Abstract
Purpose
We examined the effects of G3139 on the interaction of heparin-binding proteins (e.g., FGF2 and collagen I) with endothelial cells. G3139 is an 18mer phosphorothioate oligonucleotide targeted to the initiation codon region of the Bcl-2 mRNA. A randomized, prospective global Phase III trial in advanced melanoma (GM301) has evaluted G3139 in combination with dacarbazine. However, the mechanism of action of G3139 is incompletely understood, as it is unlikely that Bcl-2 silencing is the sole mechanism for chemo-sensitization in melanoma cells.
Experimental Design
The ability of G3139 to interact with and protect heparin-binding proteins was quantitated. The effects of G3139 on the binding of FGF2 to high affinity cell surface receptors, and the induction of cellular mitogenesis and tubular morphogenesis in HMEC-1 and HUVEC cells were determined.
Results
G3139 binds with picomolar affinity to collagen I. By replacing heparin, the drug can potentiate the binding of FGF2 to FGFR1 IIIc, and it protects FGF from oxidation and from proteolysis. G3139 can increase endothelial cell mitogenesis and tubular morphogenesis of HMEC-1 cells in 3D collagen gels, increases the mitogenesis of HUVEC cells similarly, and induces vessel sprouts in the rat aortic ring model.
Conclusions
G3139 dramatically affects the behavior of endothelial cells. There may be a correlation between this observation and the treatment interaction with LDH observed clinically.
INTRODUCTION
G3139 (Genasense) is an 18mer phosphorothioate (PS) oligonucleotide that is targeted to the initiation codon region of the Bcl-2 mRNA (1). Lipid-mediated transfection of G3139 into a wide variety of tumor cells grown on plastic tissue culture plates can silence Bcl-2 mRNA and protein expression. This has been ostensibly associated with increased sensitivity to a wide variety of chemotherapeutic agents (2).
Recently, a Phase III global, multi-center, randomized trial of DTIC with/without G3139 (GM301) was reported; 771 patients were randomized (3). Strikingly, a strong relationship was found between pre-treatment plasma lactate dehydrogenase (LDH) and overall survival after G3139 treatment (S. Agarwala et al., submitted). G3139 plus DTIC is clearly an active combination (vs. DTIC alone) in patients with baseline LDH < or = 1.1 × the Upper Limit of Normal (ULN; 508 of 771 patients randomized; P = 0.018 for overall survival). The survival difference is even more pronounced for patients with baseline LDH < or = 0.8 × ULN (274 of 771 patients randomized, P = 9 × 10−4; HR = 0.64).
Nevertheless, the mechanism of action of G3139 is not completely clear. It seems unlikely that downregulation of the basal expression of Bcl-2 is the sole mechanism responsible for extensive chemo-sensitization in melanoma cells. This is especially true in light of recent results demonstrating that multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases (4).
Treatment of melanoma cells in tissue culture by lipid-transfected G3139 causes Bcl-2-independent apoptosis via the intrinsic pathway (5), characterized by lack of synergy with such cytotoxic chemotherapeutic agents as cisplatin, taxotere and thapsigargin (6). Bcl-2 silencing by RNAi in melanoma cells also did not produce synergy with cytotoxic chemotherapy (6), although in other laboratories, in vitro synergy with chemotherapy in melanoma cells has been reported (7). The combination of G3139 plus cytotoxic chemotherapy treatment can indeed produce responses in human melanoma xenografts in SCID mice (8). However, experiments with phosphorothioate (PS) oligomers in SCID mice are difficult to interpret with respect to determination of mechanism (8). This is because of the TLR9-active properties of virtually all PS oligomers (9), even those that do not contain CpG motifs; G3139 contains two such motifs. Thus, tumor inhibition due to immunostimulation (leading to increased levels of tumoricidal IFN-α and IL-12) cannot readily be distinguished from tumor inhibition due to gene silencing.
In vivo, the endothelial cell is exposed to steady-state plasma levels of oligonucleotide (for up to five days in the GM301 trial) that may approach 1μM (10). This cell type is critical to the growth and metastasis of tumors, and in tumors is exposed to the highest levels of oligonucleotide (11). However, the effects of G3139 on this cell type have not, to our knowledge, been elucidated. While it is known (12) that compounds closely related to G3139 can bind FGF2, the consequences of such binding have never been explored appropriately. Herein, we present evidence that G3139 may non-specifically bind to several heparin-binding proteins (e.g., FGF2, collagen I) that dramatically affect endothelial cell function. We also demonstrate that G3139 can increase endothelial cell mitogenesis and tubular morphogenesis in 3D collagen gels, and can induce vessel sprout formation in the aortic ring assay.
MATERIALS AND METHODS
Cells
SV40-transformed HMEC (HMEC-1) cells were obtained from the Centers for Disease Control (Atlanta, GA), and grown in MCDB 131 media supplemented with 10% heat inactivated fetal bovine serum, 10 ng/mL EGF, 1 μg/mL hydrocortisone, 100 U/ml penicillin G sodium and 100 μg/ml streptomycin sulfate. The stock culture was maintained at 37°C in a humidified 5% CO2 incubator. BaF3 cells transfected with FGFR1 IIIc (C11 clone) were a kind gift of Dr. D. M. Ornitz (Wash. U. St. Louis) and were grown in RPMI 1640 medium + 10% newborn bovine serum, 0.5 ng/mL murine-recombinant interleukin-3, 2 mM L-glutamine, penicillin-streptomycin, 50 nM β-mercaptoethanol, and G418 (600 mg/mL). The stock cultures were maintained at 37°C in a humidified 5% CO2 incubator.
Materials
Recombinant human FGF2, VEGF165, PDGF BB and HB-EGF were from R&D Systems (Minneapolis, MN). MCDB 131 and FBS were obtained from Invitrogen (Carlsbad, CA). 125I-FGF2 and 125I-VEGF were purchased from PerkinElmer (Boston, MA). The IMUBIND Total TFPI ELISA kit was from American Diagnostica (Stanford, CT). The human FGF2 ELISA kit was purchased from R&D Systems (Minneapolis, MN). Collagen I was from BD Biosciences (Bedford, MA). Rhodamine-phalloidin was from Invitrogen-Molecular Probes (Eugene, OR)
Modification of heparin-binding proteins by ClRNH32P-OdT18
The probe, radiolabeled, alkylating, oligodeoxynucleotide ClRNH32P-OdT18 (“the probe”) was synthesized as per Guvakova, et al (12). Determination of Km for the binding of the probe for PDGF BB, VEGF165 and collagen I was by the method of Yakubov, et al. (13). For Kc determination, either FGF2 (50 nM), PDGF BB (50 nM), VEGF165 (150 nM) or collagen I (30 nM), was incubated in 0.1 M Tris-HCl, pH 7.4, containing 10–20 μM ClRNH32P-OdT18. PS oligo was added at increasing concentrations as competitors of the binding of the probe oligomer. After 1 h at 37° C, SDS-PAGE was performed, and the gel allowed to expose Kodak X-ray film until bands were visualized. These were quantitated by laser densitometry, the IC50 of competition determined, and Kc calculated by the Cheng-Prusoff (14) relationship Kc = IC50/(1 + [ClRNH32P-OdT18]/Kd.
