Abstract
Ritonavir, a protease inhibitor drug, is commonly used in AIDS therapy. As with other chemotherapeutic drugs that cause gastrointestinal adverse effects, ritonavir treatment is associated with significant nausea and vomiting. This study investigated whether Scutellaria baicalensis, and its active flavonoid constituent, baicalein, attenuate the gastrointestinal effects of ritonavir. The effects of herb administration were evaluated in ritonavir-treated rats using a rat pica model, which simulates nausea and vomiting in humans. The effects of herb administration on gastric emptying in rats were also measured. Ritonavir treatment resulted in increased kaolin intake or severe pica, the intensity of which was reduced significantly with S. baicalensis administration (1 mg kg−1; P < 0.05). High-performance liquid chromatography analysis of S. baicalensis showed the presence of an extremely potent flavonoid constituent, baicalein. The study aimed to determine if baicalein contributed to the anti-pica effect of the extract. It was observed that baicalein dose-dependently decreased pica in ritonavir-treated rats (P < 0.001). In addition to inducing pica, ritonavir also significantly delayed gastric emptying, which could contribute to ritonavir-induced gastrointestinal dysfunction. When S. baicalensis extract was administered to ritonavir-treated rats the delayed gastric emptying was significantly attenuated (P < 0.05). The results suggest that S. baicalensis and the constituent baicalein reduce the gastrointestinal dysfunction caused by ritonavir. It is concluded that S. baicalensis may potentially have a role to play in reducing drug-induced adverse effects.
Introduction
Protease inhibitors are commonly used, potent anti-HIV drugs, which act by inhibiting viral replication (Elperin & Sax 1996; Mangum & Graham 2001). Drugs in this class, especially ritonavir, cause unpleasant gastrointestinal side-effects such as nausea and vomiting (Elperin & Sax 1996). Ritonavir is currently used in anti-HIV therapy as an adjuvant to other protease inhibitors since ritonavir inhibits the hepatic CYP 3A enzyme, thereby increasing the plasma concentration and bioavailability of other antiviral drugs (Ernest et al 2005; Motwani & Khayr 2006). Although the ritonavir dose required for adjuvant effects is lower than that required for a direct antiviral effect, nausea and emesis continue to be reported in at least 20% of the patients (Barlett 2004).
Recent research on other pharmacological effects of protease inhibitors demonstrated their ability to stimulate release of reactive oxygen species in cultured cells. Ritonavir and other protease inhibitors stimulate endothelial cells in culture to produce excessive amounts of superoxide radicals, resulting in cell injury and dysfunction (Chai et al 2005; Chen et al 2005). Recently published data suggested that ritonavir can increase oxidative stress in the human body (Duong et al 2006). Since oxidant damage of the gastrointestinal mucosa has been linked to serotonin release and consequent nausea and emesis, it was postulated that ritonavir might cause gastrointestinal side-effects through a similar mechanism (Cubeddu 1992; Davis et al 2002).
Nausea and emesis caused by oxidant aetiology is responsive to treatment with antioxidants (Matsuki 1996; Yang et al 1999). Thus, treatment with antioxidant herbs may attenuate ritonavir induced-gastrointestinal effects. The current study evaluated the effect of treatment with Scutellaria baicalensis, and its active constituent, baicalein, on ritonavir-induced gastrointestinal side-effects using two rat models, the pica model and the gastric emptying model.
Materials and Methods
Animals
The experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Chicago. Male Wistar rats (Harlan Sprague Dawley, Indianapolis, IN, USA), 150–300 g, were used in this study. The animals were housed in environmentally controlled conditions with a 12-h light–dark cycle. Rats were allowed free access to water and standard laboratory rat chow (Harlan-Teklad, Madison, WI, USA).
Preparation and analysis of S. baicalensis extract
S. baicalensis root was obtained from Beijing Chinese Herbal Medicine Company, China. The herb sample was tested by Applied Consumer Services (Hialeah Gardens, FL, USA) and was found to be free from toxic contaminants.
To prepare the extract, S. baicalensis root was soaked in cold water for 2 h and then cut into small pieces (approx. 2 mm3), which were treated with hot water (approx. 95°C) for 1 h. The filtrate obtained following the hot water treatment was evaporated under vacuum and lyophilized. For animal treatment, the dried extract powder was dissolved in normal saline and centrifuged (400 g; 5 min) to remove particulate residue, and the supernatant was used.
