Abstract
The studies were undertaken to evaluate feasibility of pulmonary delivery of liposomaly encapsulated tacrolimus dry powder inhaler for prolonged drug retention in lungs as rescue therapy to prevent refractory rejection of lungs after transplantation. Tacrolimus encapsulated liposomes were prepared by thin film evaporation technique and liposomal dispersion was passed through high pressure homogenizer. Tacrolimus nano-liposomes (NLs) were separated by centrifugation and characterized. NLs were dispersed in phosphate buffer saline (PBS) pH 7.4 containing different additives like lactose, sucrose, and trehalose, and L-leucine as antiadherent. The dispersion was spray dried and spray dried powders were characterized. In vitro and in vivo pulmonary deposition was performed using Andersen Cascade Impactor and intratracheal instillation in rats respectively. NLs were found to have average size of 140 nm, 96% ± 1.5% drug entrapment, and zeta potential of 1.107 mV. Trehalose based formulation was found to have low density, good flowability, particle size of 9.46 ± 0.8 μm, maximum fine particle fraction (FPF) of 71.1 ± 2.5%, mean mass aerodynamic diameter (MMAD) 2.2 ± 0.1 μm, and geometric standard deviation (GSD) 1.7 ± 0.2. Developed formulations were found to have in vitro prolonged drug release up to 18 hours, following Higuchi’s Controlled Release model. In vivo studies revealed maximal residence of tacrolimus within lungs of 24 hours, suggesting slow clearance from the lungs. The investigation provides a practical approach for direct delivery of tacrolimus encapsulated in NLs for controlled and prolonged retention at the site of action. It may play a promising role as rescue therapy in reducing the risk of acute rejection and chronic rejection.
Keywords: lung transplant rejection, tacrolimus, nano-liposomes, spray drying, dry powder inhaler
Introduction
Lung transplantation (LT) is a widely accepted technique for the treatment of end-stage pulmonary disease (Letsou et al 1999). Short-term outcome after LT has improved significantly over the last decade, but long-term survival remains limited by the development of bronchiolitis obliterans syndrome, which is believed to be a manifestation of chronic allograft rejection and occurs in 30% to 50% of patients at 2 years after LT (Boehler et al 2000; Hertz et al 2002). The acute rejection is the primary risk factor for bronchiolitis obliterans syndrome (Boehler et al 2000; Hertz et al 2002).
The cyclosporine is administered either orally or systemically in prevention and treatment of acute and chronic transplant rejection and found 1-year survival rates to reach 70% (Hosenpud et al 1996). Unfortunately, many patients still suffer from significant morbidity and mortality, primary graft failure, acute and chronic rejection, (Griffith et al 1994) and severe cardiovascular, hepatic, renal complications (Lubetkin et al 1996). Because the severity, frequency, and duration of acute rejection have been linked to the risk of chronic allograft rejection, controlling persistent or recurrent acute rejection is paramount (Horning et al 1998). However, long-term results are not optimal; improved methods of immunosuppression and new therapeutic agents are constantly under investigation for better control of the situation. In LT recipients, because of the high risk of LT rejection the optimal selection, dosage and delivery of immunosuppressants is critical (Reams et al 2002).
Tacrolimus a primary immunosuppressant found to be effective, potent, safe and superior than cyclosporine and showed promising results in reducing risk of acute rejection and obliterative bronchiolitis in LT (Griffith et al 1994; Onsager et al 1999; Treede et al 2001) and lower toxicity (Knoop et al 2005). LT recipients with refractory rejection or intolerance or resistant to conventional immunosuppressant, steroid and antibody-resistant rejection may respond to rescue therapy with tacrolimus (Mentzer et al 1998; Flynn et al 2001). Currently, there is no consensus among the lung transplant community regarding the optimal immunosuppressive regimen, but there is a trend towards more frequent use of the tacrolimus (Wagner et al 1997). The clinical utility of tacrolimus is hindered due to dose-related efficacy and toxicity, narrow therapeutic index, potential drug interactions, and large inter-/intra-patient variability in pharmacokinetics of available oral or parental formulations (Ihara et al 1995). To overcome these constraints various novel formulations have been developed, such as cyclodextrin complexes (Arima et al 2001), biodegradable microspheres (Wang et al 2004), liposomes (Cañadas 2004; Alemdar et al 2004) etc.
Another possibility for improving the clinical results in LT is the application of currently used medications via an aerosolized route (Waldrep 1998). Although aerosolized immunosuppressant formulations is not commercially available at present, the administration of aerosolized cyclosporine after LT improved survival and decreased chronic rejection due to local immunosuppressive effect with minimal systemic toxicities (Iacono et al 1996; Arppe et al 1998; Letsou et al 1999). Pulmonary administration has expanded the potential for more effective utilization with an array of potent and effective immunosuppressant in prevention of acute and chronic rejection in LT. The utilization of liposomes for aerosol delivery has many potential advantages, including universal carrier suitability for most lipophilic drugs, aqueous compatibility, sustained release or depot and intracellular delivery (Waldrep 1998). Among pulmonary drug delivery systems, dry powder inhaler formulations (DPIs) stand out because of the stability of drugs and formulations (Prime et al 1997). Liposomal drug DPIs have many promising features for pulmonary administration particularly with respect to the controlled delivery, increased potency, reduced toxicity, can uniformly deposit drugs locally, propellant free, patient compliance, high dose carrying capacity, stability, patent protection (Letsou et al 1999; Joshi and Misra 2001, 2003; Shah and Misra 2004; Lo et al 2004; Lu and Hickey 2005; Vermehren et al 2006).
To the best of our knowledge, this is the only literature explaining the use of nano-liposomal delivery of tacrolimus to provide prolonged drug release directly to the lungs. Therefore, the aim of this study was to encapsulate tacrolimus within NLs, incorporate NLs into DPIs by spray drying and to evaluate in vitro and in vivo performance of the developed DPIs for drug release, pulmonary deposition, and fate of drug in lungs. It was hypothesized that spray dried NLs DPIs will provide stable, high aerosolization efficiency to deep lungs, prolonged drug release, slow systemic dilution, and avoid macrophage uptake of encapsulated drug by carrier based delivery of nano-range liposomes. Hence, it is expected to provide synergistic combinations of localized and sustained action within the lungs to provide multisite immunosuppression; thereby may enhance acceptance of transplanted lungs by the patient and reduce associated systemic toxicities.
Materials and methods
Materials
Tacrolimus was received as a gift from Concord Biotech Ltd (Ahemedabad, India), Hydrogenated phosphatidylcholine (HSPC), and cholesterol was obtained from Lipoid (Lipoid GmBH, Ludwigshafen, Germany) and S. D. Fine chemicals (Vadodara, India) respectively. L-leucine was received as a gift from Alembic limited (Vadodara, India). Dialysis Bag (mol. cut off weight 10,000) was obtained from Sigma Chemical Co. (Milwaukee WI 53209, United States). Inhalator brev I. S. F. was provided by Panacea Biotec Ltd, Lalru, Punjab, India for this research studies. All chemicals were of an analytical grade or spectroscopic grade.
