Abstract
Children immunized with a formalin-inactivated respiratory syncytial virus (RSV) vaccine experienced enhanced disease and exhibited pulmonary eosinophilia upon natural RSV infection. BALB/c mice immunized with either FI-RSV or a recombinant vaccinia virus (vacv) expressing the RSV attachment (G) protein develop extensive pulmonary eosinophilia after RSV challenge that mimics the eosinophilic response observed in the children during the 1960s vaccine trials. Fas-ligand (FasL) is a major immune effector molecule that can contribute to the clearance of respiratory viruses. However, the role of FasL in the development of RSV vaccine-enhanced disease has not been elucidated. RSV challenge of vacvG-immunized gld mice, that lack functional FasL, results in diminished systemic disease as well as pulmonary eosinophilia. The magnitude of the secondary RSV G-specific CD4 T cell response was diminished in gld mice as compared to wild-type controls. Furthermore, we show that CD4 T cells isolated after RSV challenge of vacvG-immunized gld mice exhibit enhanced expression of Annexin V and caspase 3/7 indicating that FasL is important for either the survival or the expansion of virus-specific secondary effector CD4 T cells. Taken together, these data identify a previously undefined role of FasL in the accumulation of secondary effector CD4 T cells and the development of RSV vaccine-enhanced disease.
Keywords: Vaccination, Th1/Th2 T cells, Lung, Eosinophils, Virus
Introduction
Respiratory syncytial virus (RSV)3 is the leading cause of lower respiratory tract infection and hospitalization in young children under 5 years of age (1). Despite the clear need, a safe and effective RSV vaccine has yet to be developed. A series of RSV vaccine trials were conducted in the 1960s using a formalin-inactivated (FI) preparation of the virus. In these trials, ~80% of FI-RSV vaccinees were hospitalized and tragically 2 children died following a subsequent RSV exposure (2–4). Histological analyses of the deceased revealed pulmonary eosinophilia (2, 4, 5). Additionally, increased levels of circulating eosinophils were detected in the peripheral blood of numerous vaccinees (5). Work performed using mouse models of RSV vaccine-enhanced disease indicate that CD4 T cells and Th2 cells are required for the development of pulmonary eosinophilia (6–8). These data suggest that the FI-RSV vaccine primed the children for a Th2 response.
A great deal of our current understanding of the underlying mechanisms that mediate the induction of RSV vaccine-enhanced pulmonary eosinophilia comes from the BALB/c mouse model. BALB/c mice immunized with either FI-RSV or a recombinant vaccinia virus (vacv) that expresses the RSV attachment (G) glycoprotein mount an RSV-specific CD4 T cell response and do not generate a detectable RSV-specific CD8 T cell response (9). RSV challenge of either FI-RSV- or vacvG-immunized mice results in a robust memory CD4 T cell response, the development of pulmonary eosinophilia, and systemic disease (i.e. weight loss), thus mimicking the enhanced disease that was observed in the FI-RSV vaccinated children (9–12). Interestingly, the secondary G-specific CD4 T cell response that occurs after RSV challenge of vacvG-immunized mice is largely oligoclonal for the Vβ14 chain of the T cell receptor (8). Furthermore, depletion of Vβ14+ CD4 T cells in vacvG-immunized mice prevents the development of pulmonary eosinophilia after RSV challenge (8). These data indicate that Vβ14+ CD4 T cells are required for the development of RSV vaccine-enhanced disease.
Fas-ligand (FasL; CD178) is a type II TNF receptor family member that plays a critical role in the control of the immune system by binding to its receptor, Fas (CD95). FasL is constitutively expressed in immune-privileged tissues (e.g. eye, uterus, and testis) where it is thought to induce the death of tissue-infiltrating immune cells that express Fas (13). Intriguing recent work has demonstrated that Fas-FasL interactions may induce bi-directional signaling, sending a death signal through Fas, as well as a signal through FasL (14). Signaling through FasL has been shown to be required for the full expansion of allogeneic CD8 T cells, suggesting that FasL signals may promote T cell proliferation and survival (15, 16).
FasL also plays a critical role in T cell-mediated clearance of respiratory pathogens and has also been previously shown to be required for the full expansion and effector function of T cells after antigenic stimulation (15–17). Interestingly, gld mice that are deficient in functional FasL exhibit delayed viral clearance and reduced morbidity after influenza virus infection as compared to wild-type (WT) mice (18). Similar to influenza virus-infected mice, RSV-infected gld mice also exhibit decreased weight loss and delayed viral clearance after primary infection as compared to WT mice (19). Taken together, these data suggest that FasL is involved in viral clearance as well as the development of immunopathology after respiratory virus infection. However, the role of FasL in the development of RSV vaccine-enhanced disease has not been examined.
In these studies we utilized gld mice to question the role of FasL in the development of RSV vaccine-enhanced disease. gld mice suffer from lymphadenopathy and systemic autoimmunity that increase in severity with the age of the mouse (20). We specifically chose to utilize gld mice over lpr mice, which lack Fas, for these studies because we were interested in also assessing the potential role of FasL expressed by CD8 T cells in mediating the inhibition of RSV vaccine-enhanced pulmonary eosinophilia (see (9)). We demonstrate here that functional FasL is required for the development of RSV vaccine-enhanced disease. FasL-defective gld mice immunized with vacvG exhibit reduced weight loss and clinical illness after RSV challenge as compared to their WT counterparts. Furthermore, vacvG-immunized gld mice also exhibit reduced levels of pulmonary eosinophilia and a diminished secondary RSV G-specific CD4 T cell response after RSV challenge. In agreement with this, FI-RSV-immunized mice also demonstrate reduced pulmonary eosinophilia and CD4 T cell responses in the lung. Both WT and gld mice exhibit similar numbers of primary RSV G-specific CD4 T cells after vacvG-immunization, however secondary memory G-specific CD4 T cells in gld mice fail to fully expand after RSV challenge. These data suggest that CD4 T cells undergoing a secondary response to antigen require functional FasL for their full expansion. Interestingly, both primary and secondary RSV-specific CD8 T cell responses in gld mice are similar to WT controls, suggesting that the expansion of memory CD4 and CD8 T cells have different requirements for FasL.
Methods
Viruses and infection of mice
The A2 strain of RSV was a gift from B.S. Graham (National Institutes of Health; NIH, Bethesda, MD) and was propagated in HEp-2 cells (American Type Culture Collections; ATCC, Manassas, VA). Recombinant vacv were a gift from T.J. Braciale (University of Virginia, Charlottesville, VA) and J.L. Beeler (U.S. Food and Drug Administration, Bethesda, MD) and were propagated in BSC-40 cells (ATCC). BALB/cAnNCr mice between 6–10 weeks of age were purchased from the National Cancer Institute (Bethesda, MD). CPt.C3-Faslgld/J (referred to hereafter as gld) mice were a gift from K.L. Legge (University of Iowa, Iowa City, IA). Mice were scarified with 3×106 PFU of recombinant vacv or a mixture of two different recombinant vacv and challenged with RSV 3 weeks later as previously described (9). Alternatively, either WT or gld mice were immunized intramuscularly with a 1:200 dilution of either FI-RSV or a formalin-inactivated mock preparation of HEp-2 cell supernatants as previously described (21). Four weeks after either mock or FI-RSV-immunization, mice were challenged intranasaly with 3×106 PFU of RSV. In some instances, mice were weighed and assigned a clinical illness score on a daily basis after RSV challenge as previously described (22). All mouse experiments have been evaluated and approved by the University of Iowa Animal Care and Use Committee.
Mononuclear cell isolation and ICS
Lung mononuclear cells and bronchial alveolar lavage (BAL) cells were harvested and prepared as previously described (9). Cytospin (Cytospin 2; Cytospin, Shandon, Pittsburgh, PA) preparations of BAL cells were stained with Diff-Quik (Baxter Healthcare, Miami, FL) prior to analysis. Differential cell counts were performed on at least 200 cells based on standard morphology and staining characteristics. In some cases, eosinophils were identified by FACS using the markers CD45 (eBioscience), CD11c (eBioscience) and Siglec F (BD-Pharmingen) as previously described (23). Cells from the spleen and mediastinal lymph nodes (MedLN) were isolated by pressing these tissues between the ends of frosted glass slides (Surgipath Richmond, IL). Peptides corresponding to the CD4 T cell epitope G183–195 and the CD8 T cell epitope M282–90 were purchased from Biosynthesis Inc. Lewisville, TX. To enumerate the number of RSV-specific CD4 and CD8 T cells, 1–2×106 lung mononuclear cells were stimulated in vitro in the presence of 1 µM peptide and 10 µg/ml brefeldin A (BFA; Sigma-Aldrich, St. Louis, MO) for 5 hours at 37°C. After stimulation cells were subsequently stained for cell surface CD4, CD8, and Thy1.2 (all mAbs from eBioscience). Cells were subsequently washed twice with cold staining buffer and then fixed for 15 minutes with FACS lysing solution (BD Biosciences, San Diego, CA). After fixation, cells were incubated at 4°C for 10 minutes in the presence of staining buffer containing 0.5% saponin to permeabilize the cells. Cells were subsequently stained for intracellular IFN-γ or IL-13 (all mAbs from eBioscience) in the presence of permeabilization buffer. Cells were then washed an additional two times with permeabilization buffer and once more with staining buffer prior to final resuspension in staining buffer. All samples were analyzed on a BD FACsCanto flow cytometer. Staining for TCR Vβ chain usage was done using a panel of Vβ antibodies (BD-Pharmingen). Data was analyzed using FlowJo software (Tree Star Inc., Ashland, OR). Peripheral blood was collected from isoflourane-anesthetized mice by eye bleed into 4% (w/v) sodium citrate. RBC in the peripheral blood were lysed with 0.84% NH4Cl and washed with RPMI.
