Abstract
Within the family of nanomaterials, carbon nanotubes (CNTs) have emerged as a new efficient scaffold for studying molecular interactions at interfaces. Poor dispersability of CNTs in any solvent presents a considerable drawback for the development of novel functional composite structures. Previous studies have demonstrated that the solubility of CNTs can be greatly enhanced by employing appropriate surfactants, some of them being biological molecules. In this work, we study the noncovalent wrapping of lipid chains onto the graphitic surface of single-walled material (SWCNTs) by electron microscopy and Raman spectroscopy. Stable and homogenous aqueous suspensions of SWCNTs in the presence of lipids have been prepared, whereas their electrophoretic mobility was confirmed by ζ-potential measurements. Raman measurements revealed that smaller diameter SWCNTs are preferentially dispersed by lipid molecules in the aqueous supernatant part of the prepared suspension.
Keywords: carbon nanotubes, Raman spectroscopy, liposomes, ζ-potential, drug delivery systems
Introduction
CNTs are considered ideal material for several applications, ranging from ultra strong fibers to electronic devices components (Baughman et al 2002). They can be imaginatively produced by rolling up a single or multiple layers of graphene sheets. Several issues, however, must be addressed before these one-dimensional nanostructures can be integrated into functional hybrids for fabrication of advanced devices. Pristine material is completely insoluble in all solvents, which is a drawback for its manipulation (Wang and Hobbie 2003). The development of efficient methodologies for the chemical decoration of CNTs has opened new avenues of applications, eg, biosensors (Wang 2005). In most of the cases, two general chemical approaches are widely employed for modification of the graphitic cylinders (Tasis et al 2006). In the first strategy, the sidewalls of as-received material or the tips of acid-treated CNTs can be covalently modified by various grafting reactions (Sun et al 2002; Dyke and Tour 2004). These treatments, however, were shown to introduce a large number of defects to the graphitic network, leading to a major alteration of its properties. Alternatively, the noncovalent adsorption or wrapping of various functional molecules results in the formation of supramolecular complexes and the fabrication of innovative systems. The main advantage of the noncovalent approach is that it is not destructive for the conjugation of the graphitic lattice. Typical examples of chemical species that can adsorb onto hydrophobic surfaces are surfactants. Such kind of functionalities has the ability to suspend CNTs individually by imparting a distribution of charges onto the graphitic surface that prevents their re-aggregation (Islam et al 2003). The assessment of CNT suspension quality can be probed directly by sophisticated techniques such as Small Angle Neutron Scattering (Wang 2004; Fagan 2006).
Despite the significant progress of suspending CNTs in organic media with the aid of surfactant-type substances, the interest in applications that require water-soluble material is growing. Aqueous suspensions of the carbon material have important implications in fields such as biochemistry and biomedical engineering (Katz and Wilner 2004). Recently, it has been found that single-tailed phospholipids can readily form supramolecular complexes with CNTs and the resulting solubility is superior to that provided by other surfactants (Richard et al 2003; Wu et al 2006). Modified CNTs with double-chain lipids are of great importance for biological assays, where they can serve as transporters of various bio-molecules (Shi Kam et al 2005).
In the present study, we describe the self assembly of an asymmetric double-chain lipid with HiPCO SWCNTs in water. The stability of the aqueous dispersions of the tubular material in the presence of lipids is investigated by measuring their zeta potential. In addition, we demonstrate that lipid-modified SWCNTs with smaller diameters are preferably suspended in aqueous media. These composite structures could lead to the development of a new class of drug delivery platforms in the future.
Materials and methods
Materials
HiPCO SWCNTs were obtained from Carbon Nanotechnology Inc. (Houston, USA). The material was purified by microwave-induced oxidation of Fe nanoparticles and subsequent washing with a 37% wt HCl solution (Vazquez et al 2002). The asymmetric double-tailed lipid 1-palmi-toyl-2-oleoyl-sn-glycero-3-phosphocholine (egg-PC) was purchased from Sigma-Aldrich (Athens, Greece). All solutions were prepared by Millipore water (conductivity lower than 0.5 μS cm−1). In all experiments, the medium used was a 10 mM NaCl aqueous solution.