Preparation of tacrolimus loaded liposomes
Nanosize liposomes of tacrolimus were prepared by medication of thin film evaporation technique (New 1990). 42 mg tacrolimus, HSPC and cholesterol (8:2) was dissolved in a mixture of methanol and chloroform (2:1) which was subjected to dry thin film formation in Rotaevaporator using 500 ml quick fit round bottom flask at 100 rpm, for a time duration of half hr, under 250 mm Hg at 65 °C temperature. The liposomes were hydrated using 30 ml of PBS pH 7.4 for a period of 1 hr. The liposomal dispersion was passed through high-pressure homogenizer (Emulsiflex®-C5, Avestin Inc., Ottawa, Canada) pre-heated to 65 °C using thermostat for two cycles at 10,000 psi for size reduction. Resultant NLs were subjected to centrifugation at 30,000 rpm, 20 °C for 20 minutes using ultracentrifuge (Sigma Laboratory centrifuge, 3K 30, Osterode, GmBH). NLs pellets were separated and washed twice with 20 ml PBS pH 7.4 and centrifuged to separate NLs. The NLs pellets were separated and characterized for vesicle size, zeta potential and percent drug entrapment. Further, percent drug entrapment within NLs and un-entrapped drug content from supernatant were analyzed by HPLC method.
Spray drying of nano-liposomal dispersion
The NLs equivalent to 40 mg of tacrolimus were dispersed in 200 ml PBS containing 20 mg/ml additive (lactose/sucrose/ trehalose), and 12% of L-leucine based on the weight of the powder at room temperature. Similarly, 40 mg of plain TL was dissolved in 200 ml methanolic PBS containing lactose (20 mg/ml) and 12% of L-leucine. Dispersion were filtered (1 μm) prior to spray drying and immediately used. A laboratory spray-dryer (LSD-48, JISL, Mumbai, India) was used for spray drying. Feed was pumped into the drying chamber at a rate of 1.7 ml/min and pneumatically atomized through a 0.7 mm nozzle using aspiration at 100% and atomization air at 2.0 kg/cm2. The inlet temperature was set at 110 °C ± 5 °C with an outlet temperature of 60 °C to 65 °C. The resultant powder was blown through the cyclone separator and collected in a receiving vessel. After the spray process, the product was filled into vials in a moisture free atmosphere. The spray dried powders equivalent to 200 ± 40 μg were filled in Quali-V®, a hydroxypropyl methylcellulose (HPMC) capsule of size 2 (Shionogi Qualicaps, S.A., Spain). These capsules were prepared for in vitro aerosolization studies.
Assay
Tacrolimus was estimated by dissolving equivalent quantity of formulations containing 500 μg of TL in methanol and passed through a 0.22 μm syringe filter before sample injections. The drug content was determined by using a Dionex HPLC system (Dionex Soffron GmbH, Germany) with UV detection. The HPLC system was composed of a pump (P-680, Dionex), a simple 20-μl loop injector (Reodyne 7125) and a UV spectrophotometric detector (UVD 170U, Dionex). The separation was carried out on a C18 column (Thermo Electron Corporation, Bellefonate, PA, USA) maintained at 65 °C using column oven having 4.6 by 25 cm (internal diameter) and particle size of 10 μm. The mobile phase comprise of water: acetonitrile: methanol (40:30:30 v/v) at a flow rate of 0.9 ml/min. The wavelength of the detector was 210 nm. The data was analyzed by Chromleon 6.5 software. The calibration curve of tacrolimus was prepared in each assay in a concentration range from 0 to 50 μg/ml. The correlation coefficient was always more than 0.99, and a detection limit of quantification was 0.5 μg/ml. The concentration of tacrolimus below detection levels was determined by spiking test solution with standard solution.
Particle size and zeta potential measurement
The size of tacrolimus NLs was measured by dynamic light scattering with a Malvern Zetasizer 3000 HS (Malvern Instruments, Malvern, UK- Sun Pharmaceutical Advanced Research Centre, Vadodara, Gujarat, India). The zeta potential was calculated by Smoluchowski’s equation from the electrophoretic mobility of TL-loaded liposomes at 25 °C (Mu and Feng 2001). The particle sizes of spray dried formulations were assessed by dispersing them in isopropyl alcohol to achieve an obscuration between 10%–20% at a stirring speed of 1000 rpm based on laser diffraction using Malvern MasterSizer SM 2000K (Malvern Instruments Inc., UK). The measurements were recorded in triplicate. The results were for volume mean diameter (VMD), which related to the mass median diameter by the density of the particles. Span value is defined from the polydispersibility of powder.
Percent drug entrapment/retention
Percent drug entrapped was analyzed by solubilizing NLs in 0.1% Triton X-100 in methanol. The drug retained in spray dried formulation was determined after rehydration of powder equivalent to 200 ± 40 μg of TL in PBS and rehydrated NLs were separated by centrifugation at 35,000 rpm, 20 °C for 20 minutes. NLs were treated with 0.1% Triton X-100 in methanol and the resultant solutions were diluted with mobile phase for HPLC analysis.
Solid state characterization
Tapped density was evaluated by mechanically tapping a measuring cylinder containing 10 g of powder sample. After observing the initial volume, the cylinder was mechanically tapped, and volume reading was taken until little to no change in volume was observed. The plateau condition was obtained after 500 taps for all samples. The pile was carefully built up by dropping the material through a funnel till the tip of the funnel (height, 2 cm). The angle of repose was calculated by inversing tangentially the ratio of height and radius of the formed pile. The Carr’s compressibility index was calculated.
The residual water content of the formulations (50 mg) was determined using Automatic Karl-Fischer Titrator (Chemito CL 48885, Mercury Labs, Baroda). Commercially available pyridine free reagent was standardized with known quantity of water (250 mg).
Surface morphology and topographical features
The surface morphology was examined by scanning electron microscopy (SEM) (JSM-5610LV, JEOL, Japan). Powder samples were adhered to sample stubs using double sided tape, and then viewed using an accelerating voltage of 15 kilovolt at the magnification of 250X to 5000X. Image analysis software (Image Proplus 5.0, Media Cybernetics, USA) was used to assess surface texture and topographical features. Image analysis of the SEM pictures was conducted on a fixed area selected on the particle flat base in order to avoid tilting angle shadow effect. Various topographical parameters were also calculated as described below:
Roundness: Reports the roundness of each object, as determined by the following formula: (perimeter2)/(4 * Π * area).
Aspect ratio: Reports the ratio between the major axis and the minor axis of the ellipse equivalent to the object (ie, an ellipse with the same area, first and second degree moments), as determined by Major Axis/Minor Axis. Three dimensional surface plots, which describe the surface topography of particles, were drawn by scanning on the selected area of the image, an up and down, line showing the variability of gray level as a function of the position. Ten surface plots were drawn on each image and topographical features viz. fractal dimension, heterogeneity, and clumpiness; the descriptors of the texture of the surface were calculated and are defined as follows
Fractal dimension: Calculated as 1 minus the slope of the regression line obtained when plotting the log of the perimeter (using a particular stride) against the log of the stride length, as calculated with multiple starting points in the outline for the strides.
Heterogeneity: Reports the fraction of pixels that vary more than 10% from the average intensity of the object.
Clumpiness: Derived from heterogeneity measurement; the fractions of heterogeneous pixels remaining in an object after an erosion process. It reflects the object texture.
Differential scanning calorimetry
The thermal properties of formulations were analyzed using differential scanning calorimetry (Mettler DSC 20, Mettler Toledo, Switzerland- Panacea Biotec Ltd, Lalru, Punjab, India) and the thermograms were analyzed using Mettler Tolerdo Star system. An empty aluminum pan was used as the reference for all measurements. A sample (2–4 mg) of sample was placed in hermetically sealed aluminum pan and scanned at a rate of 10 °C/min from 0 °C to 250 °C.
