Abstract
Background and Purpose
Approximately 25% of ischemic stroke patients awaken with their deficits. The last-seen-normal time is defined as the time the patient went to sleep, which places these patients outside the window for thrombolysis. The purpose of this study was to describe our center’s experience with off-label, compassionate thrombolysis for wake-up stroke (WUS) patients.
Methods
A retrospective review of our database identified 3 groups of ischemic stroke patients: (1) WUS treated with thrombolysis; (2) nontreated WUS; and (3) 0- to 3-hour intravenous tissue plasminogen activator-treated patients. Safety and clinical outcome measures were symptomatic intracerebral hemorrhage, excellent outcome (discharge modified Rankin score, 0–1), favorable outcome (modified Rankin score, 0–2), and mortality. Outcome measures were controlled for baseline NIHSS using logistic regression.
Results
Forty-six thrombolysed and 34 nonthrombolysed WUS patients were identified. Sixty-one percent (28/46) of the treated WUS patients underwent intravenous thrombolysis alone whereas 30% (14/46) were given only intra-arterial thrombolysis. Four patients received both intravenous and intra-arterial thrombolysis (9%). Two symptomatic intracerebral hemorrhages occurred in treated WUS (4.3%). Controlling for NIHSS imbalance, treated WUS had higher rates of excellent (14% vs 6%; P=0.06) and favorable outcome (28% vs 13%; P=0.006), but higher mortality (15% vs 0%) compared to nontreated WUS. A second comparison controlling for baseline NIHSS between treated WUS and 174 intravenous tissue plasminogen activator patients treated within 3 hours of symptoms showed no significant differences in safety and clinical outcomes.
Conclusion
Thrombolysis may be safe in WUS patients. Our center’s experience supports considering a prospective, randomized trial to assess the safety and outcome of thrombolysis for this specific patient population.
Keywords: awakening, ischemic, sleep, stroke, thrombolysis
The only Food and Drug Administration-approved therapy for acute ischemic stroke, intravenous tissue plasminogen activator (IV tPA), is limited to patients who present with a known symptom onset of <3 hours.1 Approximately 16% to 28% of ischemic stroke patients awaken with their deficits.2–7 In these wake-up strokes (WUS), the onset of symptoms is defined as the last-seen-normal (LSN) time. Because this is the time the patient went to sleep, unfortunately it usually places these patients outside the window for thrombolysis or entry into reperfusion clinical trials.
Numerous case series and clinical trials have demonstrated an early-morning peak occurrence of ischemic strokes.2,4–11 Similar to acute myocardial infarction and sudden death,12,13 the predominant stroke onset time has been shown to occur during the waking hours. In a meta-analysis of 31 publications reporting the circadian timing of 11 816 strokes (8250 ischemic), the onset of symptoms was 55% more likely to occur between 6:00AM and noon.14 If a portion of the morning-onset strokes occur near the time of awakening, reperfusion therapies may still be of potential benefit, provided patients present and are triaged emergently.
Some authors3,6,15 have reported similar clinical and radio-graphic features between WUS and known-onset ischemic strokes. Fink et al3 reported similar rates of diffusion-weighted imaging/perfusion-weighted imaging mismatch in both WUS and strokes of known onset. Another group found no significant differences in early ischemic changes in blinded assessment of hyperacute noncontrast CT scans between stroke of known onset and stroke at awakening.15 Nadeau et al16 recently published their center’s experience with WUS. Although their data were derived from an incomplete stroke registry (missing 60% of their stroke admissions), patients who awoke with their stroke seemed to have worse outcomes. However, after excluding subarachnoid cases and focusing only on ischemic WUS, there was no significant difference between WUS and non-WUS in terms of poor outcome. These studies lead to the hypothesis that some WUS patients may be candidates for thrombolysis. However, there are no published studies to our knowledge on thrombolytic therapy for this patient population.
The objective of this study was to collect all WUS patients treated with off-label thrombolysis from our institutional stroke database and report the baseline patient demographics, safety, and early clinical outcomes from this cohort. In addition, records of consecutive nontreated WUS patients and standard-of-care 0- to 3-hour IV tPA patients were collected for comparison.