Plaque assays
Lungs were harvested from either WT or gld vacv-immunized mice on day 4 or 7 post-RSV challenge in 1 ml of serum-free RPMI. Lung tissue was disrupted using a tissue homogenizer (Ultra-Turrax T25, IKA, Wilmington, NC) and lung homogenates were then centrifuged at 2000 rpm for 10 minutes. Cell-free supernatants from these samples were flash frozen in liquid nitrogen and stored at −80°C. Dilutions of thawed lung homogenates were incubated on Vero cells (ATCC) in 6-well plates (BD Falcon) for 1.5 hours at 37°C with gentle rocking. Cells were subsequently overlaid with 4 ml of 1% Seakem ME agarose (Cambrex, Rockland, ME) in Eagle’s minimal essential media (EMEM) (Cambrex) and allowed to incubate for 5 days at 37°C. After 5 days of incubation, cells were overlaid again with 2 ml of 1% agarose in EMEM containing a final concentration of 0.01% neutral red (Sigma) and allowed to incubate an additional 24 hours. The number of plaques was counted with the aid of a light box.
Activated caspase 3/7 and Annexin V staining
Mononuclear (1×106) cells from each tissue were stained with either Annexin V (BD Pharmigen) or for activated caspases 3/7 (Vybrant Fam, Molecular Probes, Eugene, OR) as per the manufacturer’s instructions. Cells stained with Annexin V were stained with antibodies specific to CD4 and Vβ14 as described above prior to staining with Annexin V. Cells stained for activated caspases 3/7 were stained after caspase staining with antibodies specific for CD4 and Vβ14 as described above.
Data analysis and statistics
Statistical analyses were performed using Prism software (GraphPad Software, SanDiego, CA). Data were analyzed using a student’s t-test or, where indicated, by ANOVA followed by a Tukey post-test. Differences were considered significant when p<0.05.
Results
Systemic disease is reduced in vacvG-immunized gld mice after RSV challenge
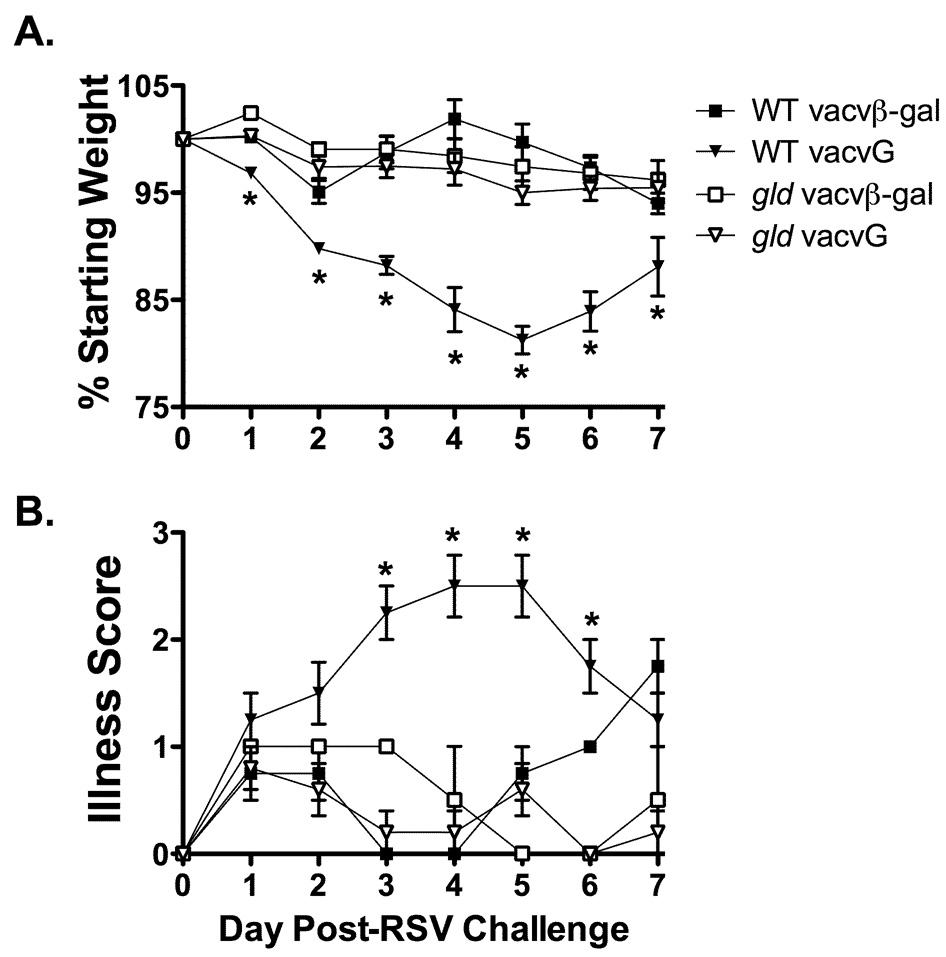
BALB/c mice exhibit weight loss and clinical illness following an acute RSV infection (19). Previous work has demonstrated that gld mice exhibit reduced weight loss and clinical illness following an acute RSV infection as compared to WT controls (19). Compared to mice undergoing an acute RSV infection, vacvG-immunized mice exhibit exacerbated weight loss and clinical illness (6, 22, 24). To determine the role of FasL in the development of weight loss and clinical illness in G-primed mice, WT and gld mice were immunized with either vacvβ-gal or vacvG followed 21 days later by RSV challenge. After RSV challenge, WT vacvG-immunized mice lost significantly more (p<0.05) weight as compared to vacvβ-gal-immunized controls peaking between day 4 and 5 post-RSV challenge (Figure 1A). This peak in weight loss also correlates with the peak in clinical illness in WT vacvG-immunized mice (Figure 1B). In contrast vacvG-immunized gld mice did not exhibit significantly enhanced weight loss or clinical illness (p>0.05) after RSV challenge as compared to vacvβ-gal-immunized gld controls (Figure 1A, B). These data indicate that FasL is required for the development of systemic disease after RSV challenge of mice previously immunized with vacvG.
FIGURE 1.
Reduced weight loss and clinical illness in vacvG-immunized gld mice after RSV challenge. WT or gld BALB/c mice were immunized with 3×106 PFU of either vacvβ-gal or vacvG and 3 weeks later challenged i.n. with 3×106 PFU of RSV. Weight loss (A) and relative illness scores (B) were recorded daily. Representative data from 1 of 4 individual experiments is shown with an n=3–4 mice per group. Error bars represent the standard error of the mean. *, significantly different than WT vacvG-immunized mice (p<0.05) as determined by ANOVA.
