Abstract
Objective
To identify the prevalence of vasomotor symptoms (VMS) in a population of premenopausal infertile women and to determine if VMS associate with enhanced bone turnover and low bone mineral density (BMD).
Design
Cross-sectional study.
Setting
Academic infertility practice.
Patients
82 premenopausal infertile but otherwise healthy women attending for routine infertility care.
Intervention
BMD testing, general health and profile of mood state (POMS) questionnaires, serum samples (cycle days 1–3).
Main Outcome Measures
VMS, specifically hot flashes-HF and night sweats-NS; BMD-Z score, BMD categorized as “Low” (Z ≤ −1.0 ) or “Normal” (Z > −1.0); ovarian reserve assessment (biochemical and ovarian dimensions on transvaginal ultrasound); serum markers of bone turnover (NTX, TRAP, BSAP) and ovarian reserve (FSH, Estradiol and Inhibin B). Multivariable regression analyses determined the associations between VMS, BMD and bone turnover (individual markers and composite turnover score).
Results
The prevalence of VMS was 12% in this relatively young population (mean age 34.53 ± SD 4.32). Symptomatic women were significantly more likely to report sleep disturbances (p<0.01), exhibit evidence of low BMD (p<0.01), enhanced bone turnover and poorer ovarian reserve parameters. Multivariable logistic regression analyses confirmed HF (p<0.01) and NS (p<0.01) as independent correlates to low BMD after adjusting for age, BMI, smoking status, menstrual regularity and ovarian reserve status. Multivariable linear regression analyses demonstrated that NS, but not HF, predicted higher bone turnover (p= 0.02) after adjusting for age, smoking, menstrual regularity and ovarian reserve.
Conclusions
We demonstrate, in a premenopausal population of infertile women, evidence of morbid accompaniments to VMS, including sleep disturbances and evidence of low BMD. Our data further suggest a state of enhanced bone turnover in association with VMS, specifically in those experiencing NS. Declining ovarian reserve may be the common pathophysiological mechanism underlying VMS and low BMD in the symptomatic population and merits further investigation.
Introduction
Vasomotor symptoms, i.e. hot flashes (HF) and night sweats (NS), while a hallmark of perimenopause (1—3), are not uncommonly encountered in the premenopausal period (4—6). Elevations in serum levels of follicle stimulating hormone (FSH), a hallmark of reproductive aging, predate these clinical stigmata of perimenopause (7). Both elevations in the pituitary gonadotropins and declining serum estradiol (E2) levels are suggested to play a pathogenic role in the occurrence of VMS (8—9).
Skeletal health is intimately related to and influenced by gonadal function (10,11). Bone mineral density (BMD) and bone metabolism or “turnover” are shown to be independent predictors of risk for fracture (12—14). Limited data accrued in the peri and post menopausal populations suggest an association between VMS and reduced BMD (15—18). The occurrence and the frequency of VMS have been shown to associate with low BMD, and with a rapid deterioration in BMD parameters in the postmenopausal as well as perimenopausal women (15—17). These data are however limited, as much by a retrospective and recall nature of the symptomatology, as by the relatively aging populations studied thus far. Data supporting an association between low BMD and VMS in the premenopausal years are strikingly sparse (19).
In the era of assisted reproduction, elevations in early follicular phase FSH and decline in inhibin B levels have emerged as reliable markers reflecting declining ovarian reserve (20). Although testing for ovarian reserve constitutes an integral component of infertility workup, and yet uncommonly utilized beyond this context at least in the premenopausal years. The infertile, yet healthy premenopausal women thus constitute an optimal population to study the relationship between VMS, ovarian reserve and BMD status. Emerging literature suggests an association between elevations in FSH levels and bone loss (21), highlighting a potential pathogenic mechanism for bone loss in the setting of declining ovarian reserve, further suggesting a relevance of ovarian reserve testing and implications, not just for assessment of reproductive potential, but also skeletal health.
This study explores the hypothesis that pre menopausal and infertile women experiencing VMS will demonstrate evidence of both low BMD and poor ovarian reserve parameters, and will demonstrate biochemical evidence of enhanced bone turnover (i.e. elevated levels of markers of bone resorption and formation) compared to those without these symptoms.
Materials and Methods
Premenopausal women with infertility attending an academic practice in the early follicular phase of the menstrual cycle (days 1–3) were offered participation in a cross sectional study. Inclusion criteria were age <42 years and generally good health, defined as the absence of known systemic diseases contraindicating pregnancy and / or known to adversely influence skeletal health (i.e. systemic lupus erythematosus, diabetes mellitus, crohns disease, renal failure, untreated or over treated thyroid disease). Eighty nine women were enrolled over a 3 year period (April 2004-April 2007). IRB approval was obtained and written consent was provided by the participants. BMD assessments were performed in 82/89 participants. In the initial 10 women, BMD was assessed by dual X-ray absorbtiometry (DXA) of the lumbar spine and hip (Lunar Prodigy, GE, Madison, WI). Secondary to recruitment constraints attributable to the logistics of participant transportation to an off-site bone density center, subsequent enrollees underwent BMD assessment by a peripheral quantitative calcaneal ultrasound device (QUS, n 72, Lunar Achilles Insight ®, GE, Madison, WI) with a known repeat measurement precision of <2% (22). The respective devices were calibrated per company guidelines utilizing the provided phantoms prior to each measurement. Anthropometeric parameters assessed included height (cm) and weight (Kilogram, Kg), and body mass index (BMI) was calculated [Wt (Kg)/Ht (meter)2].
