Abstract
The present study investigates the effect of perinatal taurine exposure on renal function in adult, female rats on a high sugar diet. Perinatal taurine depleted (TD), supplemented (TS) or untreated control (C) female offspring were fed normal rat chow and tap water (CW,TDW or TSW) or tap water with 5% glucose (CG, TDG or TSG) after weaning. At 7–8 weeks of age, renal function was studied in the conscious, restrained rats. Mean arterial pressure was significantly higher in TDW, TDG, and TSG rats. Plasma sodium concentration was significantly lower in all glucose treated animals, but the greatest decrease was in TDW rats. Basal renal blood flow was lowest in TSW and TSG, and the responses to a saline load were also lowest in those two groups. These changes were consistent with increased renal vascular resistance. The basal glomerular filtration rate was lowest in TSW, but the responses to a saline load were similar in all of the groups. Water excretion was lower in TSG and TSW, consistent with increased renal tubular water reabsorption. These data suggest that perinatal taurine exposure alters normal renal function and renal responses to dietary sugar in adult female offspring.
1. INTRODUCTION
The kidney is a major regulator of long-term arterial pressure; as such, renal dysfunction or damage can lead to sustained hypertension (Cusi and Bianchi 1991; Rettig et al. 1990). Renal dysfunction can be genetically inherited, as shown by renal transplantation studies in spontaneously hypertensive and normotensive rats (Grisk et al. 2002; Rettig and Grisk 2005). The normotensive recipients of spontaneously hypertensive rat kidneys develop hypertension as adults while the hypertensive donors receiving kidneys from normotensive rats remain normotensive. Prenatal food imbalances can result in low birth weight in neonates and in adults, produce a high risk of cardiovascular disease, insulin resistance, obesity, and hypertension (Barker et al. 2002; Harding 2001; Langley-Evans et al. 2003b). Decreases in nephron number and excretory function also result from early malnutrition (Langley-Evans et al. 2003c; Sturman 1993) and may underlie hypertensive development in adult offspring. Perinatal taurine depletion appears to induce low birth weight, multiple organ damage (Sturman 1993) and low tissue taurine content in many organs and plasma (Aerts and Van Assche 2002). While the mechanisms underlying the adverse effects of perinatal taurine exposure remain ambiguous, a decrease in taurine content likely leads to adult diseases.
Taurine is a free sulphur-containing beta-amino acid found in various organs including brain, the heart, and the kidney (Aerts and Van Assche 2002). Taurine content is highest during the perinatal period and then subsequently declines with advancing age (Sturman 1993). Renal dysfunction with aging, diabetes mellitus, hypertension, and obesity are inversely correlated to body taurine content (Cruz et al. 2000; Dawson, Jr. et al. 1999a; Eppler and Dawson, Jr., 2001; Militante and Lombardini 2002). Thus, taurine supplementation could prevent age-related renal damage (Dawson, Jr. et al. 1999b), sugar-induced hypertension (Anuradha and Balakrishnan, 1999; Harada et al. 2004), ethanol-induced hypertension (Harada et al. 2000), and drug-induced diabetes (Franconi et al. 2006; Mozaffari et al. 2003; Tenner, Jr. et al. 2003). In addition, perinatal taurine supplementation could prevent cardiovascular diseases in the adult offspring following maternal malnutrition (Militante and Lombardini 2002; Zelikovic et al. 1990) and in spontaneously hypertensive rats (Racasan et al. 2004). Our previous experiments indicate that changes in either prenatal or postnatal taurine exposure alter renal hemodynamics in the adult, male offspring. However, the renal diuretic and natriuretic responses to an acute saline load remain within the normal control range (Roysommuti et al. 2004). Further, perinatal taurine depletion by addition of beta-alanine to the drinking water increases the pressor effect of a high sugar diet in these animals (Suvanich et al. 2006). Sympathetic nerve overactivity and baroreceptor reflex dysfunction underlie these alterations. Moreover, dietary sugar supplementation may induce renal dysfunction before hypertension and glucose intolerance in male Sprague-Dawley rats (Roysommuti et al. 2002), suggesting that renal dysfunction is not due to kidney damage from chronic hypertension. The present study tests the hypothesis that perinatal taurine exposure alters renal function in the adult, female offspring, and this change predisposes them to sugar-induced renal dysfunction.
2. MATERIALS AND METHODS
Sprague-Dawley (SD) rats were bred in the animal unit of the Faculty of Medicine, Khon Kaen University and maintained at constant humidity (60 ± 5%), temperature (24 ± 1 °C), and light cycle (0600–1800 h). Female Sprague-Dawley rats were fed normal rat chow with 3% beta-alanine (taurine depletion, TD), 3% taurine (taurine supplementation, TS) or water alone (Control, C) from conception to weaning. Female offspring were fed normal rat chow with either 5% glucose in tap water (TD with glucose, TDG; TS with glucose, TSG; C with glucose, CG) or tap water alone (TDW, TSW or CW) throughout the experiment. Experimental protocols and animal care were approved and monitored by the Universities Animal Care and Use Committee and were in conformity with the Guide for the Care and Use of Animals and the US National Institutes of Health guidelines.
At 7–8 weeks of age, all female rats were placed under thiopental anesthesia and then implanted with femoral arterial, femoral venous, and urinary bladder catheters. Forty-eight hours later, arterial pressure, heart rate, basal renal function, and renal function after an acute intravenous saline load (a mixture of 0.5% inulin and 0.5% p-aminohippuric acid (PAH) in isotonic saline, 5% of body weight, 0.5 ml/min) were studied in conscious, restrained rats, that had been acclimated to the rat restrainer three hours per day for a week prior to the experiment (Roysommuti et al. 2002). Two days later, blood glucose and glucose tolerance were tested after an overnight fast. At the end of the experiment, all rats were sacrificed and kidney (KW) and heart (HW) weights were determined.