The gld mice exhibit reduced pulmonary eosinophilia and CD4 T cell responses
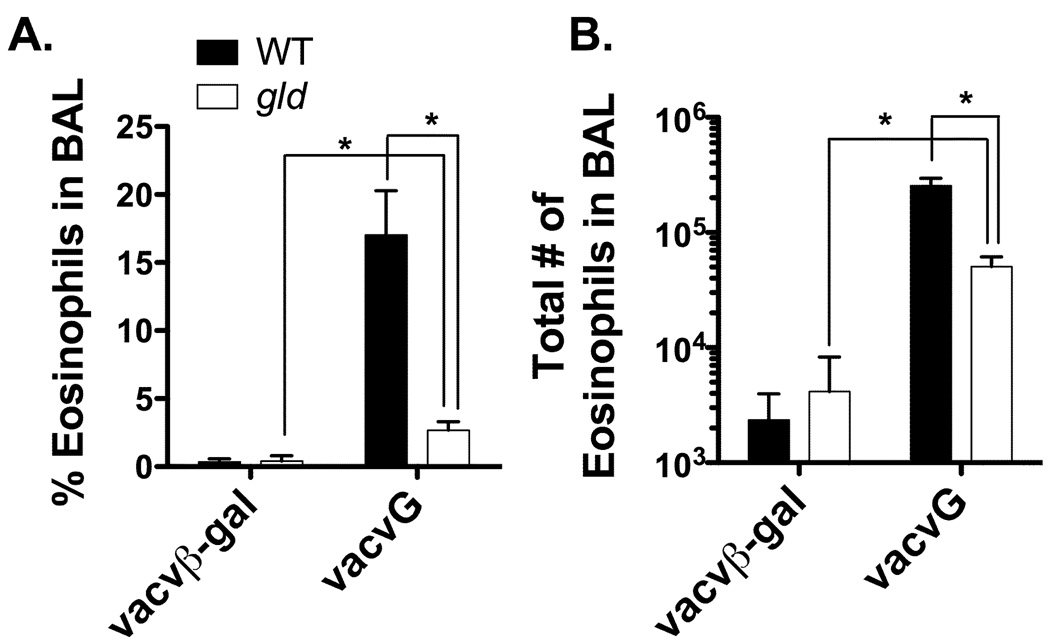
CD4 T cells play a prominent role in the development of RSV vaccine-enhanced disease by inducing systemic disease and pulmonary pathology (7, 8). For instance, adoptive transfer of in vitro stimulated CD4 T cells from vacvG-immunized mice results in both weight loss and the development of pulmonary eosinophilia after RSV challenge (25). Furthermore, depletion of Vβ14+ CD4 T cells from vacvG-immunized mice results in decreased pulmonary eosinophilia and systemic disease (8). As noted above, vacvG-immunized gld mice exhibited decreased systemic disease as compared to their WT counterparts. We therefore questioned if vacvG-immunized gld mice would also exhibit decreased levels of pulmonary eosinophilia. Figure 2 demonstrates that neither vacvβ-gal-immunized WT nor gld mice develop pulmonary eosinophilia after RSV challenge. WT mice immunized with vacvG develop extensive pulmonary eosinophilia after RSV challenge, but interestingly the frequency (Figure 2A) and total number (Figure 2B) of eosinophils in the BAL is significantly (p<0.05) reduced in vacvG-immunized gld mice. However, there is a significant increase (p<0.05) in both the frequency (Figure 2A) and total number of eosinophils (Figure 2B) in vacvG-immunized gld mice as compared to vacvβ-gal-immunized gld control mice.
FIGURE 2.
Reduced pulmonary eosinophilia in vacvG-immunized gld mice after RSV challenge. WT or gld BALB/c mice were immunized with 3×106 PFU of either vacvβ-gal or vacvG and 3 weeks later challenged i.n. with 3×106 PFU of with RSV. BAL was harvested from all mice at day 7 post-RSV challenge and analyzed for the (A) frequency and (B) total number of eosinophils by quantitative morphometry. Representative data from 1 of 4 individual experiments is shown with an n=3–4 mice per group. Error bars represent the standard error of the mean. *, significantly different than WT vacvG-immunized mice (p<0.05).
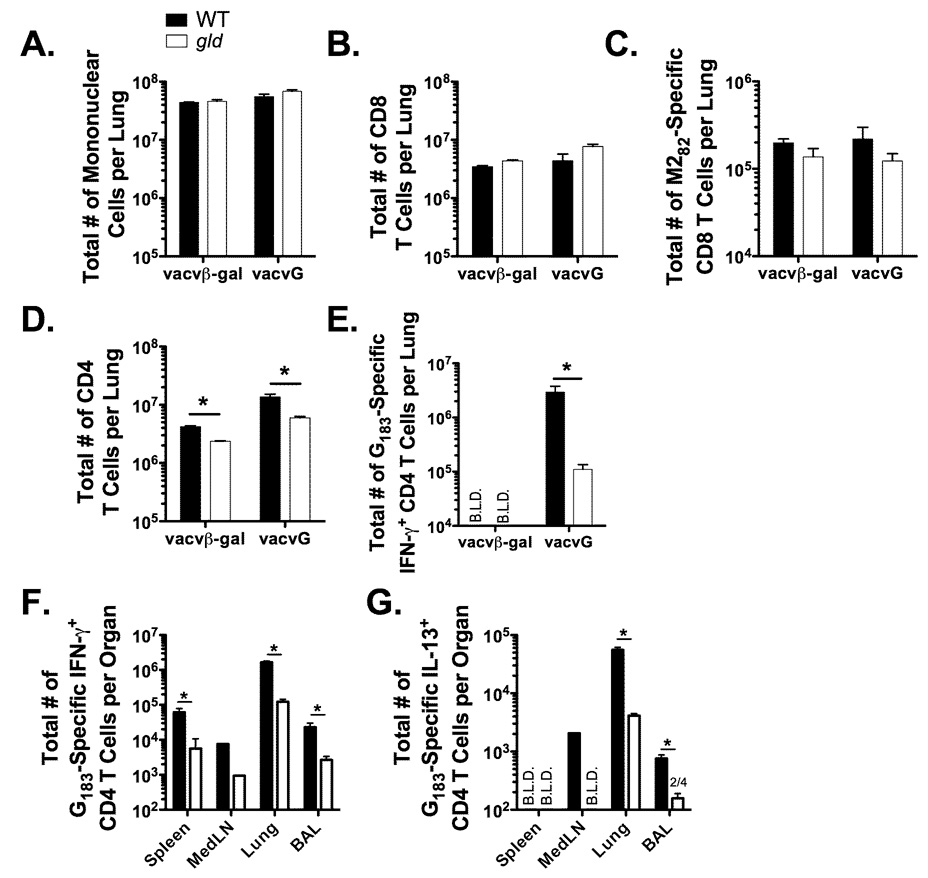
Because T cells are associated with both systemic disease and pulmonary pathology, we examined the T cell responses following RSV challenge of vacv-immunized WT and gld mice. Total mononuclear cell infiltration was similar in both vacvβ-gal and vacvG-immunized WT and gld mice at day 7 post-RSV challenge (Figure 3A). The RSV G protein contains a single CD4 T cell epitope that lies between amino acids 183–195 (26). The RSV M2 protein contains an immunodominant CD8 T cell epitope that lies between amino acids 82–90 (27). The total number of CD8 T cells (Figure 3B) and RSV M282-specific CD8 T cells (Figure 3C) was similar in WT and gld mice. However, there was a significant (p<0.05) reduction in the total number of CD4 T cells (Figure 3D) and RSV G183-specific CD4 T cells (Figure 3E) in the lungs of vacvG-immunized gld mice as compared to WT controls. The total number of CD4 T cells in the lungs is decreased 2- to 3-fold in vacvG-immunized gld mice as compared to vacvG-immunized WT controls (Figure 3D). However, the total number of RSV G183-specific CD4 T cells in the lung is decreased approximately 10-fold in vacvG gld mice as compared to vacvG-immunized WT controls (Figure 3E). These data indicate that the decrease in CD4 T cells in vacvG-immunized gld mice is largely antigen-specific. To determine if the decrease in the total number of RSV G183-specific CD4 T cells was observed only in the lung, we examined the total number of these cells in other tissues. There were also significantly (p<0.05) fewer G183-specific CD4 T cells in the spleen, MedLN, and BAL in vacvG-immunized gld mice as compared to vacvG-immunized WT controls. These data suggest that the magnitude of the secondary RSV-specific CD4 T cell response is decreased in mice lacking functional FasL.
FIGURE 3.
Memory G183-specific CD4 T cell responses are diminished in vacvG-immunized gld mice after RSV challenge. WT or gld BALB/c mice were immunized with either 3×106 PFU of vacvβ-gal or vacvG and 3 weeks later challenged i.n. with 3×106 PFU of RSV. Lung mononuclear cells were harvested at day 7 post-RSV challenge. The total number of (A) lung mononuclear cells (B) CD8 T cells and (D) CD4 T cells was determined by flow cytometry. The total number of (C) M282-specific CD8 T cells and (E) G183-specific CD4 T cells was determined by IFN-γ ICS in the presence of M282–90 or G183–195 peptide and brefeldin A followed by flow cytometric analysis. The total number of (F) IFN-γ-producing and (G) IL-13-producing RSV G183-specific CD4 T cells in the spleen, mediastinal lymph nodes (MedLN), lung and BAL was determined by ICS as described in Panel E. Representative data from 1 of 3 individual experiments is shown with an n=3–4 mice per group. Error bars represent the standard error of the mean. *, significantly different than WT vacvG-immunized mice (p<0.05). B.L.D.= Below the limit of detection (<104 cells).
We have previously demonstrated that IL-13 is required for the development of pulmonary eosinophilia after RSV challenge of mice previously immunized with vacvG (22). We therefore examined the total number of IL-13-producing RSV G183-specific CD4 T cells by ICS after RSV challenge of either WT or gld mice that had been previously immunized with vacvG. gld mice had a significantly reduced (p<0.05) total number of IL-13+ G183-specific CD4 T cells in the lung and BAL at day 7 post-RSV challenge (Figure 3G). The total number of IL-13-producing G183-specific CD4 T cells fell below the level of detection (<1×104 cells) in the MedLN in gld mice as compared to WT controls (Figure 3G). We were unable to detect IL-13-producing RSV G183-specific CD4 T cells in the spleens of both vacvG-immunized WT and gld mice (Figure 3G). Interestingly, the decrease in the total number of IFN-γ-producing (Th1) and IL-13-producing (Th2) cells is similar (~10-fold) in gld mice as compared to WT mice (Figure 3F, 3G) suggesting that FasL plays a similar role in the expansion of these two different CD4 T cell subsets.