In vitro release studies
In vitro release studies of developed DPIs and spray dried plain tacrolimus with lactose (STL) were evaluated in a customized and validated diffusion cell across cellophane membrane (10,000 MCO) for 18 hours using 50 ml of methanolic PBS as diffusion medium. The hydrodynamic characteristics of the diffusion cell were established using the benzoic acid disc method (Mojaverian et al 1996). The STL was dispersed in 1 ml of PBS, similarly developed DPIs of tacrolimus (equivalent to 15 doses; 200 μg × 15) dispersed in 1 ml of PBS. Formulations to be compared were separately transferred to the donor compartment and stirred at 50 rpm while the receptor compartment was stirred at 100 rpm. 1ml of the sample was withdrawn from the receptor compartment at definite time intervals and equivalent amount of fresh medium was replaced to the receptor compartment. Samples were evaluated for tacrolimus content using HPLC method. All experiments were carried out in triplicates. The curve of percent drug released Vs time was plotted to understand the release behavior. To examine the drug release kinetics and mechanism, the data were treated with following equations:
-
Percent Drug Diffused
The percent drug diffused was determined by the formula
Where,(1) - Cr
- Conc. of drug in receptor compartment.
- Vr
- Volume of the receptor compartment.
- Cd
- Conc. of drug in donor compartment.
- Vd
- Volume of donor compartment.
-
Kinetics of Release
The order of drug release was determined by performing regressions over the mean values of percent drug release Vs t and percent drug release Vs √t.
-
Mean Steady State Flux
The flux across the membrane was calculated using the following formula:
Where,(2) - J
- flux of the drug across the membrane.
- Vr
- Volume of receptor compartment.
- Rate of change of concentration.
Mean steady state flux is the mean of individual flux values at all sampling points.
-
Diffusion coefficient
The Diffusion coefficient of the drug at every sampling point was calculated using Equation no 3.
Where,(3) - h
- thickness of the membrane (0.02 cm)
- t
- time (sec)
- D
- Diffusion coefficient (cm2/ sec)
The diffusion coefficient used for the discussion is the mean of the value (D) obtained at each sampling point.
Characterization of aerosol performance
Aerodynamic particle sizing of developed formulations was assessed using an eight stage, nonviable Anderson Cascade Impactor with a preseparator (Graseby-Andersen, Atlanta, GA, USA) operating at an airflow rate of 28.3 lpm. The impaction plates were pre-coated with a 1.5% w/v of HPMC (4000 cps) gel in water to overcome particle bounce and re-entrainment phenomenon. A size ‘2’ Quali-V® capsule (Shionogi Qualicaps, S.A., Spain) was filled with powder equivalent to 200 ± 40 μg of tacrolimus and aerosolized using Inhalator brev I. S. F. Ten capsules were actuated for each impaction with each capsule for 10 secs. The drug content deposited in different parts such as induction port, preseparator, individual impaction plates, and powder remaining in capsule and inhaler device was rinsed with methanol. From drug deposition data the emitted dose, fine particle dose, FPF, MMAD, and GSD were calculated according to USP 27 NF 22.
Stability studies
Comparative stability studies was performed of the potential SLDPIT formulations at accelerated conditions (40 °C ± 2 °C, 75% ± 5% RH), intermediate storage (30 °C ± 2 °C, 65% ± 5% RH controlled room temperature (25 °C ± 2 °C) conditions as per ICH guidelines. SLDPIT formulations containing 200 ± 40 μg of tacrolimus was filled into HPMC capsule shells (Size “2”). These capsules were packed in HDPE bottles under nitrogen cover and the bottle was sealed with PVC coated aluminum foil. The bottles also contained silica bags as dehumactant and were resealed with flush of nitrogen after each sampling. Set of 50 capsules from a batch were filled in the HDPE bottles for each condition. The study was done with three batches of same composition. During sampling, one bottle containing 50 capsules was withdrawn at definite time interval, rehydrated with distilled water for 30 minutes. The SLDPIT formulations were also examined visually for the evidence of caking and discoloration. The content of the capsule are tested for assay, degradation, water content, PDR, emission and FPF.
Pulmonary pharmacokinetics
In vivo studies of promising DPIs were performed using intra-tracheal instillations technique as per earlier reports (Joshi and Misra 2003), six albino rats (220–240 g; obtained from a local supplier) were selected in each cohort for each time interval. Rats were housed in individual plastic cages at a constant temperature and allowed free access to water and rat chow and prior to experimentation rats were fasted for overnight. Rats of either sex were selected randomly and anaesthetized using urethane solution (1.2 gm/kg) by intraperitoneal administration. The trachea was exposed by blunt dissection of the sternohyoideus muscle and a small midline incision was made over the trachea between the fifth and sixth tracheal rings using a 20 guage needle followed by cannulated with PE200 tubing (5–7 cm) with the tip positioned approximately at the bifurcation of trachea. The PE50 (10–15 cm) tubing connected to a glass Hamilton syringe (Waters, Banglore, India) was inserted into the cannula up to bifurcation of the trachea. 200 μg of tacrolimus equivalent in STL or developed DPIs were administered. Animals that were to be killed at 3, 6, 9, 12, and 24 hours after administration had the cannula secured with sutures and the access cannula excised to leave a 1 cm protrusion. As control, Sham animals receiving PBS were included along with the experimental groups on the day of the experiment. BAL was performed on anaesthetized and recannulated animals with 12 ml PBS, prewarmed to 37 °C. To perform the lavage, the Hamilton syringe connected to the PE50 tubing was replaced with a three-way stopcock attached to two 20 ml syringes. Approximately 12 ml sterile (0.22 μm filtered) PBS was injected slowly in fractions to fill the lungs. The fluid was withdrawn by gentle aspiration; this BAL yielded between 7 – 11 ml liquid, which was centrifuged at 2000 rpm for 5 min. The supernatant was mixed with 1% Triton X-100 in a ratio of 9:1 and analyzed by HPLC to determine unreleased tacrolimus. The lungs and portions of the trachea below the instillation site were excised and homogenized in 10 ml PBS containing 1% Triton X-100. Deprotenization was performed with 10% sulphosalicylic acid and the tacrolimus released was analyzed in the supernatant after 10 times dilution with mobile phase by HPLC using sirolimus as an internal standard. The pulmonary pharmacokinetic parameters were determined using BAL and LH data. The mean pulmonary pharmacokinetic parameters were calculated on the basis of following definitions:
- Cmax
Maximum concentration of drug attained in lungs during the study ie, the drug concentration in the lungs is the drug estimated in LH.
- Tmax
The time point at which maximum drug concentration is attained in LH (ie, the time interval of Cmax).
- AUC24h0
The area under the curve of drug concentration in LH Vs time, over the period of study.
- T1/2
Pulmonary half-life of drug is calculated by, calculating the sum of the values of drug concentration in BAL and LH at individual sampling points, regressing the calculated sum over the entire duration of study and deriving the time point at which the sum of drug level is 50% compared to instilled quantity (ie, deriving the median of the regression line).
Statistical analysis: Each batch was prepared six times and data from all experiments were expressed as the mean ± standard deviation (S. D.). Data were compared using ANOVA and Student’s t-test and p < 0.05 was considered significant.
Result and discussions
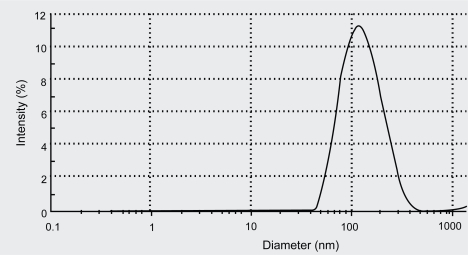
Liposomes were prepared using saturated phospholipids due to its physical and chemical stability compared to unsaturated soya phospholipids. The results of particle size distribution measurement are summarized in Table 1. Tacrolimus loaded NLs were found to have average size of 140.3 nm, span value of 0.47, and drug percent entrapment of 96% ± 1.5%. The particle size distribution pattern of NLs vesicles determined by Zetasizer was graphically represented in Figure 1.
