Abstract
Colorectal cancer remains one of the most prevalent cancer and a leading cause of cancer related death in the US. Many currently used chemotherapeutic agents are derived from botanicals. Identifying herbal sources, including those from ginseng family, to develop better anti-cancer therapies remains an essential step in advancing the treatment of the cancer. In this article, potential roles of ginseng herbs, especially American ginseng and notoginseng, in colorectal cancer therapeutics are presented. The major pharmacologically active constituents of ginsengs are ginsenosides, which can be mainly classified as protopanaxadiol and protopanaxatriol groups. Structure-activity relationship between their chemical structures and pharmacological activities are discussed. In addition, various steaming temperature and time treatment of the ginseng herbs can change ginsenoside profiles, and enhance their anti-cancer activities. This heat treatment process may increase the role of ginseng in treating colorectal cancer.
Keywords: Colorectal Cancer, Herbal Medicines, American Ginseng, Notoginseng, Ginsenosides, Rg3, Rh2, Protopanaxadiol
Introduction
Human colorectal cancer is a leading cause of cancer related death in the US, and the second most prevalent cancer worldwide. Half of all patients diagnosed with colorectal cancer eventually die from the disease, while only less than 10% of patients with metastatic colorectal cancer will survive more than 5 years after diagnosis (Goldberg et al., 2004; Jemal et al., 2008). Several controlled clinical trial data supported a multimodal and multidisciplinary approach, including combination of treatments and schedule in which they are administered, to treating both early and advance stage colorectal cancers (Goldberg et al., 2004, Hurwitz et al., 2004). Studies also showed that patients with cancer often resort to complementary and alternative medical means to treat cancer, cancer-related symptoms, and/or to reduce the adverse effects of chemotherapy (Ott, 2002; Lee et al., 2006; Wu et al., 2007).
There is compelling evidence that patients in this country resort to supplements or substitute them for conventional pharmacotherapy. Several national surveys indicate that at least one third of American adults take some form of dietary supplement, and botanicals comprise approximately 25% of the supplement market (Barnes et al., 2004). Botanicals have also been the major source of therapy in many traditional medical systems and have been used clinically for the treatment of a variety of diseases (Mashour et al., 1998; Xie et al., 2006; Wicks et al., 2007). Botanical ingredients in natural products contain bioactive constituents with medical benefits (Akerele, 1993; Leung, 2007; Zhou et al., 2007; Li and Zhang, 2008). Furthermore, botanicals have contributed significantly to cancer therapy, and it is likely that extracts and active constituents from herbal medicine will continue to play an important role in cancer therapeutics (Liu and Jiang, 2006; Ng et al., 2006a and b; Shieh et al., 2006; Ozaslan et al., 2007). In this article, we will discuss potential roles of ginseng herbs in the treatment of colorectal cancer.
Medicinal Use of Botanicals in Ginseng Family
Panax L. is a small genus of the family Araliaceae. Nearly all species in the genus Panax, such as Panax ginseng C. A. Meyer (Asian ginseng), Panax quinquefolius L. (American ginseng), and Panax notoginseng (Burk.) F. H. Chen (notoginseng), are important herbs used for different medical conditions (Chen et al., 2001; Wang et al., 2007c). Asian ginseng and notoginseng are considered as Chinese herbal medicines, and American ginseng is one of the most commonly used botanicals in the US (Wang et al., 1999; Ng, 2006a and b).
It is generally believed that the active compounds in Asian ginseng, American ginseng and notoginseng are triterpene glycosides or dammarane saponins, commonly referred to as ginseng saponins (ginsenosides and notoginsenosides). These ginseng saponins are the major active ingredients in the herb, and their levels can be used to develop quality controls for these herbs (Fuzzati, 2004; Chao et al., 2006; Wang et al., 2006a). There are over 50 different known ginseng saponins, and they are characterized by a 4 trans-ring rigid steroid aglycone skeleton and attached sugar moieties. Based on the aglycone skeleton, ginseng saponins can be divided into protopanaxadiol group and protopanaxatriol group, except for ginsenoside Ro, which is derived from oleanolic acid group (Fig. 1).
Figure 1.