Displacement of extracellular matrix (ECM)-bound 125I-FGF2 by G3139
Bovine eyes were obtained from Morris Insel Cohen (Newark, NJ), and ECM was prepared by previous methods (15). 5% dextran T-40 was included in the growth medium and the cells were incubated for 10–12 days at 37°C in 10% CO2. After NH4OH treatment to remove the corneal cells, the ECM was stored at 4°C.
For release experiments, ECM was incubated (3 h, room temperature) with 125I-FGF2 (2.5 × 104 cpm/0.3 mL/well) in PBS + 0.02% gelatin. Unbound radio-labeled protein was removed by washing × 3 with PBS + 0.02% gelatin, and the ECM was incubated with increasing concentrations of PS oligo at room temperature for 3 h. The amount of released 125I-FGF2 was determined by γ-counting. The remaining ECM was incubated (3 h, 37°C) with 1N NaOH and counted. The percentage of released 125I-FGF2 was calculated from the total ECM-associated radioactivity. For all experiments, measurements were made in duplicate (each duplicate was repeated thrice) with average differences no greater than 5% between the replicates.
Protection of FGF2 by PS oligos against protease digestion
FGF2 or VEGF165 (~0.5 μg in total volume 20 μL, pH7.4) with tracer amounts of 125I-FGF2 or 125I-VEGF165 in the presence or absence of G3139 (5 μM) were equilibrated at 37°C for 5 min. Trypsin or chymotrypsin was added to a final concentration of 1 μg protease/50 μg FGF2 or VEGF165, and digestion was allowed to proceed at 37°C for 4 h. SDS gel sample buffer was then added, the samples were heated at 100°C for 5 min, and SDS-PAGE was performed. The gels were dried and allowed to expose Kodak X-ray film until bands were visualized, which were quantitated by laser densitometry. To air oxidize FGF2, 20 ng/mL in complete media were incubated at 37°C in room air for 3 h. Under these conditions, as described in the Results, all FGF2 activity is lost.
FGF2-induced mitogenesis in C11 cells
C11 (FGFR1 IIIc-overexpressing) cells were washed twice with RPMI media lacking IL-3 and plated at 2.2 × 104 cells per well in 48-well plates. FGF2 (1 nM), PS oligo or heparin (1 μg/mL) were added in a total volume 200 μL. The cells were then incubated for 2–3 days, fixed, and stained with sulforhodamine blue (SRB). The absorbance at λ= 530 nm was taken as proportional to cell number.
Three-Dimensional Collagen I Gels
Collagen I gels were formed by mixing M199 medium containing 10% FBS + penicillin/streptomycin (basal media), 1 N NaOH (0.023 × volume of collagen I) and collagen I at a final concentration of 0.6 mg/mL (4°C). 0.25 mL aliquots of the mixture were dispensed into 24-well plates and allowed to gel at 37°C for 2 h. HMEC-1 cells were seeded onto the collagen I gels (10 × 104 cells/well) in basal media. Three hours later, the attached cells were overlaid with a second layer of collagen I, and 0.5 mL basal media +/− PS was added above the second layer. PS oligomer was added every 3 days.
After 6 days, cells were fixed with 4% paraformaldehyde + 0.25% glutaraldehyde in PBS at room temperature overnight, and permeabilized with 0.2% Triton X-100 for 30 min. After washing, the cells were stained with rhodamine phalloidin (160 nM). Tubular morphogenesis was monitored by confocal microscopy, and quantitated by Image J software (n = 3). To liberate cells, the 3D collagen gels were treated with 0.1% collagenase type I in PBS for 30–40 min at 37°C, washed with PBS and centrifuged. Cells were counted by trypan blue exclusion (n = 3).
Radial Invasion of Matrix by Aggregated Cells (RIMAC)
A modification of the method of Vernon and Sage (16) was employed. Dispersed HMEC-1 cells (180 × 104 cells/mL in basal media) were cultured in 40 μL drops suspended upside down from 12-well plate lids lined with Parafilm M. After 2 days in culture, aggregates of cohesive cells (one aggregate per drop) were transferred into wells (12-well plates) which had been pre-filled with 0.5 mL of a 0.6 mg/mL collagen type I gel. Aggregate transfer was accomplished by depressing, and therefore stretching, the parafilm until the drops containing the cells touched the polymerized collagen I. The cell aggregates were then overlaid with a second layer of collagen gel, and 1 mL basal media with or without either FGF2 (20 ng/mL), or PS oligo (1 μM), or a combination of both, was added above the second layer. The cells were cultured for 2–5 days at 37°C in a humidified 5% CO2 incubator, and photographed daily. The average distances of migration of the HMEC cells into the surrounding collagen, calculated from 9–12 replicates, were averaged to yield the final values of radial invasion.
Confocal Microscopy
Images were collected with a BioRad Radiance 2000 laser scanning confocal microscope (Zeiss, Thornwood, NY) with a Kr/Ar laser for excitation at 568 nm, narrow band filters for emission and a Nikon 20X PlanApo optics on an Eclipse 200 laser-safe microscope. Images were analyzed by the use of Image J software1.
Rat Aortic Ring Assay
Following established procedures (17), thoracic aortas were obtained from CO2-sacrificed 1- to 2-month-old Fisher 344 male rats. Aortas were sectioned into 1 mm rings, rinsed in 12 consecutive washes of serum-free medium, and the rings embedded in 0.6 mL of collagen type I gel (1 mg/mL) in M131 complete media + 10 % FBS. One hour later, the cultures with the embedded aortas (n = 6) were treated with 1 μM G3139, and the media changed, with new G3139 added, every other day. Phase-contrast photomicrographs were taken daily after treatment. The numbers of vessels were counted and their lengths (the five longest vessels in five rings) measured (17) visually with a micrometer scale in the microscope eyepiece. Data is expressed as the average vessel number or length +/− s.d. P values were calculated by the Mann-Whitney test.
Statistics
Unless otherwise mentioned, experiments were performed at least in triplicate, and data are presented as the average +/− the s.d. P values were determined by a two-sided Students t-test with unequal variance, with P < 0.05 considered significant.