Preparation of stable dispersions
Dispersions of SWCNTs were prepared as following: 8 mg of carbon material was added into an aqueous solution of phospholipid (0.8% w/w). The concentration of the lipid was kept above its critical micelle concentration (CMC) value of 0.1% wt. The ionic strength was kept at 10 mM using NaCl. The dispersions were sonicated in a Branson bath-type sonicator for 4 hours. As controls, SWCNTs were dispersed in an aqueous medium containing 10 mM NaCl and treated as described previously.
Zeta potential measurements
The electrophoretic mobility of the SWCNTs samples was measured as a function of pH at 25 °C using a Zetasizer (Malvern Nanosizer ZS, Malvern Instruments, UK). The ζ-potential of the dispersions was calculated by the instrument according to the Helmholtz-Smoluchowski equation
where μ is the electrophoretic mobility, η is the viscosity and D is the dielectric constant of the medium in the boundary layer.
The pH values of the dispersions were measured by a combination electrode and adjusted with dropwise addition of 0.1 M NaOH or HCl solutions. An aliquot of the supernatant was placed to the cuvette and measured immediately. Each measurement repeated at least three times and the mean values were calculated.
Electron microscopy studies
Starting and lipid-modified SWCNTs were visualized by Scanning Electron Microscopy (Leo 1530 FESEM, Gemini). A buffered mixture of CNTs and lipid was sonicated for 1 min. The concentration of the lipid was above the critical micelle concentration (CMC) limit of 0.1% wt. The supernatant part of the suspension was casted on a mica surface, while the solvent was evaporated at room temperature. All specimens were sputtered with gold.
Raman spectroscopy studies
The carbon material suspended in the supernatant part of the lipid solution after a short time sonication was characterized by Raman spectroscopy, while unmodified tubes were considered as the reference sample. The spectra were recorded using a micro-Raman system equipped with a Peltier cooled charge coupled device (CCD) for light detection. A 514.5 nm excitation line of an argon laser was used, while the laser power was 4 mW measured directly before the sample.
Results and discussion
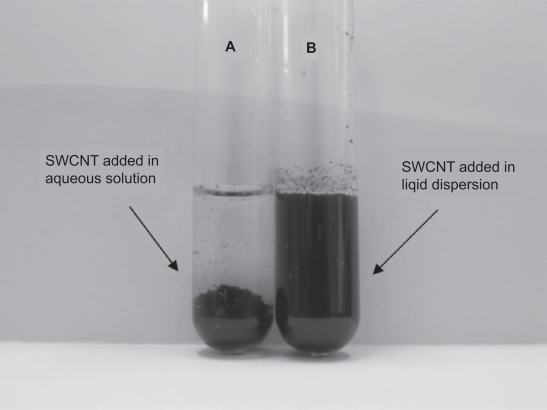
The suspension stability in aqueous solutions was studied by visual inspection over time. Stable dispersions of SWCNTs were obtained in the presence of the lipid (see Figure 1). On the contrary, unmodified tubes were precipitated within the first six hours after the sonication process. The stabilization effect of the lipid functionality could be attributed to electrostatic repulsions among the hydrophilic head group against van der Waals attractive forces between graphitic surfaces.
Figure 1.
(A) SWCNTs added in aqueous solution of 10 mM NaCl (B) SWCNTs added in an aqueous dispersion containing 0.8 w/v egg-PC.
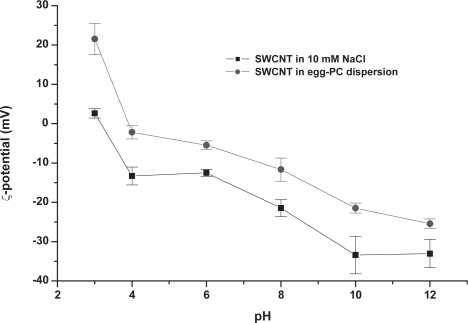
Zeta potential studies of the graphitic material as a function of pH in the presence and absence of egg-PC are presented in Figure 2. The isoelectric point (IEP) of unmodified material is approximately at pH 2. Generally, the surface charge values observed may be attributed to the purification protocol, which includes microwave-induced oxidation and introduces acidic functions at defect sites (Jiang et al 2003). The presence of zwitterionic egg-PC has induced a significant increase in absolute values of the zeta potential in the SWCNT dispersion. The zeta potential values enhancement implies that the PC molecules are bound to the CNT surface. The presence of egg-PC shifted the isoelectric point of the SWNT dispersion to 4.