Table 1.
Summary reports of particle size distribution, drug entrapment and drug retention of liposomal formulation (mean ± SD, n = 3)
| Formulations | Particle size VMD (μm) | Span | Drug retention (%) |
|---|---|---|---|
| TL NLs | 0.140 ± 0.02 | 0.47 ± 0.1 | —— |
| STL | 8.4 ± 1.1 | 1.6 ± 0.3 | 97 ± 0.4 |
| SLDPIL | 10.2 ± 0.7 | 1.3 ± 0.1 | 98 ± 1.3 |
| SLDPIT | 9.46 ± 0.8 | 1.4 ± 0.1 | 98 ± 1.1 |
| SLDPIS | 12.4 ± 0.9 | 1.5 ± 0.2 | 98 ±1.4 |
Figure 1.
Particle size distribution pattern of liposomal vesicles determined by Zetasizer.
The zeta potential of NLs was found to be 1.107 mV. Liposomal encapsulation of tacrolimus found to have enhanced immunosuppressant activity (Yang et al 2002). Nano-size range of liposome helps to achieve its uniform distribution in the bulk of dry powder formulations.
Characterization of spray dried NLs formulations
In development of novel DPIs, the spray drying technique offers a number of potential advantages over lyophilization technique (Elversson et al 2003; Bosquillon et al 2004; Nguyen et al 2004). Spray drying technique was utilized for stabilization of NLs and development of uniform particles size particles with desired properties for pulmonary administration to overcome constraints associated with lyophilization technique, such as formation of hard cake, need of micronization, addition of coarse carriers for aersolization, and heterogeneous size distribution pattern. Plain tacrolimus with lactose and L-leucine was also spray dried to compare and justify the performance of developed NLs DPIs. Spray drying parameters plays an important role on DPIs properties such as particle size, shape, topographical features, density, moisture content, and drug retention (Vanbever et al 1999; Elversson et al 2003). Hence, operating conditions were optimized for development of DPIs and operating parameters were kept constant during preparation of all DPIs of this investigation. Powder yield was observed in between 60% and 70%. Spray dried NLs with lactose (SLDPIL), spray dried NLs with trehalose (SLDPIT), and spray dried NLs with sucrose (SLDPIS) were found to have VMD of 10.2 ± 0.7 μm, 9.46 ± 0.8 μm, and 12.4 ± 0.9 μm respectively, whereas STL was found to have VMD of 8.4 ± 1.1 μm (Table 1).
Non significant effect (p < 0.05) of operational parameters was observed on particle size distribution of developed DPIs using different carriers. Above 98% of drug retention was found after spray drying (Table 1). Recently, researchers explored various novel techniques for development of aerodynamically light particles which resulted in maximum drug deposition in to the deep lung (Vanbever et al 1999; Steckel and Brandes 2004). Formulations having a tap density less than 0.4 g/cm3 and relatively large mean diameter between 5 μm to 30 μm, but pusses MMAD in the range of 1 μm to 5 μm. NLs DPIs developed in this investigation found to have particles size > 5 μm and a tap density less than 0.4 g/cm3 suggestive of formation aerodynamically light particles. Such DPIs were reported to be more capable of escaping inertial and gravitational deposition in the oropharyngeal region, and get targeted to the deep lung or airways (Vanbever et al 1999). The tapped density, flow properties and residual moisture content of developed formulations are summarized in Table 2. Trehalose based spray dried formulation were found to have the lowest density (0.15 ± 0.04 g/cc), good flowability (Angle of repose 24.9 ± 1.4°, Carr’s compressibility index 40.4 ± 1.4%), and low residual water content of 2.8 ± 0.3. Whereas, SLDPIL pusses density of 0.27 ± 0.07, angle of repose of 29.7 ± 1.6, Carr’s compressibility index of 36.7 ± 2.2, and residual water content of 4.2 ± 0.7, and SLDPIS pusses density of 0.33 ± 0.08, angle of repose of 31.6 ± 1.7, Carr’s compressibility index of 34.8 ± 2.3, and residual water content of 5.2 ± 0.7. Developed NLs DPIs were found to have low density, good flowability and low residual moisture content as is observed from Table 2.
Table 2.
Solid state characterization and residual water content of spray dried liposomal formulations (mean ± SD, n = 3)
| Formulations | Tapped density (g/cc) | Angle of repose (°) | Carr’s compressibility index | Residual water content (%) |
|---|---|---|---|---|
| STL | 0. 8 ± 0.2 | 43.1 ± 2.1 | 26.2 ± 1.8 | 5.6 ± 0.5 |
| SLDPIL | 0. 27 ± 0.07 | 29.7 ± 1.6 | 36.7 ± 2.2 | 4.2 ± 0.7 |
| SLDPIT | 0. 15 ± 0.04 | 24.9 ± 1.4 | 40.4 ± 1.4 | 2.8 ± 0.3 |
| SLDPIS | 0. 33 ± 0.08 | 31.6 ± 1.7 | 34.8 ± 2.3 | 5.2 ± 0.7 |
Phospholipids were component of pulmonary surfactants and were reported to facilitate droplet formation in the atomization step of spray drying, decrease particle surface energy, powder cohesiveness, and reduce residual water content (Steckel and Brandes 2004). Carriers formed the backbone structure of the solid particles during the spray-drying process and facilitate pulmonary delivery of NLs. An amino acid, L-leucine, was used as anti-adherent to prevent tendency of the particles to bond strongly and result into light particles with good flow behavior, deaggregation properties, and dose reproducibility of DPIs. Various techniques were used for preparing an aerodynamic light porous particle for enhancing FPF of therapeutics (Tsapis et al 2002; Steckel and Brandes 2004). In this investigation, an attempt was made to develop aerodynamically light particles of liposomal tacrolimus having a tap density below 0.4 g/cc, mean geometrical diameter above 5 μm and mean MMAD of the particles is between 2–3 μm. Formation of aerodynamically light particles may attribute to inclusion of NLs and L-leucine as antiadherent. Due to the hygroscopic behavior of sucrose, spray-drying with sucrose resulted into DPI with higher moisture content and larger particle size than DPIs with trehalose or lactose, this report is in consistence with earlier findings (Lo et al 2004).
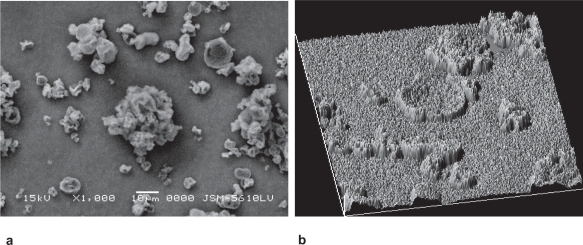
The SEM of SLDPIT was shown in Figure 2a and reveals smooth and porous surface of developed particles. Topographical features such as roundness, aspect ratio, fractal dimension, heterogeneity, clumpiness of developed formulations were derived from image analysis of SEM photographs by Image Proplus 5.0 and are shown in Table 3.
Figure 2.
(a) Scanning electron microphotograph and (b) surface texture analysis of trehalose based spray dried formulation.
Table 3.