Serum samples were collected and stored at −80 C until assessment of serum levels of markers of interest. Biomarkers of ovarian reserve that were assessed included follicle stimulating hormone (FSH, mIU/ml, DELFIA, Pharmacia, Gaithersburg, MD, DELFIA, intra-assay CV 3.2% and interassay CV 8.7%), estradiol (E2, pg/ml, DELFIA, Pharmacia, Gaithersburg,, MD, sensitivity 10pg/ml, intra-assay CV 4.2% and inter-assay CV 9.0%), and inhibin B (pg/ml. Oxford bioinnovations, Oxford shire, UK, sensitivity <15pg/ml, intra-assay and inter-assay CV <7%). As per the guidelines followed in clinical practice, the maximal historical FSH level for each patient was considered to reflect the OR status. In a subset of patients, markers of bone turnover were assessed including a formation marker, bone specific alkaline phosphatase (BAP µg/L, ELISA, IDS, Inc., Fountain Hills, Arizona, sensitivity 1.0ng/ml, intra-assay CV <10%, inter-assay CV <10% in 65/82), and resorption markers, tartrate resistant acid phosphatase (TRAP, U/L, ELISA IDS, Inc., Fountain Hills, Arizona, sensitivity <0.5 U/liter, intra-assay CV <9%, inter-assay CV <10% in 64/82), and collagen N-terminal telo-peptide (NTX, nM bone collagen equivalents- BCE, ELISA, Wampole Laboratories, Raritan, New Jersey, standard range: 3.2 to 40.0 nM per bone collagen equivalents, intra-assay CV 7.3%, inter-assay CV 6.9% in 50/82), using commercial kits.
The participants were provided with a questionnaire addressing medical, social, family and personal histories. Specific questions were phrased to enquire about occurrence of VMS: “are you bothered by night sweats (Yes/No)”, “are you bothered by hot flashes – (Yes/No)”. Additional questions asked to specify the frequency of occurrence of VMS as: less than once a day, 1–2 times per day, 3–4 times per day and equal to or more than 5 times per day. Specific questions enquired about age at menarche, regularity of menstrual cycles (Yes/No) & current smoking status (Yes/No). Two specified questions enquired about “regular exercise” (Yes/No) and ‘regular weight bearing exercise” (Yes/No) and a single question asked whether the participant was experiencing disturbed sleep (Yes/No).
An assessment of dysphoric mood parameters was performed utilizing the profile for mood state (POMS) questionnaire (23—24). Briefly, a 60 item validated tool requesting responses ranging from “0-very little” to “5-extremely” evaluates the participant responses across six dimensions of mood; five of these represent “negative mood states”, namely “tension”, “anger”, “depression”, “fatigue” and “confusion”. The sixth is a positive mood, “vigor”. The questionnaires were scored by a single investigator (KB) blinded to the participant’s vasomotor symptomatology. A total dysphoric mood score is calculated based on the sum of negative mood scores minus the vigor scores. Higher total mood scores thus reflect greater degree of dysphoria.
Bone density Z-scores were regarded as the BMD parameter of interest, given the premenopausal study population (25). BMD was categorized as “low-LBMD” if Z score ≤ −1.0 (equal to or lower than 1 SD below the age and gender matched population mean) or “normal-NBMD if Z score >−1.0 (based on age and gender matched populations utilized for standardization of the respective device).
In addition to the specified biomarkers reflecting ovarian reserve status, measurements of the individual ovarian dimensions (width, length and mean ovarian diameter) procured by transvaginal ultrasound (Aloka 1400, Phillips, 7.5 MHz) performed as a part of routine clinical care within first 3 days of the menstrual cycle were additional parameters reflective of ovarian reserve, as previously described (26).
Data Analysis
The distributions of continuous data were evaluated by Shapiro-Wilk normality test. Correlation between continuous data (age, BMI, FSH, bone turnover markers and Z score) were assessed by Pearson’s (for data demonstrating normal distribution) or Spearman (for skewed data) correlation analyses. Attempts to normalize skewed data by log transformation were employed (FSH, TRAP and BAP levels). Non parametric Mann Whitney-U rank sum test (for skewed data including BMI, POMS scores, ovarian dimensions, FSH, E2) or Student’s t test (for data demonstrating a Gaussian distribution, i.e. age, NTX, Inhibin B levels and Z scores) were employed to assess the associations between continuous variables of interest with LBMD and VMS. Associations between categorical variables (infertility diagnoses, menstrual regularity, smoking, race, sleep disturbances) with LBMD and VMS were assessed using chi-square analyses.
Multivariable logistic regression analyses was conducted to evaluate the relationship between LBMD and VMS after adjusting for biologically plausible parameters that are recognized to influence BMD in premenopausal years (i.e. menstrual regularity, BMI, smoking and ovarian reserve status as reflected by maximal FSH levels). Because of the relatively small number of events of interest, i.e. LBMD (n=19), a propensity score analysis was employed to adjust for covariates of import without unduly burdening the statistical models (27).Briefly, a propensity score derived from separate multivariable model (linear or logistic as appropriate) incorporating the adjustment covariates of interest was utilized as a single adjustment variable (summarizing the included covariates) in the logistic regression models determining an association between LBMD and VMS. The strength of associations between VMS and LBMD is presented as odds ratio (OR) ± 95% confidence intervals (CI). The association between VMS and BMD parameters was further analyzed utilizing Z-scores as a continuous variable.
A “composite bone turnover score” was generated for each individual, reflecting the sum of the evaluated bone markers (i.e. BAP+TRAP+NTX). Multivariable linear regression analyses were conducted, adjusting for age, histories of smoking, menstrual regularity and ovarian reserve as reflected by maximal FSH levels to determine independent correlates of bone turnover status (i.e. individual biomarkers as well as the composite bone turnover score). STATA 8.2 (StataCorp, TX) was utilized for analyses; p values are reported to the third decimal place, and p<0.050 was considered statistically significant.
Results
Table 1 presents the patient characteristics, the total dysphoric mood scores, BMD and ovarian reserve parameters according to the presence or absence of VMS. Continuous data are presented as mean ± standard deviation (SD), and categorical data are shown as number (percentage).
Table 1.