RESULTS
PS oligos interact with heparin- binding proteins
We used the Cheng-Prusoff relationship to determine the Kc (competition constant) of binding, of G3139 and other PS oligos to several heparin-binding proteins; these included including FGF2, VEGF165, PDGF-BB and collagen I. The determination of Kc initially required the measurement of the Michaelis constant (KM) of binding to each test protein of a probe oligonucleotide. This probe is an alkylating, 32P-labeled phosphodiester 18mer homopolymer of thymidine (ClRNH32P-OdT18). For FGF2, KM = 0.5 μM (12). As previously demonstrated (18) for the other proteins above, the binding of the probe was approximately saturable, and was described by a single-site binding equation of the Michaelis-Menton type. −1/KM was then determined by double- reciprocal Lineweaver-Burke plots. By least squares analysis, these plots, for each protein were linear. The values of KM for VEGF165, PDGF-BB and collagen I were respectively 34 μM, 4.5 μM and 0.6 μM.
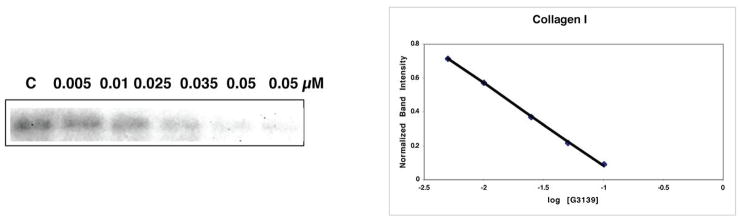
In Fig. 1, competition for probe binding to collagen I (Fig. 1A), FGF2 (Fig. 1B) PDGF-BB (Fig. 1C) and VEGF165 (Fig. 1D) is shown. The IC50s of competition were determined, and the values of Kc for each protein are given in Table I. The Kc of binding of G3139 to FGF2, based on our previous experiments (12) is approximately 50 nM.
Fig. 1.
Competition by G3139 for binding of ClRNH32P-OdT18 to collagen I (A), FGF2 (B), PDGF BB (C) and VEGF (D). G3139 was used as a competitor at the stated concentrations for the binding of the probe (ClRNH32P-OdT18) to the respective heparin-binding proteins, as described in the Materials and Methods (left, 12% PAGE). After determination of the IC50 of competition from the plot of normalized band intensity versus log [G3139] (right), values of Kc were determined by the Cheng-Prusoff equation.
Due to its high lysine content, collagen I is a basic protein. Collagen I, in monomeric form, bound with high affinity to the probe oligonucleotide (KM = 0.6 μM; R2 = 0.95). When G3139 was used as a competitor of probe binding, Kc = 400 pM (R2 = 0.99). This, we believe, is the highest affinity interaction for any non-specific oligonucleotide-protein binding yet determined, and is probably indicative of strong electrostatic interactions between polyanionic PS oligonucleotides and basic collagen I.
The Kc of G3139 competition for the binding of the probe to VEGF165 (Kc = 285 nM, R2 = 0.92; Fig. 1D) is, in contrast, much greater than that of both collagen I and FGF2. Relatively low affinity interactions were also observed between laminin and G3139, (Kc = 115 nM; R2 = 0.97; not shown). HB-EGF also interacted with G3139 (Kc = 20 nM, R2 = 0.99).
G3139 releases 125I-FGF2 from low-affinity binding sites on extracellular matrix
Extracellular matrix contains numerous low-affinity sites (KD = 1–10 nM) that interact with FGF2 (19). The protein can be readily isolated from polyanion-treated matrix (20), and mobilization of matrix-bound FGF2 is thought to promote endothelial proliferation (21).
We adsorbed 125I-FGF2 to bovine corneal endothelial matrix-coated dishes, which were then treated with G3139. The maximal release of 125I-FGF2 was compared to release by SdC28, a 28mer phosphorothioate homopolymer of cytidine: This was arbitrarily assigned a value of 1. The release of 125I-FGF2 bound to the ECM by SdC28 and 2M NaCl were identical. As shown in Fig. 2, release was G3139 concentration-dependent. However, there was little or no release of ECM-bound 125I-VEGF165 by G3139 at any concentration evaluated (up to 100 μM) (not shown). These data are consistent with our previous experiments demonstrating the low affinity of G3139 for VEGF165.
Fig. 2.
Release of ECM-bound 125I-FGF2 by G3139 and T18. ECM-coated wells were incubated (3 h, room temperature) with 125I-FGF2 (2.5 × 104 cpm/well). The ECM was washed 3 times and incubated (3 h, room temperature) with increasing concentrations of G3139 or T18. Released radioactivity is expressed as the percent of total ECM-bound 125I-FGF2, and is compared to release by 25 μM SdC28. Each assay was conducted in duplicate, and the differences in the duplicate measurements were < 10%. Experiments were repeated 3 times.
G3139 potentiates the binding of FGF2 to FGFR1 IIIc (C11 clone)
It was our hypothesis that G3139 could substitute for heparin, and thus increase the mitogenic effects of FGF2 on endothelial cells. FGF2 binds with high affinity to FGFR1 (22); BAF3 cells transfected with FGFR1 IIIc require heparin or heparin-like activity (which on occasion can be found in serum) to proliferate in response to FGF2 (23). As determined by SRB staining, cellular mitogenesis was evaluated in the presence of 1 nM FGF2 and G3139 (1 μM). Proliferation of the transfectants was increased by 3.5 +/− 0.03 fold compared to the non-G3139/non-FGF2 treated cells (n = 3; P = 0.002), and was approximately twice that observed after FGF2 treatment alone (Fig. 3). No increase in proliferation was produced by heparin (1 μg/mL) or G3139 alone (1 μM). Treatment of HMEC-1 cells cultured on plastic with 10–50 ng/mL FGF2 increased proliferation by approximately 40–50% after 6 days. However, the FGF2-induced increase in HMEC-1 cell proliferation was not changed by G3139.
Fig. 3.
G3139 substitutes for heparin, which is required for the biological activity of FGF2. C11 cells were washed twice with media without IL-3, and seeded (2.2 × 104 cells/well in 48-well plates) in media containing 10% FBS but not IL-3. The cells were then treated with 1 nM FGF2 + G3139 (1 μM). After 2 days, proliferation was evaluated by SRB staining. Data are presented as the average +/− S.D, n = 3. [FGF2 + Hep vs. FGF2: P < 0.001; FGF2 + G3139 vs. FGF2: P < 0.002; G3139 or Hep vs. C, P = NS]. C = control, Hep = Heparin.
These experiments definitively demonstrate that G3139 can substitute for heparin in promoting the proliferation of FGF2-dependent cells. In addition, they also prove that G3139, despite its interactions with low-affinity binding sites on FGF2, does not interfere with the binding of this protein to high-affinity (Kd = 2–20 × 10−11 M (24)) cell surface receptors.