Arterial pressure pulse was continuously recorded during the experiment using the Biopac system, and mean arterial pressure and heart rate were analyzed offline using Acknowledge software (version 3.8.1, Biopac system, CA). Urine volumes were measured gravitationally, urine and plasma potassium and sodium were determined using flame photometry, hematocrit by a standard method, urine and plasma inulin and PAH by colorimetric methods and blood glucose by glucostrips and a glucometer (Accuchek®, Germany). The glomerular filtration rate (GFR) was estimated by inulin clearance, effective renal blood flow (ERBF) by PAH clearance and hematocrit, and renal vascular resistance (RVR) was calculated by MAP/ERBF. The filtration fraction was calculated from the ratio of GFR to effective renal plasma flow (PAH clearance). Fractional Na excretion was calculated from the ratio of Na excretion per filtered Na load (GFR × plasma Na) while water excretion was the ratio of urinary flow per GFR. All renal excretory parameters were expressed as a gram of KW (gKW).
All data were expressed as means ± SEM and were statistically analyzed using one-way ANOVA and appropriate post hoc tests (Duncan’s Multi-Range) with significant criteria of p < 0.05.
3. RESULT
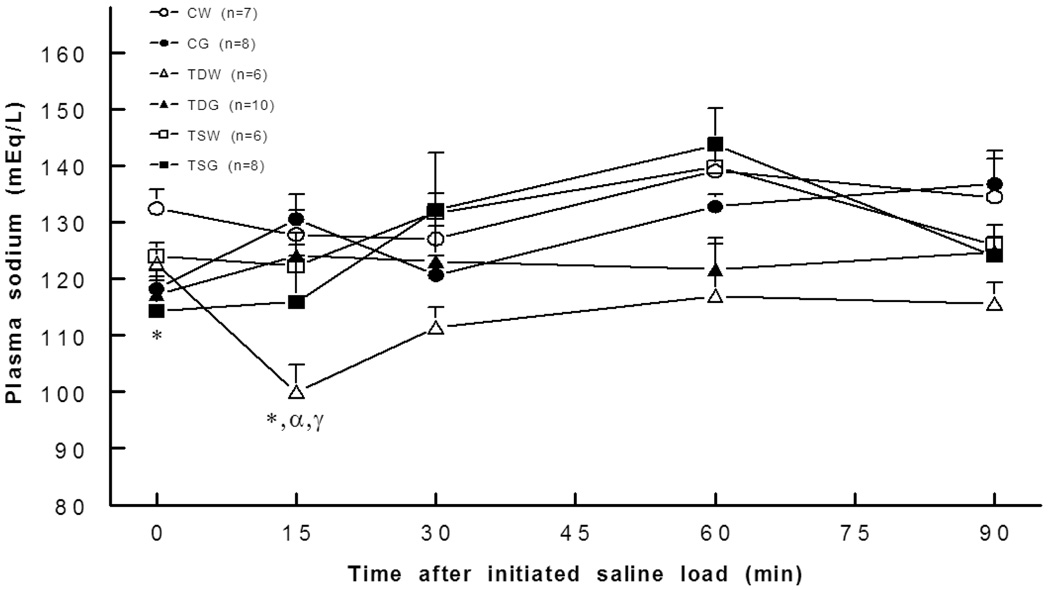
Compared to the control, body weights significantly decreased (P<0.05) only in perinatal taurine depleted rats on glucose supplementation since weaning (CW 194±5 g, CG 197±5 g, TDW 196±2 g, TDG±179 5 g, TSW 197±4 g, TSG 191±5 g). Kidney weights significantly increased (P<0.05) in offspring of perinatal taurine supplemented rats (CW 1.66±0.04 g, CG 1.61±0.04 g, TDW 1.72±0.08 g, TDG 1.59±0.02 g, TSW 1.88±0.07, 1.64±0.04 g). Heart weights were not significantly different among the groups. All experimental groups displayed similar fasting blood sugar and glucose tolerance. Basal plasma sodium concentrations were significantly decreased in all glucose treated animals, but in response to a glucose challenge, plasma sodium significantly decreased only in the TDW rats (Fig. 1). Hematocrit was not different among the groups throughout the experiment.
Fig. 1.
Plasma sodium concentration was significantly lower in controls in CG, TDG and TSG and at 15 min in TDW rats. *, P< 0.05 compared to CW; α, compared to CG; γ, compared to TSW; see text for abbreviations.
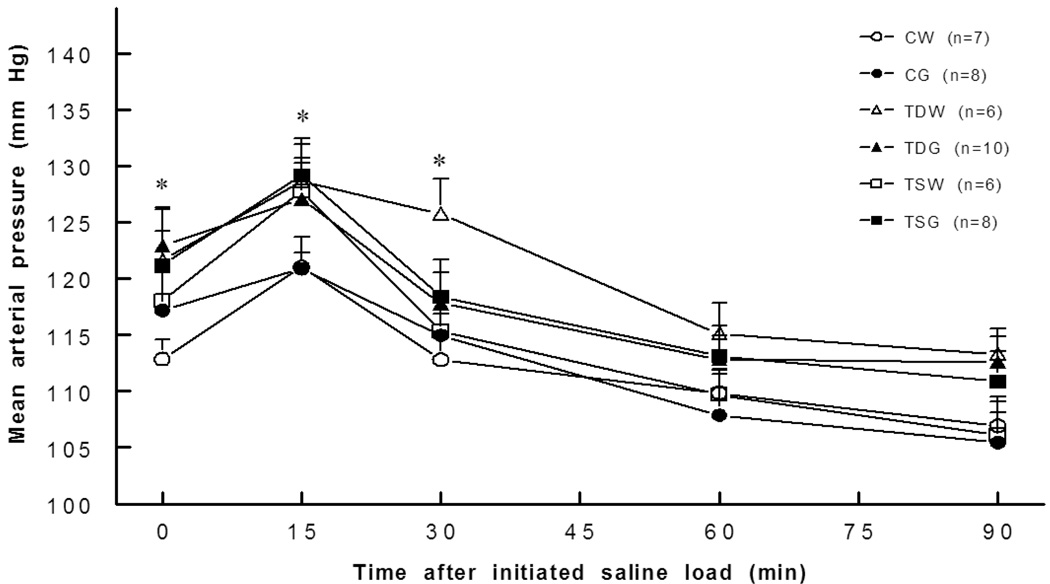
Basal mean arterial pressures were significantly higher in TDW (121.7±4.7 mm Hg), TDG (123.0±3.2 mm Hg), and TSG (121.2±3.0 mm Hg), than in CW (112.8±1.8 mm Hg) rats while arterial pressure in TSW (118.0±2.5 mm Hg) was not significantly different from that of the CW rat. The arterial pressure responses to an acute intravenous saline load were similar in CG, TDG, TSW, TSG, and CW rats (Fig. 2). Perinatal taurine depletion or supplementation did not increase the pressor effects of a high sugar diet at rest or following an acute saline load (TDW versus TDG or TSW versus TSG was not significantly different). Basal heart rate was not significantly different among the groups and remained relatively constant after an acute saline load.
