Abstract
Background and Purpose
Studies have established a relation between recanalization and improved clinical outcome in acute ischemic stroke patients; however, intra-arterial clot size has not been routinely assessed. The aim of the study was to determine the impact of intra-arterial thrombus burden on intra-arterial treatment (IAT) and clinical outcome.
Methods
A retrospective review of our IAT stroke database included procedure time, recanalization, symptomatic intracranial hemorrhage, poor outcome (modified Rankin Scale score ≥4 at discharge), and mortality. The modified Thrombolysis in Myocardial Infarction thrombus grade was dichotomized into grades 0 to 3 (no clot or moderate thrombus, <2 vessel diameters) versus grade 4 (large thrombus, >2 vessel diameters).
Results
Data were collected on 135 patients with thrombus grading. The baseline median National Institutes of Health Stroke Scale score was higher in patients of grade 4 compared with grades 0 to 3 (19 vs 17, P=0.012). Grade 4 thrombi required longer (median, range) times for IAT (113, 37 to 415 minutes vs 74, 22 to 215 minutes, respectively; P<0.001) and higher rates of mechanical clot disruption (wire, angioplasty, snare, stent, or Merci retriever) compared with grades 0 to 3 (76% vs 53%, P=0.005). There were no differences in rates of symptomatic intracranial hemorrhage (6.6% vs 4.1%, P=0.701) or recanalization (50% vs 61%, P=0.216) in grade 4 versus grades 0 to 3. Multivariate analysis adjusted for age, baseline National Institutes of Health Stroke Scale score, and artery of involvement showed that grade 4 thrombi were independently associated with poor outcome (odds ratio=2.4; 95% CI, 1.06 to 5.57; P=0.036) and mortality (odds ratio=4.0; 95% CI, 1.2 to 13.2; P=0.023).
Conclusions
High thrombus grade as measured by the modified Thrombolysis in Myocardial Infarction criteria may be a risk factor that contributes to poor clinical outcome.
Keywords: thrombus burden, thrombosis, thrombolysis, stroke, angiography, endovascular treatment, outcome
The primary goal of acute intervention for ischemic stroke remains tissue reperfusion by arterial recanalization. Successful recanalization has repeatedly been shown to be correlated with improved clinical outcome in ischemic stroke.1–7 A recent meta-analysis confirmed the role of recanalization in early clinical improvement by demonstrating a 4- to 5-fold increase in the odds of a favorable functional outcome and a 4- to 5-fold reduction in the odds of death.8 Similarly, a lack of reperfusion results in higher rates of poor outcome.5 For example, none of the patients with terminal internal carotid artery (ICA) occlusion in the Multi-MERCI trial who failed to recanalize experienced a significant recovery.1 Other accepted variables that influence outcome in ischemic stroke include age, glucose values, baseline National Institutes of Health Stroke Scale (NIHSS) score, symptom onset to treatment time, artery of involvement, and the presence and state of collaterals.9–11
Although endovascular trials routinely evaluate recanalization, thrombus size has not been systematically studied. One retrospective study found that the length of occlusion in 51 basilar artery occlusions independently affected mortality.9 Large thrombi, such as those present in the terminal ICA and proximal middle cerebral artery (MCA), have been shown to be resistant to thrombolysis and mechanical interventions.5,12 Presumably, the larger clot volume results in a smaller surface area-to-volume ratio, thus decreasing the effectiveness of thrombolysis.
To our knowledge, neither thrombus size nor any grading scales have been investigated with respect to their influence on endovascular interventions and clinical outcomes. Sheehan et al13 developed the Thrombolysis in Myocardial Ischemia (TIMI) scale, which was modified by Qureshi et al14 to include a 5-point angiographic thrombus burden grading scale. We hypothesized that large thrombi (independent of affected vessel) may affect rates of recanalization and lead to increased time to recanalization during intra-arterial therapy (IAT). This may contribute to worse clinical outcomes in acute ischemic stroke patients. We therefore reviewed our intra-arterial stroke database to compare radiographic and clinical outcomes with respect to the thrombus burden score.