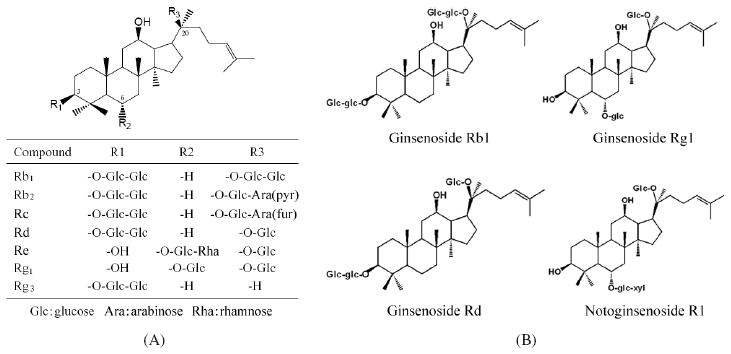
(A) Ginsenosides in American ginseng. (B) Saponins in notoginseng.
Ginseng has many reported health benefits (Attele et al., 1999; Liu et al., 2006a and b; Yamakage et al., 2006; Yoo et al., 2006). Regarding its anti-cancer effects, a case-control study on over a 1,000 subjects in Korea showed that Asian or Korean ginseng intakers had a decreased risk for many different cancers compared with nonintakers (Yun and Choi, 1995; Yun and Choi, 1998). It also suggested that ginseng has a non-organ specific preventive effect against cancer (Yun, 2003).
Regarding responsible anti-cancer constituents from Asian ginseng, published studies showed that some saponins could reduce proliferation of cancer cells and sensitize cancer cells to chemotherapeutic agents in vitro (Lee and Huemer, 1971; Kim et al., 2007; Koo et al., 2007). Several investigators found anti-tumor properties and other pharmacological activities of ginseng, and ginsenosides Rg3 and Rh2 are recognized as active anti-cancer saponins (Helms, 2004). Jia et al. (2004) noted that ginsenoside Rh2 inhibited proliferation and induced apoptosis in cancer cell lines, and sensitized drug-resistant breast cancer cells to paclitaxel. Kim et al. studied 11 ginsenosides and determined that Rg3 and Rh2 inhibited proliferation of prostate cancer cells (Kim et al., 2004). Iishi et al. (1997) used a rat model to determine the effects of ginsenoside Rg3 in inhibiting colon cancer cell proliferation.
American Ginseng
Ginseng root has been used for centuries in Oriental medicine as a panacea that promotes longevity (Attele et al., 1999; Fuzzati, 2004). However, relatively few studies focus on American ginseng, which is a popular herbal supplement in US consumers and patients (Attele et al., 1999; Helms, 2004).
American ginseng is an obligate shade perennial plant native to eastern North America. The commonly used part of the plant is the root, which is harvested after several years’ cultivation. The largest growing area in the US is in Wisconsin. The bioactive constituents of American ginseng are ginsenosides, which are present in the root, leaf, stem and berry of the plant. More than 30 ginsenosides such as Rb1, Rb2, Rc, Rd, Re, Rg1 and Rg3 have been identified (Wang et al., 1999; Assinewe et al., 2003; Wang et al., 2006b) in American ginseng (Fig. 1(A)). Several previous studies of American ginseng were focused on its activities on the cardiovascular system, such as anti-ischemic, antiarrhythmic and antihypertensive effects (Attele et al., 1999; Kim and Park, 2003). These pharmacological effects are, to a significant extent, considered to be linked to the antioxidant properties of the herb (Kitts et al., 2000; Wang et al., 2007c).
American ginseng extracts were found to inhibit the growth of breast cancer cells (Corbit et al., 2006). We previously investigated the effects of several herbal extracts on reducing chemotherapeutic side-effects and found that American ginseng can attenuate cisplatin-induced nausea and vomiting in a rat model, while not affecting its anti-cancer properties in human cancer cells (Mehendale et al., 2005; Aung et al., 2007). In addition, the extract from American ginseng enhanced the anti-proliferation effect of cisplatin on human breast cancer cells, suggesting that it possesses its own anti-cancer activity (Aung et al., 2007). Our group also showed that after steaming treatment of American ginseng, anti-proliferative effects were improved significantly possibly due to the altered ginsenoside profile (Wang et al., 2006c; Wang et al., 2007a).
Notoginseng
Notoginseng is a Chinese herbal medicine that has a long history of use in China and other Asian countries. This herb is distributed in the southwest of China, Burma, and Nepal. Notoginseng is cultivated commercially in the southwest of China, especially in Yunnan Province. The portion of the plant commonly used in remedies is the root, which is dug up after the fruit has ripened.