Fig. 2.
Mean arterial pressure was significantly elevated in TDW (0,30 min), TDG (0 min), TSW (15 min) and TSG (0,15 min) rats compared to controls. *, P<0.05 compared to CW; see text for abbreviations.
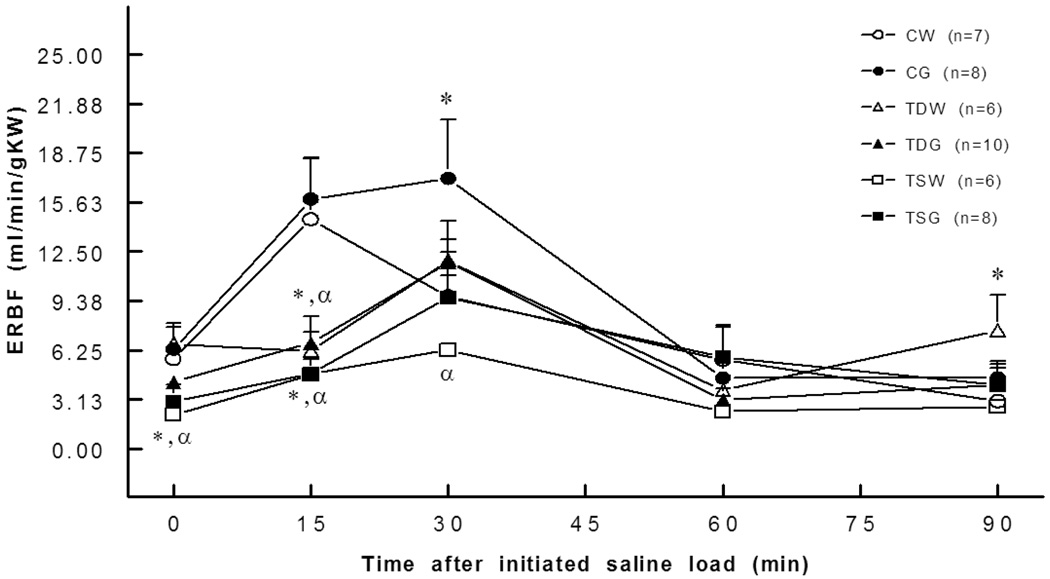
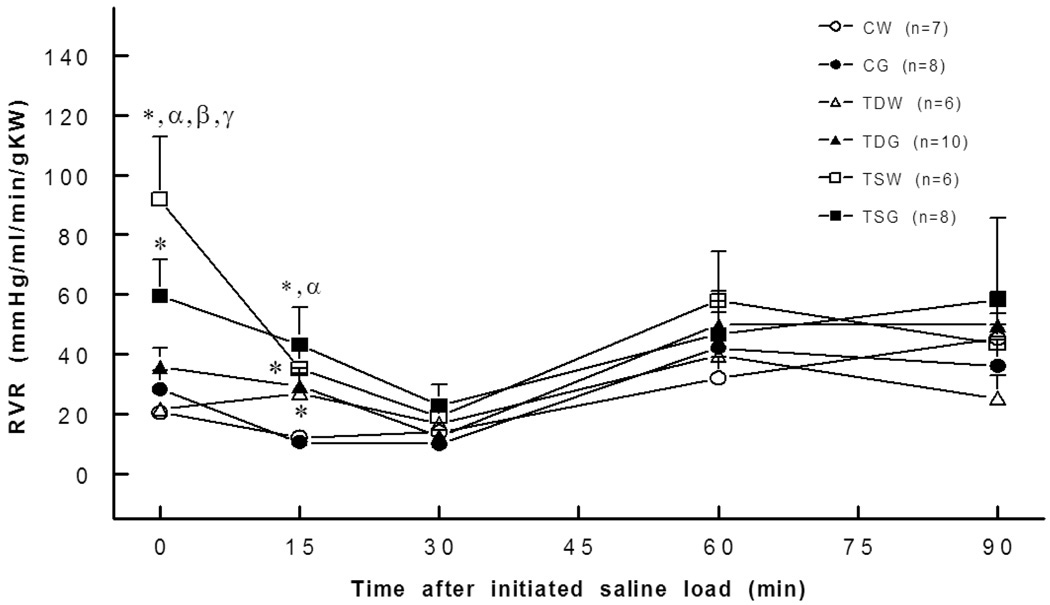
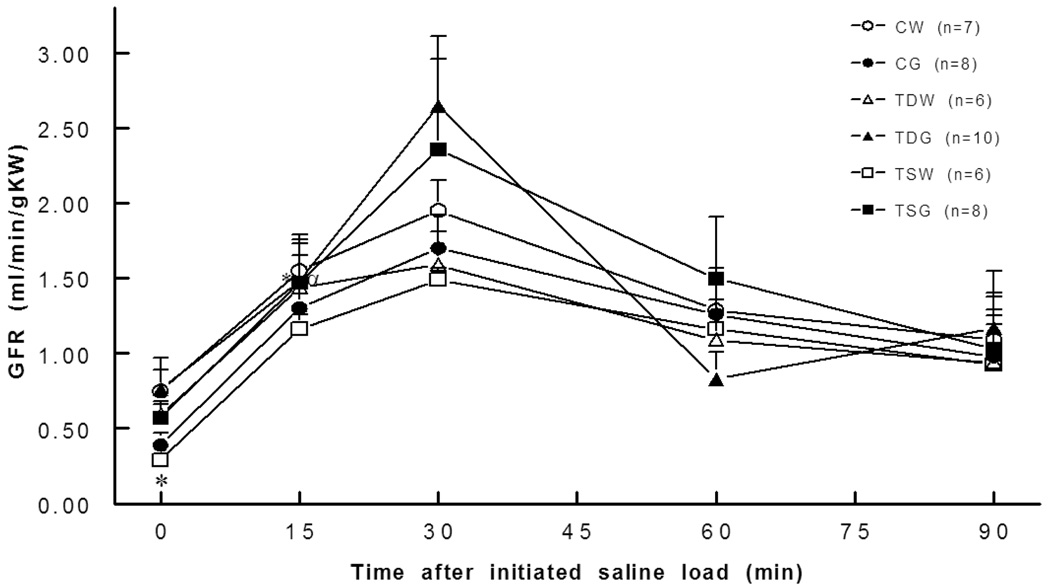
Basal ERBF was significantly lower in TSW (2.2±0.8 ml/min/gKW) and TSG (3.0±1.0 ml/min/gKW) than in the CW (5.7±0.3 ml/min/gKW) or CG groups (6.3±1.6 ml/min/gKW). Moreover, blunted renal blood flow responses to an intravenous saline load were observed in TDW, TDG, TSW, and TSG compared to the responses in the control group (Fig. 3). These changes were consistent with increased renal vascular resistance. A significant increase in basal renal vascular resistance was present in TSW but not in TDW rats (Fig. 4). Compared to CW (0.8±0.2 mm/min/gKW), the basal glomerular filtration rate was lower in TSW (0.3±0.0 mm/min/g KW), but the responses to a saline load were not significantly different among the groups (Fig. 5).
Fig. 3.
Effective renal blood flow was lower in TDW (15 min), TDG (15 min), TSW (0,15 min), and TSG (0,15 min) rats than in the controls. *, P<0.05 compared to CW; α, compared to CG; see text for abbreviations.