HPLC analysis of the extract was performed using a Shimadzu system on a Phenomenex Prodigy ODS(2) column (150 × 3.2 mm, 5μm; Phenomenex, Torrance, CA, USA) at a flow rate of 0.8 mL min−1, and the absorbance was measured at 280 nm. A sample volume of 20 μL was injected onto the column using an automatic injector. A binary gradient solvent system of acetonitrile (eluent A) and 0.03% (v/v) phosphoric acid in water (eluent B) was used as follows: 85% B (0.01 min), 72% B (12 min), 65% B (23 min), 50% B (30 min), 5% B (32 min), 5% B (34 min), 85% B (37 min), 85% B (42 min). Calibration curves of important flavonoid constituents of S. baicalensis, scutellarin, baicalein and wogonin (National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China) were obtained from standards dissolved in methanol using a series of concentrations (2–100 μg mL−1). Linear regression of the peak areas of the standard against respective concentrations of standards was used to calculate baicalein concentration in the extract. Baicalein, which is considered one of the important active constituents in S. baicalensis (Gao et al 1999; Shao et al 1999, 2002; Shieh et al 2000), was also used in the animal experiments.
Measurement of pica (kaolin intake)
Rats react to nauseous and emetic stimuli with pica, which is manifested by increased intake of non-nutritive substances such as kaolin, a type of clay (Takeda et al 1993). Kaolin pellets were prepared based on the method described previously (Aung et al 2003). Briefly, pharmacological grade kaolin (or hydrated aluminum silicate; Fisher, Fair Lawn, NJ, USA) and acacia (or Gum Arabic; Fisher, Fair Lawn, NJ, USA) were mixed using a 99:1 ratio in distilled water. The kaolin paste was rolled and cut into pieces similar in shape to regular rat chow pellets. The pellets were dried at room temperature for 72 h.
Rats were placed in individual isolation cages (45cm × 35cm × 25cm) and were allowed access to both regular food and kaolin during a 3-day adaptation period prior to the study period. There were between five and eight rats in each group, that is the vehicle group, the ritonavir group, and the ritonavir plus S. baicalensis or baicalein groups (two doses). Rats received ritonavir (20 mg kg−1; Abbott Laboratories, North Chicago, IL, USA) orally using a gavage tube in the morning on 2 consecutive days (0 h and 24 h) (Denissen et al 1997; Yamaji et al 1999; Shibata et al 2002). S. baicalensis extract (dissolved in normal saline), baicalein (dissolved in 0.2% DMSO in normal saline) or vehicle pre-treatment were administered intraperitoneally (Aung et al 2003) 30 min prior to each ritonavir administration. Rats were observed immediately, at 2 h, and daily for any signs of distress.
Kaolin and food pellets were weighed to the nearest 0.1 g and placed in separate containers within the cage each morning. The kaolin and food remaining from the previous day was collected, dried for 72 h and weighed. Daily kaolin and food intake was measured as described for 5 days following the first ritonavir treatment.
Measurement of gastric emptying
Rats were placed in individual cages with free access to food and water. The animals were fasted for 24 h before the experiment but allowed free access to water until 2 h before the experiment. There were between five and eight rats in each group, that is the vehicle group, the ritonavir group, and the ritonavir plus S. baicalensis groups (two doses).
At time 0 h, ritonavir (20 mg kg−1) was administered orally using a gavage tube. The herb treatment (0.7 mg kg−1 or 2 mg kg−1S. baicalensis extract, or vehicle) was given intraperitoneally twice, at 30 min before time 0 and at time 120 min. Gastric emptying was determined according to the method described previously (Scarpignato et al 1980). Briefly, 1.5 mL of a test meal (1.5% aqueous methylcellulose solution containing 0.5% phenol red as an indicator) was administered using a gavage tube at 3 h following oral ritonavir. At 30 min after the meal administration, the animals were killed by CO2 inhalation. The stomach was removed rapidly and carefully by laparotomy and homogenized in 100 mL of 0.1 m NaOH to measure gastric emptying. After letting the homogenate stand for 1 h at room temperature, proteins (in 5 mL of homogenate) were precipitated with 0.5 mL trichloroacetic acid (20% w/v), centrifuged and separated out. The supernatant was mixed with an equal volume of 0.5 m NaOH and the sample absorbance (corresponding to phenol red concentration) was read at 560 nm using a spectrophotometer. Rats providing the standard stomach (or 0% gastric emptying) required for the gastric emptying calculation were killed immediately after the test meal was fed.
The percentage of gastric emptying was calculated as follows: gastric emptying (%)= (1 − (concentration of phenol red in test stomach)/(concentration of phenol red in standard stomach)) × 100 (Kishibayashi et al 1993). Thus, the higher the percentage of gastric emptying, the better is the emptying.
Statistical analysis
Data were analysed in Sigmastat 3.0 using an analysis of variance. Significance was assumed at P < 0.05.
Results
HPLC of S. baicalensis extract
A chromatogram of the S. baicalensis extract is shown in Figure 1B. The peaks in the chromatogram were determined to be scutellarin, baicalein and wogonin following comparison with HPLC of a standard flavonoid solution (Figure 1A). HPLC analysis showed that 1 mg of S. baicalensis extract contained approximately 9.3 μg of baicalein, one of the most active flavonoids found in the extract (Figure 1B). The flavonoids scutellarin and wogonin were present at a concentration of 3.2 μg and 3.3 μg (mg of extract)−1, respectively.