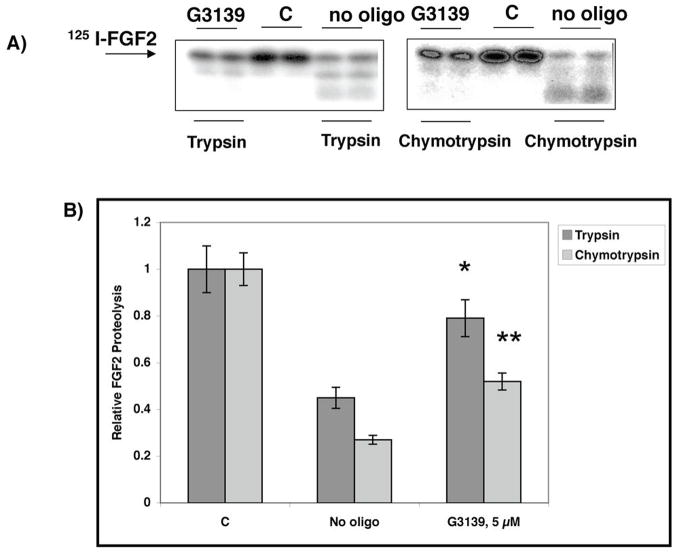
G3139 protects FGF2 from enzymatic digestion and air oxidation
In the interior of a poorly vascularized tumor, cellular necrosis can occur, and necrotic cells can release proteases. Therefore, we wanted to determine the extent to which the binding of G3139 to FGF2 could protect it against proteolytic degradation. Two potent proteases were employed (Fig. 4A,B). 125I-FGF2 was degraded by trypsin (37°C, 2 h, pH 7.4) but was partially protected from cleavage by the protease in the presence of G3139 or other PS oligomers, including L-G3139 (1 μM; not shown), which is the L-enantiomer of D-G3139. FGF2-G3139 complexes were also exposed to chymotrypsin (37°C, 2 h). In the absence of G3139, the FGF2 was degraded by chymotrypsin. In the presence of G3139, the FGF2 was partially protected from degradation. We also investigated the degradation of VEGF165-G3139 complexes by trypsin, and showed that there was no protection (not shown). This experiment is consistent with the low affinity of VEGF165 for G3139 relative to FGF2.
Fig. 4.
Preincubation with G3139 protects FGF2 from degradation by trypsin and chymotrypsin. FGF2 (A, B) (~0.5 μg in 20 μL, pH 7.4) containing tracer amounts of [125I]-FGF2 was digested with either 2% trypsin or chymotrypsin (wt/wt) in the absence or presence of 5 μM G3139 (37°C, 4 h). The digestion products were mixed with sample buffer, boiled for 5 min, and analyzed by 12% SDS-PAGE. Gel bands were quantitated by laser densitometry. Data are presented as the average +/− S.D, n = 3. * = P (vs. no oligo) < 0.05; ** = P (vs. no oligo) < 0.03. C = no trypsin, no G3139.
(C) G3139 protects FGF2 from inactivation at 37°C. FGF2 was incubated at 37°C for 3.5 h with or without 5 μM G3139, and added to the C11 FGFR1c-overexpressing cells (2.2 × 104 cells/well in 48-well plate) in the presence of heparin. Cell proliferation was measured after 3 days by SRB. FGF2 (1 nM) without preincubation at 37°C for 3.5 h was used as a positive control. Data are presented as the average +/− S.D, n = 3. * = P (vs. FGF2 + preincubation at 37°C for 3.5 h) < 0.03.
In addition, after exposure to air at 37°C, FGF is inactivated relatively rapidly, probably due to oxidation at cysteine thiol, as has been demonstrated for FGF1 (25). We incubated FGF2 in complete media at 37°C for 3.5 h with or without 5 μM G3139, and then used this mixture, in the presence of heparin, to treat the FGFR1-transfected BAF3 cells. If FGF2 was not pre-incubated with G3139, it was presumably susceptible to air oxidation; in this case there was no increase in the proliferation of the FGFR1 IIIc-transfectants in the presence of heparin vs. control cells not treated with FGF2. However, if we used FGF2 that had been pre-treated with G3139, FGF2-dependent proliferation threefold vs. control (Fig. 4C), to an essentially normal level.
G3139 stimulates tubular morphogenesis and mitogenesis of HMEC-1 cells in 3D collagen I gels
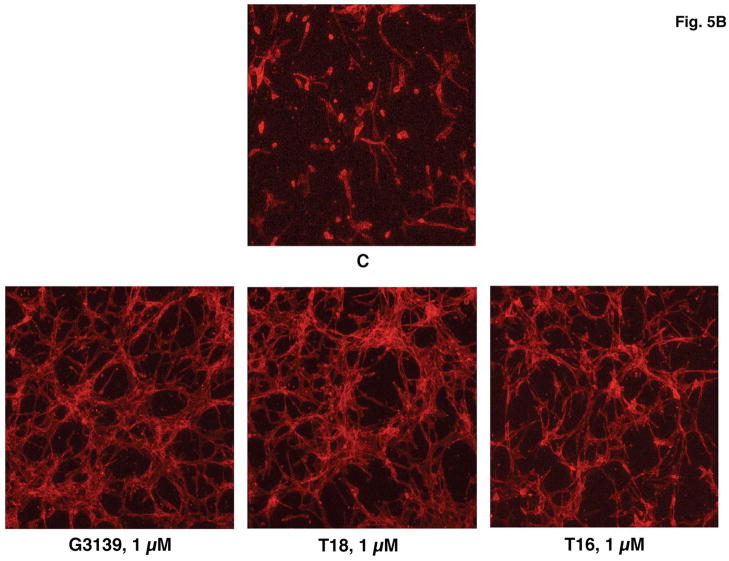
Only minimal morphologic effects or changes in cell proliferation were produced after treatment of HMEC-1 cells on plastic plates or in 2D collagen I or collagen IV gels (up to 40 μM G3139, 6 days). However, in 3D collagen I gels + complete media, the number of HMEC-1 cells, after treatment with 1 μM G3139 every three days for 6 days, increased by 1.8 +/− 0.4 fold (n = 3; P < 0.015) vs. control, non-G3139 treated cells (Fig. 5A). (In the absence of collagen I, the increase in proliferation in similarly treated cells is only approximately 20%). In addition, by 6 days, the G3139-treated, but not the untreated cells underwent tubular morphogenesis (Fig. 5B). Examination of the time course of this effect showed that by 1–2 days the cells extended fine, long apical branches, by 3–4 days the cells lined up, and along these branches; at about the same time, cell body fusion could be observed. By 6–9 days, cells became organized into tube-like structures. After 9–11 days, the tubes began to dissolve. However, the effect is not sequence specific, as T18 is as active as G3139, but it is length dependent as T16 is less active than T18.
Fig. 5.