Fig. 4.
Renal vascular resistance was significantly higher in TDW (15 min), TSW (0,15 min), TSG (0,15 min) rats than in the controls. *, P<0.05 compared to CW; α, compared to CG; β, compared to TDW; γ, compared to TSW; see text for abbreviations.
Fig. 5.
Glomerular filtration rates were similar in all groups. *. P<0.05 compared to CW at rest; see text for abbreviations.
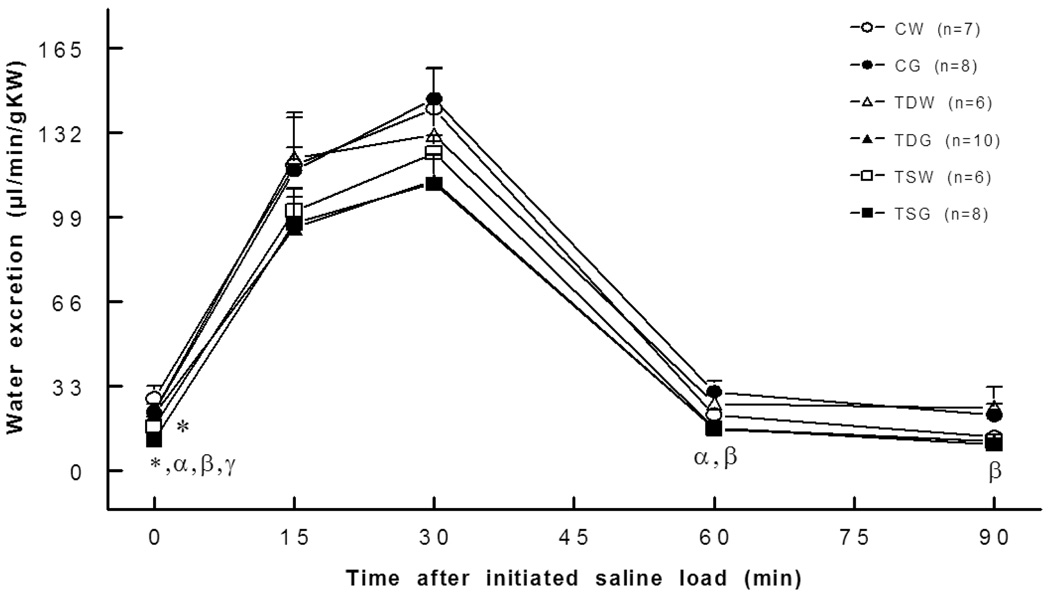
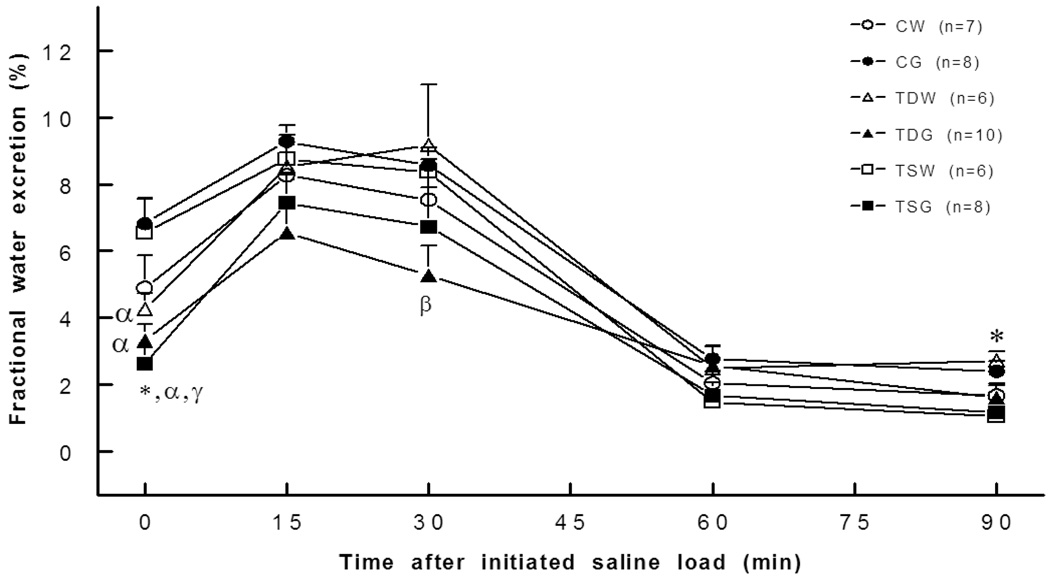
Renal water excretion at rest was significantly reduced in both TSW (17.4±3.3 µl/min/gKW, P<0.05) and TSG (11.9±2.2 µl/min/gKW; P<0.05) when compared to CW (28.2±4.7 µl/min/gKW) or CG (23.0±2.3 µl/min/g KW) rats, and they tended to be lower than the CW or CG rats throughout the experiment (Fig. 6). The reduction in water excretion of the TSG rat was consistent with an increase in renal tubular water reabsorption, as indicated by a significant decrease in fractional water excretion (Fig. 7). In addition, fractional water excretion of both TDG and TSG rats but not of the TDW and TSW rats was significantly lower than that of the CG rats.