Figure 1.
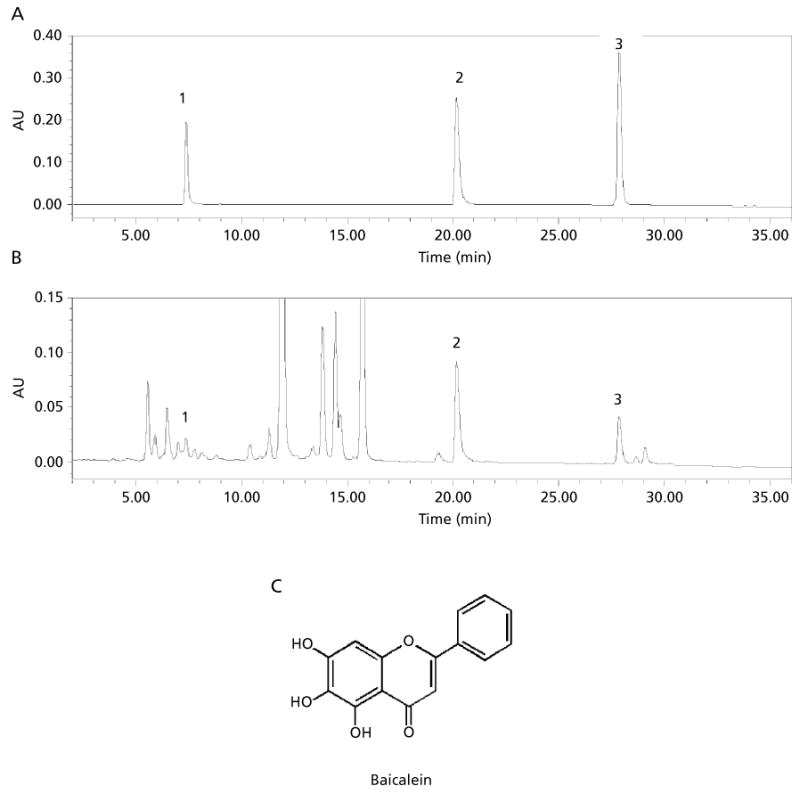
A. HPLC chromatogram of flavonoid standards. B. HPLC chromatogram of Scutellaria baicalensis extract. Flavonoid peaks: 1, scutellarin; 2, baicalein; 3, wogonin. C. Structure of baicalein, one of the important flavonoids found in S. baicalensis.
Effect of S. baicalensis on ritonavir-induced pica
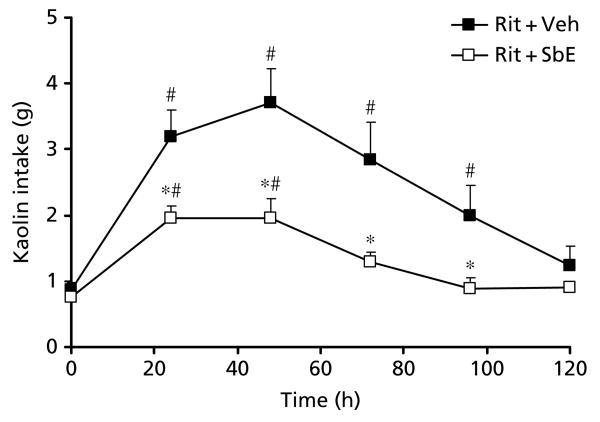
Our earlier work showed that ritonavir at a dose of 20 mg kg−1 induces significant kaolin intake (Aung et al 2005). We confirmed that significant pica was induced from 24 to 96 h following oral ritonavir (20 mg kg−1) (Figure 2; P < 0.05). We also confirmed that S. baicalensis reduces ritonavir-induced pica. In this study, we found that pre-treatment with 1 mg kg−1S. baicalensis significantly reduced both the intensity and duration of pica induced by ritonavir (P < 0.05). Food intake was not significantly affected.
Figure 2.
Effect of treatment with Scutellaria baicalensis extract (1 mg kg−1) on increased kaolin intake (pica) induced by ritonavir. Ritonavir (20 mg kg−1)-induced pica decreased significantly when treated with S. baicalensis extract (P < 0.001; n = 6–8). #Significantly different compared with time 0 h (P < 0.05); *significantly different compared with the ritonavir group (P < 0.05). Veh, vehicle; Rit, ritonavir; SbE, S. baicalensis extract.
