcyanoacrylate nanoparticles were prepared by anionic polymerization of the monomer (isobutylcyanoacrylate) in the presence of magnetite nanoparticles of between 10 and 50 nm. Final vectors included co-encapsulated dactinomycin drug and had a diameter smaller than 0.3 μm. These nanovectors responded to a magnetic field after intravenous administration in mice. A few years later, Hung prepared polymer-coated submicron magnetic nanoparticles carrying the anticancer agent methotrexate (Hung et al 1990) by in situ polymerization of polyglutaraldehyde. Methotrexate is used in the treatment of various solid tumors. Their results demonstrated that the magnetic nanoparticles contained about 8% (w/w) of Fe3O4 and the drug loading rates varied between 40 to 94 μg per mg of nanoparticles. In conclusion, the authors suppose that these magnetic nanoparticles may offer active drug targeting for cancer chemotherapy, but it should be necessary to increase the entrapment of magnetite to allow efficient retention of the carrier at the target site.
More recently, Arias reported a method to prepare colloidal composite particles consisting of a magnetite core and a biodegradable polymeric shell of poly(ethyl-2-cyanoacrylate) by anionic polymerization (Arias et al 2001). Despite the fact that the polyalkylcyanoacrylate family is considered of high interest as polymeric substrate in the realization of injectable nanoparticulate delivery systems (because of their mechanical properties, biodegradability and biocompatibility), their biodegradation products may be more toxic than those of other biodegradable/bio-compatible polymers.
In 1999, Zaitsev synthesized stable polymer coated magnetite nanoparticles by seed precipitation polymerization of methacrylic acid and hydroxyethyl methacrylate in the presence of nanophase magnetite (Zaitsev et al 1999). Deng described the preparation of magnetic polymeric particles via inverse microemulsion polymerization using acrylamide as water-soluble monomer (Deng et al 2003). They obtained water-dispersible magnetic polymeric nanoparticles with a size ranging from 80 to 180 nm. According to the authors, the magnetite concentrations in polymer particles were between 5% and 23% (w/w). However, the way in which magnetite content was determined from the values of saturation magnetization is not detailed.
Another polymer useful for encapsulation of magnetite particles is biodegradable poly(D,L-lactide) (PLA). This completely amorphous polymer is widely used in medical and pharmaceutical applications due to its very low toxicity and immunological response (Visscher et al 1985).
Müller described coating magnetite nanoparticles with polylactide and polylactide/glycolide (PLA and PLAGA) by introducing under stirring an ethanolic magnetite dispersion into the melted polymers (Müller et al 1996). After solidification, the mass was ground to reduce the particle size to 570 nm. The relatively low cytotoxicity of these magnetite-loaded PLA and PLAGA nanoparticles was demonstrated in vitro on human granulocytes.
Gomez-Lopera prepared PLA-magnetite composite nanoparticles using big magnetite crystals with a size of about 160 nm (Gomez-Lopera et al 2001). The average diameter of coated particles was 180 nm. It seems possible that in this case the coverage of magnetite by PLA was very thin and incomplete. Thereafter, much interest has been focused on the use of composite nanoparticles prepared with poly(D,L-lactide-co-glycolide) (PLGA) polymer. The degradation of this FDA-approved polymer could be controlled by molecular weight, crystallinity and the ratio of lactide to glycolide.
One of the technical limitations with hydrophobic polymers like PLA or PLGA is the difficulty to entrap high concentrations of hydrophilic ferrofluids. To circumvent this problem, our group has modified the surface of magnetite nanoparticles by adsorption of oleic acid before encapsulation in PLGA by a modified solvent emulsification/evaporation technique (Ngaboni Okassa et al 2005). This allowed the magnetite encapsulation efficiency to be increased up to 60%. Nevertheless, the magnetite loading was rather low – about 1% (w/w) – and should be increased. The composite nanoparticles were found to have a mean diameter within the range of 270–370 nm.
At the same time, Lee prepared magnetic poly(D,L-lactide-co-glycolide) nanoparticles by an emulsification-diffusion method (Lee et al 2005). Iron oxide nanoparticles (8–20 nm) were embedded in the PLGA matrix and the resulting iron oxide nanoparticles encapsulated have a size varying from 90 to 180 nm. Magnetic susceptibility of these nanoformulations increased as their size decreased. This was apparently related to the volume fraction of magnetic nanoparticles which increases when the size of the final particles decreases. Unfortunately, no indication was given on the iron oxide content.