Fig. 6.
Basal water excretion was lower in TSW and TSG rats. *, P<0.05 compared to CW; α, compared to CG; β, compared to TDG; γ, compared to TSW; see text for abbreviations.
Fig. 7.
Fractional water excretion was lower in TDW (0 min), TDG (0 min), TSG (0,30 min), TSG and TSW rats. *, P<0.05 compared to CW; α, compared to CG; β, compared to TDW; γ, compared to TSW; see text for abbreviations.
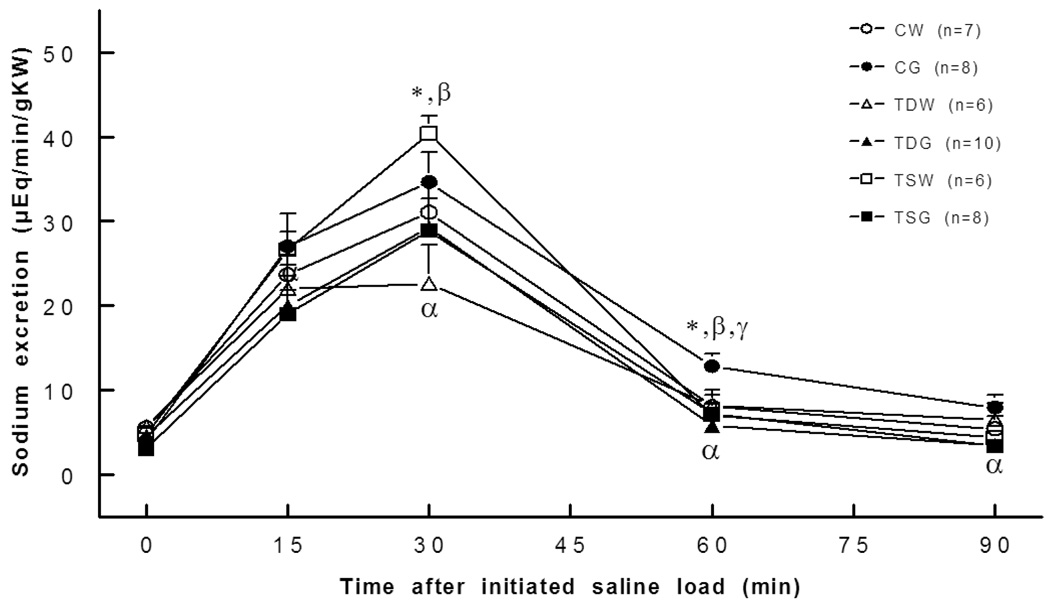
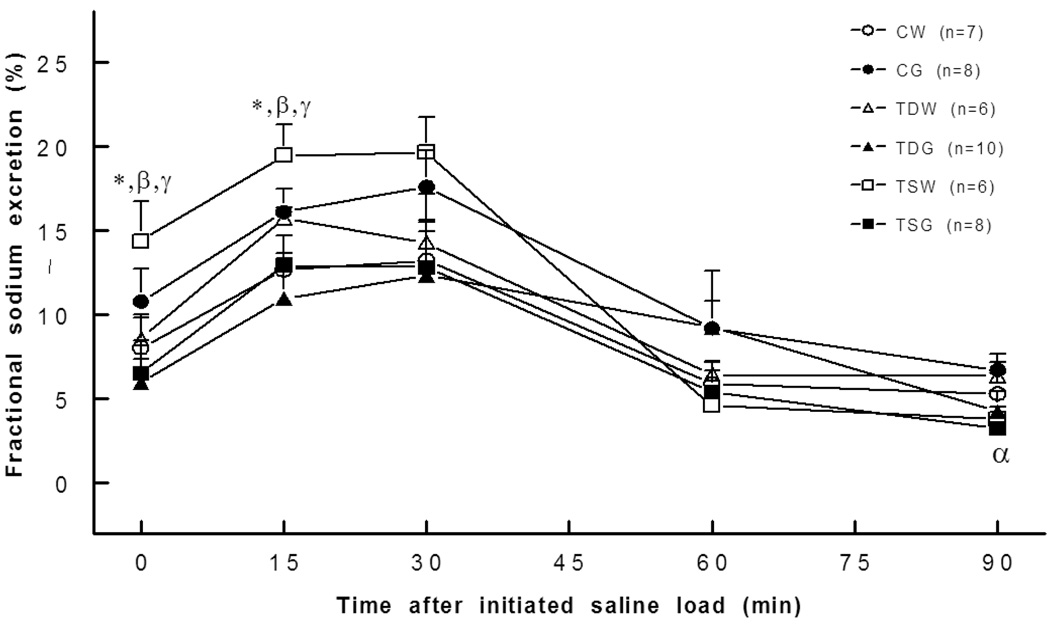
Sodium excretion at rest was not significantly different between the groups, but the natriuretic response to an acute saline load was higher at 30 minutes for the TSW rat and at 60 minutes for the CG rat compared to the CW rat (Fig. 8). Moreover, compared to the CG rat, TDG and TSG rats displayed slightly blunted natriuretic responses to an acute saline load. Fractional Na excretion at rest and following an acute saline load was higher only in the TSW rat compared to the CW or TSG groups (Fig. 9). Though the fractional Na excretions of TDG and TSG rats were not significantly different from the other groups, they tended to be lower than those of the CG rats.