The earliest scientific description of notoginseng was in Materia Medica, a dictionary of Chinese herbs, written by Li Shi Zhen (1518–1593 AD). In Materia Medica, notoginseng was also called “more valuable than gold,” indicating the significance of this herb in traditional Chinese medicines. Notoginseng is regarded as the emperor herb in treatment of different types of wounds because it is the most commonly used medicine for both internal and external hemorrhage (Ng, 2006a and b; Wang et al., 2006a).
Modern pharmacological researches on notoginseng have found that notoginseng exerts various effects on the cardiovascular system, central nervous system, endocrine system, inflammation response (Sun et al., 2005; Ng, 2006a and b). In line with the hemostatic effect of notoginseng reported in ancient China, recent studies showed that the alcohol extract of notoginseng resulted in reducing bleeding time and provides better hemostatic effects than without treatment, placebo treatment, or treatment with hydrophilic or lipophilic extracts (White et al., 2001). Notoginseng can also decrease blood pressure, improve blood supply and protect against shock, and protect the cardiovascular system and brain vasculature. Its protective mechanism could be partly due to protection against damage by oxygen free radicals, and also by binding to the estrogen receptor, as ginsenosides sharing many of the protective actions of estrogen in various body systems. Pharmacokinetic and pharmacodynamic studies have shown that intranasal preparation of notoginseng saponins is a promising development and may be beneficial for the treatment of Alzheimer’s disease. Notoginseng extracts were also found to possess the capacity to adjust energy metabolism and treat diabetes (Ng, 2006a and b).
Some studies also showed that notoginseng has anti-tumor effects (Chenet al., 2001; Ng, 2006a and b). Recently, we found that notoginseng extract can increase the effects of cancer chemotherapy. Using HCT-116 human colorectal cancer cell line, the anti-proliferative effect of notoginseng extract combined with 5-FU was investigated. Compared with control, when cells were treated with 5-FU or notoginseng separately, cell proliferation was reduced by 31% and 25%, respectively. The combination of 5-FU and notoginseng reduced cell proliferation by 59%, suggesting that combining notoginseng with 5-FU can reduce the dose of 5-FU, while significantly increase the anti-proliferation effect on the cancer cells. Since it is well-known that 5-FU has cytotoxic effects on primary cells, this synergistic effect between notoginseng and 5-FU makes it possible to reduce the dose of 5-FU in combination with notoginseng and thereby further decrease dose-related toxicity (Wang et al., 2007b).
Notoginseng has a very distinct saponin profile compared to that of American ginseng (Chen et al., 2001; Sun et al., 2005). The main bioactive compounds in notoginseng are saponins, which are dammarane saponins. Oleanane-type saponin, present in Asian ginseng and American ginseng, is not found in notoginseng. To date, 56 saponins have been isolated from the notoginseng plant. Of these notoginseng saponins, 35 belong to protopanaxadiol group, while 21 belong to protopanaxatriol group (Wang et al., 2006a). Ginsenosides Rb1, Rg1, Rd and notoginsenoside R1 are the main saponins in notoginseng root (Fig. 1(B)).
Saponin Structure-Activity Observation and Heat-Treatment of Ginsengs
Ginseng saponins belong to a family of triterpene glycosides or triterpene saponins. Ginseng saponins (except ginsenoside Ro) possess the 4 trans-ring rigid steroid skeleton, with a modified side chain at C-20. Sugar residues are attached to the -OH of the aglycon. As mentioned above, ginsenosides can be mainly classified as protopanaxadiol and protopanaxatriol groups. For the protopanaxadiol group, sugar residues are attached to the β-OH at C-3 and another -OH at C-20 of the aglycon, e.g., ginsenosides Rb1, Rb2, Rc, Rd, Rg3 and Rh2. For protopanaxatriol group, sugar residues are attached to the α-OH at C-6 and another -OH at C-20 of the aglycon, e.g., ginsenosides Re, Rg1, Rh1 and notoginsenoside R1 (Fig. 1).