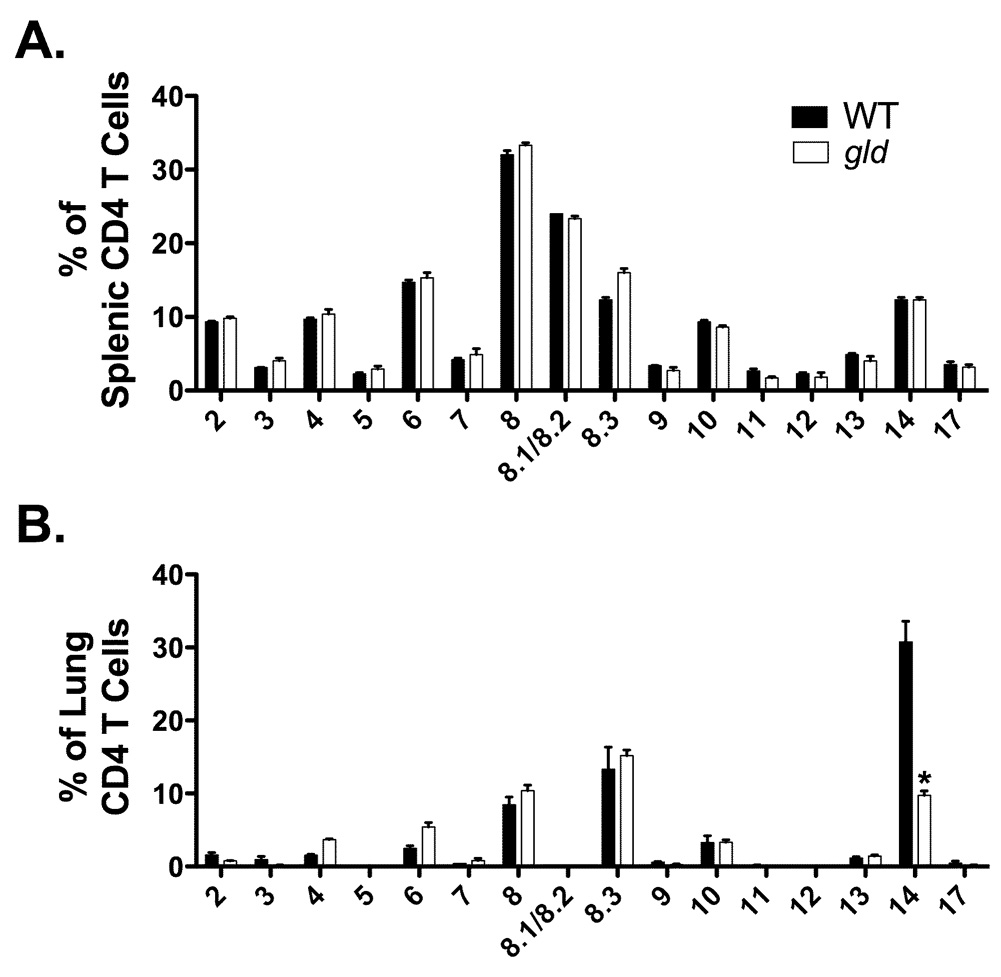
Previous work has demonstrated that RSV challenge of mice previously immunized with vacvG elicits a RSV G183-specific memory CD4 T cell response that is largely oligoclonal and expresses the Vβ14 chain of the TCR (8). Furthermore, these Vβ14+ CD4 T cells are required for the development of RSV vaccine-enhanced disease (8). Figure 1 and Figure 2 demonstrated that vacvG-immunized gld mice exhibited diminished systemic disease as well as decreased numbers of eosinophils in the lung airway as compared to WT controls. We therefore hypothesized that the Vβ14+ CD4 T cell response would also be diminished in these mice. Figure 4 demonstrates that both naïve WT and gld mice exhibit a similar distribution of Vβ-usage among naïve CD4 T cells in the spleen as there were not enough cells in the lung of naïve mice to analyze (Figure 4A). However, after RSV challenge of vacvG-immunized mice there was a significant (p<0.05) decrease in the frequency (Figure 4B) of TCR Vβ14+ CD4 T cells in the lung. Notably, this decrease in Vβ14+ CD4 T cells was not compensated for by expression of alternative Vβ chains (Figure 4B). Taken together, these data strongly suggest a role for FasL in the expansion or survival of secondary memory CD4 T cells. These data also demonstrate that the lack of systemic disease and pulmonary pathology in gld mice correlates with a decreased number of disease-causing Vβ14+ CD4 T cells (8).
FIGURE 4.
Vβ chain usage in naive and in pulmonary CD4 T cells in WT and gld mice. (A) Naïve, splenic CD4 T cells from either WT or gld mice were stained with a panel of Vβ antibodies and analyzed by flow cytometry. Representative data from 1 of 2 individual experiments is shown with an n=3–4 mice per group. Error bars represent the standard error of the mean. (B) WT or gld BALB/c mice were immunized with 3×106 PFU of vacvG and 3 weeks later challenged i.n. with 3×106 PFU of RSV. Lung mononuclear cells were harvested from these mice at day 7 post-RSV challenge and CD4 T cells were stained with a panel of Vβ antibodies and analyzed via flow cytometry. Representative data from 1 of 3 individual experiments is shown with an n=3–4 mice per group. Error bars represent the standard error of the mean. *, significantly different than WT vacvG-immunized mice (p<0.05)
Only RSV-specific memory CD4 T cell responses are defective in gld mice
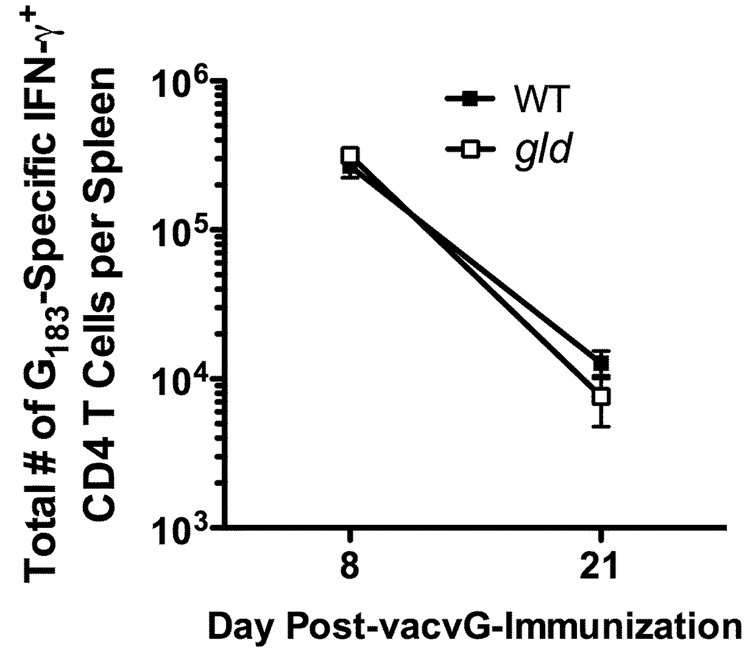
We have demonstrated that the secondary RSV G183-specific CD4 T cell response in the lung is dramatically decreased in vacvG-immunized gld mice as compared to WT mice (Figure 3E). To further elucidate the mechanism controlling the decreased total number of secondary RSV G183-specific CD4 T cells in vacvG-immunized gld mice after RSV challenge, we quantified the total number of G183-specific CD4 T cells early after vacvG immunization (day 8) and just prior to RSV challenge (day 21 after vacvG-immunization). There were similar total numbers of RSV G183-specific CD4 T cells in the spleens of WT and gld vacvG-immunized mice at both 8 and 21 days after vacvG-immunization (Figure 5). These data suggest that there is no defect in the ability to generate a primary RSV G183-specific CD4 T cell response in the absence of functional FasL. These data instead indicate that FasL is required for the generation of the RSV G183-specific secondary memory CD4 T cell response.
FIGURE 5.
Primary RSV G183-specific CD4 T cell responses in vacvG-immunized WT and gld mice. WT and gld BALB/c mice were immunized with 3×106 PFU vacvG by scarification. Eight and 21 days post-vacvG immunization, spleens were harvested and splenic cells were incubated in the presence of G183–195 peptide and BFA for 5 hours. The total number of RSV G183-specific CD4 T cells was analyzed by IFN-γ ICS. Representative data from 1 of 2 individual experiments at day 8 and 1 of 3 individual experiments at day 21 post-vacvG immunization is shown with an n=3–4 mice per group. Error bars represent the standard error of the mean.