Patients and Methods
Patient Selection
We retrospectively identified 3 historical groups of ischemic stroke patients from our database between March 2003 and January 2008: (1) WUS patients treated with off-label thrombolysis; (2) WUS patients not treated with thrombolysis; and (3) 0- to 3-hour standard IV-tPA patients. All patients were assessed by staff stroke neurologists or stroke fellows at a single academic emergency department. WUS patients all met the following criteria: (1) patients were neurologically normal when LSN before going to sleep and were either witnessed with deficits on awakening or could report that they awoke with stroke symptoms; (2) patients had a disabling neurological deficit that would usually be treated with tPA in 0- to 3-hour onset patients; and (3) hypodensity <one-third middle cerebral artery (MCA) territory on noncontrast cranial CT scan.
In situations in which the onset time was unclear (eg, vague or intermittent symptoms), inclusion was adjudicated among our stroke faculty. If a consensus was reached among stroke faculty that the onset of symptoms was at awakening (and not the day before), then those patients were included in the cohort. WUS patients whose LSN time was unknown were also included in the analysis provided they woke-up with symptoms and were LSN before going to bed. For determination of the subtype of ischemic stroke, the TOAST (Trial of ORG 10172 in Acute Stroke Treatment) criteria were used.17
At the time the cohort began, multimodal neuroimaging (CT perfusion or MRI diffusion-weighted imaging/perfusion-weighted imaging mismatch) was not part of our treatment protocol. Our treatment algorithm has since grown to incorporate additional diagnostic studies. Multimodal imaging was captured when it was obtained for treatment and the results were not used to select treatment strategies. Mismatch was determined to be present when there was at least a 20% larger perfusion deficit compared with the diffusion-weighted deficit on MRI. Similarly, if CT perfusion was used, mismatch was defined as 20% greater perfusion (prolongation of time to peak) abnormality compared with critically low cerebral blood volume deficit.
Our stroke center offers compassionate thrombolysis to patients with moderately severe, disabling symptoms on a patient-by-patient basis using the aforementioned criteria. We offer this treatment to patients who do not meet inclusion criteria into other extended-window clinical research studies and because no proven therapies exist for these unfortunate patients. Only patients who could provide consent (or had suitable family members as surrogates) were offered treatment.
Treatment
With the exception of the known onset-to-treatment time within 3 hours, all IV tPA-treated WUS patients were selected using the National Institute of Neurological Disorders and Stroke tPA Stroke Trial (NINDS) inclusion/exclusion criteria for thrombolysis and treated with standard IV tPA 0.9 mg/kg. Consent was granted by patients or their next-of-kin for off-label treatment. Our center had no formal criteria to determine the type of thrombolysis (IV vs endovascular) in WUS patients who were offered off-label treatment. Because there was no predetermined protocol, the types of treatments were left to the decision of the treating stroke fellow or stroke attending. Patients who received intra-arterial therapy (IAT) alone or combination full-dose IV tPA and IAT also gave consent for these procedures. Medical management followed the National Institute of Neurological Disorders and Stroke tPA Stroke Trial protocol.
Baseline data collected for all patients included age, gender, ethnicity, LSN time, wake-up time, NIHSS, vascular risk factors, baseline noncontrast head CT findings, and door-to-needle time. All patients were admitted to a specialized stroke unit or neurological intensive care unit with stroke-trained nursing staff. For patients treated with intravenous tPA, antiplatelet therapy was started no earlier than 24 hours after the procedure, along with no evidence of ICH on the 24-hour CT scan. The retrospective chart review was approved by the institutional review board at the University of Texas-Houston.
Outcome Assessment
Safety was assessed by the development of symptomatic intracerebral hemorrhage (sICH), defined as any intracerebral hemorrhage associated with a ≥4-point increase in the NIHSS. Clinical outcomes were defined as excellent outcome (discharge modified Rankin score, 0–1), favorable outcome (discharge modified Rankin score, 0–2), and mortality. Data were abstracted from charts by 1 author (A.D.B.).
Statistical Analysis
Categorical variables were analyzed using χ2 and Fisher exact test when appropriate. Continuous variables were analyzed using independent samples t test or Mann–Whitney U when appropriate. Treated WUS were compared to standard 0- to 3-hour IV tPA patients and nontreated WUS. Outcome measures were controlled for baseline NIHSS using multivariate logistic regression. The analysis was performed using SPSS for Windows, version 15 (SPSS Inc).