Structure-activity relationship elucidates the relations between chemical structure and their pharmacological activity for a series of compounds (Ooi et al., 2006; Benjamin et al., 2008). The anti-cancer activities of ginseng saponins are related with the type of aglycons and sugar residues (Helms, 2004; Wang et al., 2007d). The main anti-cancer saponins so far identified are from the protopanaxadiol group. The 3 most potent compounds in this group are Rg3, Rh2 and their aglycon, protopanaxadiol, and the latter 2 may have stronger effects (Popovich and Kitts, 2002; Wang et al., 2007d). Other compounds in the protopanaxadiol group showed less or no anti-cancer activities probably due to the fact that the sugar residues are attached to the -OH at C-20.
Ginsenoside Rg3 was isolated from Asian ginseng, American ginseng and notoginseng (Xu et al., 1987; Chen et al., 2002). However, Rg3 is only a trace saponin in different species of genus Panax (Fuzzati, 2004). The Rg3 can also be obtained from mild acidic hydrolysis of protopanaxadiol group saponins, such as Rb1, Rb2 and Rc (Fig. 2). Since Rg3 was found to effectively inhibit the growth of cancer cells (Mochizuki et al., 1995), studies of Rg3 sources were emphasized. In 2003, the Rg3 was approved as a new anti-cancer drug in China (Lu et al., 2008). Although this saponin can be obtained by biological transformation and chemical synthesis, the process is complicated, the yield is limited and thus, the cost of the product is high. As shown in Fig. 2, Rh2 and protopanaxadiol are also derived from the protopanaxadiol group saponins. In Asia, Asian ginseng root can be prepared as (1) air-dried to white ginseng, or (2) steamed at approximately 100°C to red ginseng. Compared with white Asian ginseng, red ginseng has stronger anti-cancer activities (Yun et al., 2001), due to relatively higher content of Rg3. It seems likely that the steaming process or heat-treatment of ginseng is a good approach to transform inactive ginsenosides to active anti-cancer compounds such as Rg3, Rh2 and protopanaxadiol.
Figure 2.
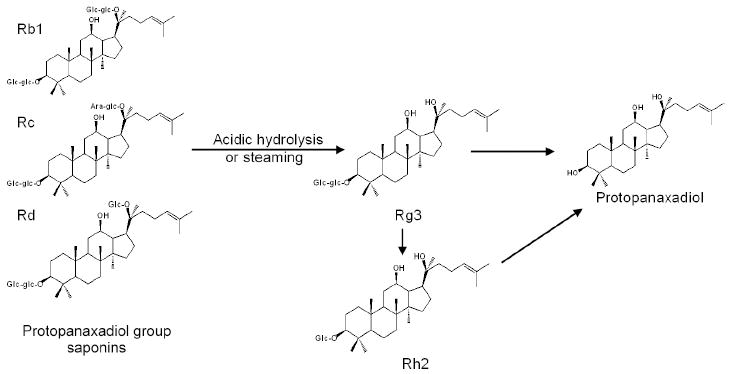
Chemical conversions starting from protopanaxadiol group saponins using acidic hydrolysis or steaming process.
Our laboratory treated American ginseng berry at various temperatures and heating time to observe the changes in ginsenoside content and anti-cancer activities on human colorectal cancer cells. We found that steamed American ginseng berry extract very significantly augmented the content of Rg3. When human colorectal cancer cells were treated with steamed berry extract (120°C, 2 hours), the anti-proliferation effects were 98% for HCT-116 and 99% for SW-480 cells. At the same treatment concentration, the effects of unsteamed extract were 34% for HCT-116 and 5% for SW-480 cells. This suggested that steamed American ginseng berry augmented Rg3 content and anti-cancer activity significantly (Wang et al., 2006c). We also steamed American ginseng root, with comparable change of the chemical constituent and anti-proliferative activities as that of steamed berry extract (Wang et al., 2007a).
Constituent changes of notoginseng after steaming treatment have also been reported (Lau et al., 2004). After the treatment, the content of Rb1, Rg1, Rd and notoginsenoside R1 decreased, while Rg3 had some increase, and the trend is similar to what we observed after the steaming treatment of American ginseng. Recently, we performed steaming treatment on notoginseng root. After the treatment, the content of Rg3 was found to be increased remarkably, and anti-proliferative effect on colorectal cancer cells was significantly increased (unpublished data).