Methods
A retrospective review was performed on a prospectively collected database of consecutive acute ischemic stroke patients treated with IAT between November 1996 and June 2005 at our center. Baseline patient demographics and intra-arterial procedural data were collected. For determination of the subtype of ischemic stroke, the original TOAST criteria were used.15 We defined the total IAT time as the time from the first diagnostic intracranial angiogram to the last angiographic film obtained before catheter removal.
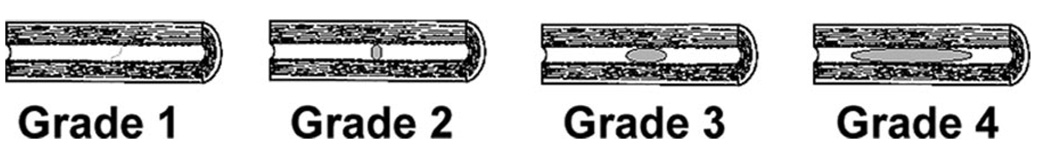
The modified TIMI criteria for thrombus grading was used to evaluate thrombus burden (the Figure).14 Grade 0 is no angiographic characteristic suggestive of thrombus; grade 1 is possible thrombus present, with angiographic characteristics such as reduced density, haziness, irregular lesion contour, or presence of smooth meniscus at the occlusion site; grade 2 is small thrombus present, with the greatest linear dimension less than half of the vessel diameter; grade 3 is moderate thrombus present, with the greatest linear dimension more than half, but less than 2, vessel diameters; and grade 4 is large thrombus present, with the greatest dimension of 2 or more vessel diameters. Pretreatment angiograms were analyzed to determine the most proximal and distal borders of the thrombus on late arterial and capillary phase images or distal microcatheter injections. Thrombus grade was determined by a single interventionalist (M.S.C.) blinded to patient outcomes. Owing to the difficulty in distinguishing between lower thrombus grades (ie, grade 2 vs 3), patients were dichotomized into grades 0 to 3 (no clot or moderate thrombus, <2 vessel diameters) versus grade 4 (large thrombus, >2 vessel diameters) for comparison. Successful recanalization was defined by a Thrombolysis in Cerebral Ischemia score ≥2b, as previously described.16
Figure.
Modified TIMI grading scale for thrombus grade. Grade 0 is no angiographic characteristic suggestive of thrombus; grade 1, possible thrombus present, with angiographic characteristics such as irregular lesion contour or presence of smooth meniscus; grade 2, small thrombus present, with greatest linear dimension less than half of the vessel diameter; grade 3, moderate thrombus present, with greatest linear dimension more than half, but less than 2, vessel diameters; and grade 4, large thrombus present, with greatest dimensions of 2 or more vessel diameters.
The IAT techniques used by our group in this study have been described previously.16 In brief, the procedure first consisted of thrombolytic infusion into the clot. If there was no response after repeated administration of the lytic agent, simple mechanical clot maceration was undertaken, which consisted of passage of the microwire through the clot multiple times. Other forms of mechanical clot disruption consisted of percutaneous angioplasty, intracranial stent deployment, snare device, or MERCI retriever.
Clinical outcomes collected included the following: (1) symptomatic intracranial hemorrhage (sICH), defined as any neurologic worsening thought to be related to the development of ICH; (2) poor clinical outcome, defined as a modified Rankin Scale (mRS) score ≥4 at discharge; (3) poor disposition at discharge, defined as discharge to a nursing home or hospice; and (4) in-hospital mortality. The mRS scores were assigned by an investigator (H.H.) blinded to the angiographic findings, study hypothesis, and design. All computed tomography (CT) scans were analyzed for hemorrhagic transformation by a neuroradiologist blinded to treatment.
For patients treated with intravenous tissue plasminogen activator (IV t-PA), medical management followed the National Institute of Neurological Disorders and Stroke t-PA Stroke Trial protocol. Antiplatelet therapy was started no earlier than 24 hours after the procedure, along with no evidence of ICH on the 24-hour CT scan. The standard guidelines of blood pressure after IV t-PA therapy were followed before and after the intervention. Routine postangiogram orders included a target blood pressure of <160 mm Hg systolic after successful recanalization (Thrombolysis in Cerebral Ischemia ≥2b). Other standard orders included acetaminophen and a cooling blanket for body temperature ≥100°F, as well as a regular insulin sliding scale with blood glucose every 4 to 6 hours. The study was approved by the institutional review board at the University of Texas at Houston.