Figure 2.
ζ-potential of SWCNTs in the presence and absence of egg-PC (0.8 w/v) as a function of pH, in an aqueous solution containing 10 mM NaCl.
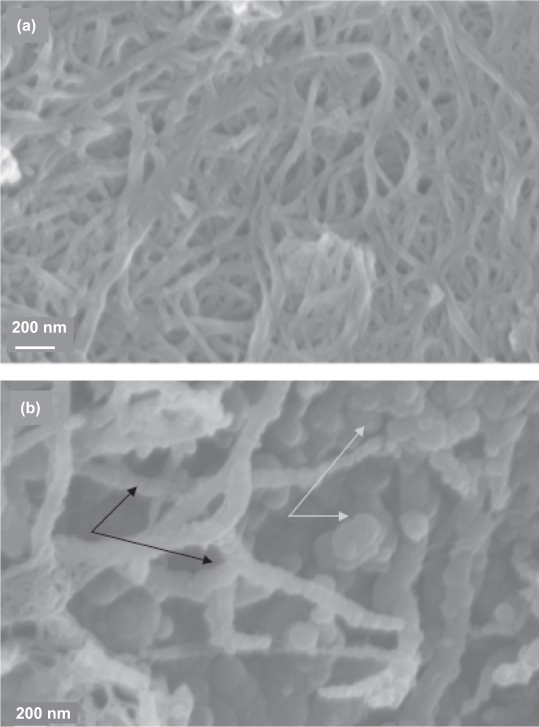
The chemical adsorption of lipid molecules on the surface of suspended SWCNTs can be envisioned by scanning electron microscopy (Figure 3). Figure 3A shows the image of starting SWCNTs added in an aqueous solution of NaCl. The tubes appear as large bundles due to their mutual van der Waals attractions. In the SWCNTs-lipid sample, a specific organization of lipid molecules on the graphitic surface can be clearly observed (Figure 3B, indicated by black arrows). This surface organization is responsible for the stabilized suspension of SWCNTs. Moreover, a significant population of vesicular superstructures appears as aggregates not interacting with the tubes (indicated with yellow arrows). Previous studies have shown that double-chain lipid bilayers can spontaneously assemble around a carbon nanotube template (Artyukhin et al 2005; Gagner et al 2006).
Figure 3.
Scanning Electron Micrographs of (a) SWCNTs added in an aqueous solution of 10 mM NaCl. (b) SWCNTs added in an aqueous dispersion containing 0.8 w/v egg-PC. Black arrows: lipid vesicles associated with CNTs. White arrows: lipid vesicles not interacting with CNTs. The scale bar of Figure 3b is similar to that in Figure 3a.