Summary
Previous studies suggested that American ginseng and notoginseng possess anti-cancer activities. We recently observed that using a special heat-preparation or steaming process, the content of Rg3, a previously identified anti-cancer ginsenoside, increased significantly and became the main constituent in the steamed American ginseng. As expected, using the steamed extract, anti-cancer activity increased significantly. Notoginseng has a very distinct saponin profile compared to that of American ginseng. Steaming treatment of notoginseng also significantly increased anti-cancer effect.
It appears that the next logical step would be to characterize the effects of the two ginseng herbs (unsteamed and steamed) and their active constituents on colorectal cancer, and their mechanisms of action. Data obtained from future studies will help develop useful products for complementary and alternative therapies in oncology.
Acknowledgments
This work was supported in part by the NIH/NCCAM grant AT003255.
References
- Akerele O. Nature’s medicinal bounty: don’t throw it away. World Health Forum. 1993;14:390–395. [PubMed] [Google Scholar]
- Assinewe VA, Baum BR, Gagnon and D, Arnason JT. Phytochemistry of wild populations of Panax quinquefolius L. (North American ginseng) J Agric Food Chem. 2003;51:4549–4553. doi: 10.1021/jf030042h. [DOI] [PubMed] [Google Scholar]
- Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- Aung HH, Mehendale SR, Wang CZ, Xie JT, McEntee E, Yuan CS. Cisplatin’s tumoricidal effect on human breast carcinoma MCF-7 cells was not attenuated by American ginseng. Cancer Chemother Pharmacol. 2007;59:369–374. doi: 10.1007/s00280-006-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Adv Data. 2004;343:1–19. [PubMed] [Google Scholar]
- Benjamin M, Kaiser E, Milz S. Structure-function relationships in tendons: a review. J Anat. 2008;212:211–228. doi: 10.1111/j.1469-7580.2008.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Z, Shoyama Y, Tanaka H. Pharmacokinetic study of ginsenosides Rb1 and Rg1 in rat by ELISA using anti-ginsenosides Rb1 and Rg1 monoclonal antibodies. Am J Chin Med. 2006;34:1069–1081. doi: 10.1142/S0192415X06004533. [DOI] [PubMed] [Google Scholar]
- Chen FD, Wu MC, Wang HE, Hwang JJ, Hong CY, Huang YT, Yen SH, Ou YH. Sensitization of a tumor, but not normal tissue, to the cytotoxic effect of ionizing radiation using Panax notoginseng extract. Am J Chin Med. 2001;29:517–524. doi: 10.1142/S0192415X0100054X. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhan E, Chen H, Duan X, Guo L. Saponins with low sugar chain from the leaves of Panax notoginseng (Burk) In: Chen FH, editor. Zhong Yao Cai. Vol. 25. 2002. pp. 176–178. [PubMed] [Google Scholar]
- Corbit R, Ebbs S, King ML, Murphy LL. The influence of lead and arsenite on the inhibition of human breast cancer MCF-7 cell proliferation by American ginseng root (Panax quinquefolius L.) Life Sci. 2006;78:1336–1340. doi: 10.1016/j.lfs.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Fuzzati N. Analysis methods of ginsenosides. J Chromatogr B. 2004;812:119–133. doi: 10.1016/j.jchromb.2004.07.039. [DOI] [PubMed] [Google Scholar]
- Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004;9:259–274. [PubMed] [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- Iishi H, Tatsuta M, Baba M, Uehara H, Nakaizumi A, Shinkai K, Akedo H, Funai H, Ishiguro S, Kitagawa I. Inhibition by ginsenoside Rg3 of bombesin-enhanced peritoneal metastasis of intestinal adenocarcinomas induced by azoxymethane in Wistar rats. Clin Exp Metastasis. 1997;15:603–611. doi: 10.1023/a:1018491314066. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Jia WW, Bu X, Philips D, Yan H, Liu G, Chen X, Bush JA, Li G. Rh2, a compound extracted from ginseng, hypersensitizes multidrug-resistant tumor cells to chemotherapy. Can J Physiol Pharmacol. 2004;82:431–437. doi: 10.1139/y04-049. [DOI] [PubMed] [Google Scholar]