Topographical features of developed liposomal formulations measured by SEM image analysis (mean ± SD, n = 10)
| Formulations | Roundness | Aspect ratio | Fractal dimension | Heterogeneity | Clumpiness |
|---|---|---|---|---|---|
| SLDPIL | 1.106 ± 0.076 | 1.356 ± 0.210 | 1.129 ± 0.064 | 0.492 ± 0.057 | 0.194 ± 0.057 |
| SLDPIT | 1.015 ± 0.065 | 1.163 ± 0.196 | 0.856 ± 0.052 | 0.351 ± 0.051 | 0.109 ± 0.047 |
| SLDPIS | 1.376 ± 0.092 | 1.453 ± 0.209 | 1.341 ± 0.064 | 0.674 ± 0.153 | 0.274 ± 0.095 |
The surface texture of SLDPIT obtained from SEM using Image Pro Plus was presented in Figure 2b. The surface fractal dimension, which represents degree of particle surface corrugation (Chew et al 2005) was determined from the texture of the images of powder surfaces and is recorded in Table 3. Figure 2b suggests varying degrees of surface roughness at different locations on surface of spray dried powder and porous nature of the particles.
The results indicate that a SLDPIT showed lower roughness compared to other formulations. Higher value of heterogeneity and clumpiness for SLDPIL and SLDPIS and also suggest high degree of roughness in contrast to SLDPIT. These variations were probably related to the composition and kinetics of particle formation.
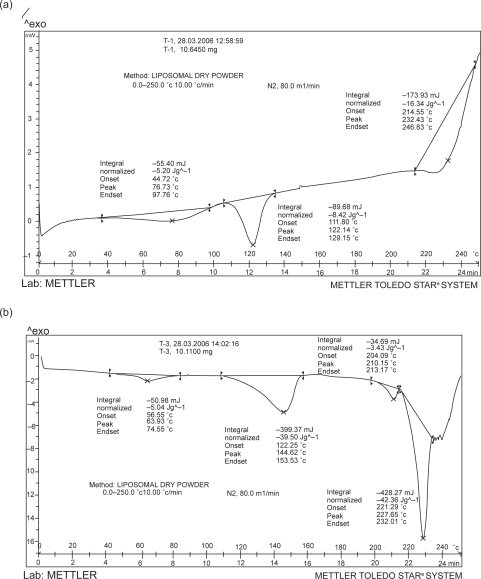
The results of DSC studies are represented in Figures 3a and 3b. The thermogram of tacrolimus showed a broad endotherm at 111.8 °C (Figure 3a), whereas, in case of physical mixture the tacrolimus showed sharp endoderm shifted to 122.25 °C and another endoderm at 221.15 °C due to additives in physical mixture (data not shown). Interestingly, the thermogram for the developed formulation showed absence of corresponding endotherm for tacrolimus and it is suggestive of encapsulation of tacrolimus in developed formulation (Figure 3b).
Figure 3.
Differential Scanning Calorimetric grams of developed plain tacrolimus (a) and SLDPIT (b).
In-vitro drug release studies
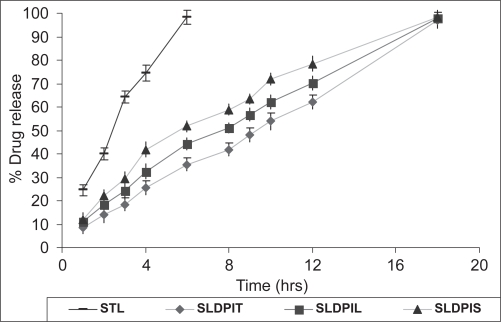
A comparison between in vitro release profiles of tacrolimus from different formulations was graphically represented in Figure 4. In vitro drug release studies showed 90% drug release within 6 hours form STL and 18 hours from SDPIS, SLDPIL, and SDPIT respectively. Statistically significant difference (p > 0.01) was observed in between in vitro release patterns of spray dried NLs DPIs and STL. The in vitro drug release parameters are tabulated in Table 4.
Figure 4.
In vitro release pattern of developed NLs DPIs and plain tacrolimus DPIs.
Table 4.
In vitro drug release parameters regression coefficient (r) of the line of percent drug released Vs square root of time, mean flux and diffusion coefficient of formulations
| Formulations | Regression coefficient (r2) | Mean Flux(μg/min) | Diffusion coefficient(cm2/sec) |
|---|---|---|---|
| STL | — | 42.83 | 1.32 E-03 |
| SLDPIL | 0.961 | 19.72 | 5.26 E-04 |
| SLDPIT | 0.957 | 15.05 | 4.32 E-04 |
| SLDPIS | 0.942 | 25.02 | 6.13 E-04 |
The regression coefficients (0.942–0.961) of the data of percent drug diffused Vs √t (Table 4) suggest a linear relationship between percent drug diffused and Vs √t confirming that the release follows Higuchi’s controlled release model and that the release rate is close to first order kinetics. The SLDPIT formulations showed more prolonged drug release compared to other formulations. Order of retardation of drug release from DPIs was found to be SLDPIT > SLDPIL > SLDPIS > STL. It suggests prolonged drug release from liposomaly encapsulated drug.
The mean flux values and diffusion coefficient of the STL were found to be higher compared to developed formulations. The mean flux values and diffusion coefficient of the STL was found to be 2.84 times and 3.05 times higher than those of SLD-PIT, 2.17 times and 2.5 times higher than those of SLDPIL, and 1.71 times and 2.15 times higher than those of SLDPIS respectively (Table 4). This is suggestive of prolonged drug release DPIs of TL may play a promising role in prevention of acute and chronic lung transplant rejection in lung transplanted patients.
Aerosol powder performances
In this investigation, we have used ‘size 2’ HPMC capsules instead of gelatin capsules due to its advantages over hard gelatin capsules such as unaffected mechanical strength at low level of moisture content and does not under go cross linking reaction at accelerated storage conditions. Table 5 illustrates aerosol powder performances of developed formulations assessed using in vitro lung model Andersen Cascade Impactor USP type II.
Table 5.
In-vitro aerosol deposition data of spray dried liposomal formulations (mean ± SD, n = 6)
| Formulations | Emitted dose (%) | Fine particle fraction (%) | Mean median aerodynamic diameter (μm) | Geometric standard deviation |
|---|---|---|---|---|
| SLDPIT | 82 ± 3.0 | 71.1 ± 2.5 | 2.2 ± 0.1 | 1.7 ± 0.2 |
| SLDPIS | 63 ± 5.5 | 53.7 ± 3.6 | 2.8 ± 0.2 | 2.3 ± 0.1 |
| SLDPIL | 74 ± 4 | 62.8 ± 3.1 | 2.6 ± 0.2 | 2.2 ± 0.1 |
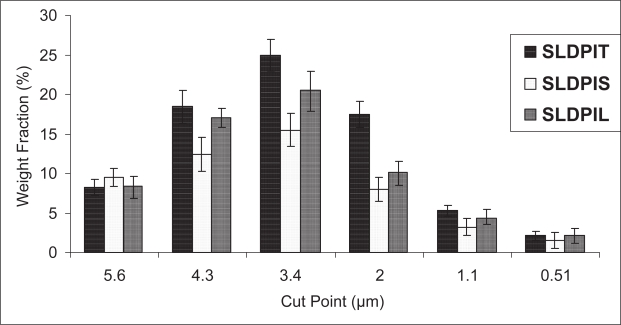
The efficiency of powder recovery from the cascade impaction test was found to be > 92%. The weight fraction according to the size distribution of the aerosolized particles from DPIs was graphically presented in Figure 5. Each bar represents the powder of certain sizes collected on a defined stage of the Andersen Cascade Impactor. The comparison of the data indicates that significant differences occurred on the aerodynamic diameter distribution among the different DPIs (p > 0.05). Shift in the deposition pattern of SLDPIT towards the lower stages of the impactor (stages 3–7) was also been noticed (Figure 5).