The analysis was performed with SPSS for Windows, version 15 (SPSS Inc, Chicago, Ill). Categorical variables were analyzed by χ2 and Fisher’s exact test, as appropriate. Continuous variables were analyzed by an independent sample t test or the Mann–Whitney U test, as appropriate. Multiple logistic regression was used to assess the association of age, baseline NIHSS score, thrombus grade group, and artery of involvement (MCAs, ICAs, and basilar arteries were entered as categorical variables) with poor outcome and mortality. A probability value <0.05 was considered statistically significant.
Results
We identified 161 IAT patients. Twenty-six patients were excluded because of incomplete radiographic or clinical data, leaving 135 patients in the final analysis. Eighty-five (63%) of the 135 patients received combination IV t-PA (0.9 mg/kg, 10% bolus and remaining infusion over 60 minutes; maximum 90 mg) and then IAT. Table 1 shows the baseline patient characteristics and thrombus location. Overall, patients with ICA or basilar artery occlusions tended to have higher thrombus grades than did patients with MCA occlusions. Angiographically, 74 had thrombus grade 4 (55%), 37 had thrombus grade 3 (27%), 13 had thrombus grade 2 (10%), 10 had thrombus grade 1 (7%), and 1 had thrombus grade 0. After dichotomization, 74 patients had grade 4 thrombi and 61, grades 0 to 3.
Table 1.
Baseline Characteristics of Patients
| Thrombus Grade |
|||
|---|---|---|---|
| Grades 0–3 n=61 | Grade 4 n=74 | P Value | |
| Age, mean±SD, y | 61.1±13.9 | 62.6±13.6 | 0.532 |
| Men, No. (%) | 32 (53) | 42 (57) | 0.618 |
| Baseline NIHSS score, median, range | 17, 1–39 | 19, 7–39 | 0.012 |
| Hypertension, No. (%) | 36 (59) | 43 (58) | 0.915 |
| Diabetes mellitus, No. (%) | 8 (13) | 16 (22) | 0.198 |
| Coronary artery disease, No. (%) | 15 (24.6) | 10 (13.5) | 0.099 |
| Hyperlipidemia, No. (%) | 10 (16) | 17 (23) | 0.342 |
| TOAST classification, No. (%) | 0.456 | ||
| Cardioembolic | 29 (48) | 37 (50) | |
| Large-artery atherosclerotic | 19 (31) | 28 (38) | |
| Unknown | 9 (15) | 5 (7) | |
| Other | 4 (6) | 4 (5) | |
| Thrombus location, No. (%) | |||
| MCA | 50 (82) | 31 (42) | <.0001 |
| Terminal ICA | 5 (8.2) | 30 (41) | |
| Basilar artery | 6 (9.8) | 13 (18) | |
Intra-Arterial Procedures
Both groups began IAT procedures at a median of 5 hours from symptom onset. Intra-arterial thrombolytic drugs used included 86 (64%) with reteplase, 29 (22%) with urokinase, 15 (11%) with t-PA, 1 (1%) with tenecteplase, and 2 (2%) with a combination. The remaining 2 patients received mechanical clot disruption alone with no infusion of intra-arterial lytic agents. Four patients received mechanical clot disruption with the MERCI retriever (2 were grade 4 and 2 were grade 3). Six ICA stents were placed in the bifurcation during intra-arterial procedures when severe tandem lesions were discovered. One intracranial stent was placed in the vertebral-basilar junction. Intra-arterial procedural data are displayed in Table 2. Grade 4 thrombi required significantly longer IAT times and higher rates of mechanical clot disruption compared with their grade 0 to 3 counterparts. Despite larger dosages of intra-arterial thrombolytics, there was no significant difference in the rate of recanalization in grade 4 thrombi versus grades 0 to 3 (50% vs 61%, P=0.216). However, in those patients who recanalized, the time from the initial diagnostic angiogram to recanalization was significantly longer in grade 4 thrombi (median of 118 vs 72 minutes, P=0.001). A total of 3 procedural complications occurred (2 in thrombus grade 4 and 1 in thrombus grades 0 to 3). One patient had an MCA rupture during balloon angioplasty and died of an sICH. One pneumobasilar occlusion was diagnosed on a postprocedure CT scan. The patient partially recanalized and received hyperbaric treatment but still developed a large, pontine infarct and was discharged to a nursing home with an mRS score of 4. The last complication was an iatrogenic proximal ICA dissection, which required emergent stenting. The patient was discharged to inpatient rehabilitation with an mRS score of 3.