Characteristics of participants (n 75) based on vasomotor symptoms are presented.
| Characteristics | Asymptomatic N 66 (87.5%) |
Symptomatic N 9 (12.5%) |
P value |
|---|---|---|---|
| Age§ (Years) | 34.63 ± 4.50 | 30.17 ± 4.41 | 0.41♪ |
| BMI§ | 27.17 ± 6.95 | 29.11 ± 7.13 | 0.20♫ |
| Smoking history n (%)σ | 10/65 (15.38) | 3/9 (33.33) | 0.18† |
| Regular cycles n (%) | 50/66 (75.76) | 6/9 (66.67) | 0.57† |
| Acknowledge disturbed sleep n (%) | 18/64 (27) | 7/9 (78) | <0.01*∫ |
| Z-score (SD)§ | 0.08 ± 1.23 | −0.08 ± 1.23 | 0.06♪ |
| POMS Scores | |||
| Total dysphoric mood | 20.11 ± 34.11 | 22.37 ± 25.28 | 0.47♫ |
| Depression | 8.31 ± 11.17 | 7.87 ± 6.77 | 0.37♫ |
| Tension | 9.18 ± 6.22 | 8.75 ± 5.47 | 0.93♫ |
| Fatigue | 6.61 ± 5.94 | 9.0 ± 5.21 | 0.13♫ |
| Anger | 6.89 ± 7.80 | 6.0 ± 3.85 | 0.64♫ |
| Vigor | 14.37 ± 4.66 | 13.50 ± 4.95 | 0.55♫ |
| Ovarian Reserve Parameters | |||
| Mean ovarian diameter (cm) | 6.20 ± 1.44 | 5.80 ± 0.54 | 0.45♫ |
| Mean ovarian length (cm) | 4.69 ± 1.08 | 4.46 ± 0.44 | 0.63♫ |
| Mean ovarian width (cm) | 3.03 ± 0.87 | 2.66 v 0.53 | 0.34♫ |
| FSH (mIU/ml) | 7.14 ± 5.02 | 9.89 ± 5.37 | 0.62♫ |
| Estradiol (pg/ml) | 35.25 ± 15.71 | 48.83 ± 26.97 | 0.39♫ |
| Inhibin B (pg/ml) | 73.18 ± 27.62 | 59.53 ± 36.28 | 0.43♪ |
Continuous data are presented as mean ± SD; Categorical data are presented as percentage
Statistical significance
T test;
Mann U Whitney;
Chi square
Specific response was not available for one participant in the asymptomatic group.
Specific response was not available for 2 participants in the asymptomatic group.
BMD assessment was not available for one participant in each group.
Eighty nine premenopausal women with infertility were enrolled over a 3 year period (April 2004-April 2007); 82/89 (92%) underwent BMD assessment. Of the 88/89 patients in whom contributory etiology/ies for infertility were clearly identified from the patient records, a single etiology for infertility was discernable in 74; in 14/88 (16%), more than one contributory factor was noted. The commonest contributors to infertility were ovulatory disturbances (19/88, 22%), diminished ovarian reserve (17/88, 19%), and male factor infertility (17/88, 19%) followed by unexplained infertility (10/88, 11%), tubal infertility (9/88, 10%) and miscellaneous causes (3/88, 3%).
Responses for VMS were available for 75/89 (84%) of the participants; Twelve percent (9/75) of the participants acknowledged experiencing one or both of the specified VMS (NS only in 2/9, HF only in 3/9 or both NS and HF in 4/9). The frequency of VMS was described as “once or twice a day” by all those who were symptomatic. Seventy one (95%) responded to the question on race/ethnicity and 73 (97%) answered to the query regarding “disturbed sleep”. The majority of participants self identified as being Caucasian (50/71, 70%); 7/71 (10%) as Black, 10/71 (14%) as Asians and 4/71 (6%) reported belonging to more than one or otherwise specified race/ethnicity. Sixty two (83% of the 75 with available VMS responses) completed the POMS questionnaire.
Although the distribution of infertility etiologies was comparable in the symptomatic versus asymptomatic women, women with unexplained infertility were significantly more likely to specifically report NS (compared to those with identified contributors to infertility, 29% versus 6%, chi square 4.95, p=0.04). Almost 17% of the participants (14/82 who responded to the specified question) acknowledged smoking. Those experiencing VMS were almost 3 times more likely, and those reporting NS almost 6 times more likely, to report current smoking (OR for VMS and NS in smokers 2.75, 95% CI 0.38–15.45 and OR 5.7, 95% CI 1.00–32.33 respectively); the relationship approached statistical significance for the association between smoking and NS (p=0.05).
Consistent with reported literature, symptomatic women were of a heavier BMI compared to those without VMS; these differences however were not statistically significant (Table 1, p>0.05). A history of menstrual irregularities was comparable between the patients in the two groups (p>0.05). Those experiencing VMS were 9 times more likely to report sleep disturbances compared to the asymptomatic group (OR 8.90, 95% CI 1.50–93.0, p<0.01). Although higher dysphoric and lower vigor scores were noted in the symptomatic women, the differences were however not of statistical significance (Table 1). The symptomatic group of women demonstrated evidence of poorer ovarian reserve parameters, i.e. smaller ovarian morphometeric dimensions as measured by transvaginal ultrasound, higher early follicular phase serum levels of FSH and E2 and lower inhibin B levels (Table 1); these associations however did not reach statistically significance.