Fig. 8.
Sodium excretion was higher in CG (60 min) and TSW (30 min) and decreased in TDW (30 min), TDG (90 min), and TSG (90 min) rats. *, P<0.05 compared to CW; α, compared to CG; β, compared to TDW; γ, compared to TSW; see text for abbreviations.
Fig. 9.
Fractional sodium excretion was higher in TSW rats (0,15 min). *, P<0.05 compared to; α, compared to CG; β, compared to TDW; γ, compared to TSW; see text for abbreviations.
4. DISCUSSION
The perinatal origin of adult function and disease has been widely accepted. Perinatal nutritional imbalances play a key role in adult diseases of the offspring, as supported by many lines of evidence in animal models and human epidemiologic studies (Barker et al. 2002; Harding 2001; Lackland et al. 2002; Langley-Evans et al. 2003b). Hypertension in adult life has been reported to be programmed during perinatal exposure to protein over- or under-nutrition, high sugar intake, high salt intake, intrauterine hypoxia, and other stressors. Animals and humans subjected in utero to such environments display decreased nephrogenesis that causes a permanent reduction in nephron number throughout adult life (Gopalakrishnan et al. 2005; Langley-Evans et al. 2003a). This decrease in nephron number results in altered renal weight and glomerular filtration rate and blunted pressure-natriuretic and diuretic responses, anomalies that are commonly observed in many forms of arterial hypertension (Hall et al. 1992). Although the present study supports the perinatal origin of adult renal function, perinatal taurine depletion or supplementation did not decrease kidney weights in female offspring, as previously reported in male offspring (Roysommuti et al. 2007). In addition, perinatal taurine supplementation increased renal weight and despite a decrease in the basal glomerular filtration rate, the kidneys responded to an acute intravenous saline load similarly in all of the groups. Moreover, these changes in kidney weight and glomerular filtration were restored to normal by maintenance of a postnatal high sugar diet. Thus, these data suggest that perinatal nutritional imbalances have long-term effects on renal growth and function in the adult offspring. Moreover, both excess sugar consumption during postnatal life and perinatal taurine alterations influence the renal functional phenotype. Similar effects are observed in response to low birth weight and adult obesity and hypertension. Low birth weight also triggers obesity and hypertension in rats on a basal diet or humans from a poor socioeconomic environment, i.e., malnourished (Barker 2007; Militante and Lombardini 2002). In addition, in the present study perinatal taurine depletion coupled with sugar supplementation since weaning led to a decrease in body weight but perinatal taurine depletion alone did not have any long-term effect on body, kidney, and heart weight.
Pressure-diuresis/natriuresis is the main mechanism by which the kidney regulates arterial pressure (Hall et al. 1996). Though mean arterial pressure of the perinatal taurine depleted rat is significantly elevated and was higher than any of the other groups at rest and following an acute saline load, their diuretic and natriuretic responses were similar. This indicates a blunted pressure-diuretic/natriuretic function in perinatal taurine depleted rats but not offspring of the perinatal taurine supplemented rats. This renal dysregulation could not be restored or heightened by maintenance on a high sugar diet. However, the natriuretic responses to a saline load are different in perinatal taurine depleted rats depending on their dietary sugar intake. Since their glomerular filtration rates were similar but plasma sodium is sharply decreased only in rats on a basal sugar diet, the maintenance of pressure-natriuresis likely resulted from decreased renal tubular sodium reabsorption; the slight increase in fractional sodium excretion observed during the initial phase of the responses to the saline load in the present study supports this interpretation. In contrast, increases in mean arterial pressure may underlie higher natriuretic but not diuretic responses in perinatal taurine supplemented rats, indicating that the offspring exhibit a normal pressure-natriuretic relationship. Thus, the high sugar diet since weaning did not restore mean arterial pressure of the TSG rats but natriuretic responses to an acute saline load were not perturbed, indicating that the high sugar diet induced a blunted pressure-natriuretic response in the perinatal taurine supplemented offspring. Though pressure-diuretic responses were similar in perinatal taurine supplemented and depleted rats, the taurine supplemented rats appear to depend on renal tubular reabsorption rather than changes in glomerular filtration to maintain homeostasis. In the perinatal taurine supplement group, the basal glomerular filtration rate was lower while fractional sodium excretion was higher.
Perinatal nutritional imbalances have been reported to induce hypertension in offspring. Exposure to diets with high protein and low carbohydrate or low protein and high carbohydrate in the perinatal period can produce hypertension in the adult offspring, likely via sympathetic nerve overactivity, insulin resistance, renin-angiotensin system overactivity, and renal dysfunction (Hall 2003; Langley-Evans et al. 2003a). Significantly, altered perinatal taurine exposure has also been reported to contribute to these abnormalities. Thus, taurine supplementation in prenatal or later life attenuates hypertension in the offspring (Militante and Lombardini 2002). However, this action of taurine may not be related to the improvement in insulin resistance or hyperinsulinemia (Hultman et al. 2007; Nandhini and Anuradha 2004). The present data support the hypothesis that an imbalance in perinatal nutrition alters adult offspring function. In addition, the present study supports our previous experiment regarding male offspring, namely, that perinatal taurine can induce hypertension independent of insulin resistance and diabetes mellitus (Roysommuti et al. 2007). Moreover, high sugar intake since weaning did not exacerbate hypertension in the perinatal taurine depletion or supplementation groups or induce hypertension in untreated control rats as previously reported in the male offspring (Roysommuti et al. 2007). Nevertheless, high sugar intake blunted renal pressure-natriuretic and diuretic function in these rats, suggesting that the perinatal taurine status interacts with dietary sugar supplementation to impair renal function before the appearance of either insulin resistance or significant hypertension.