Effect of baicalein on ritonavir-induced pica
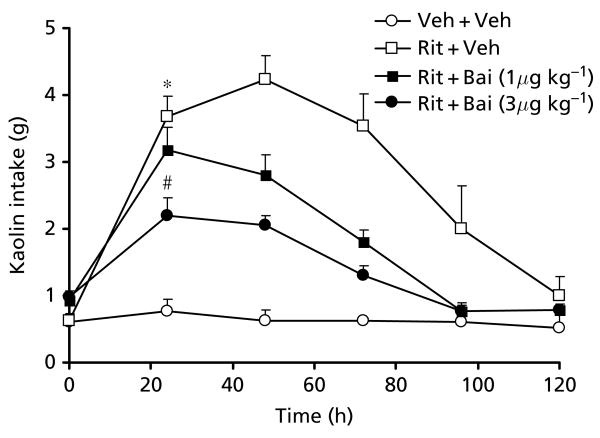
Baicalein, an active flavonoid constituent found in S. baicalensis, was tested for its anti-pica effect and its contribution to the actions of S. baicalensis extract. Our data showed that baicalein significantly decreased ritonavir-induced pica in a dose-related manner (P < 0.01; Figure 3). The area under the curve (AUC) for kaolin intake from time 0 h to 120 h for the vehicle group, the ritonavir (20 mg kg−1) group, the baicalein (1 μg kg−1) plus ritonavir group, and the baicalein (3 μg kg−1) plus ritonavir group were 82 ± 15 g h, 355 ± 32 g h, 237 ± 13 g h and 184 ± 12 g h, respectively. Food intake was not significantly affected in any group.
Figure 3.
Effect of treatment with baicalein (μg kg−1) on ritonavir-induced pica. Ritonavir-induced pica decreased significantly with baicalein pre-treatment in a dose-related manner (P < 0.001; n = 5–6). The area under the curve for all groups was compared. The vehicle group (Veh + Veh) was significantly different compared with the ritonavir-treated group (Rit + Veh; P < 0.05). The ritonavir-treated group was significantly different compared with the group receiving ritonavir and baicalein (Rit + Bai; P < 0.05), suggesting that baicalein treatment at a dose of 3 μg kg−1 reversed ritonavir-induced pica. *AUC significantly different compared with the Veh + Veh group; #AUC significantly different compared with the Rit + Veh group.
Effect of S. baicalensis on ritonavir-induced decrease in gastric emptying
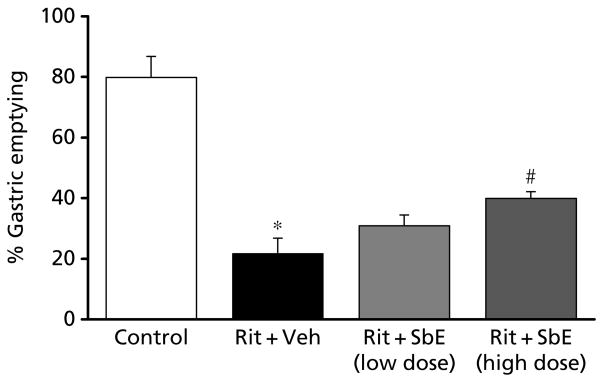
As shown in Figure 4, in control animals, the normal gastric empting was 80%, suggesting that 80% of the test meal had emptied from the stomach at 30 min. In ritonavir-treated rats, gastric emptying was significantly decreased to 21.6 ± 5% from 79.8 ± 6% in control rats at 30 min. When ritonavir-treated rats were also administered S. baicalensis extract, the impairment in gastric emptying induced by ritonavir was significantly reversed in a dose-related manner (P < 0.05).
Figure 4.
Effect of treatment with Scutellaria baicalensis extract on delayed gastric emptying induced by ritonavir in rats. When compared with normal gastric emptying, ritonavir (20 mg kg−1) significantly reduced gastric emptying (P < 0.001). Administering S. baicalensis extract to ritonavir-treated rats attenuated the delayed gastric emptying significantly in a dose-dependent manner, suggesting that the extract reduces gastrointestinal dysfunction induced by ritonavir (P < 0.05; n = 5–8). Veh, vehicle; Rit, ritonavir; SbE, S. baicalensis extract; SbE low dose, 0.7 mg kg−1; SbE high dose, 2 mg kg−1. *Significantly different compared with control; #significantly different compared with the Rit + Veh group.
Discussion
Protease inhibitor drugs are efficacious antiretroviral drugs that produce several adverse effects, including nausea and vomiting. Considering that compliance is a prerequisite for effective antiviral therapy in AIDS, drug-induced adverse effects that prevent compliance need to be treated. However, the compounds chosen to reduce drug-induced adverse effects should ideally be devoid of additional adverse effects. Herbs and botanicals, with their repertoire of multiple active constituents, are considered to be milder, non-toxic alternatives to actual drugs and may find use in the treatment of drug-induced adverse effects. In the current study, we tested whether ritonavir-induced gastrointestinal side-effects may be attenuated by treatment with the botanical S. baicalensis.