Bone Mineral Density
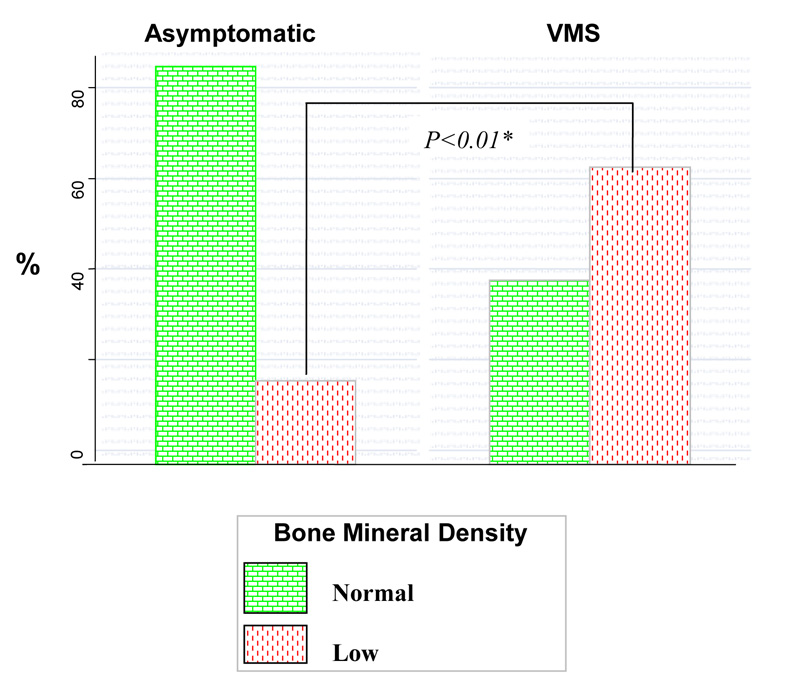
Of the 82 enrollees on whom BMD measurements were available, responses to VMS were completed by 73 (89%). Twenty three percent (19/82) of the premenopausal infertile women demonstrated evidence of LBMD. Patients experiencing VMS demonstrated lower BMD Z scores (Table 1). A significantly higher proportion of premenopausal women experiencing VMS demonstrated evidence of LBMD (as previously defined) compared to those without these symptoms (62.5% versus 15.38%, p<0.01, Figure 1). This association with LBMD was most robust for NS followed by VMS and HF in descending order of magnitude for the association (Table 2).
Figure 1.
Higher prevalence of low BMD in premenopausal women experiencing vasomotor symptoms.
Table 2.
An increased likelihood of low BMD (reported as odds ratio, OR ± 95% CI) is seen in association with vasomotor symptoms.
| Unadjusted OR | P value | Adjusted† OR | P value | |
|---|---|---|---|---|
| VMS | 9.17, (1.88–44.59) | <0.01* | 9.94 (1.89–46.76) | <0.01* |
| Hot flash | 6.67 (1.30–34.06) | 0.02* | 7.09 (1.33–37.84) | 0.02* |
| Night sweat | 20.36 (2.07–200.00) | 0.01* | 20.51 (2.04–205.76) | 0.01* |
Statistical significance.
propensity score analysis adjusting for age, BMI, smoking status, regular menstrual cycles and ovarian reserve status (FSH levels).
Multivariable logistic regression analyses after adjusting for age, BMI, smoking, menstrual regularity, and ovarian reserve status (as reflected by maximal FSH levels) confirmed both HF and NS as independent correlates of LBMD in these young women, (Table 2). Multivariable linear regression analysis utilizing BMD as a continuous variable (Z scores) confirmed this independent and inverse association between VMS and LBMD (R2 for the model 0.25, β coefficient for the association between Z score and VMS −1.20, SE 0.42, 95% CI −2.03 to −0.35, p<0.01); 25% of the variability in Z scores in this population was explained by this statistical model.
Bone Metabolism
Serum NTX, BAP and TRAP levels were available respectively for 46/73 (63%), 59/73 (81%) and 58/73 (79%). Bone metabolism, as reflected by the levels of markers of bone formation (BAP), resorption (NTX, TRAP), and specifically by the cumulative turnover score, was noted to inversely correlate with patient’s age (r= −0.31, p=0.02 for an association between age and NTX; r= −0.23, p=0.06 for association of age with BAP). Smokers exhibited higher serum levels of bone turnover markers (NTX 15.14 ± 3.0 versus 13.19 ± 2.90 nM BCE and TRAP 9.75 ± 5.60 versus 9.53 ± 8.72, compared to the non-smokers; this association approached statistical significance for NTX (p=0.06).
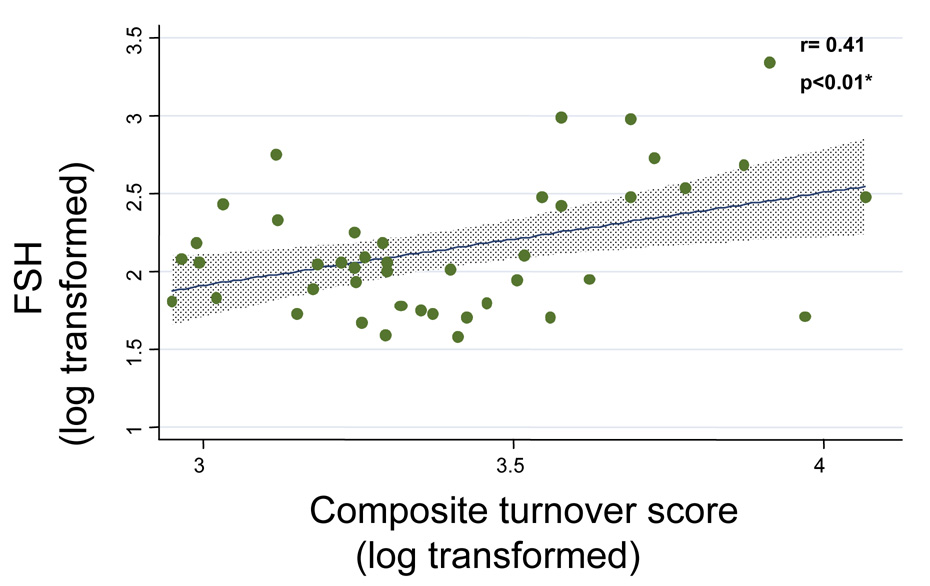
A relationship between enhanced bone turnover (reflected by higher levels of bone markers) and worsening ovarian reserve was noted; maximal FSH levels (log transformed) were observed to significantly correlate with TRAP (log transformed, r= 0.29, p=0.02) and with the composite turnover score (log transformed, r= 0.41, p<0.01) (Figure 2). Those reporting irregular menstrual cycles demonstrated marginally higher levels of NTX, BAP and the composite turnover score (data not shown), these differences were not of statistical significance (p >0.05).
Figure 2.