Previous experiments indicate that inappropriate taurine exposure during prenatal or postnatal periods affects renal hemodynamics in male offspring (Roysommuti et al. 2004). The present data further suggest that perinatal taurine status also affects these parameters in female offspring. Perinatal taurine supplementation decreased renal blood flow at rest and following an acute saline load, consistent with a rise in renal vascular resistance. These changes could not be restored to control levels, suggesting a permanent deficit in these animals. Though perinatal taurine depletion did not alter resting renal blood flow, its response to an acute saline load was attenuated (slight increase in renal vascular resistance). Mean arterial pressure is unlikely to underlie these abnormalities. In contrast, a rise in mean arterial pressure in the perinatal taurine supplemented groups may contribute to increased renal vascular resistance.
In summary, the present data indicate that a modification in perinatal taurine exposure alters renal function and blood pressure in adult female offspring and these effects enable high sugar diets to impair kidney function in later life. The complexity of this phenomenon is dependent on a change in perinatal programming and the subsequent development and adaptation in later life. Thus, perinatal taurine status should be considered carefully in pregnant animals and humans.
ACKNOWLEDGEMENTS
This work was supported by grants from the Faculty of Medicine, Khon Kaen University and the National Institutes of Health (AT 00477 (JMW) from the National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Complementary and Alternative Medicine, the Office of Dietary Supplements, or the National Institutes of Health.
Abbreviations
- KW
kidney weight
- ERBF
effective renal blood flow
- GFR
glomerular filtration rate
- RVR
renal vascular resistance
Contributor Information
Sanya Roysommuti, Department of Physiology, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand.
Wichaporn Lerdweeraphon, Department of Physiology, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand.
Pisamai Malila, Department of Physiology, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand.
Dusit Jirakulsomchok, Department of Physiology, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand.
J. Michael Wyss, Department of Cell Biology, School of Medicine, University of Alabama at Birmingham, Birmingham, AL 35294, USA.
REFERENCES
- Aerts L, Van Assche FA. Taurine and taurine-deficiency in the perinatal period. J Perinat Med. 2002;30:281–286. doi: 10.1515/JPM.2002.040. [DOI] [PubMed] [Google Scholar]
- Anuradha CV, Balakrishnan SD. Taurine attenuates hypertension and improves insulin sensitivity in the fructose-fed rat, an animal model of insulin resistance. Can J Physiol Pharmacol. 1999;77:749–754. [PubMed] [Google Scholar]
- Barker DJ. Obesity and early life. Obes Rev 8 Suppl. 2007;1:45–49. doi: 10.1111/j.1467-789X.2007.00317.x. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- Cruz CI, Ruiz-Torres P, del Moral RG, Rodriguez-Puyol M, Rodriguez-Puyol D. Age-related progressive renal fibrosis in rats and its prevention with ACE inhibitors and taurine. Am J Physiol Renal Physiol. 2000;278:F122–F129. doi: 10.1152/ajprenal.2000.278.1.F122. [DOI] [PubMed] [Google Scholar]
- Cusi D, Bianchi G. The kidney in the pathogenesis of hypertension. Semin Nephrol. 1991;11:523–527. [PubMed] [Google Scholar]
- Dawson R, Jr, Liu S, Eppler B, Patterson T. Effects of dietary taurine supplementation or deprivation in aged male Fischer 344 rats. Mech Ageing Dev. 1999b;107:73–91. doi: 10.1016/s0047-6374(98)00138-9. [DOI] [PubMed] [Google Scholar]
- Eppler B, Dawson R., Jr Dietary taurine manipulations in aged male Fischer 344 rat tissue: taurine concentration, taurine biosynthesis, and oxidative markers. Biochem Pharmacol. 2001;62:29–39. doi: 10.1016/s0006-2952(01)00647-5. [DOI] [PubMed] [Google Scholar]
- Franconi F, Loizzo A, Ghirlanda G, Seghieri G. Taurine supplementation and diabetes mellitus. Curr Opin Clin Nutr Metab Care. 2006;9:32–36. doi: 10.1097/01.mco.0000196141.65362.46. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan GS, Gardner DS, Dandrea J, Langley-Evans SC, Pearce S, Kurlak LO, Walker RM, Seetho IW, Keisler DH, Ramsay MM, Stephenson T, Symonds ME. Influence of maternal pre-pregnancy body composition and diet during early-mid pregnancy on cardiovascular function and nephron number in juvenile sheep. Br J Nutr. 2005;94:938–947. doi: 10.1079/bjn20051559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisk O, Kloting I, Exner J, Spiess S, Schmidt R, Junghans D, Lorenz G, Rettig R. Long-term arterial pressure in spontaneously hypertensive rats is set by the kidney. J Hypertens. 2002;20:131–138. doi: 10.1097/00004872-200201000-00019. [DOI] [PubMed] [Google Scholar]
- Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]
- Hall JE, Guyton AC, Brands MW. Pressure-volume regulation in hypertension. Kidney Int Suppl. 1996;55:S35–S41. [PubMed] [Google Scholar]