S. baicalensis is a potent herb that has been popularly used in traditional Chinese medicine for its antimicrobial, antipyretic and anti-inflammatory effects (Bensky & Gamble 1993). In recent years, S. baicalensis was found to contain several active flavonoids (Shieh et al 2000; Shao et al 2002). In the current study, we tested whether S. baicalensis may attenuate ritonavir-induced pica (measure of nausea/vomiting in rats) and delayed gastric emptying. We showed that S. baicalensis treatment significantly attenuated the gastrointestinal side-effects caused by ritonavir.
In this study, we demonstrated that baicalein, one of the important flavonoids found in S. baicalensis, significantly contributed to the action of the herb. HPLC analysis of S. baicalensis extract showed that baicalein is present at a concentration of 10 μg mg−1. If baicalein was an important contributor to the total bioactivity of the extract, then a 10 μg kg−1 dose of baicalein should produce an anti-pica effect equivalent to that produced by 1 mg kg−1S. baicalensis extract. From our unpublished work, we know that a dose of 10 μg kg−1 in rats is toxic. Thus, we chose to use a lower dose of baicalein and tested the anti-pica effect at doses of 3 μg kg−1 and 1 μg kg−1. We demonstrated an equivalent attenuation of the ritonavir-induced pica response in the groups treated with 3 μg kg−1 baicalein and with 1 mg kg−1S. baicalensis (AUC: 184 ± 12 g h and 172 ± 13 g h, respectively). Thus, baicalein at a 300-times lower dose than in the S. baicalensis extract was equally efficacious, suggesting that baicalein is an important active constituent responsible for the activity of S. baicalensis. The results also suggest other constituents in the herbal extract that may act to counter the effectiveness of baicalein. Such a dynamic may be considered typical of a herbal extract composed of several active ingredients that may have opposing actions and possibly contributing to reducing the toxicity caused by extremely potent active ingredients.
The rat pica model was used to evaluate the symptoms of nausea and emesis. Rats exposed to a variety of emetic stimuli experience induced feeding of non-nutritive substances such as clay, a phenomenon that has been termed pica. Pica in rats is thus analogous to nausea and vomiting in humans and other species (Mitchell et al 1976; Takeda et al 1993). It was also demonstrated that pica in rats is mediated by similar mechanisms and receptors, involving serotonin and dopamine, to nausea and vomiting in other species, including humans (Takeda et al 1993, 1995). The model has been used extensively and validated in several studies researching anti-emetic drugs (Takeda et al 1995; Aung et al 2004). Our earlier study demonstrated a dose-dependent pica response induced by ritonavir (Aung et al 2005). In this study, we used the pica model to confirm that treatment with S. baicalensis and its constituent antioxidant flavonoid, baicalein, significantly reduced ritonavir-induced pica.
We also studied the effect of ritonavir on gastric emptying, since delayed gastric emptying is known to lead to nausea and vomiting. Drug-induced nausea and vomiting could be caused by gastric stasis and resultant delay in gastric emptying (Perkel et al 1980; Wengrower et al 1991). Drugs such as cisplatin, a commonly used chemotherapeutic agent with a significant emetic effect, result in gastric stasis caused by 5-HT release from the enterochromaffin cells in the intestinal mucosa (Kishibayashi et al 1993). Apart from mediating the neural pathway of nausea and vomiting to a significant extent, 5-HT also slows gastric motility (Buchheit et al 1985), which could be a physical cause of nausea and vomiting (Perkel et al 1980; Wengrower et al 1991). This is confirmed by the fact that 5-HT3 antagonists can reverse cisplatin-induced delay in gastric emptying (Ozaki & Sukamoto 1999). We demonstrated that ritonavir at a dose that produces a significant pica response also delayed gastric emptying in rats. The slowing of gastric emptying with ritonavir was supported by a study using mice as the animal model (Huisman et al 2003). We further observed that treatment with S. baicalensis extract improved the delayed gastric emptying caused by ritonavir. Ritonavir administration could be linked to oxidant tissue damage of the gut, with subsequent release of 5-HT (Cubeddu 1992; Chai et al 2005) and, thus, probably mediates gastrointestinal symptoms via mechanisms similar to cisplatin.
To date, the underlining mechanism of S. baicalensis and baicalein in attenuating ritonavir-induced gastrointestinal side-effects is not fully understood. It has been shown that nausea and vomiting could be directly related to an increase in oxidative stress (Nicolson & Conklin 2006). For example, cisplatin is known to generate free radicals (Satoh et al 2003). Recently, Guney et al (2007) observed a close correlation between plasma lipid peroxidation (reflecting reactive oxygen species activity) and antioxidant levels in pregnant women suffering from severe emesis. Furthermore, ritonavir has been shown to increase oxidative stress in humans. After HIV-infected patients were administered ritonavir, their circulating oxidized LDL increased significantly (Duong et al 2006), oxidized LDL being one of the established parameters of oxidative stress (Maziere et al 2000; Ceriello et al 2005). Since S. baicalensis possesses strong antioxidant activity, the observed effects using pica and gastric emptying models could contribute to the herb's antioxidant activity. Related mechanistic studies will be conducted in future investigations.