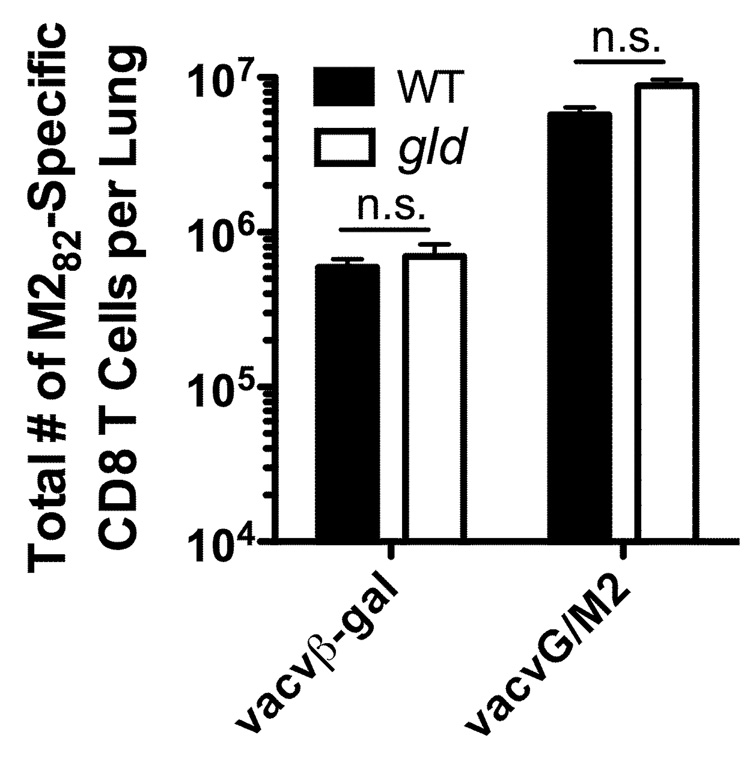
Our earlier results also demonstrated that the generation of a primary RSV M282-specific CD8 T cell response is unaltered in the lung after RSV challenge of either vacβ-gal or vacvG-immunized WT and gld mice (Figure 3C). Therefore, we next questioned if the generation of a memory CD8 T cell response also requires FasL. In these experiments, mice were immunized with a recombinant vacv that expresses a chimeric G protein that contains the immunodominant M282–90 CD8 T cell epitope (vacvG/M2) (28). Mice previously immunized with vacvG/M2 generate a robust M282-specific CD8 T cell response after RSV challenge (9, 28). In contrast to the RSV G183-specific CD4 T cell response, neither the primary (vacvβ-gal-immunized) nor the memory (vacvM2-immunized) M282-specific CD8 T cell response was significantly altered (p>0.05) in gld mice as compared to WT mice (Figure 6). The RSV G183-specific CD4 T cell response was not significantly different in WT or gld vacvG/M2-immunized mice (data not shown). This is likely due to the already decreased total number of RSV G183-specific CD4 T cells observed in vacvG/M2-immunized mice after RSV challenge (9). These data suggest that CD4, but not CD8, T cells require functional FasL to fully expand and/or survive upon secondary exposure to antigen.
FIGURE 6.
Memory RSV M282-specific CD8 T cell responses in WT and gld mice. WT or gld BALB/c mice were immunized with either 3×106 PFU of vacvβ-gal or vacvG and 3 weeks later challenged i.n. with 3×106 PFU of RSV. Lung mononuclear cells were then stained with RSV M282-specific tetramers and Abs to Thy1.2 and CD8 and the total number of M282-specific CD8 T cells was determined by flow cytometry. Representative data from 1 of 2 individual experiments is shown with an n=3–4 mice per group. Error bars represent the standard error of the mean. n.s.= not significantly different (p>0.05).
Viral titers in vacv-immunized WT and gld mice
As described above, we observed a dramatic decrease in the total number of RSV G183-specific CD4 T cells in the lungs of gld vacvG-immunized mice as compared to their WT counterparts after RSV challenge. Because the clearance of RSV is delayed after acute infection of gld mice, we determined if viral clearance was similar after RSV challenge of WT and gld mice that had been previously immunized with vacvG. As demonstrated previously (9), vacvG-immunized WT mice clear virus more efficiently than vacvβ-gal-immunized mice at day 4 post-RSV challenge (Figure 7). Furthermore, RSV is completely cleared from the lungs of both WT vacvG- and vacvβ-gal immunized mice by day 7 post-RSV challenge (Figure 7). In contrast, vacvG-immunized gld mice exhibited significantly higher (p<0.05) virus titers than WT mice at day 4 post-infection. In contrast to WT mice, we were still able to detect virus in the lungs of vacvG-immunized gld mice at day 7 post-RSV challenge. Likewise, vacvβ-gal-immunized gld mice failed to clear virus by day 7 post-infection (Figure 7). These data suggest that the diminished CD4 T cell response observed in vacvG-immunized gld mice correlates with delayed viral clearance.
FIGURE 7.
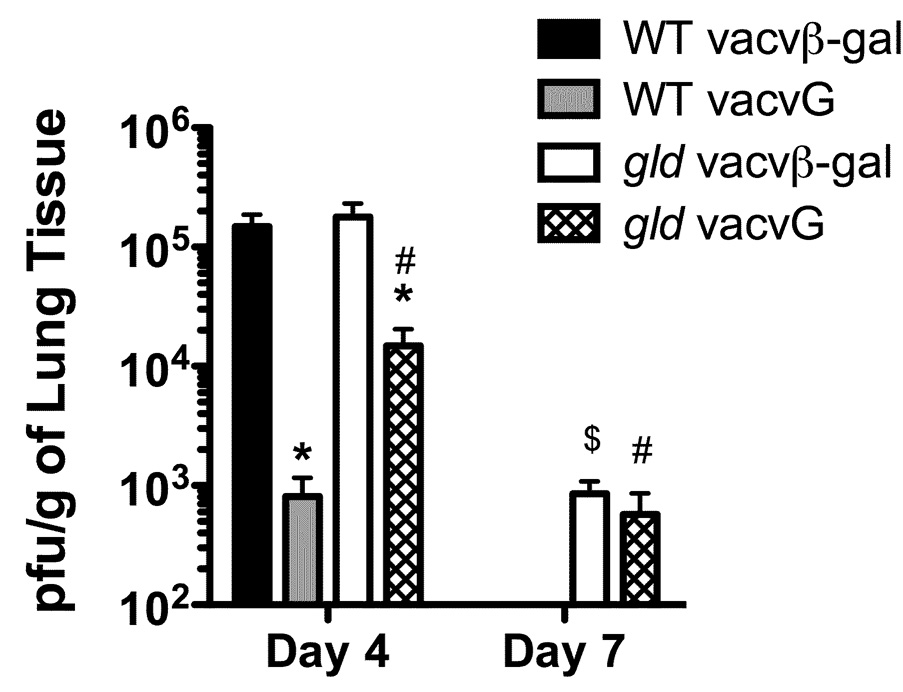
Viral clearance in vacv-immunized WT and gld mice. WT or gld BALB/c mice were immunized with 3×106 PFU of either vacvβ-gal or vacvG and at least 3 weeks later challenged i.n. with 3×106 PFU of RSV. Lungs were harvested at either day 4 or 7 post-RSV challenge, homogenized, and subsequently snap frozen in liquid nitrogen until analysis by plaque assay on Vero cells. Pooled data from 3 individual experiments with an n= 3–4 mice per group in each individual experiment is shown. Error bars represent the standard error of the mean. *, significantly different than any vacvβ-gal-immunized mice (p<0.05). #, significantly different than WT vacvG-immunized mice (p<0.05). $, significantly different than WT vacvβ-gal-immunized mice (p<0.05) as determined by ANOVA.