Results
During the study timeframe, a total of 1253 ischemic stroke patients were admitted to our center and 338 received IV tPA alone (27%). This does not include patients treated with IAT therapy or combination IV plus IAT. After removing patients treated within research protocols and off-label treatments, 174 remained who were treated within 0 to 3 hours. This group served as the standard-of-care 0- to 3-hour IV tPA arm in the study.
A total of 80 consecutive WUS patients were identified from our database (6.4% of all ischemic strokes). Of these, 46 received off-label thrombolysis (treated WUS) and 34 did not receive any intravenous or endovascular treatments (non-treated WUS). In treated WUS patients, 28 received full-dose (0.9 mg/kg, maximum 90 mg) IV tPA alone; 14 received IAT alone; and 4 received combination full-dose IV tPA plus IA thrombolysis. Only 2 patients had preceding vague symptoms requiring adjudication for inclusion into the thrombolysed WUS group. Of the 46 WUS patients, 7 woke-up with stroke symptoms but lacked an exact LSN time. Their chart documentation noted a LSN time of the previous evening.
For comparison, 34 nonthrombolysed WUS patients were identified. Half (17/34) of the nontreated WUS patients had an unknown exact LSN time but were known to have been neurologically normal before going to sleep the previous evening.
In the thrombolysed WUS patients, 16 underwent pretreatment multimodal neuroimaging either with CT perfusion (n=10) or with MRI diffusion-weighted imaging/perfusion-weighted imaging (n=6). Two CT perfusion studies were nondiagnostic because of poor contrast bolus and 1 CT perfusion study was normal. The remaining patients were treated using noncontrast CT alone to exclude hemorrhage or hypodensity >one-third MCA territory. Emergent vascular imaging was performed before treatment in 30 of the 46 thrombolysed WUS patients. This included 14 CT angiograms, 10 transcranial Doppler ultrasounds, and 6 MR angiograms. Eighty-six percent (26/30) of patients who had vascular imaging had documented occlusions of intracranial arteries.
Baseline demographic and radiological data for group comparisons are displayed in Table 1 and Table 2. All 18 patients who received IAT were given thrombolytics (11 reteplase, 6 urokinase, and 1 tenecteplase). Five IAT patients underwent embolectomy with the MERCI retriever system (Concentric Medical, Inc). Two IAT patients received balloon angioplasty and intracranial stent placement.
Table 1.
Baseline Clinical and Radiographic Characteristics of Thrombolysed WUS vs Nonthrombolysed WUS Patients
| Thrombolysed WUS |
Nonthrombolysed WUS |
P | |
|---|---|---|---|
| N | 46 | 34 | . . . |
| Age, mean±SD | 62±14 | 64±13 | 0.51 |
| % Male | 39 | 44 | 0.69 |
| NIHSS median (range) | 16 (3–24) | 10.5 (2–26) | 0.003 |
| Ethnicity, % | 0.52 | ||
| White | 52 | 35 | |
| Black | 34 | 45 | |
| Hispanic | 12 | 17 | |
| Asian | 2 | 3 | |
| Hypertension, % | 65 | 65 | 0.96 |
| Diabetes mellitus, % | 21 | 33 | 0.31 |
| Coronary artery disease, % | 17 | 15 | 0.75 |
| Hyperlipidemia, % | 27 | 27 | 0.79 |
| TOAST classification, % | 0.17 | ||
| Cardioembolic | 44 | 41 | |
| Large artery atherosclerosis | 33 | 21 | |
| Small vessel | 2 | 15 | |
| Unknown | 11 | 18 | |
| Other | 10 | 5 | |
| Early changes on baseline CT (%) | 13/46 (28) | 15/34 (44) | 0.16 |
| Hypodensity on baseline CT (%) | 9/46 (20) | 12/34 (35) | 0.13 |
Table 2.