Conclusion
Since ritonavir produces symptoms of nausea and vomiting as a result of its oxidant toxicity, we tested whether S. baicalensis could treat these symptoms in two rat models with simulated gastrointestinal dysfunction. The results demonstrate that S. baicalensis and its constituent, baicalein, could be useful in treating ritonavir-induced gastrointestinal dysfunction. Systematic investigation of potentially efficacious herbs for clinical use could help identify low-cost alternatives to conventional pharmacological treatments, which may help improve the quality of life of patients with chronic disease.
Acknowledgments
Funding: This work was supported in part by NIH/NCCAM grants AT002445 and AT003255.
Contributor Information
Sangeeta Mehendale, Tang Center for Herbal Medicine Research, The Pritzker School of Medicine, University of Chicago, Chicago, IL 60637, USA; Department of Anesthesia and Critical Care, The Pritzker School of Medicine, University of Chicago, Chicago, IL 60637, USA.
Han Aung, Tang Center for Herbal Medicine Research, The Pritzker School of Medicine, University of Chicago, Chicago, IL 60637, USA; Department of Anesthesia and Critical Care, The Pritzker School of Medicine, University of Chicago, Chicago, IL 60637, USA.
Chong-Zhi Wang, Tang Center for Herbal Medicine Research, The Pritzker School of Medicine, University of Chicago, Chicago, IL 60637, USA; Department of Anesthesia and Critical Care, The Pritzker School of Medicine, University of Chicago, Chicago, IL 60637, USA.
Robin Tong, Tang Center for Herbal Medicine Research, The Pritzker School of Medicine, University of Chicago, Chicago, IL 60637, USA; Department of Anesthesia and Critical Care, The Pritzker School of Medicine, University of Chicago, Chicago, IL 60637, USA.
Adela Foo, Tang Center for Herbal Medicine Research, The Pritzker School of Medicine, University of Chicago, Chicago, IL 60637, USA; Department of Anesthesia and Critical Care, The Pritzker School of Medicine, University of Chicago, Chicago, IL 60637, USA.
Jing-Tian Xie, Tang Center for Herbal Medicine Research, The Pritzker School of Medicine, University of Chicago, Chicago, IL 60637, USA; Department of Anesthesia and Critical Care, The Pritzker School of Medicine, University of Chicago, Chicago, IL 60637, USA.
Chun-Su Yuan, Tang Center for Herbal Medicine Research, The Pritzker School of Medicine, University of Chicago, Chicago, IL 60637, USA; Department of Anesthesia and Critical Care, The Pritzker School of Medicine, University of Chicago, Chicago, IL 60637, USA; Committee on Clinical Pharmacology and Pharmacogenomics, The Pritzker School of Medicine, University of Chicago, Chicago, IL 60637, USA.
References
- Aung HH, Dey L, Mehendale S, Xie JT, Wu JA, Yuan CS. Scutellaria baicalensis extract decreases cisplatin-induced pica in rats. Cancer Chemother Pharmacol. 2003;52:453–458. doi: 10.1007/s00280-003-0694-9. [DOI] [PubMed] [Google Scholar]
- Aung HH, Mehendale SR, Xie JT, Moss J, Yuan CS. Methylnaltrexone prevents morphine-induced kaolin intake in the rat. Life Sci. 2004;74:2685–2691. doi: 10.1016/j.lfs.2003.08.047. [DOI] [PubMed] [Google Scholar]
- Aung H, Mehendale S, Chang WT, Wang CZ, Xie JT, Yuan CS. Scutellaria baicalensis decreases ritonavir-induced nausea. AIDS Res Ther. 2005;2:12. doi: 10.1186/1742-6405-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlett JG. HIV disease management guide. Thompson PDR; Montvale, NJ, USA: 2004. HIV: current diagnosis, management, and treatment options; pp. 101–122. [Google Scholar]
- Bensky D, Gamble A. Chinese herbal medicine Materia Medica. Eastland Press; Seattle: 1993. [Google Scholar]
- Buchheit KH, Engel G, Mutschler E, Richardson B. Study of the contractile effect of 5-hydroxytryptamine (5-HT) in the isolated longitudinal muscle strip from guinea-pig ileum. Evidence for two distinct release mechanisms. Naunyn Schmiedebergs Arch Pharmacol. 1985;329:36–41. doi: 10.1007/BF00695189. [DOI] [PubMed] [Google Scholar]