Relationship between worsening ovarian reserve (i.e. higher FSH levels) and bone metabolism:
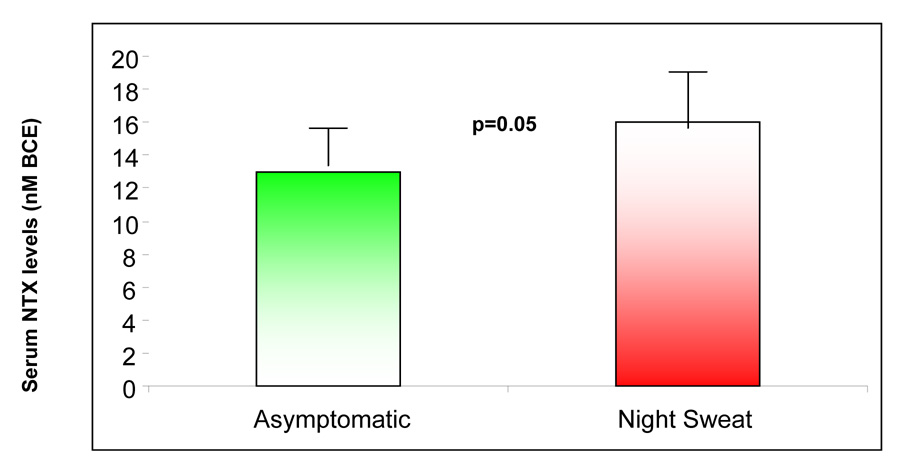
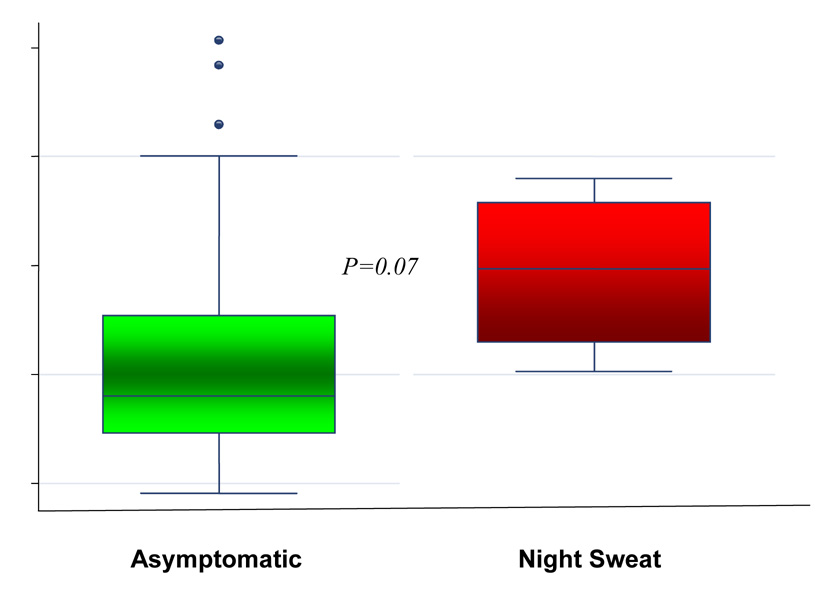
A state of up regulated bone turnover, albeit insignificantly so, was appreciated in association with VMS; these parameters approached statistical significance in women specifically reporting NS (Figure 3). Multivariable linear regression analyses, utilizing the propensity scores for the covariates (age, smoking, menstrual regularity and ovarian reserve status as reflected by maximal FSH levels) demonstrated symptom of NS, as an independent determinant of significantly higher serum NTX levels (R2 0.07, β coefficient 2.81, SE 1.31, t 2.14 95% CI 0.16 − 5.46, p=0.04); a similar, albeit non significant, association between NS and composite turnover score was also noted (R2 0.20, β coefficient 0.24, SE 0.13, t 1.8, 95% CI −0.03 to 0.51, p=0.08). Multivariable analyses failed to demonstrate a relationship between NS and the additional evaluated bone markers (i.e. BAP and TRAP).
Figure 3.
An up regulation of bone turnover, as reflected by serum levels of N-telopeptide (3a) and the composite bone turnover score (3b), is noted in premenopausal infertile women experiencing night sweats.
The box represents the inter quartile range of data (between 25th and 75th percentile); the horizontal line represents the median value and the whiskers represent the 95% confidence intervals; the individual dots represent the outliers.
Discussion
The prevalence of LBMD (23%) in our premenopausal and dominantly Caucasian population is somewhat higher than the expected 15% per the Gaussian population distribution (25). Our findings of LBMD and increased bone turnover in association with VMS, specifically NS, are consistent with those reported by Salamone et al. (16), albeit in an older population of pre-menopausal women (ages 44–50); similar associations have hitherto not been described in such a young population (mean age 34 years). Lee et al. (15), in a similar older population, demonstrated an association between recalled premenstrual and postmenopausal VMS and vertebral fractures, suggesting a relationship between VMS and osseous integrity. These observations are however in contrast to those reported by Von Muhlen et al. (28) The authors, in the Rancho Bernardo Study, failed to demonstrate any association between recalled VMS at menopause and BMD in 894 postmenopausal women (mean age 73 ± 9.5 years, range 47–97). Of note, consistent with our findings however, these authors reported a significantly higher prevalence of smokers amongst women experiencing NS. Similarly, Scoutellas et al. (29), were unable to demonstrate an association between recalled symptoms and vertebral fractures in a population based study of postmenopausal women aged 50–64 years. The older age of the enrolled women, the excessive reporting of postmenopausal estrogen therapy use by women acknowledging a history of VMS in the Rancho Bernardo study, and the long period of time elapsed since the occurrence of VMS all may have explained the inconsistencies in findings in observations noted in these latter studies when compared to our data.
The prevalence of VMS in our premenopausal and infertile population (12%), while identical to what was recently reported by Ohayon (30), is less than described in the SWAN study; of the 4497 premenopausal women aged 40–55 years screened for eligibility for enrollment in the SWAN study, 19.4% reported any HF or NS (3). Differences in the ages and the ethnic composition of the enrolled populations in the mentioned studies may partly explain the discrepancies in the prevalence of VMS in premenopausal years.