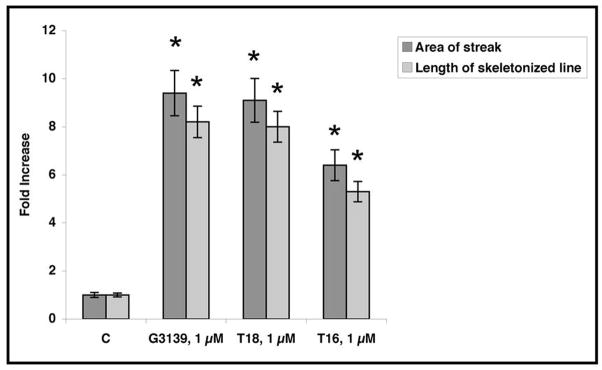
(A) G3139 increases HMEC-1 proliferation in 3D-collagen I gels. Cells (45 × 104 cells/well in 6-well plate) were seeded in 3D collagen I gels (0.6 mg/mL) and treated with G3139 (5 μM). Cells were counted on day 6. Each data point is the average of triplicate wells +/− S.D. Relative HMEC-1 proliferation = 1.8 +/− 0.18 fold, n = 3 [P < 10−2; G3139 (5 μM) vs. C (= no treatment)]. (B) Confocal micrograms of HMEC-1 cells in 3D collagen I gels after 6 days in culture. HMEC-1 cells (10 × 104 cells/well) were seeded in collagen I (0.6 mg/mL) and treated with G3139, T18 or T16 (1 μM), or were untreated (= C). At the end of treatment (6 days), the cells were fixed permeabilized and stained with rhodamine phalloidin (160 nM). (C, D) Image J skeletonization and quantitation of the images in (B). Fold increase of skeletonized line = 9.0+/− 1.0 (1 μM G3139 or 1 μM T18), and 6.0 +/− 0.4 (1 μM T16). * = P (vs. C [control, untreated]) < 0.05 (1 μM G3139 or T18); P (vs. C) < 0.02 (1 μM T16).
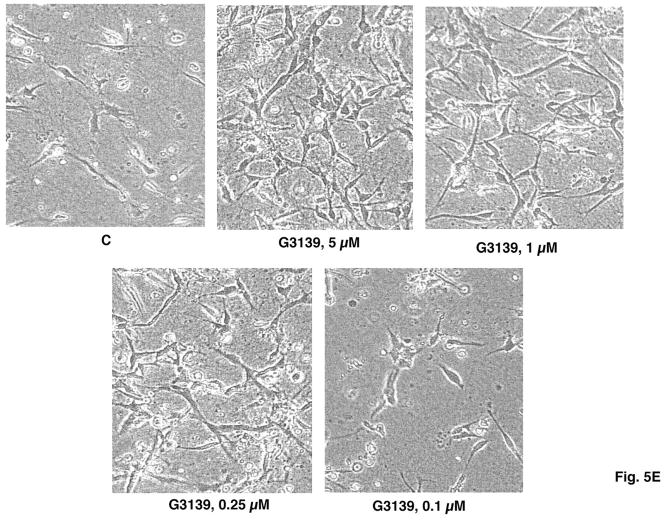
(E) Light microscopic images of the concentration dependence of HMEC-1 tubular morphogeneis in 3D collagen gels as induced after 6 days by G3139. (F, G) Quantitation of skeletonization by Image J of the confocal micrograms of HMEC-1 cells in 3D collagen I gels in (E). Each experiment was performed in triplicate. * = P (vs. C [control, untreated]) < 0.05 (5, 1 or 0.25 μM G3139); 0.1 μM G3139 vs. C, P = NS.
Quantitation of the extent of tubular structure formation in the rhodamine-phalloidin-stained cells, after fixation, was accomplished by confocal microscopy and Image J software. A “streak” pattern was obtained via the initial analysis: This pattern was skeletonized (Fig. 5B) and subsequently quantitated (Fig. 5C). The tubular morphogenesis of HMEC-1 cells in 3D collagen I gels was increased approximately eightfold by either G3139 or T18 (1 μM) after skeletonization vs. the non-G3139 treated control cells (Fig. 5D). The effects of G3139 are highly concentration dependent (Fig. 5E, F, G), and are lost at [G3139] = 0.25 μM.
G3139-protected FGF2 promotes HMEC-1 mitogenesis in 3D collagen I gels, but does not promote radial cellular outgrowth
The ability of FGF2 to induce the mitogenesis of FGFR1 IIIc-transfected BAF-3 cells (C11 cells) was completely lost when the protein was incubated in air at 37°C for 3.5 hours, as described above. When HMEC-1 cells in 3D collagen I gels were incubated with air-oxidized FGF2 (20 ng/mL), mitogenesis was similarly blocked. In contrast, FGF2-induced mitogenesis was restored to normal levels (Fig. 6A, B) when the HMEC-1 cells embedded in collagen I were treated with pre-incubated FGF2. Here, G3139 (5 μM) had been added to the pre-incubation buffer only.
Fig. 6.
G3139-protected FGF2 promotes HMEC-1 mitogenesis in 3D collagen I gels. Cells (10 × 104 cells/well in 24-well plate) were seeded in collagen I (0.6 mg/mL) and treated with either nothing (= C), FGF2, or FGF2 + G3139, or FGF2 pre-incubated in air (37°C, 3.5 h) with or without G3139. The cells were cultured for 7 days, photographed (A), and counted (B). Each data point is the average of triplicate wells +/− S.D. * = P <0.025, FGF2 + (G3139 in pre-incubation mix) vs. FGF2 (without G3139 in pre-incubation mix).
Nevertheless, in the induction of radial invasion of matrix (here 3D collagen I gels), G3139 does not protect FGF2. In the RIMAC model, after 2–3 days, FGF2 (20 ng/mL) induced measurable HMEC-1 radial invasion (2.5 fold vs. control; average 0.75 mm vs. 0.3 mm; n = 6). However, radial invasion was not induced by either G3139 alone (1 μM) or FGF2 pre-incubated in air (37°C, 3.5 h) (Fig. 1S). We had initially hypothesized that FGF2 pre-incubated with 1 μM G3139 would be protected in its ability to induce radial invasion of HMEC-1 cells in 3D collagen I gels. However, this was not the case, since we observed only a minimal to no increase in radial invasion (vs. no added FGF2). Therefore, it is possible that FGF2 has two distinct functions. G3139, which binds at the heparin-binding site, protects induction of cellular mitogenesis by FGF2. G3139, however, does not protect the induction of radial migration by FGF2, perhaps implying that the molecular epitope responsible for this is possibly distant and distinct from the heparin-binding site.