- Kim SH, Park KS. Effects of Panax ginseng extract on lipid metabolism in humans. Pharmacol Res. 2003;48:511–513. doi: 10.1016/s1043-6618(03)00189-0. [DOI] [PubMed] [Google Scholar]
- Kim HS, Lee EH, Ko SR, Choi KJ, Park JH, Im DS. Effects of ginsenosides Rg3 and Rh2 on the proliferation of prostate cancer cells. Arch Pharm Res. 2004;27:429–435. doi: 10.1007/BF02980085. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Jeong HJ, Yi BJ, Kang TH, An NH, Lee EH, Yang DC, Kim HM, Hong SH, Um JY. Transgenic Panax ginseng inhibits the production of TNF-alpha, IL-6, and IL-8 as well as COX-2 expression in human mast cells. Am J Chin Med. 2007;35:329–339. doi: 10.1142/S0192415X07004850. [DOI] [PubMed] [Google Scholar]
- Kitts DD, Wijewickreme AN, Hu C. Antioxidant properties of a North American ginseng extract. Mol Cell Biochem. 2000;203:1–10. doi: 10.1023/a:1007078414639. [DOI] [PubMed] [Google Scholar]
- Koo HN, Jeong HJ, Choi IY, An HJ, Moon PD, Kim SJ, Jee SY, Um JY, Hong SH, Shin SS, Yang DC, Seo YS, Kim HM. Mountain grown ginseng induces apoptosis in HL-60 cells and its mechanism have little relation with TNF-alpha production. Am J Chin Med. 2007;35:169–182. doi: 10.1142/S0192415X07004710. [DOI] [PubMed] [Google Scholar]
- Lau AJ, Seo BH, Woo SO, Koh HL. High-performance liquid chromatographic method with quantitative comparisons of whole chromatograms of raw and steamed Panax notoginseng. J Chromatogr A. 2004;1057:141–149. doi: 10.1016/j.chroma.2004.09.069. [DOI] [PubMed] [Google Scholar]
- Lee KD, Huemer RP. Antitumoral activity of Panax ginseng extracts. Jpn J Pharmacol. 1971;21:299–302. doi: 10.1254/jjp.21.299. [DOI] [PubMed] [Google Scholar]
- Lee TI, Chen HH, Yeh ML. Effects of chan-chuang qigong on improving symptom and psychological distress in chemotherapy patients. Am J Chin Med. 2006;34:37–46. doi: 10.1142/S0192415X06003618. [DOI] [PubMed] [Google Scholar]
- Leung PC. The efficacy of Chinese medicine for SARS: a review of Chinese publications after the crisis. Am J Chin Med. 2007;35:575–581. doi: 10.1142/S0192415X07005077. [DOI] [PubMed] [Google Scholar]
- Li XJ, Zhang HY. Western-medicine-validated anti-tumor agents and traditional Chinese medicine. Trends Mol Med. 2008;14:1–2. doi: 10.1016/j.molmed.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Liu R, Xing D, Lu H, Wu H, Du L. Pharmacokinetics of puerarin and ginsenoside Rg1 of CBN injection and the relation with platelet aggregation in rats. Am J Chin Med. 2006a;34:1037–1045. doi: 10.1142/S0192415X06004508. [DOI] [PubMed] [Google Scholar]
- Liu XS, Jiang J. Molecular mechanism of matrine-induced apoptosis in leukemia K562 cells. Am J Chin Med. 2006b;34:1095–1103. doi: 10.1142/S0192415X06004557. [DOI] [PubMed] [Google Scholar]
- Lu P, Su W, Miao ZH, Niu HR, Liu J, Hua QL. Effect and mechanism of ginsenoside Rg3 on postoperative life span of patients with non-small cell lung cancer. Chin J Integr Med. 2008;14:33–36. doi: 10.1007/s11655-007-9002-6. [DOI] [PubMed] [Google Scholar]
- Mashour NH, Lin GI, Frishman WH. Herbal medicine for the treatment of cardiovascular disease: clinical considerations. Arch Intern Med. 1998;158:2225–2234. doi: 10.1001/archinte.158.20.2225. [DOI] [PubMed] [Google Scholar]