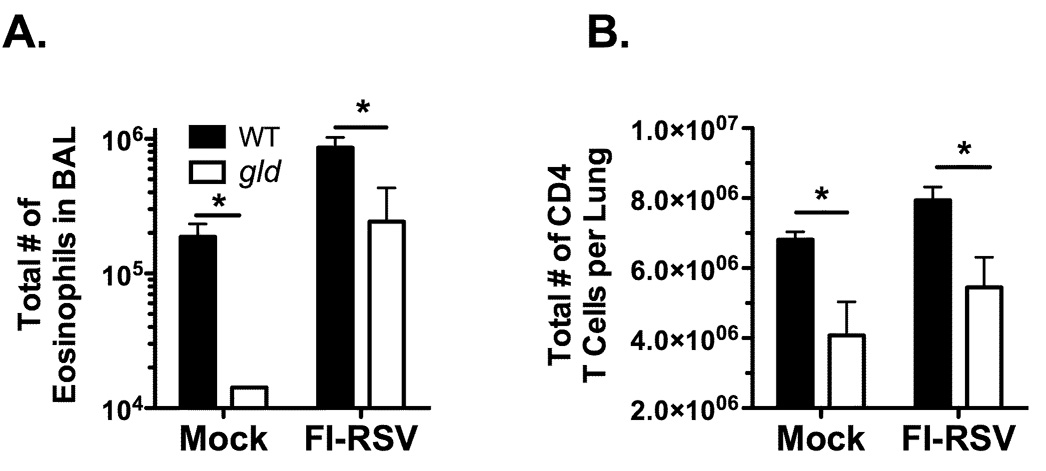
Decreased eosinophilia and CD4 T cell responses in FI-RSV-immunized gld mice
Our results depicted in Figure 4B demonstrate a reduced Vβ14+ CD4 T cell response in vacvG-immunized gld mice as compared to their WT counterparts. Previous work has shown that this Vβ14+ CD4 T cell response is required for the development of pulmonary eosinophilia after RSV challenge of vacvG-immunized mice, but not in mice previously immunized with FI-RSV (8, 29). Therefore, we questioned if gld mice immunized with FI-RSV would also develop reduced pulmonary eosinophilia and CD4 T cell responses after RSV challenge as compared to FI-RSV-immunized WT mice. As expected, WT FI-RSV-immunized mice develop extensive pulmonary eosinophilia after RSV challenge as compared to mock-immunized controls (Figure 8A). In contrast, gld mice previously immunized with either FI-RSV or a mock control exhibited a significant (p<0.05) reduction in the total number of eosinophils in the BAL after RSV challenge as compared to their WT counterparts (Figure 8A). The specificity of CD4 T cells in FI-RSV immunized mice after RSV challenge is currently unknown (29). Therefore we measured the total number of CD4 T cells in the lung at day 7 post-RSV challenge of either mock- or FI-RSV-immunized mice. Consistent with our observations in vacvG-immunized WT and gld mice (Figure 3D), there was a significant reduction (p<0.05) in the total number of CD4 T cells in the lungs after RSV challenge of both mock- and FI-RSV-immunized gld mice as compared to their WT counterparts (Figure 8B). Taken together, these data suggest that the decreased memory CD4 T cell response observed after RSV challenge of either vacvG- or FI-RSV-immunized gld mice is independent of the antigen specificity of the memory CD4 T cells.
FIGURE 8.
Reduced numbers of pulmonary eosinophils and CD4 T cells in FI-RSV-immunized gld mice after RSV challenge. WT or gld mice were intramuscularly immunized with a 1:200 dilution of a mock (formalin-inactivated HEp 2 cell supernatant) or a FI-RSV preparation. Four weeks after immunization all mice were challenged i.n. with 3×106 PFU of RSV. (A) At day 7 after RSV challenge, BALs were harvested and analyzed for the presence of eosinophils by quantitative morphometry. (B) Lung mononuclear cells were also harvested at day 7 post-RSV challenge and analyzed for the number of CD4 T cells by flow cytometry. Representative data from 1 of 2 individual experiments is shown with an n=3–4 mice per group. Error bars represent the standard error of the mean. *, p<0.05 significantly different that WT FI-RSV-immunized mice.
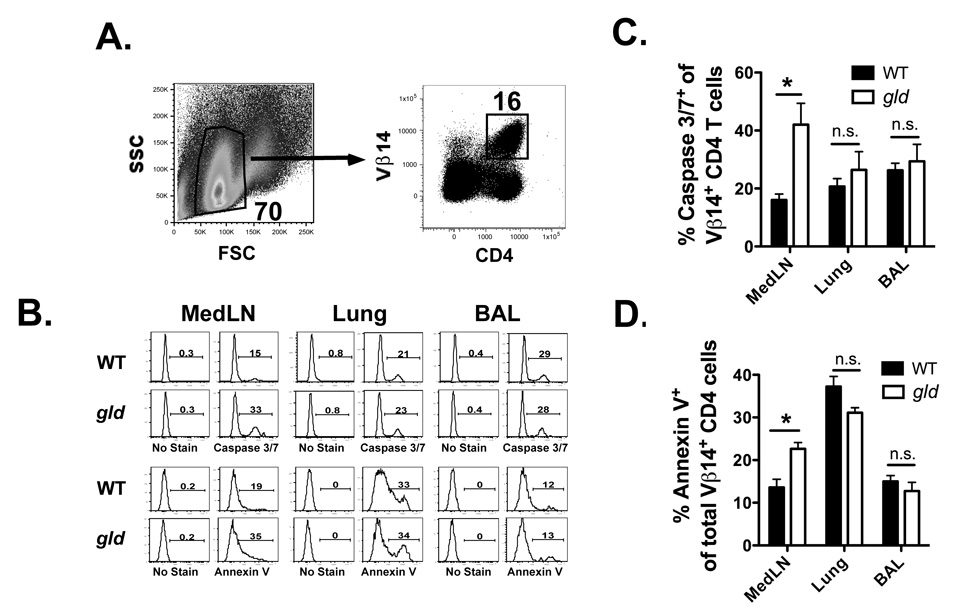
Increased frequency of apoptotic CD4 T cells in secondary lymphoid tissues of gld mice
The gld mice exhibit decreased total numbers of RSV G183-specific CD4 T cells after RSV challenge as compared to WT mice (Figure 3E). It is unclear if this difference is due to the inability of FasL-deficient memory CD4 T cells to either fully expand or survive after RSV challenge. The Vβ14 chain of the TCR is expressed on the majority of RSV G183-specific CD4 T cells and has been previously used as a surrogate to identify RSV G183-specific CD4 T cells after RSV challenge of vacvG-immunized mice (30). To determine if Vβ14+ CD4 T cells fail to accumulate after RSV challenge due to enhanced apoptosis, we measured the frequency of apoptotic Vβ14+ CD4 T cells in either vacvG-immunized WT or gld mice after RSV challenge. Figure 9 demonstrates that a greater frequency (p<0.05) of Vβ14+ CD4 T cells in the MedLN of gld mice stained positive for activated caspases 3/7 and Annexin V, indicating that a greater proportion of these cells were apoptotic as compared to WT mice at day 5 after RSV challenge (Figure 9B, C, D). However, there was no significant difference (p>0.05) between the frequency of either caspase 3/7+ or Annexin V+ Vβ14+ CD4 T cells in either the lung or the BAL. Taken together these data suggest that secondary effector CD4 T cells lacking functional expression of FasL fail to expand to their full capacity because of an increased rate of apoptosis in the draining lymph nodes.
FIGURE 9.
RSV G183-specific CD4 T cells in gld mice exhibit enhanced apoptotic phenotype. BALB/c WT or gld mice were immunized with 3×106 PFU of vacvG and 21 days later challenged i.n. with 3×106 PFU of RSV. Lymphocytes from the mediastinal lymph nodes (MedLN), lung and BAL were harvested at day 5 after RSV challenge. Cells isolated from the lung, and BAL were analyzed from each animal. Cells from the MedLN were analyzed as a pool representing 3 or 4 animals per group in each individual experiment. (A) Dot plots represent the gating strategy used to identify Vβ14+ CD4 T cells. Lymphocytes were gated based on forward scatter (FSC) and side scatter (SSC) properties. These cells were subsequently gated on Vβ14+ CD4 T cells. Depicted are representative dot plots generated from the lung of vacvG-immunized WT mice at day 5 post-RSV challenge. (B) Representative staining of caspase 3/7 (top panel) or Annexin V (bottom panel) on Vβ14+ CD4 T cells from the MedLN, lung, or BAL. (C) Quantification of caspase 3/7 staining or (D) Annexin V staining on Vβ14+ CD4 T cells. Representative data from 1 of 3 individual experiments is shown with an n=3–4 mice per group. Error bars represent the standard error of the mean. *, p<0.05.
Discussion
CD4 T cells are critical for mediating the development of RSV vaccine-enhanced disease including pulmonary eosinophilia and systemic disease (as measured by weight loss) (6–8, 25, 31). We demonstrate here that FasL is required for the development of RSV vaccine enhanced disease. Both FI-RSV- and vacvG-immunized gld mice exhibit reduced levels of pulmonary eosinophilia as compared to their WT counterparts (Figure 1 and Figure 3). Furthermore, vacvG-immunized gld mice have dramatically decreased numbers of pulmonary RSV G183-specific IFN-γ- and IL-13-producing CD4 T cells as compared to WT mice after RSV challenge (Figure 3). Taken together these data suggest that FasL is required for the expansion and/or the survival of RSV-specific secondary effector CD4 T cells that are necessary for the development of RSV vaccine-enhanced disease.
FasL has been demonstrated to play a role in the development of immunopathology and viral clearance after acute RSV infection. gld mice lose significantly less weight and exhibit a delay in viral clearance after acute infection as compared to WT mice (19). Interestingly, this is also accompanied by prolonged production of inflammatory chemokines and IFN-γ, but not TNF-α. It is currently unclear how these specific inflammatory mediators are elicited in the absence of functional FasL or how these differences play a role in viral clearance and of systemic disease. In a CD8 T cell adoptive transfer system, RSV-specific CD8 T cells lacking functional FasL did not differ in their ability to either induce immunopathology or clear virus as compared to WT CD8 T cells, suggesting that CD8 T cell expression of FasL is not required for either CD8 T cell-mediated immunopathology or reduction of viral load (32). However, our data suggests that FasL expression on CD4 T cells may play a role in the induction of CD4 T cell-mediated immunopathology.