G3139 promotes the growth of human umbilical vein endothelial (HUVEC) cells
It was exceptionally difficult to coax HUVEC cells to grow on either plastic, or on plastic but underneath, a collagen I gel (2 mg/mL), and in 3D collagen I gels, cell growth was minimal. We plated cells on plastic and underneath a collagen I gel and treated them with G3139 (20 μM; Figure 2S). Cellular proliferation, as determined after collagenase digestion and counting by trypan blue exclusion (n = 4, P vs. untreated for all experiments), increased by 2.2 +/− 0.2-fold (2.2 vs. 1.1 × 104 cells/well, P < 10−4). This increase was only slightly less than what we observed with heparin, the standard growth additive for HUVEC cells (2.7 +/− 0.2-fold, P < 10−4). When plated on plastic alone, G3139 (20 μM) increased cellular mitogenesis by only 1.7 +/− 0.03-fold (P < 10−7), as compared to 2.7 +/− 0.05-fold, (P < 10−6) for heparin. Qualitatively, these data are similar to what we observed in G3139-treated HMEC-1 cells. The increase in proliferation was accompanied by a dramatic increase in F-actin stress fibers, observable after rhodamine-phalloidin staining, as shown in Fig. 2S
G3139 promotes vessel sprout formation in the rat aortic ring assay
When 1 mm-thick rat aortic rings were embedded in 3D collagen I gels and treated with 1 μM G3139 with media changes every other day, on average 3.5-fold more vessel sprouts emerged after 4 days from each G3139-treated ring than from control, untreated specimens (35 +/− 9 vs. 10 +/− 6 [n = 6], P = 5 × 10−3; Fig. 3S). Vessel sprouting was significantly (about 50%) diminished when the G3139 concentration was raised to 5 μM vs. 1 μM, probably due to toxicity. In addition, the vessels in the 1 μM G3139-treated aortas were substantially longer (41 +/− 12 μm, n = 30 vessels measured) than those in the control, untreated aortas (24 +/− 12, n = 24; P < 10−4).
DISCUSSION
The complete mechanism of action of G3139, a drug been studied in advanced melanoma, is not yet known. Data presented in this study indicate that G3139 can potentiate the binding of FGF2 to its high-affinity cell surface receptors, here FGFR1 IIIc. Re-examination of seemingly contradictory early data suggesting inhibition of high-affinity receptor binding (12) actually reveals that inhibition is confined to longer PS oligomers at higher concentrations than employed here, and is in any case minimal.
In addition, G3139, at a physiologically attainable concentration for HMEC-1 cells (HUVEC cells require higher concentrations), dramatically increases endothelial cell mitogenesis and tubular morphogenesis in 3D collagen I gels. The potential significance of this observation has been described by Liu and Senger (26). When endothelial precursor cells contact collagen I-containing matrix in the embryo, they align into solid pre-capillary cord-like structures that are interconnected to form a polygonal network. This morphogenic program can be mimicked in mature endothelial cells after degradation of basement membrane and subsequent contact with collagen I. Collagen I then drives the morphogenesis of new vessel sprouts, in part by inducing G-actin to F-actin polymerization in the endothelial cells (27), as we observed in primary HUVECs.
The interaction of G3139 and FGF2 affects endothelial cell function. Mobilization of FGF2 from its low-affinity glycosaminoglycan binding sites on ECM increases the radius of its activity (28). FGF2 has long been known to promote microvessel formation (29), and, unlike VEGF, also produces chemoresistance in endothelial cells by promotion of a complex between Raf-1 and ASK1 (30). This complex neutralizes the pro-apoptotic activity of ASK-1 by blocking its translocation to the mitochondrial outer membrane.
Addition of FGF2 to endothelial cells (31) or stromal cells (32) in culture can also result in the expression of the highly pro-angiogenic protein VEGF. However, FGF2 and VEGF have different impacts on blood vessel maturation and function (33). For example, gene transcripts are differentially modified by the two growth factors (34), and increased endothelial fenestration is observed in VEGF-over-expressing cells, but not FGF-over-expressing cells (35).
Thus, agents that potentiate the effects of FGF2 stimulate angiogenesis. G3139 mobilizes FGF2 from its low-affinity binding sites on extracellular matrix, potentiates FGF2 binding to FGFR1 IIIc, and protects FGF2 against proteolysis. The effects of G3139 on endothelial cells in vitro may be mediated by its very high affinity for collagen I, one of the most important protein inducers of angiogenesis. Taken in toto, the behavior of the 18mer G3139 is very similar to that of Defibrotide (DF), which is also an angiogenesis-altering agent. DF is a polydisperse mixture of single-stranded phosphodiester oligonucleotides (range 9–80mer; average 50mer) derived from porcine intestines. As demonstrated in several Phase II clinical trials (e.g., ref. 36), DF can be curative for patients with chemotherapy-induced severe hepatic veno-occlusive disease, an entity characterized by profound hepatic sinusoidal angiotoxicity, which leads to hepatic hypoxia, dysfunction, and death.
This discussion raises a question. Usually, it is anti-angiogenic, not pro-angiogenic strategies that are thought important in anti-cancer therapeutics. However, as noted by Jain (37, 38) this is the paradox of anti-angiogenic therapy. If the vasculature of a tumor is destroyed, the delivery of oxygen is diminished, producing hypoxia, which would diminish the effectiveness of cytotoxic chemotherapy. This diminution may occur possibly because of induction of the HIF-1α transcription factor, which is a major hypoxia sensor (39) responsible for the upregulation of numerous anti-apoptotic (e.g., ADM, IGF2, TGFA), and pro-invasion and pro-metastasis genes (e.g., UPAR, MMP2). HIF-1α also stimulates VEGF and VEGFR2 expression (40), which induce abnormalities (e.g., vascular leakiness) in the tumor vasculature. Interestingly, Bcl-2 overexpression in melanoma cells also appears to increase HIF-1α, and hence VEGF, activity (41).
Hypoxia can also induce genetic instability that can select for tumor cells with increased metastatic potential (42) and can, via c-met protooncogene activation, lead to cells that are more aggressive and invasive (43). Diminished blood flow and low pH can also compromise the functions of tumor-infiltrating immune effector cells and cytokines. Clinical studies (44) have demonstrated that the presence of hypoxic regions within tumors correlates with poor prognosis and increased metastatic risk regardless of treatment—viz., what is observed in advanced melanoma.
What relevance do these facts have to the clinical situation in advanced melanoma patients treated with G3139 as part of the GM301 trial? We raise the possibility that transient, local, revascularization or vascular maintenance by G3139-mediated, FGF2-dependent endothelial cell mitogenesis and tubular morphogenesis may produce an increase in oxygen and nutrient delivery to local cancer cells. This, in turn, might raise local pH, diminish the number of cells in Go, decrease HIF-1α expression and thus VEGF activity, and in general diminish vascular leakiness and promote vascular “normalization”. Clinically, it is possible that this may also promote chemo-sensitization to DTIC, as in the GM301 trial (3). How much “revascularization” might be required? The answer is unknown, but it has been suggested that as little as 22% endothelial cell death might cause a significant decrease in microvascular density (45). The inverse may be true as well.
The stimulatory effects of G3139 on endothelial cells may also suggest a possible explanation for the relationship of overall survival to pre-treatment plasma levels of LDH (S. Agrawala, et al., submitted).