- Mehendale S, Aung H, Wang A, Yin JJ, Wang CZ, Xie JT, Yuan CS. American ginseng berry extract and ginsenoside Re attenuate cisplatin-induced kaolin intake in rats. Cancer Chemother Pharmacol. 2005;56:63–69. doi: 10.1007/s00280-004-0956-1. [DOI] [PubMed] [Google Scholar]
- Mochizuki M, Yoo YC, Matsuzawa K, Sato K, Saiki I, Tono-oka S, Samukawa K, Azuma I. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of red ginseng. Biol Pharm Bull. 1995;18:1197–1202. doi: 10.1248/bpb.18.1197. [DOI] [PubMed] [Google Scholar]
- Ng LT, Chiang LC, Lin YT, Lin CC. Antiproliferative and apoptotic effects of tetrandrine on different human hepatoma cell lines. Am J Chin Med. 2006a;34:125–135. doi: 10.1142/S0192415X06003692. [DOI] [PubMed] [Google Scholar]
- Ng TB. Pharmacological activity of sanchi ginseng (Panax notoginseng) J Pharm Pharmacol. 2006b;58:1007–1019. doi: 10.1211/jpp.58.8.0001. [DOI] [PubMed] [Google Scholar]
- Ooi LS, Li Y, Kam SL, Wang H, Wong EY, Ooi VE. Antimicrobial activities of cinnamon oil and cinnamaldehyde from the Chinese medicinal herb Cinnamomum cassia Blume. Am J Chin Med. 2006;34:511–522. doi: 10.1142/S0192415X06004041. [DOI] [PubMed] [Google Scholar]
- Ott MJ. Complementary and alternative therapies in cancer symptom management. Cancer Pract. 2002;10:162–166. doi: 10.1046/j.1523-5394.2002.103004.x. [DOI] [PubMed] [Google Scholar]
- Ozaslan M, Didem Karagoz I, Kalender ME, Kilic IH, Sari I, Karagoz A. In vivo antitumoral effect of Plantago major L. extract on Balb/C mouse with Ehrlich ascites tumor. Am J Chin Med. 2007;35:841–851. doi: 10.1142/S0192415X07005314. [DOI] [PubMed] [Google Scholar]
- Popovich DG, Kitts DD. Structure-function relationship exists for ginsenosides in reducing cell proliferation and inducing apoptosis in the human leukemia (THP-1) cell line. Arch Biochem Biophys. 2002;406:1–8. doi: 10.1016/s0003-9861(02)00398-3. [DOI] [PubMed] [Google Scholar]
- Shieh DE, Cheng HY, Yen MH, Chiang LC, Lin CC. Baicalin-induced apoptosis is mediated by Bcl-2-dependent, but not p53-dependent, pathway in human leukemia cell lines. Am J Chin Med. 2006;34:245–261. doi: 10.1142/S0192415X06003801. [DOI] [PubMed] [Google Scholar]
- Sun H, Ye Y, Pan Y. Immunological-adjuvant saponins from the roots of Panax notoginseng. Chem Biodivers. 2005;2:510–515. doi: 10.1002/cbdv.200590032. [DOI] [PubMed] [Google Scholar]
- Wang CZ, McEntee E, Wicks S, Wu JA, Yuan CS. Phytochemical and analytical studies of Panax notoginseng (Burk.) In: Chen FH, editor. J Nat Med. Vol. 60. 2006a. pp. 97–106. [Google Scholar]
- Wang CZ, Wu JA, McEntee E, Yuan CS. Saponins composition in American ginseng leaf and berry assayed by high-performance liquid chromatography. J Agric Food Chem. 2006b;54:2261–2266. doi: 10.1021/jf052993w. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Zhang B, Song WX, Wang A, Ni M, Luo X, Aung HH, Xie JT, Tong R, He TC, Yuan CS. Steamed American ginseng berry: ginsenoside analyses and anticancer activities. J Agric Food Chem. 2006c;54:9936–9942. doi: 10.1021/jf062467k. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Aung HH, Ni M, Wu JA, Tong R, Wicks S, He TC, Yuan CS. Red American ginseng: ginsenoside constituents and antiproliferative activities of heat-processed Panax quinquefolius roots. Planta Med. 2007a;73:669–674. doi: 10.1055/s-2007-981524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Luo X, Zhang B, Song WX, Ni M, Mehendale S, Xie JT, Aung HH, He TC, Yuan CS. Notoginseng enhances anti-cancer effect of 5-fluorouracil on human colorectal cancer cells. Cancer Chemother Pharmacol. 2007b;60:69–79. doi: 10.1007/s00280-006-0350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Mehendale SR, Yuan CS. Commonly used antioxidant botanicals: active constituents and their potential role in cardiovascular illness. Am J Chin Med. 2007c;35:543–558. doi: 10.1142/S0192415X07005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhao Y, Rayburn ER, Hill DL, Wang H, Zhang R. In vitro anti-cancer activity and structure-activity relationships of natural products isolated from fruits of Panax ginseng. Cancer Chemother Pharmacol. 2007d;59:589–601. doi: 10.1007/s00280-006-0300-z. [DOI] [PubMed] [Google Scholar]