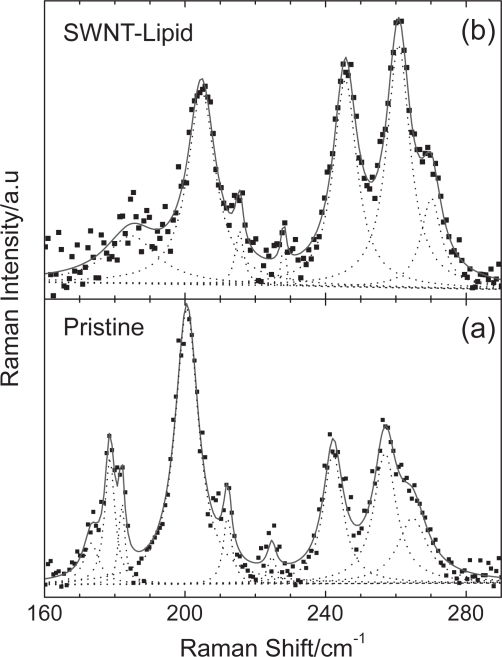
Raman spectroscopy is a valuable tool for the characterization of CNT-based composites. The strength of adherence of various functionalities to the surface of CNTs can be monitored by this spectroscopy. Figure 4 presents the Raman spectra of the starting and the lipid-coated samples in the radial breathing mode (RBM) frequency region, excited with the 514.5 nm laser line. In the spectrum of starting SWCNTs material, many RBM peaks are observed with the most intense ones located at 201, 242 and 257 cm−1. Using the well known relation ωRBM(cm−1) = (223.5/dt(nm)) + 12.5 (Bachilo et al 2002), the tube diameters dt can be estimated from the experimentally determined RBM frequency positions. Therefore, the diameters of the unmodified sample are in the range of 0.9–1.4 nm. However, it should be noted that due to the resonant nature of the Raman scattering in CNTs (Dresselhaus et al 2005), the use of additional laser lines is required to fully investigate the tube diameter distribution.
Figure 4.
Raman spectra in the RBM frequency region of (a) starting and (b) lipid-coated SWCNTs excited with 514.5 nm. Dashed lines indicate Lorentzian fits to the spectra.
As it is evident from Figure 4, significant changes occur in the Raman spectrum of lipid-coated sample compared to that of starting SWCNTs. More specifically, the RBM frequencies of the lipid-coated sample exhibit a blue shift of ∼4 cm−1. For example, the above mentioned strong bands of the starting material shift to 205, 245 and 261 cm−1, respectively. A similar change in RBM frequencies has been reported for CNTs coated with different peptides (Dieckmann 2003). Therefore, the presence of the functionality affects substantially the symmetric radial vibrations of the hollow cylinders. This concomitant mode stiffening can be attributed to the interactions between the CNTs and the wrapped lipid molecules. Furthermore, using a typical pressure slope value of ∼8 cm−1/GPa for the RBMs of SWCNTs obtained from high-pressure Raman experiments under hydrostatic conditions (Loa 2003), we can roughly estimate that SWCNTs experience a stress of ∼0.5 GPa after lipid adsorption.
On the other hand, a substantial Raman intensity attenuation of the lower (<230 cm−1) frequency RBM peaks relative to the higher ones is observed. Also, the radial band of the starting material extended from 160 to 190 cm−1 and comprising of three distinct peaks undergoes a dramatic intensity reduction and cannot be resolved clearly in the lipid-coated sample (Figure 4). These results lead to the possibility that the tube diameter distribution has been affected by the modification process and suggest that smaller diameters CNTs are preferentially suspended by lipid molecules (Yang 2006).
Conclusion
We showed that double-chain lipids can spontaneously wrap around SWCNT templates. By this noncovalent approach, stable suspensions of the carbon material can be prepared in aqueous media. In addition, we observed that smaller diameter nanotubes are preferentially dispersed by lipid molecules in the aqueous supernatant part. The biocompatibility of lipid membranes may have important implications to the development of lipid-coated CNTs as drug delivery systems in the future.