Elevations in serum FSH levels are a hallmark of the period of perimenopause (7). We (31) and others (32) have previously provided evidence in support of influences of ovarian reserve on BMD parameters in the premenopausal years; ; furthermore, existing data, in the perimenopausal women, are supportive of a linear correlation between ovarian reserve and BMD parameters, and of an inverse relationship between ovarian reserve and bone turnover (33,34). Recent reports provide evidence of direct effects of FSH on osteoclasts (21)
A decline in estrogen levels is suggested as a biological mechanism contributing to VMS. Significantly lower circulating levels of E2 and its metabolites are reported in women experiencing NS (8) and indeed NS are considered more profound of the two VMS. A hypoestrogenic milieu may thus explain the observed relationship between VMS and LBMD. Although the symptomatic patients in our study demonstrated evidence of lower ovarian reserve, i.e. lower levels of inhibin B and higher levels of FSH, and smaller ovarian dimensions compared to the asymptomatic group (Table 1), the early follicular phase E2 levels were actually higher in the symptomatic women (log transformed, t= −1.51, p=0.14). This latter finding is of interest as higher E2 levels in the early follicular phase are recognized as a marker of declining ovarian reserve (20). The failure to achieve statistical significance to the observed associations is a likely reflection of power constraints, given the small study sample. We conjecture that poorer ovarian reserve in premenopausal women experiencing VMS is a plausible mechanism that can explain the occurrence of VMS, and the low BMD in the setting of elevated bone turnover; in this context, future studies are needed to better elucidate these mechanisms.
Given this association between VMS and declining ovarian reserve, the significantly higher prevalence of VMS in patients with unexplained infertility is of interest. A single population based retrospective cohort study (35) suggested a history of infertility, specifically unexplained infertility, as a risk for vertebral fractures. Concerns regarding a subtle decline in ovarian reserve have been suggested in association with unexplained infertility (36) and may account for the noted association. Although the serum biomarkers reflecting ovarian reserve status (FSH and Inhibin B) did not suggest poor ovarian reserve in patients with unexplained infertility (data not shown), the early follicular phase E2 levels (log transformed) were higher (t −1.97, p=0.05) compared to those in whom an etiology for infertility was appreciated, thus providing some suggestion that issues with ovarian reserve may indeed exist in association with unexplained infertility.
Our finding of increased bone turnover (p=0.06 for NTX) in smokers, is in agreement with the known adverse influences of smoking on bone metabolism (37—38); adverse influences of smoking on ovarian reserve were additionally suggested, as reflected by higher (albeit not statistically significant) levels of FSH (9.62 ± 4.50 versus 8.92 ± 4.32 in nonsmokers, p=0.52) and smaller ovarian size (mean ovarian diameter 5.60 ± 1.3 versus 6.12 ± 1.3 cm in nonsmokers, p=0.31). While reduced ovarian reserve, and therefore a state of relative hypoestrogenism is a plausible mechanism explaining both the occurrence of VMS and the enhanced bone turnover in our premenopausal smokers, we are however unable to establish this notion in the present study.
Evidence of LBMD in a young individual at a single time point raises the question regarding whether this observation reflects a suboptimal peak bone attainment in an otherwise healthy individual, or if this is a sequel to an exaggerated bone loss. While postmenopausal exacerbation in bone loss and an enhanced fracture risk following menstrual cessation are recognized (12—13), the implications of low BMD in the mid-reproductive years are somewhat unclear. What is obvious however is that these young women will enter menopause with a lower “skeletal reserve,” that is destined to further decline during the early postmenopausal years. Our findings of LBMD and elevated bone turnover status in premenopausal women experiencing VMS support a notion that increased bone turnover may be a contributor to the low bone mass in these young women; we are however unable to substantiate this impression given the cross sectional nature of this study, and propose a need for future longitudinal studies to better address the mechanisms at play.
There are several limitations of our study that preclude extrapolation of our findings to the general and non-infertile population at large. The relatively small sample size, the low prevalence of vasomotor symptoms, the categorical nature of our symptom data, which does not provide any information regarding the severity of the VMS, all limit our ability to understand the nuances of how these phenomenon relate to BMD. Given that the frequency of VMS was identical in the symptomatic population, we are unable to assess for an association, if any, between the frequency of VMS, ovarian reserve, bone mass and metabolism. Although the small sample size of our population does not preclude a probability that the statistically significant associations between VMS, BMD and bone turnover may represent an alpha error, the consistency, the magnitude and a biological plausibility to the demonstrated associations is reassuring. We acknowledge the limitations intrinsic to a lack of uniformity in BMD assessments; as stated earlier in methods, this decision was necessitated by a need to facilitate recruitment. Although not regarded as a “gold standard” modality for BMD assessment, the sensitivity and reliability of QUS in evaluating BMD is well established and shown to be comparable to DXA (39).
In summary, our findings provide evidence that VMS in premenopausal years auger adverse health implications. Specifically, infertile women experiencing VMS, especially NS, are significantly more likely to be smokers, experience disturbed sleep, demonstrate low BMD and enhanced bone turnover, associations thus far unappreciated in such a young population of otherwise healthy women. Our data furthermore suggest a relationship between declining ovarian reserve and enhanced bone turnover, thus implying a unifying pathophysiological mechanism that can explain the symptomatology as well as the exaggerated bone loss, and low bone mass in premenopausal women experiencing VMS. Evidence of excessive bone turnover as well as lower ovarian reserve parameters in the premenopausal smokers reiterates’ the adverse influences of smoking on both ovarian and bone physiology. These findings underscore the intimate relationship between reproductive health and bone metabolism in the premenopausal years. Although both low BMD and an enhanced bone turnover are recognized as risk factors for fracture in the post and the perimenopausal years (13), extrapolating this conjecture to a young premenopausal population merits further assessment by appropriately designed longitudinal studies. We propose that VMS in the premenopausal years be recognized as risk factors for low bone density. We believe that these data will help clinicians in offering appropriate patient screening and counseling aimed at strategies to stabilize osseous integrity, and minimize adverse influences on the skeletal as well as reproductive health, i.e. smoking cessation and lifestyle modifications. Indeed, a benefit of BMD testing and subsequent counseling in facilitating adoption of positive life style changes has been previously demonstrated in the premenopausal population (40). BMD testing and bone health education was shown to positively influence smoking, alcohol intake and use of caffeinated drinks, as well as improve compliance with calcium and vitamin D supplement intake in premenopausal women diagnosed with low bone density (40).