The reduced immunopathology, decreased level of pulmonary eosinophilia, and delayed viral clearance exhibited by vacvG-immunized gld mice correlates with the diminished magnitude of the secondary RSV G183-specific CD4 T cell response after RSV challenge as compared to WT controls (Figure 3E). Interestingly, vacvG-immunized WT and gld mice have similar total numbers of RSV G183-specific CD4 T cells 8 and 21 days post-vacvG immunization (Figure 5). These data suggest that the secondary expansion of RSV-specific memory CD4 T cells requires functional FasL. However, it is currently unclear if FasL expression on RSV-specific memory CD4 T cells is required for their full secondary expansion after RSV challenge, or if FasL expression on other cells (i.e. dendritic cells) is required for this full expansion. Legge and Braciale (18) demonstrated that IL-12p40-regulated expression of FasL on lymph node dendritic cells suppressed proliferation of influenza virus-specific CD8 T cells after influenza virus infection. Furthermore, gld mice infected with influenza virus exhibited an enhanced CD8 T cell response as compared to WT control mice (18). This contrasts with our data demonstrating that the secondary RSV G-specific CD4 T cell response is suppressed in the absence of FasL (Figure 3). These results may suggest that FasL expression on lymph node DCs does not directly affect the secondary RSV G-specific response, but rather FasL expression on RSV G-specific CD4 T cells is important for their secondary expansion and survival.
Previous studies have demonstrated that both Fas-mediated caspase cleavage and bi-directional signaling through FasL are required for full proliferative responses of T cells in various systems (16, 33–39). In Figure 9 we examined the frequency of apoptotic Vβ14+ CD4 T cells after RSV challenge of vacvG-immunized WT or gld mice. An enhanced frequency of Vβ14+ CD4 T cells in the MedLN of gld mice after RSV challenge exhibit activated caspase 3/7 and are AnnexinV+ as compared to their WT counterparts suggesting that a higher frequency of these cells are apoptotic. Interestingly, there was no difference in the frequency of apoptotic Vβ14+ CD4 T cells in either the lungs or the BAL of vacvG-immunized WT or gld mice after RSV challenge (Figure 9). Wissinger et al (30) demonstrated that memory RSV G183-specific CD4 T cells, as identified by their expression of the Vβ14 TCR chain, are re-activated in the lung draining lymph nodes and then traffic to the lungs and proliferate after RSV challenge of mice previously immunized with vacvG. These data may indicate that RSV-specific memory CD4 T cells migrate to the draining lymph nodes and subsequently receive survival signals via FasL prior to their migration to the lung.
Recent work from our laboratory has demonstrated that eosinophils elicited during the development of RSV vaccine-enhanced disease do not contribute to systemic illness (i.e. weight loss or clinical illness) (40). Our current results further support these findings. gld mice exhibit reduced CD4 T cell responses which correlates with reduced weight loss and clinical illness scores indicating that the RSV-specific memory CD4 T cell response is an important determinant in mediating systemic disease in this model (Figure 1 andFigure 3).
In Figure 3 we demonstrated that RSV-specific memory CD4 T cells require FasL for full expansion after RSV challenge. Interestingly, the secondary expansion of RSV-specific memory CD8 T cells did not require the expression of functional FasL (Figure 6). This may be partially explained by differential FasL expression patterns in CD4 and CD8 T cells. Upon primary activation of a CD8 T cell through the TCR, FasL is produced and stored in the secretory lysosome (41). Upon subsequent contact with an infected host cell, these FasL-containing lysosomes are transported to the cell membrane where FasL is subsequently expressed. In this scenario, FasL would be unavailable for signaling upon initial contact with a Fas+ antigen-bearing APC suggesting that CD8 T cells may have evolved other mechanisms to efficiently expand after initial antigen encounter. FasL expression on Th1 CD4 T cell clones is largely on the cell surface after initial antigen activation (41–45). Thus when an antigen-specific memory CD4 T cell encounters antigen a second time, FasL is on the cell surface and receptive to a FasL-induced survival signal. However, previous studies have demonstrated a pronounced role for FasL in the primary expansion of CD8 T cells and a minimal role for FasL expression on the primary expansion of CD4 T cells (15–17). These studies utilized either in vitro stimulation of CD8 T cells isolated from either WT or gld mice or adoptive transfer of a large number (>1×106) of Ova-specific OT-1 TCR transgenic T cells (15, 16). Our studies examined endogenous RSV-specific CD4 and CD8 T cell populations. It is possible that utilizing a high number of TCR transgenic T cells may accentuate a role for FasL in the primary expansion of CD8 T cells that is not present at endogenous T cell precursor frequencies.
gld mice develop a lymphoproliferative disease that results in a large population of CD4 and CD8 double negative (DN) T cells expressing B220 (20). These cells have been demonstrated in vitro to inhibit the proliferation of T cells by inhibiting their ability to respond to IL-2 signals (46). Although this may be occurring in vacvG-immunized gld mice, it only affects RSV-specific memory CD4 T cells, as memory RSV-specific CD8 T cell responses are unaltered (Figure 6). Furthermore, studies examining the regulatory ability of DN T cells have noted that their regulatory ability requires direct activation through their TCR (46, 47). In our experiments, DN T cells did not produce IFN-γ after in vitro stimulation with either G183–195 or M282–90 peptides (data not shown). These data indicate that it is less likely that these B220+ DN T cells are inhibiting RSV-specific memory T cell responses.
Memory CD4 T cells cause immunopathology in a number of model systems including experimental autoimmune encephalomyelitis (EAE). Similar to our data that FasL is required for the development of CD4 T cell-mediated pathology in RSV vaccine-enhanced disease, the severity of EAE in gld mice is markedly reduced as compared to WT controls (48). These data suggest that FasL may be required for full expansion of CD4 T cells that cause immunopathology in other model systems such as EAE. Therefore, therapeutic blockade of FasL in disease states mediated by memory CD4 T cells may reduce immunopathology and improve clinical outcome.
ACKNOWLEDGEMENTS
The authors would like to thank John Harty and Kevin Legge for critical review of this manuscript. Additionally, we would like to thank Stacey Hartwig for her excellent technical support.
This work was supported by National Institutes of Health Grant AI 063520
Footnotes
This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
Abbreviations used in this paper: RSV, respiratory syncytial virus; FI-RSV, formalin inactivated-RSV; vacv, vaccinia virus; G, RSV attachment protein; M2, RSV transcription anti-termination factor; ICS, intracellular cytokine stain; BAL, bronchial alveolar lavage; MedLN, mediastinal lymph node; BFA, brefeldin A; WT, wild-type; DN, double negative; EAE, experimental autoimmune encephalomyelitis.
