At the time of harvesting organs from cadaveric donors, we have also removed long segments of the iliac arteries and veins, inferior venacava and aorta. The vessels are placed in a sterile plastic container containing cold normal saline solution or lactated Ringer's solution and kept in a commercial refrigerator at 4 degrees C.
Although use of the vascular grafts is almost never planned, an unexpected need for extra vessel length has developed in several renal and hepatic recipients and has been thereby met. Examples are shown in Figure 1.
FIG. 1.
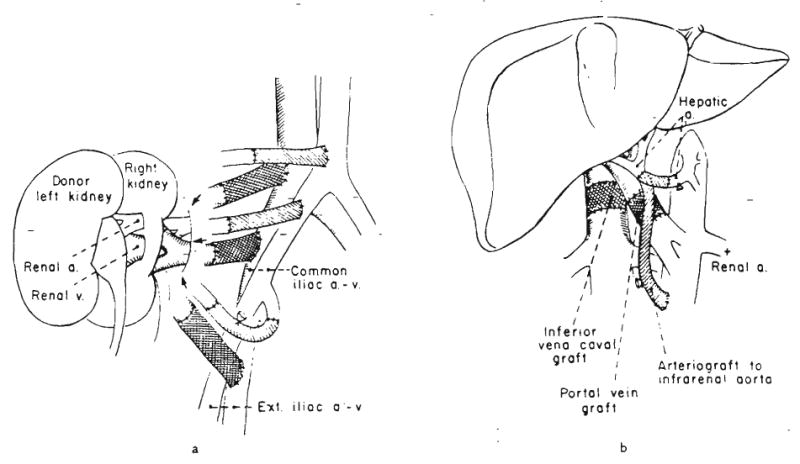
Uses to which vascular grafts have been, or might be, put. a and b, The arterial grafts have a single cross hatch and the venous grafts are double cross hatched. b, The portal vein graft was obtained from the common iliac vein from the donor.
After an unexpected vascular problem is encountered in an organ recipient, it is tragic to realize that the means of easily rectifying the situation has already been lost with removal of the cadaver from the operating room and for lack of a few extra minutes' efforts by the harvest team. Therefore, we recommend the simple security measure of donor vessel removal and short term storage in every cadaveric donor. This ensures the ability to deal with almost any conceivable vascular exigency that might be encountered in the recipient.
Acknowledgments
The work was supported by research Grants No. MRIS 8118-01 and No. 7227-01 from the Veterans Administration; by Grants No. AM-17260 and No. AM-07772 from the National Institutes of Health, and by Grants No. RR-00051 and No. RR-00069 from the General Clinical Research Centers Program of the Division of Research Resources, National Institutes of Health.