Baseline Clinical and Radiographic Characteristics of Thrombolysed WUS vs 0- to 3-Hour IV tPA-Treated Patients
| Thrombolysed WUS |
0- to 3-Hour IV tPA |
P | |
|---|---|---|---|
| N | 46 | 174 | . . . |
| Age, mean±SD | 62±14 | 65±15 | 0.27 |
| % Male | 39 | 56 | 0.043 |
| NIHSS median (range) | 16 (3–24) | 11 (1–35) | 0.001 |
| Ethnicity (%) | 0.60 | ||
| White | 52 | 41 | |
| Black | 34 | 41 | |
| Hispanic | 12 | 16 | |
| Asian | 2 | 2 | |
| Hypertension, % | 65 | 72 | 0.36 |
| Diabetes mellitus, % | 21 | 28 | 0.58 |
| Coronary artery disease, % | 17 | 22 | 0.68 |
| Hyperlipidemia, % | 27 | 20 | 0.84 |
| TOAST classification, % | 0.026 | ||
| Cardioembolic | 44 | 27 | |
| Large artery atherosclerosis | 33 | 29 | |
| Small vessel | 2 | 14 | |
| Unknown | 11 | 23 | |
| Other | 10 | 7 | |
| Early changes on baseline CT (%) | 13/46 (28) | 20/174 (12) | 0.009 |
| Hypodensity on baseline CT (%) | 9/46 (20) | 13/174 (7) | 0.02 |
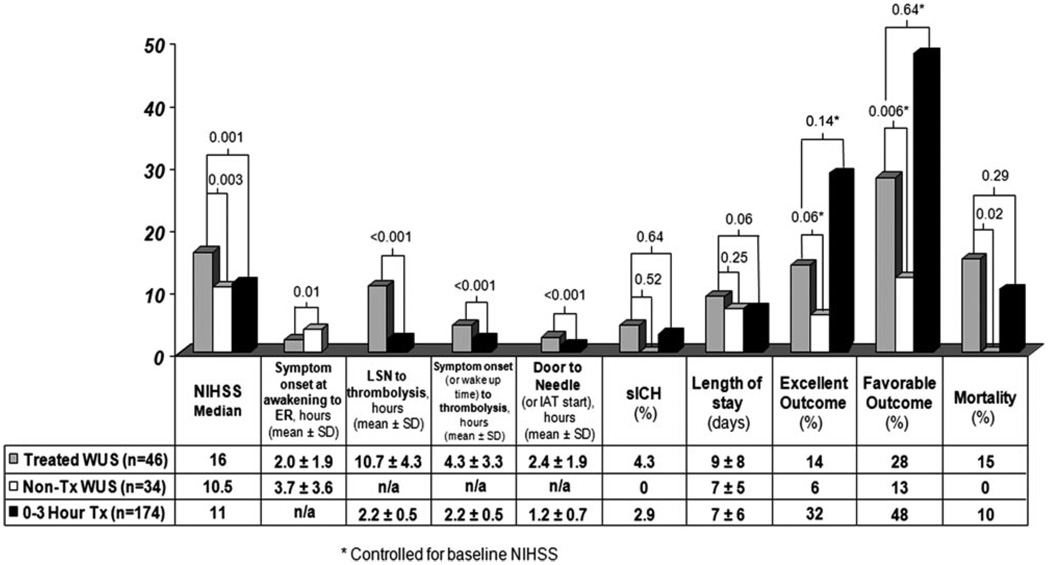
The timing parameters (eg, door-to-needle time), incidence of sICH, and clinical outcomes are displayed in Figure 1. Seven of the 46 WUS patients died: 3 deaths were the result of malignant MCA syndrome, 1 was from a basilar thrombosis that led to massive brain stem infarction, and 1 patient was given comfort care per family wishes after large right hemispheric infarction unresponsive to tPA and no hemorrhage. The remaining 2 patients died from sICH.
Figure 1.
Time parameters and clinical outcome data.
Thrombolysed vs Nonthrombolysed WUS
After controlling for the baseline NIHSS imbalance, thrombolysed WUS patients had higher rates of excellent (OR, 9.0; 95% CI, 0.9 –90; P=0.06) and favorable outcomes (OR, 9.2; 95% CI, 0.1.9–45; P=0.006). In unadjusted (χ2) analysis, thrombolysed WUS had a nonsignificant increase in sICH (P=0.64), but a higher rate of death (P=0.02). A regression to control for the NIHSS imbalance was not able to be performed because the nonthrombolysed WUS patients experienced zero deaths.