What is the origin of the increased plasma levels of LDH in melanoma, or in patients with any tumor? LDH is a product of anaerobic glycolysis (46), which is frequently enhanced in relatively hypoxic tumors (47). Levels of the LDH-A can be upregulated due to activation of its upstream transcription factor, the hypoxia sensor HIF-1α (48). LDH is a long-recognized, standard marker of cellular necrosis, where, unlike in apoptosis, the cell membrane ruptures and intracellular contents spill into the surrounding space. Cells tend to undergo necrosis when they can no longer produce sufficient ATP to cover their metabolic requirements (49). This can occur in the setting of profound hypoxia, suggesting that tumor cell necrosis, and LDH spillage, depends on the balance between tumor growth and the ability to supply nutrients and oxygen. In turn, this balance depends on the state of tumor vascularization. Because of effects of G3139 on endothelial cells, we speculate that G3139 may affect the balance between the rate of tumor growth and the rate of oxygen and nutrient delivery. This could produce complex effects on tumor biology, and hence possibly on patient prognosis. However, confirmation of this speculation requires extensive in vivo measurements of intratumoral angiogenesis, blood flow, and tumor hypoxia that are beyond the scope of the current study.
Supplementary Material
Acknowledgments
This work was funded by National Institutes of Health [CA108415 to C.S.]
Footnotes
Translational Relevance
A randomized, global Phase III trial of G3139 in combination with dacarbazine vs. dacarbazine alone was completed in patients with advanced melanoma, and confirmatory trial of G3139 plus dacarbazine is currently underway in patients with advanced melanoma and low-normal baseline LDH. G3139 was designed to target and silence the Bcl-2 mRNA, producing, in theory, chemo-sensitization. It appears, however, that this mechanism may, in fact, be one of several that contribute to the drug’s effectiveness. Given the clinical interest in G3139, it is thus of critical importance to the continued development of oligonucleotide therapeutics for cancer to further elucidate the basis of this activity.
References
- 1.Klasa R, Gillum A, Klem R. Oblimersen Bcl-2 antisense: Facilitating apoptosis in anticancer treatment. Antisense Nucleic Acid Drug Dev. 2002;12:193–212. doi: 10.1089/108729002760220798. [DOI] [PubMed] [Google Scholar]
- 2.Kitada S, Takayama S, Riel K, Reed J. Reversal of chemoresistance of lymphoma cells by antisense-mediated reduction of bcl-2 gene expression. Antisense Res Dev. 1994;4:71–9. doi: 10.1089/ard.1994.4.71. [DOI] [PubMed] [Google Scholar]
- 3.Bedikian A, Millward M, Pehamberger H, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: The oblimersen study group. J Clin Oncol. 2006;24:4738–45. doi: 10.1200/JCO.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- 4.Smalley K, Haass M, Brafford P, et al. Multiple signaling pathways must be targeted to overcome drug resistance in cell lines derived from melanoma metastases. Mol Cancer Ther. 2006;5:1136–44. doi: 10.1158/1535-7163.MCT-06-0084. [DOI] [PubMed] [Google Scholar]
- 5.Lai J, Benimetskaya L, Khvorova A, et al. Induction of apoptosis in 518A2 melanoma cells by G3139. Mol Cancer Ther. 2005;4:305–15. [PubMed] [Google Scholar]
- 6.Benimetskaya L, Lai J, Wu S, et al. Relative Bcl-2 independence of drug induced cytotoxicity and resistance in 518A2 melanoma cells. Clin Cancer Res. 2004;10:8371–9. doi: 10.1158/1078-0432.CCR-04-1294. [DOI] [PubMed] [Google Scholar]
- 7.Wacheck V, Losert D, Gunsberg P, et al. Small interfering RNA targeting bcl-2 sensitizes malignant melanoma. Oligonucleotides. 2003;13:393–400. doi: 10.1089/154545703322617078. [DOI] [PubMed] [Google Scholar]
- 8.Gekeler V, Gimmnich P, Hofmann HP, et al. G3139 and other CpG-containing immuno-stimulatory phosphorothioate oligodeoxynucleotides are potent suppressors of the growth of human tumor xenografts in nude mice. Oligonucleotides. 2006;16:83–93. doi: 10.1089/oli.2006.16.83. [DOI] [PubMed] [Google Scholar]
- 9.Ballas Z, Rasmussen W, Krieg A. Induction of natural killer activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacteria DNA. J Immunol. 1996;157:1840–5. [PubMed] [Google Scholar]
- 10.Lopes de Menezes A, Hudon N, McIntosh N, et al. Molecular and pharmacokinetic properties associated with the therapeutics of bcl-2 antisense oligonucleotide G3139 combined with free and liposomal doxorubicin. Clin Cancer Res. 2000;6:2891–902. [PubMed] [Google Scholar]
- 11.Santel A, Aleku M, Keil O, et al. A novel si-RNA-lipoplex technology for RNA interference in the mouse vascular endothelium. Gene Ther. 2006;13:1222–34. doi: 10.1038/sj.gt.3302777. [DOI] [PubMed] [Google Scholar]
- 12.Guvakova M, Yakubov L, Vlodavsky I, et al. Phosphorothioate oligodeoxynucleotides bind to basic fibroblast growth actor, inhibit its binding to cell surface receptors, and remove it from low affinity binding sites on extracellular matrix. J Biol Chem. 1995;270:2620–7. doi: 10.1074/jbc.270.6.2620. [DOI] [PubMed] [Google Scholar]
- 13.Yakubov L, Khaled Z, Zhang L-M, et al. Oligodeoxynucleotides interact with recombinant CD4 at multiple sites. J Biol Chem. 1993;268:18818–23. [PubMed] [Google Scholar]
- 14.Cheng Y, Prusoff W. Relationship between the inhibition constant and the concentration of inhibitor which causes 50% inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 15.Ishai-Michaeli R, Svahn C, Weber M, et al. Importance of size and sulfation of heparin in release of basic fibroblast growth factor from the vascular endothelium and extracelllar matrix. Biochem. 1992;31:2080–8. doi: 10.1021/bi00122a027. [DOI] [PubMed] [Google Scholar]
- 16.Vernon RB, Sage EH. A novel, quantitative model for study of endothelial cell migration and sprout formation within three-dimensional collagen matrices. Microvasc Res. 1999;5:118–33. doi: 10.1006/mvre.1998.2122. [DOI] [PubMed] [Google Scholar]
- 17.Nicosia R, Zhu W-H. Rat Aortic Ring assay of Angiogenesis. Methods Endothelial Cell Biol. 2004;13:125–43. [Google Scholar]
- 18.Benimetskaya L, Wu S, Voskresenskiy A, et al. Angiogenesis-alteration by Defibrotide: Implications for its mechanism of action in severe hepatic veno-occlusive disease. doi: 10.1182/Blood-2008-04-149682. [DOI] [PubMed] [Google Scholar]
- 19.Rapraeger A, Guimond S, Krufka A, Olwin B. Regulation by heparan sulfate in fibroblast growth factor signaling. Methods Enzymol. 1994;245:219–40. doi: 10.1016/0076-6879(94)45013-7. [DOI] [PubMed] [Google Scholar]