Acknowledgements
Supported in part by NIH K12 (to LP).
The authors would like to extend their appreciation to Stacea Bowen, MD. for assistance with recruitment. Our appreciation and thanks to Goli Adel, MS. and to Jun Shu, MD (Alice) for facilitating and conducting the immunoassays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Capsule: Low BMD and enhanced bone turnover accompany vasomotor symptoms in infertile premenopausal women.
This work was presented as an oral abstract at the Annual Meeting of the American Society for Reproductive Medicine, New Orleans, October 2006 and was recognized as a prize paper by the Society for Reproductive Endocrinology and Infertility.
References
- 1.Randolph JF, Jr, Sowers M, Bondarenko I, Gold EB, Greendale GA, Bromberger JT, et al. The relationship of longitudinal change in reproductive hormones and vasomotor symptoms during the menopausal transition. J Clin Endocrinol Metab. 2005;90:6106–6112. doi: 10.1210/jc.2005-1374. [DOI] [PubMed] [Google Scholar]
- 2.Gold EB, Colvin A, Avis N, Bromberger J, Greendale GA, Powell L, Sternfeld B, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women's health across the nation. Am J Public Health. 2006;96:1226–1235. doi: 10.2105/AJPH.2005.066936. Epub 2006 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gold EB, Sternfeld B, Kelsey JL, Brown C, Mouton C, Reame N, et al. Relation of demographic and lifestyle factors to symptoms in a multiracial/ethnic population of women 40–55 years of age. Am J Epidemiol. 2000;152:463–473. doi: 10.1093/aje/152.5.463. [DOI] [PubMed] [Google Scholar]
- 4.Hahn PM, Wong J, Reid RL. Menopausal-like hot flashes reported in women of reproductive age. Fertil Steril. 1998;70:913–918. doi: 10.1016/s0015-0282(98)00281-7. [DOI] [PubMed] [Google Scholar]
- 5.Juang KD, Wang SJ, Lu SR, Lee SJ, Fuh JL. Hot flashes are associated with psychological symptoms of anxiety and depression in peri- and post- but not premenopausal women. Maturitas. 2005;52:119–126. doi: 10.1016/j.maturitas.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Freeman EW, Sammel MD, Rinaudo PJ, Sheng L. Premenstrual syndrome as a predictor of menopausal symptoms. Obstet Gynecol. 2004;103:960–966. doi: 10.1097/01.AOG.0000124804.81095.7f. [DOI] [PubMed] [Google Scholar]
- 7.Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, et al. Executive Summary: Stages of Reproductive Aging Workshop (STRAW) Fertile Steril. 2001;76:874–878. doi: 10.1016/s0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- 8.Erlik Y, Meldrum DR, Judd HL. Estrogen levels in postmenopausal women with hot flashes. Obstet Gynecol. 1982;59:403–407. [PubMed] [Google Scholar]
- 9.Meldrum DR, Defazio JD, Erlik Y, Lu JK, Wolfsen AF, Carlson HF, et al. Pituitary hormones during the menopausal hot flash. Obstet Gynecol. 1984;64:752–756. [PubMed] [Google Scholar]
- 10.Uygur D, Sengul O, Bayar D, Erdinc S, Batioglu S, Mollamahmutoglu L. Bone loss in young women with premature ovarian failure. Archives of Gynecology and Obstetric. 2005;273:17–19. doi: 10.1007/s00404-005-0029-7. [DOI] [PubMed] [Google Scholar]
- 11.White CM, Hergenroeder AC, Klish WJ. Bone mineral density in 15- to 21-year-old eumenorrheic and amenorrheic subjects. American journal of diseases of children. 1992;46:31–35. doi: 10.1001/archpedi.1992.02160130033016. [DOI] [PubMed] [Google Scholar]
- 12.Kroger H, Huopio J, Honkanen R, Tuppurainen M, Puntila E, Alhava E, et al. Prediction of fracture risk using axial bone mineral density in a perimenopausal population: a prospective study. J Bone Miner Res. 1995;10:302–306. doi: 10.1002/jbmr.5650100218. [DOI] [PubMed] [Google Scholar]
- 13.Melton LJ, 3rd, Khosla S, Atkinson EJ, O’Fallon WM, Riggs BL. Relationship of bone turnover to bone density and fractures. J Bone Miner Res. 1997;12:1083–1091. doi: 10.1359/jbmr.1997.12.7.1083. [DOI] [PubMed] [Google Scholar]
- 14.Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res. 2000;15:1526–1536. doi: 10.1359/jbmr.2000.15.8.1526. [DOI] [PubMed] [Google Scholar]
- 15.Lee SJ, Kanis JA. An association between osteoporosis and premenstrual symptoms and postmenopausal symptoms. Bone Miner. 1994;24:127–134. doi: 10.1016/s0169-6009(08)80150-x. [DOI] [PubMed] [Google Scholar]
- 16.Salamone LM, Gregg E, Wolf RL, Epstein RS, Black D, Palermo L, et al. Are menopausal symptoms associated with bone mineral density and changes in bone mineral density in premenopausal women? Maturitas. 1998;29:179–187. doi: 10.1016/s0378-5122(98)00019-x. [DOI] [PubMed] [Google Scholar]
- 17.Naessen T, Persson I, Ljuanghall S, Bergstrom R. Women with climacteric symptoms: a target group for prevention of rapid bone loss and osteoporosis. Osteoporos Int. 1992;2:225–231. doi: 10.1007/BF01624146. [DOI] [PubMed] [Google Scholar]
- 18.Oldenhave A, Jaszmann LJB. The Climacteric: Absence or presence of hot flashes and their relation to other complaints. Prog Basic Clin Pharmacol. 1991;6:6. [Google Scholar]
- 19.Menon RK, Okonofua FE, Agnew JE, Thomas M, Bell J, O'Brien PM, Dandona P. Endocrine and metabolic effects of simple hysterectomy. Int J Gynecol Obstet. 1987;25:459. doi: 10.1016/0020-7292(87)90062-2. [DOI] [PubMed] [Google Scholar]
- 20.Evers JL, Slaats P, Land JA, Dumoulin JC, Dunselman GA. Normal basal levels of follicle-stimulating hormone undergoing in vitro fertilization. Fertil Steril. 1998;69:1010–1014. doi: 10.1016/s0015-0282(98)00080-6. [DOI] [PubMed] [Google Scholar]
- 21.Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, et al. FSH directly regulates bone mass. Cell. 2006;125:247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 22. http://www.gehealthcare.com/euen/bone-densitometery/docs/Achilles-ins.pdf.