Asmatulu reported model studies on magnetic control of magnetite nanoparticles embedded in poly(L-lactide), poly(D,L-lactide) and poly(ɛ-caprolactone) (Asmatulu et al 2005). The magnetic retention of composite nanoparticles in a flexible tube was measured as a function of various parameters such as magnetic saturation, magnet distance, fluid speed, particle size and solid content. All these parameters were proven to influence the capture of the magnetic particles by an external magnetic field but, unfortunately, no indication was given on the range of values needed to attain this objective in vivo.
Although biocompatible, PLA or PLGA coating is not furtive because of its hydrophobic surface. Therefore, to obtain furtive nanovectors, the PLGA particles need an additional surface treatment with hydrophilic polymers. This remark leads one to the necessity to develop protocols of chemical bonding of hydrophilic polymers to the nanoparticle surface. In fact, such bonding can also be applied directly to iron oxide nanoparticles.
Treatment of the iron oxide surface for enhanced furtivity and reduced agglomeration
PEG-modified iron oxide nanoparticles
To increase both the stability and furtivity of magnetic suspensions, iron oxide nanoparticles are often coated with hydrophilic polymers adsorbed or chemically attached to their surface. Polyethylene glycol (PEG) seems to be one of the most appropriate ones because of its unique properties such as hydrophilicity, flexibility, nontoxicity, and non immunogenicity.
Data from physico-chemical characterization indicate that coating with PEG efficiently reduces nanoparticle agglomeration (Gupta and Gupta 2005). A first study to show that polyethylene oxide exhibits low protein adsorption was performed by Jeon and Andrade (Jeon and Andrade 1991). Consecutively to this study, many authors tried to link such polymers to the surface of different kinds of nanoparticles with the hope to decrease their uptake by the mononuclear phagocyte system (Storm et al 1995).
Zhang et al (2002) immobilized silane-modified PEG on the surface of magnetite nanoparticles. A rather similar approach for coating PEG to magnetic nanoparticles was proposed by Butterworth et al (2001).
Using fluorimetry, confocal fluorescence microscopy and inductively coupled plasma emission spectroscopy (ICP), Zhang et al (2002) measured the uptake of their magnetic nanoformulations in breast cancer cells (BT20cells) and in mouse macrophage cells (RAW 264.7). The results of this study suggest that PEG coating of iron oxide nanoparticles limits protein adsorption on their surface and avoids their recognition by macrophages. Furthermore, PEG-modified surfaces facilitate incorporation of nanoparticles by cancer cells, probably due to a high affinity of the polymer to cellular membranes.
The main difficulty with using PEG for the formulation of nanovectors is that, except for the terminal (hydroxyl) group, this long-chain polymer lacks functional groups useful to attach therapeutic agents and/or specific ligands. Zhang circumvented this difficulty by introducing PEG modified with a trifluoroethylester-silane terminus (Kohler et al 2004). This modification allowed them to bind a folic acid ligand to PEG-treated magnetic nanoparticles. Since folate receptors are overexpressed on membranes of many cancer cells, the folic acid ligand should provide better access of nanovectors to tumors. In this way, the targeting efficacy should be additionally increased because the therapeutic vectors will be retained by both the recognition of a ligand and the magnetic field. As interesting as this strategy is, it seems to be less appropriate for grafting an anticancer agent, because it will provide limited drug loading rates.
Gupta also studied iron oxide nanoparticles modified with PEG and found them to be internalized within lysosomes of fibroblasts (Gupta and Curtis 2004). In a different way, Acar covered iron oxide nanoparticles with PEGylated polymers (Acar et al 2005). In that case, the coating consisted of two layers, the inner layer consisting of an ionic surfactant such as 10-undecenoic acid, and the outer layer made of PEG modified by esterification with the same surfactant. These particles were synthesized according to various modes of preparation and present really interesting sizes for a possible administration in vivo. Furthermore, this way of synthesis offers interesting perspectives for fixation of various water-soluble polymers.