Table 2.
IAT Data and Procedures
| Thrombus Grade |
|||
|---|---|---|---|
| Grades 0–3 | Grade 4 | P Value | |
| Symptom onset to IAT start, median, range; min | 300, 66–894 | 300, 154–1215 | 0.760 |
| Total IAT time, median, range; min | 74, 22–215 | 113, 37–415 | <0.001 |
| Mechanical clot disruption, n/total (%) | 32/61 (53) | 56/74 (76) | 0.005 |
| Simple guide wire | 15/32 (47) | 29/56 (52) | |
| Balloon angioplasty alone | 11/32 (34) | 17/56 (30) | |
| Balloon and snare | 3/32 (10) | 6/56 (11) | |
| MERCI retriever | 2/32 (6) | 2/56 (3) | |
| Snare alone | 1/32 (3) | 1/56 (2) | |
| Balloon and intracranial stent | 0/32 | 1/56 (2) | |
| IAT dosage | |||
| Reteplase units, median, range | 2, 0.4–5 (n=39) | 2.3, 0.6–8 (n=47) | 0.074 |
| Urokinase units, median, range | 155K, 50K–1.5 million (n=16) | 250K, 30K–1.9 million (n=13) | 0.182 |
| t-PA mg, mean±SD | 10.7±6.8 (n=6) | 14.2±6.0 (n=9) | 0.313 |
| Recanalization, n/total (%; final TICI score ≥2b) | 37/61 (61) | 37/74 (50) | 0.216 |
| Time to recanalization, median, range; min | 72, 26–185 (n=37) | 118, 37–415 (n=37) | 0.001 |
| Combination IV/IA therapy, n/total (%) | 37/61 (60.7) | 48/74 (64.9) | 0.614 |
TICI indicates Thrombolysis in Cerebral Ischemia.
Clinical Outcomes
Clinical outcomes are shown in Table 3. The median time of mRS assessment was identical in both groups at 6 days. Thrombus grade did not significantly affect the rate of sICH. After controlling for age, baseline NIHSS score, and artery of involvement, patients with grade 4 thrombi were 2.4 times more likely to experience a poor clinical outcome (95% CI, 1.06 to 5.57; P=0.036) and 4 times more likely to die (95% CI, 1.2 to 13.2; P=0.023) when compared with their grade 0 to 3 counterparts. When we substituted poor discharge disposition for clinical outcome, patients with grade 4 thrombi were 2.7 times more likely to experience discharge to a nursing home or hospice (95% CI, 1.1 to 6.5; P=0.029). The same regression analysis was performed after removal of data for the 4 MERCI-treated patients, and there was no change in the level of significance for any of the clinical outcomes (sICH P=0.4; poor clinical outcome P=0.023; mortality P=0.014). In the 37 patients from each group who achieved recanalization, there were no differences in rates of sICH. However, in a univariate analysis (χ2), grade 4 thrombi patients still had greater rates of poor clinical outcomes (65% vs 35%, P=0.011) and death (22% vs 5%, P=0.041) despite successful recanalization.
Table 3.