- 20.Folkman J, Klagsbrun M, Sasse J, et al. A heparin-binding angiogenic protein-basic fibroblast growth factor-is stored within basement membrane. Amer J Pathol. 1988;130:393–400. [PMC free article] [PubMed] [Google Scholar]
- 21.Baird A, Ling N. Fibroblast growth factors are present in the extracellular matrix produced by endothelial cells in vitro: Implications for a role of heparinase-like enzymes in the neovascular response. Biochem Biophys Res Commun. 1987;142:428–35. doi: 10.1016/0006-291x(87)90292-0. [DOI] [PubMed] [Google Scholar]
- 22.Powers C, McLeskey S, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Related Cancer. 2000;7:165–97. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- 23.Ornitz D, Yayon A, Flanagan J, et al. Heparin is required for cell-free binding of basic fibroblast growth factor to a soluble receptor and for mitogenesis in whole cells. Mol Cell Biol. 1992;12:240–7. doi: 10.1128/mcb.12.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yayon A, Klagsbrun M, Esko J, et al. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64:841–8. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- 25.Volkin D, Tsai P, Dabora J, et al. Physical stabilization of acidic fibroblast growth factor by polyanions. Arch Biochem Biophys. 1993;300:30–41. doi: 10.1006/abbi.1993.1005. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Senger D. Matrix-specific activation of Src and Rho initiates capillary morphogenesis of endothelial cells. FASEB J. 2004;18:457–68. doi: 10.1096/fj.03-0948com. [DOI] [PubMed] [Google Scholar]
- 27.Whelan M, Senger D. Collagen I initiates endothelial cell morphogenesis by inducing actin polymerization through suppression of cyclic AMP and protein kinase A. J Biol Chem. 2003;278:327–34. doi: 10.1074/jbc.M207554200. [DOI] [PubMed] [Google Scholar]
- 28.Flaumenhaft R, Moscatelli D, Rifkin D. Heparin and heparan sulfate increase the radius of diffusion and action of basic fibroblast growth factor. J Cell Biol. 1990;111:1651–5. doi: 10.1083/jcb.111.4.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akimoto T, Hammerman M. Fibroblast growth factor 2 promotes microvessel formation from mouse embryonic aorta. Am J Physiol Cell Physiol. 2003;284:C371–7. doi: 10.1152/ajpcell.00193.2002. [DOI] [PubMed] [Google Scholar]
- 30.Alavi A, Acevedo L, Min W, Cheresh D. Chemoresistance of endothelial cells induced by basic fibroblast growth factor depends on raf-1-mediated inhibition of the proapoptotic kinase, ASK1. Cancer Res. 2007;67:2766–72. doi: 10.1158/0008-5472.CAN-06-3648. [DOI] [PubMed] [Google Scholar]
- 31.Seghezzi G, Patel S, Ren C, et al. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: An autocrine mechanism contributing to angiogenesis. J Cell Biol. 1998;141:1659–73. doi: 10.1083/jcb.141.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claffey K, Abrams K, Shih S-C, et al. Fibroblast growth factor 2 activation of stromal cell vascular endothelial growth factor expression and angiogenesis. Lab Invest. 2001;81:61–75. doi: 10.1038/labinvest.3780212. [DOI] [PubMed] [Google Scholar]
- 33.Presta M, Dell-Era P, Mitola S. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Revs. 2005;16:159–78. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Jih Y, Lien W, Tsai W, et al. Distinct regulation of genes by FGF2 and VEGF-A in endothelial cells. Angiogenesis. 2001;4:313–21. doi: 10.1023/a:1016080321956. [DOI] [PubMed] [Google Scholar]
- 35.Ribatti D, Nico B, Morbidelli L, et al. Cell mediated delivery of fibroblast growth factor-2 and vascular endothelial growth factor onto the chick chorioallantoic membrane: endothelial fenestration and angiogenesis. J Vasc Res. 2001;38:389–97. doi: 10.1159/000051070. [DOI] [PubMed] [Google Scholar]
- 36.Richardson P, Murakami C, Jin Z, et al. Multi-institutional use of Defibrotide in 88 patients after stem cell transplant with severe veno-occlusive disease and multi-system organ failure: Response without significant toxicity in a high-risk population and factors predictive of outcome. Blood. 2002;100:4337–43. doi: 10.1182/blood-2002-04-1216. [DOI] [PubMed] [Google Scholar]
- 37.Jain R. Normalization of tumor vasculature: An emerging concept in anti-angiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 38.Jain R. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradism for combination therapy. Nat Med. 2001;7:987–9. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 39.Semenza G. Targeting HIF-1 for cancer therapy. Nature Rev Can. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 40.Iervolino A, Trisciuolgio D, Ribatti D. Bcl-2 overexpression in human melanoma cells increases angiogenesis through VEGF mRNA stabilization and HIF-1 mediated transcriptional activity. FASEB J. 2002;16:1453–5. doi: 10.1096/fj.02-0122fje. [DOI] [PubMed] [Google Scholar]
- 41.Cairns R, Kalliomaki K, Hill R. Acute (cyclic) hypoxia enhances spontaneous metastasis of KHT murine tumors. Cancer Res. 2001;61:8903–8. [PubMed] [Google Scholar]
- 42.Rofstad E, Rasmusse H, Galappathi K, et al. Hypoxia promotes lymph node metastasis in human melanoma xenografts by upregulating the urokinase type plasminogen activator receptor. Cancer Res. 2002;62:1847–53. [PubMed] [Google Scholar]
- 43.Pennacchietti S, Michieli P, Galluzzo M, et al. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–61. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 44.Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol. 2004;14:198–206. doi: 10.1016/j.semradonc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Dong Z, Zeitlin D, Song W, et al. Level of endothelial cell apoptosis required for a significant decrease in microvessel density. Exp Cell Res. 2007;313:3645–57. doi: 10.1016/j.yexcr.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fantin V, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology and tumor maintenance. Cancer Cell. 2006;9:425–34. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 47.Corn P, Ricci M, Scata K, et al. Mxi1 is induced by hypoxia in a HIF-1 dependent manner and protects cells from c-myc-induced apoptosis. Cancer Biol Ther. 2005;4:1285–94. doi: 10.4161/cbt.4.11.2299. [DOI] [PubMed] [Google Scholar]
- 48.Ebert B, Bunn H. Regulation of transcription by hypoxia requires a multiprotein complex that includes hypoxia-inducible factor 1, an adjacent transcription factor and p300/CREB binding protein. Mol Cell Biol. 1998;18:4089–96. doi: 10.1128/mcb.18.7.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nasu R, Kimura H, Akagi T, et al. Blood flow influences vascular growth during tumor angiogenesis. Br J Cancer. 1999;79:780–6. doi: 10.1038/sj.bjc.6690125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.