Twenty-three of the 46 thrombolysed WUS (50%) were treated within 3 hours of awakening. There were no significant differences in outcomes between those thrombolysed within 3 hours of awakening with their deficits compared to those treated after 3 hours (sICH: P=0.76; excellent outcome: P=0.31; favorable outcome: P=0.54; death: P=0.5). Eleven patients had mismatch on multimodal imaging, 4 of whom had an excellent or favorable clinical outcome. There were no significant differences between treated WUS with mismatch compared to all other treated WUS patients in any of the outcome measures (sICH: P=0.44; excellent outcome: P=0.61; favorable outcome: P=0.62; death: P=0.57). Similarly, the presence of hypodensity on CT scan did not influence the study outcome measures (sICH: P=0.36; excellent outcome: P=0.32; favorable outcome: P=0.56; death: P=0.61).
Two patients (both received IV tPA alone) experienced sICH: 1 patient presented with a left MCA occlusion and baseline NIHSS of 20 and the other presented with a right MCA occlusion and NIHSS of 14. No sICH occurred in IAT-treated patients.
Thrombolysed WUS vs 0- to 3-Hour IV tPA Patients
After controlling for the higher median NIHSS scores in the thrombolysed WUS patients, treated WUS experienced similar rates of excellent outcomes (14% vs 32%; OR, 0.48; 95% CI, 0.18 –1.27; P=0.14) and favorable outcomes (28% vs 48%; OR, 0.64; 95% CI, 0.3–1.38; P=0.64) compared to the standard-of-care 0- to 3-hour IV tPA-treated patients.
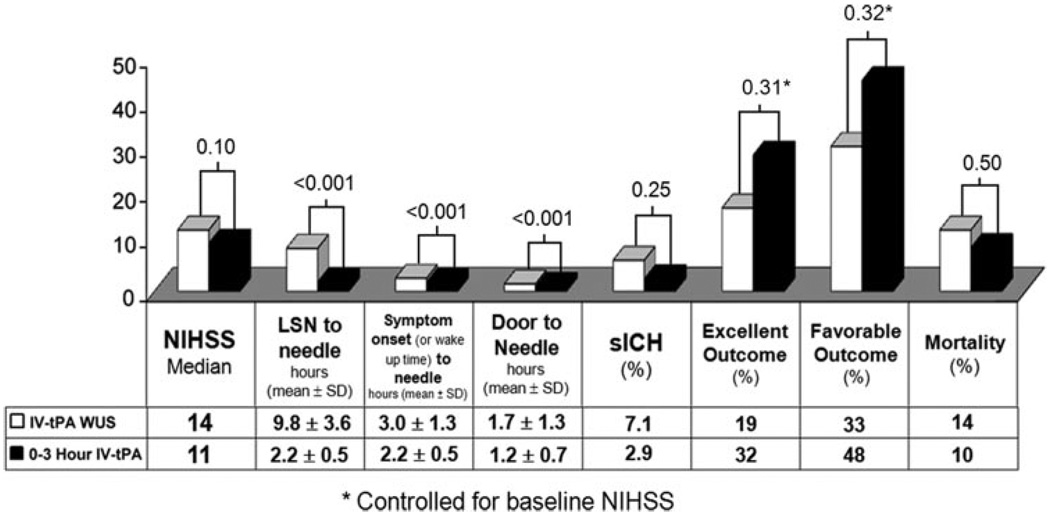
WUS patients who received only IV tPA (n=28) were compared to the 0- to 3-hour standard of care group (Table 3). IV tPA-treated WUS patients had longer onset-to-needle and door-to-needle times, but after controlling for baseline NIHSS, still experienced similar rates of excellent outcomes (19% vs 32%; OR, 0.57; 95% CI, 0.19 –1.68; P=0.31) and favorable outcomes (33% vs 48%; OR, 0.63; 95% CI, 0.25–1.57; P=0.32; Figure 2).
Table 3.