Clinical Outcome Data
| Thrombus Grade |
||||
|---|---|---|---|---|
| Grades 0–3 | Grade 4 | P Value | ||
| sICH, n/total (%) | 4/61 (6.6) | 3/74 (4.1) | 0.701 | |
| Poor clinical outcome (mRS score ≥4), n/total (%) | 26/61 (43) | 55/74 (74) | OR=2.4; 95% CI, 1.06–5.57; 0.036* | OR=2.25; 95% CI, 0.96–5.3; 0.062† |
| Poor discharge disposition (discharge to nursing home or hospice care), n/total (%) | 23/61 (38) | 49/73 (67) | OR=2.7; 95% CI, 1.1–6.5; 0.029* | OR=2.5, 95% CI, 1.0–6.2; 0.046† |
| Mortality, n/total (%) | 6/61 (10) | 24/74 (32) | OR=4.0; 95% CI, 1.2–13.2; 0.023* | OR=3.84, 95% CI, 1.1–13.2; 0.033† |
OR indicates odds ratio.
Regression analysis was controlled for age, baseline NIHSS score, and artery of involvement.
Regression analysis was controlled for age, baseline NIHSS score, artery of involvement, and recanalization.
The association between grade 4 thrombi and poor outcome was no longer statistically significant once recanalization was included in the regression model (odds ratio=2.25; 95% CI, 0.96 to 5.29; P=0.062), whereas the association between grade 4 thrombi and discharge disposition (odds ratio=2.5; 95% CI, 1.018 to 6.206; P=0.046) and mortality (odds ratio=3.84; 95% CI, 1.12 to 13.19; P=0.033) remained statistically significant.
Discussion
Our study illustrates the importance of thrombus burden from both a procedural and a clinical perspective. Because the modified TIMI thrombus burden score can be rapidly assessed after the diagnostic angiogram, it can help guide treatment decision making during IAT. Our decision to dichotomize the thrombus burden scores into large (grade 4) versus all others (grades 0 to 3) was performed a priori before any data analysis. This decision was made for 2 reasons. First, there was difficulty in angiographically differentiating the lower grades. Second, the collective experience of our intervention team indicated that very large thrombi were more resistant to intra-arterial interventions and thus, probably more clinically important.
The overall recanalization rate of 54% in our study is similar to that in other intra-arterial trials. For instance, the recanalization rate was 64% in the phase I MERCI study (which included patients who also received intra-arterial lytics) and 60% in IMS-II (all patients who received IV and intra-arterial t-PA).3,17 Importantly, the endovascular procedures undertaken in our cohort reflect the variety of methods used in “real-world” (ie, nonclinical trial) scenarios to achieve the best IAT outcome. It is likely that thrombus burden confounds recanalization due to their relation; ie, larger thrombi are resistant to intra-arterial techniques and delay recanalization. Our numbers may be too small to make definitive conclusions, but they are suggestive that grade 4 thrombi may still be an independent risk factor for poor outcome.
Procedural Implications
A large thrombus burden predicted a slower response to intra-arterial procedures and prolonged time to recanalization. In addition, there was a strong trend for larger total doses of intra-arterial thrombolytics in patients with grade 4 thrombi. Because a delay in recanalization has been shown to result in larger territory infarctions, our findings support the use of more aggressive IAT techniques when interventionalists are faced with grade 4 thrombi.18 For example, higher initial doses of intra-arterial thrombolytics as well as earlier and/or more aggressive mechanical clot disruption may result in faster clot dissolution and avoid delays in tissue reperfusion.
With the exception of 4 patients, the MERCI retriever was unavailable at our institution during the studied retrospective timeframe. Hypothetically, if the retriever is more effective than previous means of mechanical disruption used in this study (ie, it leads to faster and more complete recanalization), it may reduce the impact of thrombus grade. An additional study of thrombus grade in a cohort of adjunctive MERCI-treated patients is warranted to further address this question.
Clinical Implications
In this study, patients with grade 4 thrombi had worse clinical outcomes and higher rates of death. Although a delay in recanalization can partially help explain the greater rates of poor outcome, other mechanisms are also likely important. For instance, more extensive thrombi may result in occlusion of important collateral pathways that could contribute to more rapid and extensive infarction. This theory was supported in our multivariate analysis: when recanalization was included in the model, there was still a significant impact on clinical outcomes and mortality.
Determination of the thrombus burden score may be important when counseling families and predicting the chances of clinical recovery. This information could be used to complement traditional predictors of poor outcome, including age, stroke severity, time to treatment, and recanalization. Neither completed2,17,19 nor major ongoing intra-arterial stroke studies20,21 have addressed the potential influence that thrombus burden may exert on clinical end points. The thrombus burden score may represent a variable that could confound the results of IAT clinical trials and may need to be controlled for as a risk factor for poor outcome.