- Wang X, Sakuma T, Asafu-Adjaye E, Shiu GK. Determination of ginsenosides in plant extracts from Panax ginseng and Panax quinquefolius L. by LC/MS/MS. Anal Chem. 1999;71:1579–1584. doi: 10.1021/ac980890p. [DOI] [PubMed] [Google Scholar]
- White CM, Fan C, Song J, Tsikouris JP, Chow M. An evaluation of the hemostatic effects of hydrophilic, alcohol, and lipophilic extracts of notoginseng. Pharmacotherapy. 2001;21:773–777. doi: 10.1592/phco.21.9.773.34561. [DOI] [PubMed] [Google Scholar]
- Wicks SM, Tong R, Wang CZ, O’Connor M, Karrison T, Li S, Moss J, Yuan CS. Safety and tolerability of Ganoderma lucidum in healthy subjects: a double-blind randomized placebo-controlled trial. Am J Chin Med. 2007;35:407–414. doi: 10.1142/S0192415X07004928. [DOI] [PubMed] [Google Scholar]
- Wu H, Yang F, Cui S, Qin Y, Liu J, Zhang Y. Hematopoietic effect of fractions from the enzyme-digested colla corii asini on mice with 5-fluorouracil induced anemia. Am J Chin Med. 2007;35:853–866. doi: 10.1142/S0192415X07005326. [DOI] [PubMed] [Google Scholar]
- Xie JT, Chang WT, Wang CZ, Mehendale SR, Li J, Ambihaipahar R, Ambihaipahar U, Fong HH, Yuan CS. Curry leaf (Murraya koenigii Spreng.) reduces blood cholesterol and glucose levels in ob/ob mice. Am J Chin Med. 2006;34:279–284. doi: 10.1142/S0192415X06003825. [DOI] [PubMed] [Google Scholar]
- Xu SX, Chen YJ, Cai ZQ, Yao XS. Studies on the chemical constituents of Panax quinquefolius Linn. Yao Xue Xue Bao. 1987;22:750–755. [PubMed] [Google Scholar]
- Yamakage M, Hattori J, Satoh J, Namiki A. Effects of the Chinese herbal medicines Bupleuri radix, Ginseng radix, and Zingiberis rhizoma on lymphatic vessel activity in rats. Am J Chin Med. 2006;34:1063–1068. doi: 10.1142/S0192415X06004521. [DOI] [PubMed] [Google Scholar]
- Yoo HH, Yokozawa T, Satoh A, Kang KS, Kim HY. Effects of ginseng on the proliferation of human lung fibroblasts. Am J Chin Med. 2006;34:137–146. doi: 10.1142/S0192415X06003709. [DOI] [PubMed] [Google Scholar]
- Yun TK. Experimental and epidemiological evidence on non-organ specific cancer preventive effect of Korean ginseng and identification of active compounds. Mutat Res. 2003;523–524:63–74. doi: 10.1016/s0027-5107(02)00322-6. [DOI] [PubMed] [Google Scholar]
- Yun TK, Choi SY. Preventive effect of ginseng intake against various human cancers: a case-control study on 1987 pairs. Cancer Epidemiol Biomarkers Prev. 1995;4:401–408. [PubMed] [Google Scholar]
- Yun TK, Choi SY. Non-organ specific cancer prevention of ginseng: a prospective study in Korea. Int J Epidemiol. 1998;27:359–364. doi: 10.1093/ije/27.3.359. [DOI] [PubMed] [Google Scholar]
- Yun TK, Lee YS, Lee YH, Kim SI, Yun HY. Anticarcinogenic effect of Panax ginseng C.A. Meyer and identification of active compounds. J Korean Med Sci. 2001;16(Suppl):S6–S18. doi: 10.3346/jkms.2001.16.S.S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Lin J, Yin Y, Zhao J, Sun X, Tang K. Ganodermataceae: natural products and their related pharmacological functions. Am J Chin Med. 2007;35:559–574. doi: 10.1142/S0192415X07005065. [DOI] [PubMed] [Google Scholar]