Literature Cited
- 1.Heilman CA. From the National Institute of Allergy and Infectious Diseases and the World Health Organization. Respiratory syncytial and parainfluenza viruses. J. Infect. Dis. 1990;161:402–406. doi: 10.1093/infdis/161.3.402. [DOI] [PubMed] [Google Scholar]
- 2.Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am. J. Epidemiol. 1969;89:435–448. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- 3.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 4.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 5.Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am. J. Epidemiol. 1969;89:449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- 6.Walzl G, Matthews S, Kendall S, Gutierrez-Ramos JC, Coyle AJ, Openshaw PJ, Hussell T. Inhibition of T1/ST2 during respiratory syncytial virus infection prevents T helper cell type 2 (Th2)- but not Th1-driven immunopathology. J. Exp. Med. 2001;193:785–792. doi: 10.1084/jem.193.7.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson TR, Rothenberg ME, Graham BS. Pulmonary eosinophilia requires interleukin-5, eotaxin-1, and CD4+ T cells in mice immunized with respiratory syncytial virus G glycoprotein. J. Leukoc. Biol. 2008;84:748–759. doi: 10.1189/jlb.0907621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varga SM, Wang X, Welsh RM, Braciale TJ. Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4(+) T cells. Immunity. 2001;15:637–646. doi: 10.1016/s1074-7613(01)00209-6. [DOI] [PubMed] [Google Scholar]
- 9.Olson MR, Varga SM. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. J. Immunol. 2007;179:5415–5424. doi: 10.4049/jimmunol.179.8.5415. [DOI] [PubMed] [Google Scholar]
- 10.Openshaw PJ, Clarke SL, Record FM. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int. Immunol. 1992;4:493–500. doi: 10.1093/intimm/4.4.493. [DOI] [PubMed] [Google Scholar]
- 11.Waris ME, Tsou C, Erdman DD, Zaki SR, Anderson LJ. Respiratory synctial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J. Virol. 1996;70:2852–2860. doi: 10.1128/jvi.70.5.2852-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson TR, Teng MN, Collins PL, Graham BS. Respiratory syncytial virus (RSV) G glycoprotein is not necessary for vaccine-enhanced disease induced by immunization with formalin-inactivated RSV. J. Virol. 2004;78:6024–6032. doi: 10.1128/JVI.78.11.6024-6032.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson TA, Green DR, Griffith TS. Cell death and immune privilege. Int. Rev. Immunol. 2002;21:153–172. doi: 10.1080/08830180212058. [DOI] [PubMed] [Google Scholar]
- 14.Sun M, Fink PJ. A new class of reverse signaling costimulators belongs to the TNF family. J. Immunol. 2007;179:4307–4312. doi: 10.4049/jimmunol.179.7.4307. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki I, Fink PJ. Maximal proliferation of cytotoxic T lymphocytes requires reverse signaling through Fas ligand. J. Exp. Med. 1998;187:123–128. doi: 10.1084/jem.187.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki I, Martin S, Boursalian TE, Beers C, Fink PJ. Fas ligand costimulates the in vivo proliferation of CD8+ T cells. J. Immunol. 2000;165:5537–5543. doi: 10.4049/jimmunol.165.10.5537. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki I, Fink PJ. The dual functions of fas ligand in the regulation of peripheral CD8+ and CD4+ T cells. Proc. Natl. Acad. Sci. U S A. 2000;97:1707–1712. doi: 10.1073/pnas.97.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Legge KL, Braciale TJ. Lymph node dendritic cells control CD8+ T cell responses through regulated FasL expression. Immunity. 2005;23:649–659. doi: 10.1016/j.immuni.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Rutigliano JA, Graham BS. Prolonged production of TNF-alpha exacerbates illness during respiratory syncytial virus infection. J. Immunol. 2004;173:3408–3417. doi: 10.4049/jimmunol.173.5.3408. [DOI] [PubMed] [Google Scholar]
- 20.Nagata S, Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol. Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 21.Peebles RS, Jr, Sheller JR, Collins RD, Jarzecka K, Mitchell DB, Graham BS. Respiratory syncytial virus (RSV)-induced airway hyperresponsiveness in allergically sensitized mice is inhibited by live RSV and exacerbated by formalin-inactivated RSV. J. Infect. Dis. 2000;182:671–677. doi: 10.1086/315783. [DOI] [PubMed] [Google Scholar]
- 22.Castilow EM, Meyerholz DK, Varga SM. IL-13 Is Required for Eosinophil Entry into the Lung during Respiratory Syncytial Virus Vaccine-Enhanced Disease. J. Immunol. 2008;180:2376–2384. doi: 10.4049/jimmunol.180.4.2376. [DOI] [PubMed] [Google Scholar]
- 23.Stevens WW, Kim TS, Pujanauski LM, Hao X, Braciale TJ. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J. Immunol. Methods. 2007;327:63–74. doi: 10.1016/j.jim.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussell T, Pennycook A, Openshaw PJ. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur. J. Immunol. 2001;31:2566–2573. doi: 10.1002/1521-4141(200109)31:9<2566::aid-immu2566>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 25.Alwan WH, Record FM, Openshaw PJ. CD4+ T cells clear virus but augment disease in mice infected with respiratory syncytial virus. Comparison with the effects of CD8+ T cells. Clin. Exp. Immunol. 1992;88:527–536. doi: 10.1111/j.1365-2249.1992.tb06482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varga SM, Wissinger EL, Braciale TJ. The attachment (G) glycoprotein of respiratory syncytial virus contains a single immunodominant epitope that elicits both Th1 and Th2 CD4+ T cell responses. J. Immunol. 2000;165:6487–6495. doi: 10.4049/jimmunol.165.11.6487. [DOI] [PubMed] [Google Scholar]
- 27.Kulkarni AB, Collins PL, Bacik I, Yewdell JW, Bennink JR, Crowe JE, Jr, Murphy BR. Cytotoxic T cells specific for a single peptide on the M2 protein of respiratory syncytial virus are the sole mediators of resistance induced by immunization with M2 encoded by a recombinant vaccinia virus. J. Virol. 1995;69:1261–1264. doi: 10.1128/jvi.69.2.1261-1264.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srikiatkhachorn A, Braciale TJ. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J. Exp. Med. 1997;186:421–432. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson TR, Varga SM, Braciale TJ, Graham BS. Vbeta14(+) T cells mediate the vaccine-enhanced disease induced by immunization with respiratory syncytial virus (RSV) G glycoprotein but not with formalin-inactivated RSV. J. Virol. 2004;78:8753–8760. doi: 10.1128/JVI.78.16.8753-8760.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wissinger EL, Stevens WW, Varga SM, Braciale TJ. Proliferative expansion and acquisition of effector activity by memory CD4+ T cells in the lungs following pulmonary virus infection. J. Immunol. 2008;180:2957–2966. doi: 10.4049/jimmunol.180.5.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alwan WH, Kozlowska WJ, Openshaw PJ. Distinct types of lung disease caused by functional subsets of antiviral T cells. J. Exp. Med. 1994;179:81–89. doi: 10.1084/jem.179.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ostler T, Davidson W, Ehl S. Virus clearance and immunopathology by CD8(+) T cells during infection with respiratory syncytial virus are mediated by IFN-gamma. Eur. J. Immunol. 2002;32:2117–2123. doi: 10.1002/1521-4141(200208)32:8<2117::AID-IMMU2117>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 33.Desbarats J, Wade T, Wade WF, Newell MK. Dichotomy between naive and memory CD4(+) T cell responses to Fas engagement. Proc. Natl. Acad. Sci. U S A. 1999;96:8104–8109. doi: 10.1073/pnas.96.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walsh CM, Wen BG, Chinnaiyan AM, O'Rourke K, Dixit VM, Hedrick SM. A role for FADD in T cell activation and development. Immunity. 1998;8:439–449. doi: 10.1016/s1074-7613(00)80549-x. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- 36.Kennedy NJ, Kataoka T, Tschopp J, Budd RC. Caspase activation is required for T cell proliferation. J. Exp. Med. 1999;190:1891–1896. doi: 10.1084/jem.190.12.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alam A, Cohen LY, Aouad S, Sekaly RP. Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells. J. Exp. Med. 1999;190:1879–1890. doi: 10.1084/jem.190.12.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun M, Lee S, Karray S, Levi-Strauss M, Ames KT, Fink PJ. Cutting edge: two distinct motifs within the Fas ligand tail regulate Fas ligand-mediated costimulation. J. Immunol. 2007;179:5639–5643. doi: 10.4049/jimmunol.179.9.5639. [DOI] [PubMed] [Google Scholar]
- 39.Sun M, Ames KT, Suzuki I, Fink PJ. The cytoplasmic domain of Fas ligand costimulates TCR signals. J. Immunol. 2006;177:1481–1491. doi: 10.4049/jimmunol.177.3.1481. [DOI] [PubMed] [Google Scholar]
- 40.Castilow EM, Legge KL, Varga SM. Cutting edge: Eosinophils do not contribute to respiratory syncytial virus vaccine-enhanced disease. J. Immunol. 2008;181:6692–6696. doi: 10.4049/jimmunol.181.10.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janssen O, Sanzenbacher R, Kabelitz D. Regulation of activation-induced cell death of mature T-lymphocyte populations. Cell Tissue Res. 2000;301:85–99. doi: 10.1007/s004419900155. [DOI] [PubMed] [Google Scholar]
- 42.Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 43.Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 44.Janssen O, Stocker A, Sanzenbacher R, Oberg HH, Siddiqi MA, Kabelitz D. Differential regulation of activation-induced cell death in individual human T cell clones. Int. Arch. Allergy. Immunol. 2000;121:183–193. doi: 10.1159/000024316. [DOI] [PubMed] [Google Scholar]
- 45.Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, et al. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature. 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 46.Hamad AR, Mohamood AS, Trujillo CJ, Huang CT, Yuan E, Schneck JP. B220+ double-negative T cells suppress polyclonal T cell activation by a Fas-independent mechanism that involves inhibition of IL-2 production. J. Immunol. 2003;171:2421–2426. doi: 10.4049/jimmunol.171.5.2421. [DOI] [PubMed] [Google Scholar]
- 47.Ford McIntyre MS, Young KJ, Gao J, Joe B, Zhang L. Cutting edge: in vivo trogocytosis as a mechanism of double negative regulatory T cell-mediated antigen-specific suppression. J. Immunol. 2008;181:2271–2275. doi: 10.4049/jimmunol.181.4.2271. [DOI] [PubMed] [Google Scholar]
- 48.Waldner H, Sobel RA, Howard E, Kuchroo VK. Fas- and FasL-deficient mice are resistant to induction of autoimmune encephalomyelitis. J. Immunol. 1997;159:3100–3103. [PubMed] [Google Scholar]