Study Limitations
Several limitations to this study deserve discussion, including its retrospective nature. First, each IAT case may have been handled differently from a procedural standpoint. For instance, our institution had 3 interventional neuroradiologists on staff during the study time period, and each may have used different thrombolytic dosing schemes or injection methods (ie, distal injection of lytic into the vascular bed before vs after intrathrombus injection). We were unable to control for these differences, because our IAT procedures did not follow a standard protocol. Despite the fact that each intervention-alist may have had a different approach, the large number of patients included in this study suggests that none of their strategies were successful in grade 4 thrombi. Second, the lack of 30- or 90-day clinical outcome data limits comparison of our results with other IAT studies, such as PROACT II or the MERCI retriever studies. Stroke recovery occurs over a longer period of time beyond initial hospitalization. Therefore, our results must be interpreted with caution.
The treating physicians’ decision to offer rescue or primary IAT during the study period was not based on results from advanced neuroimaging findings, such as multimodal magnetic resonance imaging or CT perfusion with penumbral estimation. Although it is possible that our patient selection included patients with no diffusion/perfusion mismatch or a malignant pattern, it remains undetermined whether these factors would have affected both groups equally.22
Summary
In summary, our results support the hypothesis that a high thrombus grade as measured by the modified TIMI criteria prolongs intra-arterial procedure duration, delays recanalization, and may be a risk factor that contributes to poor short-term clinical outcome in acute ischemic stroke patients. Further studies are necessary to determine its role in long-term stroke outcomes.
Acknowledgment
We thank Miriam Morales for reviewing the statistical studies in this report.
Source of Funding
A.D.B. was supported by NIH training grant T32NS04712 to the University of Texas Health Science Center at Houston Vascular Neurology program.
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
None.
References
- 1.Flint AC, Duckwiler GR, Budzik RF, Liebeskind DS, Smith WS. Mechanical thrombectomy of intracranial internal carotid occlusion: pooled results of the MERCI and multi MERCI part I trials. Stroke. 2007;38:1274–1280. doi: 10.1161/01.STR.0000260187.33864.a7. [DOI] [PubMed] [Google Scholar]
- 2.Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, Pessin M, Ahuja A, Callahan F, Clark WM, Silver F, Rivera F. Intra-arterial prourokinase for acute ischemic stroke: the PROACT II study: a randomized controlled trial: PROlyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 3.Gobin YP, Starkman S, Duckwiler GR, Grobelny T, Kidwell CS, Jahan R, Pile-Spellman J, Segal A, Vinuela F, Saver JL. MERCI 1: a phase 1 study of mechanical embolus removal in cerebral ischemia. Stroke. 2004;35:2848–2854. doi: 10.1161/01.STR.0000147718.12954.60. [DOI] [PubMed] [Google Scholar]
- 4.Labiche LA, Al-Senani F, Wojner AW, Grotta JC, Malkoff M, Alexandrov AV. Is the benefit of early recanalization sustained at 3 months? a prospective cohort study. Stroke. 2003;34:695–698. doi: 10.1161/01.STR.0000055940.00316.6B. [DOI] [PubMed] [Google Scholar]
- 5.Saqqur M, Molina CA, Salam A, Siddiqui M, Ribo M, Uchino K, Calleja S, Garami Z, Khan K, Akhtar N, O’Rourke F, Shuaib A, Demchuk AM, Alexandrov AV. Clinical deterioration after intravenous recombinant tissue plasminogen activator treatment: a multicenter transcranial Doppler study. Stroke. 2007;38:69–74. doi: 10.1161/01.STR.0000251800.01964.f6. [DOI] [PubMed] [Google Scholar]
- 6.Smith WS. Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke: results of the Multi mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial, part I. AJNR Am J Neuroradiol. 2006;27:1177–1182. [PMC free article] [PubMed] [Google Scholar]