- Ceriello A, Piconi L, Quagliaro L, Wang Y, Schnabel CA, Ruggles JA, Gloster MA, Maggs DG, Weyer C. Effects of pramlintide on postprandial glucose excursions and measures of oxidative stress in patients with type 1 diabetes. Diabetes Care. 2005;28:632–637. doi: 10.2337/diacare.28.3.632. [DOI] [PubMed] [Google Scholar]
- Chai H, Yang H, Yan S, Li M, Lin PH, Lumsden AB, Yao Q, Chen C. Effects of 5 HIV protease inhibitors on vasomotor function and superoxide anion production in porcine coronary arteries. J Acquir Immune Defic Syndr. 2005;40:12–19. doi: 10.1097/01.qai.0000172368.05327.7b. [DOI] [PubMed] [Google Scholar]
- Chen C, Lu XH, Yan S, Chai H, Yao Q. HIV protease inhibitor ritonavir increases endothelial monolayer permeability. Biochem Biophys Res Commun. 2005;335:874–882. doi: 10.1016/j.bbrc.2005.07.155. [DOI] [PubMed] [Google Scholar]
- Cubeddu LX. Mechanisms by which cancer chemotherapeutic drugs induce emesis. Semin Oncol. 1992;19:2–13. [PubMed] [Google Scholar]
- Davis DA, Read-Connole E, Pearson K, Fales HM, Newcomb FM, Moskovitz J, Yarchoan R. Oxidative modifications of kynostatin-272, a potent human immunodeficiency virus type 1 protease inhibitor: potential mechanism for altered activity in monocytes/macrophages. Antimicrob Agents Chemother. 2002;46:402–408. doi: 10.1128/AAC.46.2.402-408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denissen JF, Grabowski BA, Johnson MK, Buko AM, Kempf DJ, Thomas SB, Surber BW. Metabolism and disposition of the HIV-1 protease inhibitor ritonavir (ABT-538) in rats, dogs, and humans. Drug Metab Dispos. 1997;25:489–501. [PubMed] [Google Scholar]
- Duong M, Petit JM, Martha B, Galland F, Piroth L, Walldner A, Grappin M, Buisson M, Duvillard L, Chavanet P, Portier H. Concentration of circulating oxidized LDL in HIV-infected patients treated with antiretroviral agents: relation to HIV-related lipodystrophy. HIV Clin Trials. 2006;7:41–47. doi: 10.1310/7381-m1yd-rtv5-4ryt. [DOI] [PubMed] [Google Scholar]
- Elperin A, Sax P. A patient's guide to protease inhibitors. AIDS Clin Care. 1996;8:83–84. [PubMed] [Google Scholar]
- Ernest CS, 2nd, Hall SD, Jones DR. Mechanism-based inactivation of CYP3A by HIV protease inhibitors. J Pharmacol Exp Ther. 2005;312:583–591. doi: 10.1124/jpet.104.075416. [DOI] [PubMed] [Google Scholar]
- Gao Z, Huang K, Yang X, Xu H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim Biophys Acta. 1999;1472:643–650. doi: 10.1016/s0304-4165(99)00152-x. [DOI] [PubMed] [Google Scholar]
- Guney M, Oral B, Mungan T. Serum lipid peroxidation and antioxidant potential levels in hyperemesis gravidarum. Am J Perinatol. 2007;24:283–289. doi: 10.1055/s-2007-981429. [DOI] [PubMed] [Google Scholar]
- Huisman MT, Smit JW, Wiltshire HR, Beijnen JH, Schinkel AH. Assessing safety and efficacy of directed P-glycoprotein inhibition to improve the pharmacokinetic properties of saquinavir coadministered with ritonavir. J Pharmacol Exp Ther. 2003;304:596–602. doi: 10.1124/jpet.102.044388. [DOI] [PubMed] [Google Scholar]
- Kishibayashi N, Ichikawa S, Yokoyama T, Ishii A, Karasawa A. Pharmacological properties of KF18259, a novel 5-HT3-receptor antagonist, in rats: inhibition of the distal colonic function. Jpn J Pharmacol. 1993;63:495–502. doi: 10.1254/jjp.63.495. [DOI] [PubMed] [Google Scholar]
- Mangum EM, Graham KK. Lopinavir-ritonavir: a new protease inhibitor. Pharmacotherapy. 2001;21:1352–1363. doi: 10.1592/phco.21.17.1352.34419. [DOI] [PubMed] [Google Scholar]
- Matsuki N. Mechanisms of cytotoxic drug-induced emesis and its prevention. Yakugaku Zasshi. 1996;116:710–718. doi: 10.1248/yakushi1947.116.9_710. [DOI] [PubMed] [Google Scholar]