Figure 5.
In vitro pulmonary deposition pattern of different spray dried liposomal formulations. Each bar represents the average of six repeats and error bars refer to S. D.
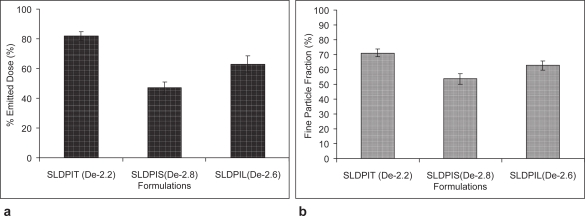
The results of emitted dose and FPF are compared in (Figures 6a and 6b). Maximum FPF of 71.1% ± 2.5% was observed with SLDPIT as compared to SLDPIS of 53.7% ± 3.6% and SLDPIL of 62.8% ± 3.1% (Table 5). The emitted dose percentage was ranged from 63% ± 5.5% for SLDPIS to 82% ± 3% for SLDPIT (Table 5 and Figure 6a). SLDPIT found to have 1.32 times and 1.13 times enhanced FPF compared to SLDPIS and SLDPIL respectively (Table 5 and Figure 6b). The mass median aerodynamic diameter and geometric standard deviation of SLDPIT are found to have 2.2 ± 0.1 and 1.7 ± 0.2 respectively (Table 5). Statistically significant change in the FPF was observed in the in vitro aerosolization performance.
Figure 6.
In vitro pulmonary deposition profile (a) emitted dose and (b) fine particle fraction of developed liposomal formulations (De – menan median aerodynamic diameter).
Results also revealed that SLDPIT exhibited the best aerosol powder performance among the DPIs prepared in this investigation in terms of emitted dose, MMAD, FPF, homogeneous size distribution. Promising aersolization results of SLDPIT may be contributing to formation of aerodynamic light and large particles, low density, good flow properties, and lower moisture content. We had selected promising SLDPIT and SLDPIT batches for in vivo studies and SLDPIT batch for stability studies.
Stability studies
The physical stability of liposomal formulations is one of the major obstacles in commercializing formulations. 1–2 years stability of liposomal formulations preferably at room temperature to be considered as pharmaceutically acceptable with high drug retention within liposome and non significant change in particle size during storage time, hence the drug leakage, particle size growth, in-vitro pulmonary deposition, and emission were studied at accelerated (40 °C 75% RH), intermediate (30 °C 65% RH) and controlled room temperature (CRT – 25 °C) conditions according to ICH guidelines for countries falling under zone III (hot, dry) and zone IV (very hot, humid). The results of stability studies are summarized in Table 6.
Table 6.
Stability data of developed SLDPIT formulation
| Stability conditions | Description | Assay (%) | Water content | Percent drug retained | Liposomal size(VMD) (μm) | Emission (%) | Fine Particle Fraction (FPF) |
|---|---|---|---|---|---|---|---|
| Initial | White free flowing powder | 101.16 ± 1.86 | 2.8 ± 0.3 | 101.38 ± 2.47 | 0.140 ± 0.02 | 82 ± 3.0 | 71.1 ± 2.5 |
| 40 ± 2 °C and 75 ± 5 % RH | |||||||
| 1M | White free flowing powder | 101.08 ± 3.24 | 2.7 ± 0.5 | 95.92 ± 3.51 | 0.145 ± 0.03 | 80.6 ± 2.2 | 70.45 ± 2.7 |
| 2M | White free flowing powder | 99.43 ± 2.97 | 3.3 ± 0.5 | 91.45 ± 3.95 | 0.152 ± 0.03 | 78.4 ± 3.0 | 67.13 ± 2.5 |
| 3M | White free flowing powder | 98.09 ± 3.95 | 3.4± 0.6 | 87.43 ± 3.91 | 0.158 ± 0.02 | 77.2 ± 2.9 | 62.91 ± 1.9 |
| 6M | White free flowing powder | 99.72 ± 3.54 | 3.0 ± 0.7 | 85.71± 3.98 | 0.161 ± 0.03 | 75.4 ± 2.6 | 60.11 ± 3.0 |
| 30 ± 2 °C and 65 ± 5% RH | |||||||
| 1M | White free flowing powder | 103.46± 2.72 | 2.8 ± 0.4 | 100.26 ± 3.29 | 0.144 ± 0.03 | 81.87 ± 3.7 | 70.93 ± 2.1 |
| 2M | White free flowing powder | 102.16 ± 2.91 | 2.9 ± 0.5 | 101.49 ± 3.96 | 0.138 ± 0.03 | 81.46 ± 3.4 | 71.08 ± 2.4 |
| 3M | White free flowing powder | 101.13 ± 2.58 | 2.9 ± 0.4 | 100.17 ± 3.21 | 0.148 ± 0.02 | 81.4 ± 3.9 | 70.84 ± 2.3 |
| 6M | White free flowing powder | 100.18 ± 3.1 | 3.0 ± 0.4 | 102.41 ± 4.23 | 0.151 ± 0.03 | 80.19 ± 3.2 | 68.42 ± 2.9 |
| 9M | White free flowing powder | 99.42 ± 2.9 | 2.9 ± 0.5 | 99.59 ± 3.91 | 0.153 ± 0.04 | 80.07 ± 3.0 | 67.82 ± 2.8 |
| 12M | White free flowing powder | 100.51 ± 3.59 | 2.9 ± 0.4 | 99.14 ± 4.1 | 0.152 ± 0.03 | 78.76 ± 3.5 | 65.19 ± 2.8 |
| 25 ± 2 °C and 60 ± 5% RH | |||||||
| 3M | White free flowing powder | 102.19 ± 2.53 | 2.8 ± 0.3 | 101.80 ± 2.95 | 0.138 ± 0.03 | 82.4 ± 2.6 | 71.20 ± 2.7 |
| 6M | White free flowing powder | 101.17 ± 3.5 | 2.8 ± 0.4 | 102.11 ± 3.44 | 0.143 ± 0.02 | 82.1 ± 3.1 | 70.12 ± 2.5 |
| 9M | White free flowing powder | 101.83 ± 3.92 | 2.9 ± 0.5 | 101.74 ± 4.28 | 0.148 ± 0.02 | 80.74 ± 2.7 | 68.17 ± 2.9 |
| 12M | White free flowing powder | 102.81 ± 4.59 | 2.9 ± 0.6 | 101.62 ± 4.38 | 0.143 ± 0.02 | 79.13 ± 3.4 | 68.28 ± 2.8 |
The percentage of tacrolimus remained entrapped for SLDPIT formulations after six months accelerated storage was found to be 85.71% and was below the acceptable level, hence as per the guideline recommendation one can not assign a shelf-life of 18 months. Hence, the product was tested on intermediate storage condition in order to assign a shelf-life at CRT. The product stability studies were conducted for one year at intermediate storage condition. The assay of formulations evaluated using HPLC method under intermediate storage conditions was found to be 97.35%, and at controlled room temperature storage was 99.46%. There was decrease in percent drug retention with the increase in the temperature of storage as evident from Table 6. The FPF of SLDPIT formulations results were observed to be in parallel to the results of liposomal size on rehydration ie, on accelerated storage the FPF found to decrease on prolonged storage (ANOVA: Single Factor p > 0.05). However the is no significant deference (p < 0.05) in FPF on intermediate and CRT storage was observed for a year (Table 6).