Polysaccharide-modified iron oxide nanoparticles
As an alternative to PEG, natural polymers such as dextran, chitosan or starch can be attached to the surface of iron oxide nanoparticles in order to minimize the adsorption of proteins and to protect them from macrophages (Gupta and Gupta 2005). Dextran is a polymer of anhydroglucose having mainly alpha-D(1-6) linkages with side chains attached to the 3-positions of the backbone glucose units. A little more than twenty years ago, Molday and Mackenzie prepared iron oxide nanoparticles modified with dextran (Molday and Mackenzie 1982). They mixed a solution of ferrous chloride and ferric chloride with dextran under alkaline conditions. Similar preparations, where dextran was physically adsorbed, were then studied by various authors (Pardoe et al 2001; Bautista et al 2005). Recently, Xu et al (2005) contributed to optimize the protocol by their study of various operating conditions. The authors reported that reaction time and size of the polymer are the main parameters essential for the quality of preparations. The high biological tolerance (Lacava et al 2004) of these modified particles has been demonstrated and justified their extensive use as contrast agents for magnetic-resonance imaging (MRI).
Incorporation of anticancer therapeutic agents in magnetic nanoformulations
The challenges described in this section are among the more difficult and important to resolve. The association of drugs with nanovectors implies three main requirements. Firstly, the drug loading should be realistic to generate efficient therapeutic concentrations. Secondly, the drug-nanovector association should be reversible and have no consequences on biological activity of the therapeutical agent. Finally, the drug release profiles should be compatible with a reasonable duration of the treatment.
Encapsulation of the drug
The association could be realized by physical encapsulation of both drug and magnetic particles in a polymer matrix. In this case, the release of the drug will depend on the degradation or swelling of the polymer in the body. Although this approach is widely used to include active molecules in polymers (Puisieux et al 1994), only a few groups have combined these polymers simultaneously with drugs and magnetic materials (Ibrahim et al 1982; Hung et al 1990).
Chemical binding of the drug
Alternatively, the drug can be incorporated into magnetic nanoformulations through chemical bonds with the iron oxide surface or the polymer coating. Many authors described the conjugation of anticancerous agents with a polymer in the absence of iron oxides. The idea was to use macromolecules as carriers for anticancer drugs mainly to improve their biodistribution and resistance to degradation and thus prolong their activity (reviewed in Kopecek et al 2000). As above, this type of drug charged polymer could one day be applied to the concept of magnetic drug targeting.
Ionic binding of the drug to magnetic nanoparticles
As an example where the drug is attached to the polymer by ionic attraction, one can cite nanoparticles loaded with epirubicin anticancer drug via interaction of its amino sugar with the anionic group of a modified polymer (Bergemann et al 1999). This nanoformulation was used in the clinical trials described above (Lübbe, Bergemann, Riess et al 1996). Following the success of the first clinical trials, the authors continued to study conditions of the drug release, distribution and mechanism of action both in vivo and in vitro. On the basis of commercial iron oxide nanoparticles, the same group developed a novel nanoformulation covered with modified starch to which the therapeutic agent mitoxantrone was ionically bound (Alexiou et al 2001). Mitoxantrone is used in the treatment of a wide spectrum of tumors. To this end, the polymer must be initially functionalized by introducing for example anionic phosphate groups. An enormous advantage of this protocol is that it allows one to bind to the nanoparticles very different organic molecules, as long as they possess positively charged groups. Under physiologically relevant conditions in vitro, mitoxantrone was released from the magnetic nanoparticles in less than one hour. Biodistribution and activity in vivo of this nanoformulation was assessed via the detection of mitoxantrone and of the nanoparticles (labeled with 59Fe and 123I) in tumors and in neighboring zones and organs (Alexiou et al 2003). Suspensions of nanovectors were injected intra-arterially for treatment of VX2 squamous cell carcinoma in rabbits, with only 50% of the usual systemic dose (Alexiou et al 2005). A magnetic field was applied over the tumor for a duration of one hour. In comparison to control experiments without magnetic retention, the drug concentrations obtained in tumors under magnetic field were largely higher and could explain remissions observed in spite of the lower amount of drug administered.
Covalent binding of the drug to magnetic nanoparticles
As described above, the main inconvenient of ionic binding is that the drug release depends on physiological parameters. To prevent this, another strategy is to couple the drug via a covalent bond to the iron oxide surface or to the polymer coating. The more current coupling agent for covalent binding directly to the iron oxide surface is aminopropylsilane (Kobayashi and Matsunaga 1991; Xu et al 1997, 1999; Zhang et al 2002). Aminopropylsilane presents the advantage to possess a reactive primary amino group that reacts for example as well with an aldehyde group to form a Schiff base as with a carboxylic acid group to form a peptide bond. For many years, proteins have been extensively immobilized on inorganic supports such as magnetite via silane coupling agents (Weetall 1993). This methodology has then been applied to grafting drugs and/or polymers on iron oxide nanoparticles, one of the best examples being the study of Zhang (2002) described above.