- 7.Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin YP, Lutsep HL, Nesbit GM, Grobelny T, Rymer MM, Silverman IE, Higashida RT, Budzik RF, Marks MP. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005;36:1432–1438. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 8.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 9.Brandt T, von Kummer R, Muller-Kuppers M, Hacke W. Thrombolytic therapy of acute basilar artery occlusion: variables affecting recanalization and outcome. Stroke. 1996;27:875–881. doi: 10.1161/01.str.27.5.875. [DOI] [PubMed] [Google Scholar]
- 10.Wechsler LR, Roberts R, Furlan AJ, Higashida RT, Dillon W, Roberts H, Rowley HA, Pettigrew LC, Callahan AS, III, Bruno A, Fayad P, Smith WS, Firszt CM, Schulz GA. Factors influencing outcome and treatment effect in PROACT II. Stroke. 2003;34:1224–1229. doi: 10.1161/01.STR.0000068782.15297.28. [DOI] [PubMed] [Google Scholar]
- 11.Baird TA, Parsons MW, Phanh T, Butcher KS, Desmond PM, Tress BM, Colman PG, Chambers BR, Davis SM. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke. 2003;34:2208–2214. doi: 10.1161/01.STR.0000085087.41330.FF. [DOI] [PubMed] [Google Scholar]
- 12.Fredieu A, Duckwiler GR, Starkman S, Kidwell CS, Jahan R, Vinuela F, Ovbiagele B, Vespa P, Selco S, Rajajee V, Saver JL. Clot burden in acute ischemic stroke: relation to occlusion site and the success of revascularization therapy. Stroke. 2005;36:449–450. [Google Scholar]
- 13.Sheehan FH, Braunwald E, Canner P, Dodge HT, Gore J, Van Natta P, Passamani ER, Williams DO, Zaret B. The effect of intravenous thrombolytic therapy on left ventricular function: a report on tissue-type plasminogen activator and streptokinase from the Thrombolysis in Myocardial Infarction (TIMI phase I) trial. Circulation. 1987;75:817–829. doi: 10.1161/01.cir.75.4.817. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi AI, Ringer AJ, Suri MF, Guterman LR, Hopkins LN. Acute interventions for ischemic stroke: present status and future directions. J Endovasc Ther. 2000;7:423–428. doi: 10.1177/152660280000700512. [DOI] [PubMed] [Google Scholar]
- 15.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., III Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST: Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 16.Noser EA, Shaltoni HM, Hall CE, Alexandrov AV, Garami Z, Cacayorin ED, Song JK, Grotta JC, Campbell MS., III Aggressive mechanical clot disruption: a safe adjunct to thrombolytic therapy in acute stroke? Stroke. 2005;36:292–296. doi: 10.1161/01.STR.0000152331.93770.18. [DOI] [PubMed] [Google Scholar]
- 17.The interventional management of stroke (IMS) II study. Stroke. 2007;38:2127–2135. doi: 10.1161/STROKEAHA.107.483131. [DOI] [PubMed] [Google Scholar]
- 18.Derex L, Nighoghossian N, Hermier M, Adeleine P, Berthezene Y, Philippeau F, Honnorat J, Froment JC, Trouillas P. Influence of pretreatment MRI parameters on clinical outcome, recanalization and infarct size in 49 stroke patients treated by intravenous tissue plasminogen activator. J Neurol Sci. 2004;225:3–9. doi: 10.1016/j.jns.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 19.Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke study. Stroke. 2004;35:904–911. doi: 10.1161/01.STR.0000121641.77121.98. [DOI] [PubMed] [Google Scholar]
- 20.Magnetic Resonance and REcanalization of Stroke Clots Using Embolectomy (MR RESCUE) trial. [Last accessed on March 20, 2008];2007 Available at www.clinicaltrials.gov. Identifier: Nct00389467.
- 21.Interventional Management of Stroke (IMS) III trial. [Last accessed on March 20, 2008];2007 Available at www.clinicaltrials.gov. Identifier: Nct00359424.
- 22.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, Bammer R, Kakuda W, Lansberg MG, Shuaib A, Coplin W, Hamilton S, Moseley M, Marks MP. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the Diffusion and perfusion imaging Evaluation For Understanding Stroke Evolution (DEFUSE) study. Ann Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]