Pulmonary pharmacokinetics
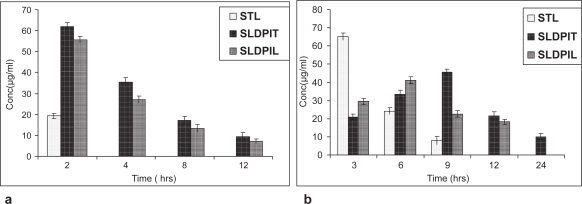
The animal experimental protocol was approved by the institutional animal ethical committee of The Maharaja Sayajirao University of Baroda, Vadodara, India. The in vivo studies were conducted in this investigation to test the possibility of obtaining prolonged localized actions of liposomal tacrolimus from spray dried NLs DPIs in lungs and the results are represented in Figures 7a and 7b. The quantity of tacrolimus estimated in the LH was considered as the drug absorbed and available for a pharmacological response. The quantity of tacrolimus present in the BAL was considered unabsorbed drug present in the lungs and still available for absorption. After intratracheal instillation of NLs DPIs, 35.47 ± 2.1% and 27.18 ± 1.63% of administered tacrolimus was recovered in BAL during the first 4 hours, eventually decreasing to 9.47 ± 1.76% and 7.09 ± 1.1 by 12 hours for SLDPIT and SLDPIL respectively; no tacrolimus was recovered after instillation of STL after 4 hours (Figures 7a and 7b). The mass balance of the tacrolimus between the percentage drug absorbed and percentage drug remaining entrapped within NLs ie, estimated in BAL was initially found to be close to 100%.
Figure 7.
Tacrolimus level in biological samples (a) Broncho alveolar lavage and (b) Lung homogenate following intratracheal instillation of developed formulations
The pulmonary pharmacokinetic parameters were calculated and are represented Table 7. On comparison of t1/2 values, a rank order increase was observed starting from STL< SLDPIL< SLDPIT. A maximum t1/2 value of 16 hours was observed with SLDPIT compared to 11 hours with SLPDIL and 3.9 hours with STL. A reverse relationship was found in the case of Cmax values. Eventually, there was an increase in AUC024h for NLs DPIs compared with the AUC0 24h of STL. SLDPIT and SLDPIL showed 1.8 times and 1.43 times higher AUC024h values than STL. The tmax values for NLs DPI were highest (SLDPIT-10 hours and SLDPIL-8 hours) compared to STL (2.1 hours), thereby confirming the maintenance of effective drug concentration with NLs DPIs in lung tissue for prolonged period compared to STL (Table 7).
Table 7.
Mean pulmonary pharmacokinetic parameters of liposomal DPIs comparative to plain drug DPIs
| DPIs | AUC024h (μg-h/ ml) | Cmax (μg) | tmax (hrs) | t1/2 (hrs) |
|---|---|---|---|---|
| STL | 827. 21 | 186.64 | 2.5 | 3.9 |
| SLDPIT | 1509.75 | 127.03 | 10 | 16 |
| SLDPIL | 1183.43 | 103.8 | 8 | 11 |
Thus, the plain tacrolimus was rapidly absorbed from lungs and then into the systemic circulation or rapidly metabolized, in contrast, the liposomal-encapsulated tacrolimus showed prolonged residence of 24 hours, this is in consistence with our earlier studies (Joshi and Misra 2003). Recovery of tacrolimus from lung tissue, after intratracheal instillation of NLs DPIs, increased with time until Cmax was achieved (Figures 7a and 7b). It was assumed that the amount of tacrolimus that could not be accounted during later stages may have been either metabolized or systemically absorbed or both.
Conclusion
A promising in vitro aerosol performance (FPF >70%) was observed for developed formulation of tacrolimus loaded NLs DPIs suggesting high deep lung deposition of tacrolimus and it was also found to prolonged drug release up to 18 hours in in vitro. The data of in vivo studies show drug residence up to 24 hours within the lungs and slow systemic dilution of tacrolimus. Prolonged and high drug residence within lungs after pulmonary administration of NLs DPIs is expected to provide prolonged local action and necessitating less frequent administration and/or dose of tacrolimus and eventually, reduced associated systemic toxicities. The investigation provides a practical approach for direct delivery of tacrolimus encapsulated in NLs for controlled and prolonged residence at the site of action. Hence, it may play a promising role as a rescue therapy in reducing the risk of acute rejection and chronic rejection after LT. However, superiority of tacrolimus NLs DPIs over conventional oral and parentral dosage forms can only be established after preclinical studies in two more animal species followed by extensive clinical trails.
Acknowledgments
The authors are thankful to Indian Council of Medical Research (ICMR), New Delhi, India for providing funding to the research project and Technology Information and Forecasting Council’s (TIFAC) Centre of Relevance and Excellence in New Drug Delivery System.
References
- Alemdar AY, Sadi D, McAlister VC, et al. Liposomal formulations of tacrolimus and rapamycin increase graft survival and fiber outgrowth of dopaminergic grafts. Cell Transplant. 2004;13:263–71. doi: 10.3727/000000004783983936. [DOI] [PubMed] [Google Scholar]
- Arima H, Yunomae K, Miyake K, et al. Comparative studies of the enhancing effects of cyclodextrins on the solubility and oral bioavailability of tacrolimus in rats. J Pharma Sci. 2001;90:690–701. doi: 10.1002/jps.1025. [DOI] [PubMed] [Google Scholar]
- Arppe J, Vidgren M, Waldrep JC. Pulmonary pharmacokinetics of cyclosporin A liposomes. Int J of Pharm. 1998;161:205–14. [Google Scholar]
- Boehler A, Estenne M. Obliterative bronchiolitis after lung transplantation. Curr Opin Pulm Med. 2000;6:133–9. doi: 10.1097/00063198-200003000-00009. [DOI] [PubMed] [Google Scholar]
- Bosquillon C, Rouxhet PG, TLimou F, et al. Aerosolization properties, surface composition and physical state of spray-dried protein powders. J Control Rel. 2004;99:357–67. doi: 10.1016/j.jconrel.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Cañadas O, Guerrero R, García-Cañero R, et al. Characterization of liposomal tacrolimus in lung surfactant-like phospholipids and evaluation of its immunosuppressive activity. Biochemistry. 2004;43:9926–38. doi: 10.1021/bi036227z. [DOI] [PubMed] [Google Scholar]
- Chew NYK, Tang P, Chan HK, et al. How much particle surface corrugation is sufficient to improve aerosol performance of powders? Pharm Res. 2005;22:148–52. doi: 10.1007/s11095-004-9020-4. [DOI] [PubMed] [Google Scholar]
- Elversson J, Millqvist-Fureby A, Alderborn G, et al. Droplet and particle size relationship and shell thickness of inhalable lactose particles during spray drying. J Pharm Sci. 2003;92:900–10. doi: 10.1002/jps.10352. [DOI] [PubMed] [Google Scholar]
- Flynn JT, Bunchman TE, Sherbotie JR. Indications, results, and complications of tacrolimus conversion in pediatric renal transplantation. Pediatr Transplant. 5:439–46. [PubMed] [Google Scholar]