Recently, Kohler bound methotrexate via this method (Kohler et al 2005). Methotrexate has structural similarity with folic acid. This similarity could be favorable to the internalization of methotrexate by cancer cells. Magnetic nanoforms of methotrexate reduced the viability of human cervical cancer cells (Hela cell line), as well as that of human breast cancer cells (MCF-7 cell line) in culture. Cellular uptake of the nanovectors in these cells was observed by TEM and quantified through measurement of iron concentrations by ICP. The data indicated that the vectors were accumulated into the lysosomes, where proteases should be able to cleave the amide bond between the drug and the magnetic particle. In fact, release of methotrexate in the presence of proteases was demonstrated in vitro (Kohler et al 2005). Internalization of the nanovectors and lysosomal release of the drug were presented as promising parameters for anticancer activity.
The necessity of internalization of the whole vector particles will depend on the general strategy adopted for the drug delivery. If the drug is covalently bound and needs specific enzymes or lower pH in lysosomes to trigger drug release, the whole particles have to enter the cell. Otherwise, simple diffusion through the polymer may be sufficient to desorb the drug from the particle. Thus, cellular uptake of magnetic nanovectors is not a necessary condition for their anticancer activity. Indeed, nanovectors could stimulate drug uptake by cancer cells by locally providing high extracellular concentrations of the drug and/or by direct action on the permeability of cellular membranes (Hong et al 2006). From this point of view, drug release from nanovectors in close vicinity of cancer cells could be sufficient to bring a real advance in efficacy of cancer chemotherapy.
Final remarks
Magnetic drug targeting is a novel drug delivery system that has been proven feasible resulting in an increase in local drug concentration and thus permitting a reduction of side effects. Nevertheless, the clinical trials highlight numerous problems still to be resolved. If the principle of magnetic drug targeting is simple, the development of magnetic vectors is complex. The critical parameters to optimize are size, magnetization, biocompatibility, and drug loading and release. Although for the time being no preparation satisfies all requirements simultaneously, most research groups succeed to produce biocompatible magnetic vectors with controllable sizes in the nanometer range. This is a source of optimism for a real possibility of magnetically targeted chemotherapy.
References
- Acar HY, Garaas RS, Syud F, et al. Superparamagnetic nanoparticles stabilized by polymerized PEGylated coatings. J Magn Magn Mater. 2005;293:1–7. [Google Scholar]
- Alexiou C, Arnold W, Hulin P, et al. Magnetic mitoxantrone nanoparticle detection by histology, X-ray and MRI after magnetic tumor targeting. J Magn Magn Mater. 2001;225:187–93. [Google Scholar]
- Alexiou C, Jurgons R, Schmid R, et al. Magnetic drug targeting – biodistribution of the magnetic carrier and the chemotherapeutic agent mitoxantrone after locoregional cancer treatment. J Drug Target. 2003;11:139–49. doi: 10.1080/1061186031000150791. [DOI] [PubMed] [Google Scholar]
- Alexiou C, Jurgons R, Schmid R, et al. In vitro and in vivo investigations of targeted chemotherapy with magnetic nanoparticles. J Magn Magn Mater. 2005;293:389–93. [Google Scholar]