- 23.Mc Nair DM, Lorr M, Droppleman LF. Manual for the Profile for Mood States (POMS) San Diego, CA: Educational and Industrial testing service; 1992. [Google Scholar]
- 24.Profile for Mood States. MHS Inc.; http://www.mhs.com/ [Google Scholar]
- 25.Lewjecki ME. Low bone mineral density in premenopausal women. Southern Medical Association. 2004;97:544–440. doi: 10.1097/00007611-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Bowen S, Norian J, Santoro N, Pal L. Simple tools for assessment of ovarian reserve (OR): Individual ovarian dimensions are reliable predictors of OR. Fertil Steril. 2007 April 3; doi: 10.1016/j.fertnstert.2006.11.175. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurth T, Walker AM, Glynn RJ, Chan KA, Gaziano JM, Berger K, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment and propensity based weighting under conditions of non union effect. Am J Epidemiol. 2006;163:262–270. doi: 10.1093/aje/kwj047. [DOI] [PubMed] [Google Scholar]
- 28.von Muhlen DG, Soroko S, Kritz-Silverstein D, Barrett-Connor E. Vasomotor symptoms are not associated with reduced bone mass in postmenopausal women: the Rancho Bernardo Study. J Womens Health Gend Based Med. 2000;9:505–511. doi: 10.1089/15246090050073585. [DOI] [PubMed] [Google Scholar]
- 29.Scoutellas V, O'Neill TW, Lunt M, Reeve J, Silman AJ. Does the presence of postmenopausal symptoms influence susceptibility to vertebral deformity? European Vertebral Osteoporosis Study (EVOS) Group. Maturitas. 1999;32:179–187. doi: 10.1016/s0378-5122(99)00025-0. [DOI] [PubMed] [Google Scholar]
- 30.Ohayon MM. Severe hot flashes are associated with chronic insomnia. Arch Intern Med. 2006;166:1262–1268. doi: 10.1001/archinte.166.12.1262. [DOI] [PubMed] [Google Scholar]
- 31.Pal L, Norian J, Hailpern S, Zeitlian G, Adel G, Freeman R, et al. Ovarian reserve parameters are predictive of bone density and metabolism in young infertile women. Boston, MA. Poster presentation, The Endocrine Society 88th annual meeting; 2006. P3-519; 768. [Google Scholar]
- 32.Vural F, Vural B, Yucesoy I, Badur S. Ovarian aging and bone metabolism in menstruating women aged 35–50 years. Maturitas. 2005;52:147–153. doi: 10.1016/j.maturitas.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Sowers M, Randolph JF, Jr, Crutchfield M, Jannausch ML, Shapiro B, Zhang B, et al. Urinary ovarian and gonadotrophin hormone levels in premenopausal women with low bone mass. J Bone Miner Res. 1998;13:1191–1202. doi: 10.1359/jbmr.1998.13.7.1191. [DOI] [PubMed] [Google Scholar]
- 34.Lukacs JL, Reame NE. Concentration of follicle stimulating hormone correlate with alkaline phosphatase and a marker for vitamin K status in the perimenopause. J Women's Health & Gender Based Medicine. 2000;9:731–739. doi: 10.1089/15246090050147709. [DOI] [PubMed] [Google Scholar]
- 35.Melton LJ, 3rd, Hesdorffer DC, Malkasian GD, Atkinson EJ, Brinton LA, O'Fallon WM. Long-term fracture risk among infertile women: a population-based cohort study. J Womens Health & Gend Based Med. 2001;10:289–297. doi: 10.1089/152460901300140040. [DOI] [PubMed] [Google Scholar]
- 36.Leach RE, Moghissi KS, Randolph JF, Reame NE, Blacker CM, Ginsburg KA, et al. Intensive hormone monitoring in women with unexplained infertility: evidence for subtle abnormalities suggestive of diminished ovarian reserve. Fertil Steril. 1997;68:413–420. doi: 10.1016/s0015-0282(97)00222-7. [DOI] [PubMed] [Google Scholar]
- 37.Shulman A, Shulman A, Ellenbogen A, Maymon R, Bahary C. Smoking out the estrogens. Hum Reprod. 1990;3:231–233. doi: 10.1093/oxfordjournals.humrep.a137077. [DOI] [PubMed] [Google Scholar]
- 38.Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, Eisman JA, et al. Smoking and fracture risk: a meta analysis. Osteopor Int. 2005;16:155–162. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 39.Gregg EW, Kriska AM, Salamone LM, Roberts MM, Anderson SJ, Ferrell RE, et al. The epidemiology of quantitative ultrasound: a review of the relationships with bone mass, osteoporosis and fracture risk. Osteoporos Int. 1997;7:89–99. doi: 10.1007/BF01623682. [DOI] [PubMed] [Google Scholar]
- 40.Jamal SA, Ridout R, Chase C, Fielding L, Rubin LA, Hawker GA. Bone mineral density testing and osteoporosis education improve lifestyle behaviors in premenopausal women: a prospective study. J Bone Miner Res. 1999;14:2143–2149. doi: 10.1359/jbmr.1999.14.12.2143. [DOI] [PubMed] [Google Scholar]