- Griffith BP, Bando K, Hardesty RL, et al. A prospective randomized trial of FK506 versus cyclosporine after human pulmonary transplantation. Transplantation. 1994;57:848–51. doi: 10.1097/00007890-199403270-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz MI, Taylor DO, Trulock EP, et al. The registry of the international society for heart and lung transplantation: nineteenth official report. J Heart Lung Transplant. 2002;21:950–70. doi: 10.1016/s1053-2498(02)00498-9. [DOI] [PubMed] [Google Scholar]
- Horning NR, Lynch JP, Sundaresan SR, et al. Tacrolimus therapy for persistent or recurrent acute rejection after lung transplantation. J Heart Lung Transplant. 1998;17:761–7. [PubMed] [Google Scholar]
- Hosenpud JD, Novick RJ, Bennett LE, et al. The Registry of the International Society for Heart and Lung Transplantation: thirteenth official report – 1996. Heart Lung Transplant. 1996;15:655–74. [PubMed] [Google Scholar]
- Iacono AT, Keenan RJ, Duncan SR, et al. Aerosolized cyclosporine in lung recipients with refractory chronic rejection. Am J Respir Crit Care Med. 1996;153:1451–5. doi: 10.1164/ajrccm.153.4.8616581. [DOI] [PubMed] [Google Scholar]
- Ihara H, Shinkuma D, Ichikawa Y, et al. Intra- and interindividual variation in the pharmacokinetics of tacrolimus (FK506) in kidney transplant recipients: importance of trough level as a practical indicator. Int J Urol. 1995;2:151–5. doi: 10.1111/j.1442-2042.1995.tb00444.x. [DOI] [PubMed] [Google Scholar]
- Knoop C, Thirty P, Saint-Marcoux F, et al. Tacrolimus pharmacokinetics and dose monitoring after lung transplantation for cystic fibrosis and other conditions. Am J of Tranplantation. 2005;5:1477–82. doi: 10.1111/j.1600-6143.2005.00870.x. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Straubinger RM, Jusko WJ. Physicochemical, pharmacokinetic and pharmacodynamic evaluation of liposomal tacrolimus (FK 506) in rats. Pharm Res. 1995;12:1055–9. doi: 10.1023/a:1016222817860. [DOI] [PubMed] [Google Scholar]
- Letsou GV, Safi HJ, Reardon MJ, et al. Pharmacokinetics of liposomal aerosolized cyclosporine a for pulmonary immunosuppression. Ann Thorac Surg. 1999;68:2044–8. doi: 10.1016/s0003-4975(99)01183-2. [DOI] [PubMed] [Google Scholar]
- Lo Y, Tsai J, Kuo J. Liposomes and disaccharides as carriers in spray-dried powder formulations of superoxide dismutase. J Controlled Release. 2004;94:259–72. doi: 10.1016/j.jconrel.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Lu D, Hickey AJ. Liposomal dry powders as aerosols for pulmonary delivery of proteins. AAPS Pharm Sci Tech. 2005;6:E641–8. doi: 10.1208/pt060480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubetkin EI, Lipson DA, Palevsky HI, et al. GI complications after orthotopic lung transplantation. Am J Gastroenterol. 1996;91:2382–90. [PubMed] [Google Scholar]
- Mentzer RM, Jahania MS, Lasley RD. Tacrolimus as a rescue immunosuppressant after heart and lung transplantation. The US Multicenter FK506 study group. Transplantation. 1998;65:109–13. doi: 10.1097/00007890-199801150-00021. [DOI] [PubMed] [Google Scholar]
- Mojaverian J, Rosen WA, Vadino S, et al. In-vivo/in-vitro correlation of four extended release formulations of pseudoephedrine sulfate. J Pharm Biomed Anal. 1997;15:439–45. doi: 10.1016/s0731-7085(96)01834-1. [DOI] [PubMed] [Google Scholar]
- Mu L, Feng SS. Fabrication, characterization and in vitro release of paclitaxel (Taxol) loaded poly (lactic-co-glycolic acid) microspheres prepared by spray drying technique with lipid/cholesterol emulsifiers. J Control Release. 2001;76:239–54. doi: 10.1016/s0168-3659(01)00440-0. [DOI] [PubMed] [Google Scholar]
- New RRC. Preparation of liposomes In liposomes a practical approach edited by RRC New. Oxford University press; New York Tokyo: 1990. pp. 33–104. [Google Scholar]
- Nguyen XC, Herberger JD, Burke1 PA. Protein powders for encapsulation: a comparison of spray-freeze drying and spray drying of darbepoetin alfa. Pharm Res. 2004;21:507–14. doi: 10.1023/B:PHAM.0000019306.89420.f0. [DOI] [PubMed] [Google Scholar]
- Onsager DR, Canver CC, Jahania MS, et al. Efficacy of tacrolimus in the treatment of refractory rejection in heart and lung transplant recipients. The J of Heart and Lung Transplantation. 1999;18:448–55. doi: 10.1016/s1053-2498(99)00016-9. [DOI] [PubMed] [Google Scholar]
- Peters DH, Fitton A, Plosker GL, et al. Tacrolimus: a review of its pharmacology, and therapeutic potential in hepatic and renal transplantation. Drugs. 1993;46:746–96. doi: 10.2165/00003495-199346040-00009. [DOI] [PubMed] [Google Scholar]
- Reams BD, Palmer SM. Sublingual tacrolimus for immunosuppression in lung transplantation: a potentially important therapeutic option in cystic fibrosis. Am J of Res Med. 2002;1:91–8. doi: 10.1007/BF03256598. [DOI] [PubMed] [Google Scholar]
- Steckel H, Brandes HG. A novel spray-drying technique to produce low density particles for pulmonary delivery. Int J Pharm. 2004;278:187–95. doi: 10.1016/j.ijpharm.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Treede H, Klepetko W, Reichenspurner H, et al. Tacrolimus versus cyclosporine after lung transplantation: a prospective, open, randomized two-center trial comparing two different immunosuppressive protocols. The J of Heart and Lung Transplantation. 2001;20:511–17. doi: 10.1016/s1053-2498(01)00244-3. [DOI] [PubMed] [Google Scholar]
- Tsapis N, Bennet D, Jackson B, et al. Trojan particles: large porous carriers of nanoparticles for drug delivery. Proc Na Acad Sci. 2002;99:12001–5. doi: 10.1073/pnas.182233999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanbever R, Mintzes J, Wan J, et al. Formulation and physical characterization of large porous particles for inhalation. Pharm Res. 1999;16:1735–42. doi: 10.1023/a:1018910200420. [DOI] [PubMed] [Google Scholar]
- Vermehren C, Frokjaer S, Aurstad T, et al. Lung surfactant as a drug delivery system. Int J Pharm. 2006;307:89–92. doi: 10.1016/j.ijpharm.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Wagner K, Webber SA, Kurland G, et al. New-onset diabetes mellitus in pedriatic thorasic organ receipients receiving tacrolimus-based immunosupression. J Heart Lung Transplant. 1997;16:275–82. [PubMed] [Google Scholar]
- Waldrep JC. New aerosol drug delivery systems for the treatment of immune-mediated pulmonary diseases. Drugs Today. 1998;34:549–61. doi: 10.1358/dot.1998.34.6.485253. [DOI] [PubMed] [Google Scholar]
- Wang Q, Uno T, Miyamoto Y, et al. A biodegradable microsphere-loaded tacrolimus enhanced the effect on mice islet allograft and reduced the adverse effect on insulin secretion. Am J of Transplantation. 2004;4:721–6. doi: 10.1111/j.1600-6143.2004.00423.x. [DOI] [PubMed] [Google Scholar]
- Yang H, Mc-Alister VC, Al-Jazaeri A, et al. Liposomal encapsulation significantly enchances the immunosuppressive effect of tacrolimus in a discordant islet xenotransplant model. Transplantation. 2002;73:710–13. doi: 10.1097/00007890-200203150-00009. [DOI] [PubMed] [Google Scholar]