- Arias JL, Gallardo V, Gomez-Lopera SA, et al. Synthesis and characterization of poly(ethyl-2-cyanoacrylate) nanoparticles with a magnetic core. J Control Release. 2001;77:309–21. doi: 10.1016/s0168-3659(01)00519-3. [DOI] [PubMed] [Google Scholar]
- Asmatulu R, Zalich MA, Claus RO, et al. Synthesis, characterization and targeting of biodegradable magnetic nanocomposite particles by external magnetic fields. J Magn Magn Mater. 2005;292:108–19. [Google Scholar]
- Baird RD, Kaye SB. Drug resistance reversal – are we getting closer? Eur J Cancer. 2003;39:2450–61. doi: 10.1016/s0959-8049(03)00619-1. [DOI] [PubMed] [Google Scholar]
- Barratt GM. Therapeutic applications of colloidal drug carriers. Pharm Sci Technol Today. 2000;3:163–71. doi: 10.1016/s1461-5347(00)00255-8. [DOI] [PubMed] [Google Scholar]
- Bautista MC, Bomati-Miguel O, del Puerto Morales M, et al. Surface characterisation of dextran-coated iron oxide nanoparticles prepared by laser pyrolysis and coprecipitation. J Magn Magn Mater. 2005;293:20–7. [Google Scholar]
- Bazile D, Prud’homme C, Bassoullet MT, et al. Stealth Me.PEG-PLA nanoparticles avoid uptake by the mononuclear phagocytes system. J Pharm Sci. 1995;84:493–8. doi: 10.1002/jps.2600840420. [DOI] [PubMed] [Google Scholar]
- Bean CP, Livingston JD. Superparamagnetism. J Appl Phys. 1959;30:120S–9S. [Google Scholar]
- Begent RHJ. Targeting cancer therapy. Brit J Cancer. 1999;80:104–9. doi: 10.1038/sj.bjc.6690328. [DOI] [PubMed] [Google Scholar]
- Bergemann C, Müller-Schulte D, Oster J, et al. Magnetic ion-exchange nano- and microparticles for medical, biochemical and molecular biological applications. J Magn Magn Mater. 1999;194:45–52. [Google Scholar]
- Butterworth MD, Illum L, Davis SS. Preparation of ultrafine silica- and PEG-coated magnetite particles. Colloid Surface A. 2001;179:93–102. [Google Scholar]
- Chourpa I, Douziech-Eyrolles L, Ngaboni-Okassa L, et al. Molecular composition of iron oxide nanoparticles, precursors for magnetic drug targeting, as characterized by confocal Raman microspectroscopy. Analyst. 2005;130:1395–403. doi: 10.1039/b419004a. [DOI] [PubMed] [Google Scholar]
- De Cuyper M, Joniau M. Magnetoliposomes. Eur Biophys J. 1988;15:311–9. doi: 10.1007/BF00256482. [DOI] [PubMed] [Google Scholar]
- Deng Y, Wang L, Yang W, et al. Preparation of magnetic polymeric particles via inverse microemulsion polymerization process. J Magn Magn Mater. 2003;257:69–78. [Google Scholar]
- Emerich DF, Thanos CG. Nanomedicine. Current Nanoscience. 2005;1:177–88. [Google Scholar]
- Fauconnier N, Bée A, Roger J, et al. Synthesis of aqueous magnetic liquids by surface complexation of maghemite nanoparticles. J Mol Liq. 1999;83:233–42. [Google Scholar]
- Gomez-Lopera SA, Plaza RC, Delgado AV. Synthesis and characterization of spherical magnetite/biodegradable polymer composite particles. J Colloid Interf Sci. 2001;240:40–7. doi: 10.1006/jcis.2001.7579. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Curtis ASG. Surface modified superparamagnetic nanoparticles for drug delivery:interactions studies with human fibroblasts in culture. J Mater Sci: materials in medicine. 2004;15:493–6. doi: 10.1023/b:jmsm.0000021126.32934.20. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Häfeli UO. Magnetically modulated therapeutic systems. Int J Pharm. 2004;277:19–24. doi: 10.1016/j.ijpharm.2003.03.002. [DOI] [PubMed] [Google Scholar]