References
- Artyukhin AB, Shestakov A, Harper J, et al. Functional one-dimensional lipid bilayers on carbon nanotube templates. J Amer Chem Soc. 2005;127:7538–42. doi: 10.1021/ja043431g. [DOI] [PubMed] [Google Scholar]
- Bachilo SM, Strano MS, Kittrell C, et al. Structure-assigned optical spectra of single-walled carbon nanotubes. Science. 2002;298:2361–6. doi: 10.1126/science.1078727. [DOI] [PubMed] [Google Scholar]
- Baughman RH, Zakhidov AA, de Heer WA. Carbon nanotubes-the route toward applications. Science. 2002;297:787–92. doi: 10.1126/science.1060928. [DOI] [PubMed] [Google Scholar]
- Dieckmann GR, Dalton AB, Johnson PA, et al. Controlled assembly of carbon nanotubes by designed amphiphilic peptide helices. J Amer Chem Soc. 2003;125:1770–7. doi: 10.1021/ja029084x. [DOI] [PubMed] [Google Scholar]
- Dresselhaus MS, Dresselhaus G, Saito R, et al. Raman spectroscopy of carbon nanotubes. Physics Reports. 2005;409:47–99. [Google Scholar]
- Dyke CA, Tour JM. Covalent functionalization of single-walled carbon nanotubes for material applications. J Phys Chem A. 2004;108:11151–9. [Google Scholar]
- Fagan JA, Landi BJ, Mandelbaum I, et al. Comparative measures of single-wall carbon nanotube dispersion. J Phys Chem B. 2006;110:23801–5. doi: 10.1021/jp0647434. [DOI] [PubMed] [Google Scholar]
- Gagner J, Johnson H, Watkins E, et al. Carbon nanotube supported single phospholipid bilayer. Langmuir. 2006;22:10909–11. doi: 10.1021/la062038g. [DOI] [PubMed] [Google Scholar]
- Islam MF, Rojas E, Bergey DM, et al. High weight fraction surfactant solubilization of single-walled carbon nanotubes in water. Nano Lett. 2003;3:269–73. [Google Scholar]
- Jiang L, Gao L, Sun J. Production of aqueous colloidal dispersions of carbon nanotubes. J Coll Int Sci. 2003;260:89–94. doi: 10.1016/s0021-9797(02)00176-5. [DOI] [PubMed] [Google Scholar]
- Katz E, Wilner I. Biomolecule-functionalized carbon nanotubes: applications in nanobioelectronics. Chem Phys Chem. 2004;5:1084–104. doi: 10.1002/cphc.200400193. [DOI] [PubMed] [Google Scholar]
- Loa I. Raman spectroscopy on carbon nanotubes at high pressure. J Raman Spectr. 2003;34:611–27. [Google Scholar]
- Richard C, Balavoine F, Schultz P, et al. Supramolecular self assembly of lipid derivatives on carbon nanotubes. Science. 2003;300:775–8. doi: 10.1126/science.1080848. [DOI] [PubMed] [Google Scholar]
- Shi Kam NW, Liu Z, Dai H. Functionalization of carbon nanotubes via cleavable disulfide bonds for efficient intracellular delivery of siRNA and potent gene silencing. J Amer Chem Soc. 2005;127:12492–3. doi: 10.1021/ja053962k. [DOI] [PubMed] [Google Scholar]
- Sun YP, Fu K, Lin Y, et al. Functionalized carbon nanotubes: properties and applications. Acc Chem Res. 2002;35:1096–104. doi: 10.1021/ar010160v. [DOI] [PubMed] [Google Scholar]
- Tasis D, Tagmatarchis N, Bianco A, et al. Chemistry of carbon nanotubes. Chem Rev. 2006;106:1105–36. doi: 10.1021/cr050569o. [DOI] [PubMed] [Google Scholar]
- Vazquez E, Georgakilas V, Prato M. Microwave-assisted purification of HIPCO carbon nanotubes. Chem Commun. 2002;20:2308–9. doi: 10.1039/b207436b. [DOI] [PubMed] [Google Scholar]
- Wang H, Hobbie EK. Amphiphobic carbon nanotubes as macroemulsion surfactants. Langmuir. 2003;19:3091–3. [Google Scholar]
- Wang H, Zhou W, Ho DL, et al. Dispersing single-walled carbon nanotubes with surfactants: A small angle neutron scattering study. Nano Lett. 2004;4:1789–93. [Google Scholar]
- Wang J. Carbon nanotube-based electrochemical biosensors: a review. Electroanalysis. 2005;17:7–14. [Google Scholar]
- Wu Y, Hudson JS, Lu Q, et al. Coating single-walled carbon nanotubes with phospholipids. J Phys Chem B. 2006;110:2475–8. doi: 10.1021/jp057252c. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang SC, Mercier P, et al. Diameter-selective dispersion of single-walled carbon nanotubes using a water-soluble biocompatible polymer. Chem Commun. 2006:1425–7. doi: 10.1039/b515896f. [DOI] [PubMed] [Google Scholar]