Baseline Clinical and Radiographic Characteristics of IV tPA-Treated Alone WUS vs 0- to 3-Hour IV tPA-Treated Ischemic Strokes
| IV tPA WUS | 0- to 3-Hour IV tPA |
P | |
|---|---|---|---|
| N | 28 | 174 | . . . |
| Age, mean±SD | 64±15 | 65±15 | 0.77 |
| % Male | 33 | 56 | 0.04 |
| NIHSS median (range) | 14 (3–24) | 11 (1–35) | 0.10 |
| Ethnicity, % | 0.53 | ||
| White | 52 | 41 | |
| Black | 37 | 41 | |
| Hispanic | 7 | 16 | |
| Asian | 4 | 2 | |
| Hypertension, % | 64 | 72 | 0.38 |
| Diabetes mellitus, % | 32 | 28 | 0.65 |
| Coronary artery disease, % | 21 | 22 | 1.0 |
| Hyperlipidemia, % | 25 | 20 | 0.62 |
| TOAST classification, % | 0.04 | ||
| Cardioembolic | 54 | 27 | |
| Large artery atherosclerosis | 21 | 29 | |
| Small vessel | 4 | 14 | |
| Unknown | 11 | 23 | |
| Other | 11 | 7 | |
| Early changes on baseline CT (%) | 8/28 (29) | 20/174 (12) | 0.03 |
| Hypodensity on baseline CT (%) | 5/28 (18) | 13/174 (7) | 0.08 |
Figure 2.
Timing parameters and clinical outcomes of IV-tPA treated WUS and 0- to 3-hour patients.
Discussion
Our study details 1 center’s experience with off-label thrombolysis in patients with WUS. Although these patients have historically been denied thrombolysis and have limited treatment options, some WUS patients may be candidates for treatment. In fact, other stroke centers have shown similar rates of poor outcome between WUS and non-WUS patients and suggest that WUS patients should be a target for further therapeutic considerations in the future.16 Whereas other investigators have described clinical and radiographic similarities between WUS and known-onset stroke patients, our study is the first to our knowledge to present the outcomes of a cohort of WUS patients treated with thrombolysis. In our study, WUS patients had more severe strokes compared with the NINDS tPA arm (median NIHSS, 16 vs 14) and our cohort of IV 0- to 3-hour tPA-treated patients (16 vs 11). The etiology in our WUS patients was predominantly cardioembolic (43%). This is in contrast to other published reports2,4 that noted only 17% to 19% cardioembolic strokes in WUS. Our incidence of cardioembolic stroke may be higher because of the small numbers of patients in the study, and because compassionate thrombolysis was offered to patients who have moderate to severe strokes without treatment options.
Because previous studies have reported an early-morning predominance of stroke and similar rates of MRI mismatch compared to those of strokes of known onset, there is evidence to suggest that WUS may occur on awakening. Therefore, rapid institution of thrombolysis for WUS may be a consideration similar to strokes of known onset. In our small cohort, the incidence of sICH was within the range of previous IV tPA studies.1,18–20 Importantly, this incidence takes into account both IV and endovascular approaches.
Twenty-six of the 28 (93%) IV tPA-alone WUS patients received treatment within 4.5 hours of awakening. Door-to-needle times were prolonged in our cohort when compared with current treatment guidelines that mandate <60 minutes. 21 Delays may have been attributable to many factors, including slower response of emergency personnel/stroke team activation after patients were confirmed to have an unknown onset time, inability to consent aphasic patients, difficulty reaching next-of-kin, or obtaining supplemental neuroimaging. If thrombolysis of WUS is to be prospectively studied, techniques to reduce these delays need to be undertaken. Nevertheless, it is possible that patients may still derive benefit from IV tPA when treated within 4.5 hours of symptom onset.22
Despite significantly higher NIHSS scores, treated WUS patients as a group experienced better clinical outcomes compared to nontreated WUS patients; however, these results must be interpreted with caution because of the low numbers of patients and retrospective nature of this study. Higher death rates in the treated WUS cohort may partially be explained by the inclusion of more severe strokes and incidence of malignant MCA syndrome.
The only available literature that exists regarding treatment of WUS is the recently published ABESTT-II trial,23 which enrolled a total of 41 WUS patients (21 treatment and 20 placebo) randomized to either abciximab or placebo within 3 hours of awakening. The stroke severity in the treated cohort from this trial was much lower than that in our thrombolysed WUS patients (median NIHSS, 10 vs 16). After 3 of the 21 (14%) abciximab-treated patients had sICH (2 fatal) develop, the study arm was suspended (1 placebo sICH). Although the cause for increased sICH in ABESTT-II is not entirely known, it may have been related to reperfusion hemorrhage after delayed recanalization.