- Hong S, Leroueil PR, Janus EK, et al. Interaction of polycationic polymers with supported lipid bilayers and cells: nanoscale hole formation and enhanced membrane permeability. Bioconjug Chem. 2006;17:728–34. doi: 10.1021/bc060077y. [DOI] [PubMed] [Google Scholar]
- Huang PS, Oliff A. Drug targeting strategies in cancer therapy. Curr Opin Genet Dev. 2001;11:104–10. doi: 10.1016/s0959-437x(00)00164-7. [DOI] [PubMed] [Google Scholar]
- Hung CT, McLeod AD, Gupta PK. Formulation and characterization of magnetic polyglutaraldehyde nanoparticles as carriers for poly-l-lysine-methotrexate. Drug Dev Ind Pharm. 1990;16:509–21. [Google Scholar]
- Ibrahim A, Couvreur P, Roland M, et al. New magnetic drug carrier. J Pharm Pharmacol. 1982;35:59–61. doi: 10.1111/j.2042-7158.1983.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Jeon SI, Andrade JD. Protein-surface interactions in the presence of polyethylene oxide. J Colloid Interf Sci. 1991;142:159–66. [Google Scholar]
- Kobayashi H, Matsunaga T. Amino-silane modified superparamagnetic particles with surface-immobilized enzyme. J Colloid Interf Sci. 1991;141:505–11. [Google Scholar]
- Kohler N, Fryxell GE, Zhang M. A bifunctional poly(ethyleneglycol) silane immobilized on metallic oxide-based nanoparticles for conjugation with cell targeting agents. J Am Chem Soc. 2004;126:7206–11. doi: 10.1021/ja049195r. [DOI] [PubMed] [Google Scholar]
- Kohler N, Sun C, Wang J, et al. Methotrexate-modified superpara-magnetic nanoparticles and their intracellular uptake into human cancer cells. Langmuir. 2005;21:8858–64. doi: 10.1021/la0503451. [DOI] [PubMed] [Google Scholar]
- Kopecek J, Kopeckova P, Minko T, et al. HPMA copolymer-anticancer drug conjugates:design, activity, and mechanism of action. Eur J Pharm Biopharm. 2000;50:61–81. doi: 10.1016/s0939-6411(00)00075-8. [DOI] [PubMed] [Google Scholar]
- Kreuter J. Nanoparticles as drug delivery systems. Encyclopedia of Nanosciences and Nanotechnology. 2004;7:161–80. [Google Scholar]
- Lacava LM, Garcia VAP, Kückelhaus S, et al. Long-term retention of dextran-coated magnetite nanoparticles in the liver and spleen. J Magn Magn Mater. 2004:272–276. 2434–5. [Google Scholar]
- Lee SJ, Jeong JR, Shin SC, et al. Magnetic enhancement of iron oxide nanoparticles encapsulated with poly(D,L-lactide-co-glycolide) Colloid Surf A: Physicochem Eng Aspects. 2005;255:19–25. [Google Scholar]
- Lottichi P, Barato C, Bersani D, et al. Fe2O3 films for χ(3) optics: Raman and XAS characterization. Opt Mater. 1998;9:368–72. [Google Scholar]
- Lübbe AS, Bergemann C, Huhnt W, et al. Preclinical experiences with magnetic drug targeting:tolerance and efficacy. Cancer Res. 1996;56:4694–701. [PubMed] [Google Scholar]
- Lübbe AS, Bergemann C, Riess H, et al. Clinical experiences with magnetic drug targeting:a phase I study with 4′-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res. 1996;56:4686–93. [PubMed] [Google Scholar]
- Lübbe AS, Alexiou C, Bergemann C. Clinical applications of magnetic drug targeting. J Surg Res. 2001;95:200–6. doi: 10.1006/jsre.2000.6030. [DOI] [PubMed] [Google Scholar]
- Lübbe AS, Bergemann C, Alexiou C. Targeting tumors with magnetic drugs. In: Pagé M, editor. Cancer drug discovery and development:tumor targeting in cancer therapy. Totowa NJ: Humana Press Inc; 2003. pp. 379–88. [Google Scholar]
- Maliksi L. Small is big business [online] 2006 Mercatornet, 03 March 2006. Accessed 19 June 2006. URL: http://www.mercatornet.com.