- Maziere C, Meignotte A, Dantin F, Conte MA, Maziere JC. Oxidized LDL induces an oxidative stress and activates the tumor suppressor p53 in MRC5 human fibroblasts. Biochem Biophys Res Commun. 2000;276:718–723. doi: 10.1006/bbrc.2000.3528. [DOI] [PubMed] [Google Scholar]
- Mitchell D, Wells C, Hoch N, Lind K, Woods SC, Mitchell LK. Poison induced pica in rats. Physiol Behav. 1976;17:691–697. doi: 10.1016/0031-9384(76)90171-2. [DOI] [PubMed] [Google Scholar]
- Motwani B, Khayr W. Pharmacoenhancement of protease inhibitors. Am J Ther. 2006;13:57–63. doi: 10.1097/00045391-200601000-00010. [DOI] [PubMed] [Google Scholar]
- Nicolson GL, Conklin KA. Molecular replacement for cancer metabolic and mitochondrial dysfunction, fatigue and the adverse effects of cancer therapy. Cancer Genomics Proteomics. 2006;3:159–168. [PubMed] [Google Scholar]
- Ozaki A, Sukamoto T. Improvement of cisplatin-induced emesis and delayed gastric emptying by KB-R6933, a novel 5-HT3 receptor antagonist. Gen Pharmacol. 1999;33:283–288. doi: 10.1016/s0306-3623(98)00286-9. [DOI] [PubMed] [Google Scholar]
- Perkel MS, Hersh T, Moore C, Davidson ED. Metoclopramide therapy in fifty-five patients with delayed gastric emptying. Am J Gastroenterol. 1980;74:231–236. [PubMed] [Google Scholar]
- Satoh M, Kashihara N, Fujimoto S, Horike H, Tokura T, Namikoshi T, Sasaki T, Makino H. A novel free radical scavenger, edarabone, protects against cisplatin-induced acute renal damage in vitro and in vivo. J Pharmacol Exp Ther. 2003;305:1183–1190. doi: 10.1124/jpet.102.047522. [DOI] [PubMed] [Google Scholar]
- Scarpignato C, Capovilla T, Bertaccini G. Action of caerulein on gastric emptying of the conscious rat. Arch Int Pharmacodyn Ther. 1980;246:286–294. [PubMed] [Google Scholar]
- Shao ZH, Li CQ, Vanden Hoek TL, Becker LB, Schumacker PT, Wu JA, Attele AS, Yuan CS. Extract from Scutellaria baicalensis Georgi attenuates oxidant stress in cardiomyocytes. J Mol Cell Cardiol. 1999;31:1885–1895. doi: 10.1006/jmcc.1999.1021. [DOI] [PubMed] [Google Scholar]
- Shao ZH, Vanden Hoek TL, Qin Y, Becker LB, Schumacker PT, Li CQ, Dey L, Barth E, Halpern H, Rosen GM, Yuan CS. Baicalein attenuates oxidant stress in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2002;282:H999–H1006. doi: 10.1152/ajpheart.00163.2001. [DOI] [PubMed] [Google Scholar]
- Shibata N, Gao W, Okamoto H, Kishida T, Iwasaki K, Yoshikawa Y, Takada K. Drug interactions between HIV protease inhibitors based on physiologically-based pharmacokinetic model. J Pharm Sci. 2002;91:680–689. doi: 10.1002/jps.10051. [DOI] [PubMed] [Google Scholar]
- Shieh DE, Liu LT, Lin CC. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anti-cancer Res. 2000;20:2861–2865. [PubMed] [Google Scholar]
- Takeda N, Hasegawa S, Morita M, Matsunaga T. Pica in rats is analogous to emesis: an animal model in emesis research. Pharmacol Biochem Behav. 1993;45:817–821. doi: 10.1016/0091-3057(93)90126-e. [DOI] [PubMed] [Google Scholar]
- Takeda N, Hasegawa S, Morita M, Horii A, Uno A, Yamatodani A, Matsunaga T. Neuropharmacological mechanisms of emesis. II. Effects of antiemetic drugs on cisplatin-induced pica in rats. Methods Find Exp Clin Pharmacol. 1995;17:647–652. [PubMed] [Google Scholar]
- Wengrower D, Zaltzman S, Karmeli F, Goldin E. Idiopathic gastroparesis in patients with unexplained nausea and vomiting. Dig Dis Sci. 1991;36:1255–1258. doi: 10.1007/BF01307518. [DOI] [PubMed] [Google Scholar]
- Yamaji H, Matsumura Y, Yoshikawa Y, Takada K. Pharmacokinetic interactions between HIV-protease inhibitors in rats. Biopharm Drug Dispos. 1999;20:241–247. doi: 10.1002/(sici)1099-081x(199907)20:5<241::aid-bdd182>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Yang Y, Kinoshita K, Koyama K, Takahashi K, Tai T, Nunoura Y, Watanabe K. Novel experimental model using free radical-induced emesis for surveying anti-emetic compounds from natural sources. Planta Med. 1999;65:574–576. doi: 10.1055/s-2006-960829. [DOI] [PubMed] [Google Scholar]