Some investigators may feel that the pursuit of invasive angiography and intra-arterial recanalization techniques in stroke patients with unknown onset time raises ethical considerations. In the absence of data from randomized studies, the decisions to pursue such approaches rests with the individual physician who has a duty to explain to the patient or the patient’s legal decision-maker about the potential benefits vs the risks of invasive diagnostic studies such as cerebral angiogram and experimental treatments.
This study has many limitations, including its retrospective nature and small numbers. The nonrandomized nature of our study limits the general application of our findings to all WUS patients. Furthermore, lack of long-term outcome data (eg, 90 days) limits comparison with other clinical trial results. The higher incidence of cardioembolic strokes in WUS patients compared to that in other studies indicates that there was a selection bias in our cohort. This bias is a major limitation to our study. Finally, treatment occurred at an experienced academic center and may not be applicable to the community setting.
In conclusion, although thrombolysis may be safe in WUS patients, future, well-designed, prospective studies with prespecified inclusion and exclusion criteria and treatment strategy are needed to further assess the safety of thrombolysis in WUS patients. For research purposes, those interested in offering tPA to WUS patients should proceed only with an institutional review board-approved protocol. In addition, further studies are needed to confirm if multimodal neuroimaging can improve selection of WUS patients likely to benefit from thrombolysis compared with noncontrast CT alone.
Acknowledgments
Sources of Funding
A.D.B was supported by training grant 5-T32-NS007412-09 from the National Institutes of Health to the University of Texas-Houston Medical School Stroke Program. S.I.S was supported by AHA #0475008N.
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
None.
References
- 1.The national institute of neurological disorders and stroke rt-pa stroke study group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi S, Adams HP, Jr, Woolson RF. Circadian variation in ischemic stroke subtypes. Stroke. 1999;30:1792–1795. doi: 10.1161/01.str.30.9.1792. [DOI] [PubMed] [Google Scholar]
- 3.Fink JN, Kumar S, Horkan C, Linfante I, Selim MH, Caplan LR, Schlaug G. The stroke patient who woke up: Clinical and radiological features, including diffusion and perfusion MRI. Stroke. 2002;33:988–993. doi: 10.1161/01.str.0000014585.17714.67. [DOI] [PubMed] [Google Scholar]
- 4.Lago A, Geffner D, Tembl J, Landete L, Valero C, Baquero M. Circadian variation in acute ischemic stroke: A hospital-based study. Stroke. 1998;29:1873–1875. doi: 10.1161/01.str.29.9.1873. [DOI] [PubMed] [Google Scholar]
- 5.Marler JR, Price TR, Clark GL, Muller JE, Robertson T, Mohr JP, Hier DB, Wolf PA, Caplan LR, Foulkes MA. Morning increase in onset of ischemic stroke. Stroke. 1989;20:473–476. doi: 10.1161/01.str.20.4.473. [DOI] [PubMed] [Google Scholar]
- 6.Serena J, Davalos A, Segura T, Mostacero E, Castillo J. Stroke on awakening: Looking for a more rational management. Cerebrovasc Dis. 2003;16:128–133. doi: 10.1159/000070592. [DOI] [PubMed] [Google Scholar]
- 7.Jimenez-Conde J, Ois A, Rodriguez-Campello A, Gomis M, Roquer J. Does sleep protect against ischemic stroke? Less frequent ischemic strokes but more severe ones. J Neurol. 2007;254:782–788. doi: 10.1007/s00415-006-0438-y. [DOI] [PubMed] [Google Scholar]
- 8.Casetta I, Granieri E, Fallica E, la Cecilia O, Paolino E, Manfredini R. Patient demographic and clinical features and circadian variation in onset of ischemic stroke. Arch Neurol. 2002;59:48–53. doi: 10.1001/archneur.59.1.48. [DOI] [PubMed] [Google Scholar]