- Massart R. Preparation of aqueous magnetic liquids inalkaline and acidic media. IEEE Trans Magn. 1981;17:1247–8. [Google Scholar]
- Molday RS, Mackenzie D. Immunospecific ferromagnetic iron-dextran reagents for the labeling and magnetic separation of cells. J Immunol Methods. 1982;52:353–67. doi: 10.1016/0022-1759(82)90007-2. [DOI] [PubMed] [Google Scholar]
- Müller RH, Maaβen S, Weyhers H, et al. Cytotoxicity of magnetite-loaded polylactide, polylactide/glycolide particles and solid lipid nanoparticles. Int J Pharm. 1996;138:85–94. [Google Scholar]
- Muller JP, Gähde J, Mehner H, et al. Plasma-assisted transformation of iron surfaces into magnetite. Surf Coat Tech. 1999:116–19. 367–9. [Google Scholar]
- Neuberger T, Schöpf B, Hofmann H, et al. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J Magn Magn Mater. 2005;293:483–96. [Google Scholar]
- Ngaboni Okassa L, Marchais H, Douziech-Eyrolles L, et al. Development and characterization of sub-micron poly(D,L-lactide-co-glycolide) particles loaded with magnetite/maghemite nanoparticles. Int J Pharm. 2005;302:187–96. doi: 10.1016/j.ijpharm.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Pardoe H, Chua-anusorn W, St Pierre TG, et al. Structural and magnetic properties of nanoscale iron oxide particles synthesized in the presence of dextran or polyvinyl alcohol. J Magn Magn Mater. 2001;225:41–6. [Google Scholar]
- Puisieux F, Barratt G, Couarraze G, et al. Polymeric micro- and nanoparticles as drug carriers. In: Dumitriu S, editor. Polymeric Biomaterials. New-York: Marcel Dekke, Inc.; 1994. pp. 749–94. [Google Scholar]
- Pulfer SK, Gallo JM. Targeting tumors using magnetic drug delivery In Torrence PF ed Biomedical chemistry:applying chemical principles to the understanding and treatment of disease. John Wiley and Sons, Inc; 2000. pp. 211–25. [Google Scholar]
- Sachdeva MS. Drug targeting systems for cancer chemotherapy. Expert Opin Inv Drug. 1998;7:1849–64. doi: 10.1517/13543784.7.11.1849. [DOI] [PubMed] [Google Scholar]
- Storm G, Belliot SO, Daemen T, et al. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv Drug Deliver Rev. 1995;17:31–48. [Google Scholar]
- Tronc E, Ezzir A, Cherkaoui R, et al. Surface-related properties of γ-Fe2O3 nanoparticles. J Magn Magn Mater. 2000;221:63–79. [Google Scholar]
- Visscher GE, Robinson RL, Maulding HV, et al. Biodegradation and tissue reaction to 50:50 poly(D,L-lactide-co-glycolide) microcapsules. J Biomed Mater Res. 1985;19:349–65. doi: 10.1002/jbm.820190315. [DOI] [PubMed] [Google Scholar]
- Weetall HH. Preparation of immobilized proteins covalently coupled through silane coupling agents to inorganic supports. Appl Biochem Biotech. 1993;41:157–88. doi: 10.1007/BF02916421. [DOI] [PubMed] [Google Scholar]
- Whelan J, Mayenne F. Drug delivery:it’s a small world. Chem Ind. 2006:18–20. [Google Scholar]
- Widder KJ, Flouret G, Senyei AE. Magnetic microspheres: synthesis of a novel parenteral drug carrier. J Pharm Sci. 1979;68:79–82. doi: 10.1002/jps.2600680124. [DOI] [PubMed] [Google Scholar]
- Widder KJ, Senyei AE, Ranney D. In vitro release of biologically active adriamycin by magnetically responsive albumin microspheres. Cancer Res. 1980;40:3512–17. [PubMed] [Google Scholar]
- Widder KJ, Morris RM, Poore GA, et al. Selective targeting of magnetic albumin microspheres containing low-dose doxorubicin: total remission in Yoshida sarcoma-bearing rats. Eur J Cancer Clin Oncol. 1983;19:135–9. doi: 10.1016/0277-5379(83)90408-x. [DOI] [PubMed] [Google Scholar]
- Xu Z, Liu Q, Finch JA. Silanation and stability of 3-aminopropyl triethoxy silane on nanosized superparamagnetic particles:I. Direct silanation. Appl Surf Sci. 1997;120:269–78. [Google Scholar]
- Xu Z, Liu Q, Finch JA. Engineering of nanosize superparamagnetic particles for use in magnetic carrier technology. Surf Sci Series. 1999;78:31–50. [Google Scholar]
- Xu XQ, Shen H, Xu JR. Core-shell structure and magnetic properties of magnetite magnetic fluids stabilized with dextran. Appl Surf Sci. 2005;252:494–500. [Google Scholar]
- Zaitsev VS, Filimonov DS, Presnyakov IA, et al. Physical and chemical properties of magnetite and magnetite-polymer nanoparticles and their colloidal dispersions. J Colloid Interf Sci. 1999;212:49–57. doi: 10.1006/jcis.1998.5993. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kohler N, Zhang M. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials. 2002;23:1553–61. doi: 10.1016/s0142-9612(01)00267-8. [DOI] [PubMed] [Google Scholar]