- 9.Cheung RT, Mak W, Chan KH. Circadian variation of stroke onset in Hong Kong Chinese: A hospital-based study. Cerebrovasc Dis. 2001;12:1–6. doi: 10.1159/000047673. [DOI] [PubMed] [Google Scholar]
- 10.Kelly-Hayes M, Wolf PA, Kase CS, Brand FN, McGuirk JM, D’Agostino RB. Temporal patterns of stroke onset. The Framingham study. Stroke. 1995;26:1343–1347. doi: 10.1161/01.str.26.8.1343. [DOI] [PubMed] [Google Scholar]
- 11.Kocer A, Ilhan A, Ince N, Bilge C. The related causes in very early morning onset of stroke. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:983–988. doi: 10.1016/j.pnpbp.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Cohen MC, Rohtla KM, Lavery CE, Muller JE, Mittleman MA. Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am J Cardiol. 1997;79:1512–1516. doi: 10.1016/s0002-9149(97)00181-1. [DOI] [PubMed] [Google Scholar]
- 13.Muller JE. Circadian variation in cardiovascular events. Am J Hypertens. 1999;12:35S–42S. doi: 10.1016/s0895-7061(98)00278-7. [DOI] [PubMed] [Google Scholar]
- 14.Elliott WJ. Circadian variation in the timing of stroke onset: A meta-analysis. Stroke. 1998;29:992–996. doi: 10.1161/01.str.29.5.992. [DOI] [PubMed] [Google Scholar]
- 15.Todo K, Moriwaki H, Saito K, Tanaka M, Oe H, Naritomi H. Early CT findings in unknown-onset and wake-up strokes. Cerebrovasc Dis. 2006;21:367–371. doi: 10.1159/000091545. [DOI] [PubMed] [Google Scholar]
- 16.Nadeau JO, Fang J, Kapral MK, Silver FL, Hill MD. Outcome after stroke upon awakening. Can J Neurol Sci. 2005;32:232–236. doi: 10.1017/s0317167100004029. [DOI] [PubMed] [Google Scholar]
- 17.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., III Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of ORG 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 18.Sobesky J, Frackowiak M, Zaro Weber O, Hahn M, Moller-Hartmann W, Rudolf J, Neveling M, Grond M, Schmulling S, Jacobs A, Heiss WD. The Cologne stroke experience: Safety and outcome in 450 patients treated with intravenous thrombolysis. Cerebrovasc Dis. 2007;24:56–65. doi: 10.1159/000103117. [DOI] [PubMed] [Google Scholar]
- 19.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, Schneider D, Diez-Tejedor E, Trouillas P. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second european-australasian acute stroke study investigators. Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 20.Wahlgren N, Ahmed N, Davalos A, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Kuelkens S, Larrue V, Lees KR, Roine RO, Soinne L, Toni D, Vanhooren G. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke-monitoring study (SITS-MOST): An observational study. Lancet. 2007;369:275–282. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 21.Alberts MJ, Latchaw RE, Selman WR, Shephard T, Hadley MN, Brass LM, Koroshetz W, Marler JR, Booss J, Zorowitz RD, Croft JB, Magnis E, Mulligan D, Jagoda A, O’Connor R, Cawley CM, Connors JJ, Rose-DeRenzy JA, Emr M, Warren M, Walker MD. Recommendations for comprehensive stroke centers: A consensus statement from the brain attack coalition. Stroke. 2005;36:1597–1616. doi: 10.1161/01.STR.0000170622.07210.b4. [DOI] [PubMed] [Google Scholar]
- 22.Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, Brott T, Frankel M, Grotta JC, Haley EC, Jr, Kwiatkowski T, Levine SR, Lewandowski C, Lu M, Lyden P, Marler JR, Patel S, Tilley BC, Albers G, Bluhmki E, Wilhelm M, Hamilton S. Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS, and NINDS rt-pa stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 23.Adams HP, Jr, Effron MB, Torner J, Davalos A, Frayne J, Teal P, Leclerc J, Oemar B, Padgett L, Barnathan ES, Hacke W. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: Results of an international phase III trial: Abciximab in emergency treatment of stroke trial (ABESTT-II) Stroke. 2008;39:87–99. doi: 10.1161/STROKEAHA.106.476648. [DOI] [PubMed] [Google Scholar]