- Hall JE, Mizelle HL, Brands MW, Hildebrandt DA. Pressure natriuresis and angiotensin II in reduced kidney mass, salt-induced hypertension. Am J Physiol. 1992;262:R61–R71. doi: 10.1152/ajpregu.1992.262.1.R61. [DOI] [PubMed] [Google Scholar]
- Harada H, Kitazaki K, Tsujino T, Watari Y, Iwata S, Nonaka H, Hayashi T, Takeshita T, Morimoto K, Yokoyama M. Oral taurine supplementation prevents the development of ethanol-induced hypertension in rats. Hypertens Res. 2000;23:277–284. doi: 10.1291/hypres.23.277. [DOI] [PubMed] [Google Scholar]
- Harada H, Tsujino T, Watari Y, Nonaka H, Emoto N, Yokoyama M. Oral taurine supplementation prevents fructose-induced hypertension in rats. Heart Vessels. 2004;19:132–136. doi: 10.1007/s00380-003-0757-1. [DOI] [PubMed] [Google Scholar]
- Harding JE. The nutritional basis of the fetal origins of adult disease. Int J Epidemiol. 2001;30:15–23. doi: 10.1093/ije/30.1.15. [DOI] [PubMed] [Google Scholar]
- Hultman K, Alexanderson C, Manneras L, Sandberg M, Holmang A, Jansson T. Maternal taurine supplementation in the late pregnant rat stimulates postnatal growth and induces obesity and insulin resistance in adult offspring. J Physiol. 2007;579:823–833. doi: 10.1113/jphysiol.2006.124610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ, Olivier NB, Mohankumar PS, Lee JS, Padmanabhan V, Fink GD. Hypertension caused by prenatal testosterone excess in female sheep. Am J Physiol Endocrinol Metab. 2007;292:E1837–E1841. doi: 10.1152/ajpendo.00668.2006. [DOI] [PubMed] [Google Scholar]
- Lackland DT, Egan BM, Syddall HE, Barker DJ. Associations between birth weight and antihypertensive medication in black and white medicaid recipients. Hypertension. 2002;39:179–183. doi: 10.1161/hy0102.100545. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Langley-Evans AJ, Marchand MC. Nutritional programming of blood pressure and renal morphology. Arch Physiol Biochem. 2003c;111:8–16. doi: 10.1076/apab.111.1.8.15136. [DOI] [PubMed] [Google Scholar]
- Militante JD, Lombardini JB. Treatment of hypertension with oral taurine: experimental and clinical studies. Amino Acids. 2002;23:381–393. doi: 10.1007/s00726-002-0212-0. [DOI] [PubMed] [Google Scholar]
- Mozaffari MS, Miyata N, Schaffer SW. Effects of taurine and enalapril on kidney function of the hypertensive glucose-intolerant rat. Am J Hypertens. 2003;16:673–680. doi: 10.1016/s0895-7061(03)00915-4. [DOI] [PubMed] [Google Scholar]
- Nandhini AT, Anuradha CV. Hoe 140 abolishes the blood pressure lowering effect of taurine in high fructose-fed rats. Amino Acids. 2004;26:299–303. doi: 10.1007/s00726-003-0003-2. [DOI] [PubMed] [Google Scholar]
- Ojeda NB, Grigore D, Robertson EB, Alexander BT. Estrogen protects against increased blood pressure in postpubertal female growth restricted offspring. Hypertension. 2007;50:679–685. doi: 10.1161/HYPERTENSIONAHA.107.091785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racasan S, Braam B, van der Giezen DM, Goldschmeding R, Boer P, Koomans HA, Joles JA. Perinatal L-arginine and antioxidant supplements reduce adult blood pressure in spontaneously hypertensive rats. Hypertension. 2004;44:83–88. doi: 10.1161/01.HYP.0000133251.40322.20. [DOI] [PubMed] [Google Scholar]
- Rettig R, Folberth C, Kopf D, Stauss H, Unger T. Role of the kidney in the pathogenesis of primary hypertension. Clin Exp Hypertens A. 1990;12:957–1002. doi: 10.3109/10641969009073513. [DOI] [PubMed] [Google Scholar]
- Rettig R, Grisk O. The kidney as a determinant of genetic hypertension: evidence from renal transplantation studies. Hypertension. 2005;46:463–468. doi: 10.1161/01.HYP.0000178189.68229.8a. [DOI] [PubMed] [Google Scholar]
- Roysommuti S, Khongnakha T, Jirakulsomchok D, Wyss JM. Excess dietary glucose alters renal function before increasing arterial pressure and inducing insulin resistance. Am J Hypertens. 2002;15:773–779. doi: 10.1016/s0895-7061(02)02974-6. [DOI] [PubMed] [Google Scholar]
- Roysommuti S, Malila P, Jirakulsomchok D, Jirakulsomchok S, Wyss JM. Perinatal taurine status influences renal hemodynamics in adult conscious rats. FASEB J. 2004;18(4 Part I):A292–A293. [Google Scholar]
- Roysommuti S, Suvanich A, Jirakulsomchok D, Wyss JM. Perinatal taurine depletion causes autonomic dysregulation in rats on a high glucose diet. FASEB J. 2007;21(6 Part II):A887. [Google Scholar]
- Sturman JA. Taurine in development. Physiol Rev. 1993;73:119–147. doi: 10.1152/physrev.1993.73.1.119. [DOI] [PubMed] [Google Scholar]
- Suvanich A, Jirakulsomchok D, Muchimapura S, Wyss JM, Roysommuti S. Perinatal taurine depletion impairs autonomic control in conscious rats. FASEB J. 2006;20(5 Part II):A1406–A1407. [Google Scholar]
- Tenner TE, Jr, Zhang XJ, Lombardini JB. Hypoglycemic effects of taurine in the alloxan-treated rabbit, a model for type 1 diabetes. Adv Exp Med Biol. 2003;526:97–104. doi: 10.1007/978-1-4615-0077-3_13. [DOI] [PubMed] [Google Scholar]
- Zelikovic I, Chesney RW, Friedman AL, Ahlfors CE. Taurine depletion in very low birth weight infants receiving prolonged total parenteral nutrition: role of renal immaturity. J Pediatr. 1990;116:301–306. doi: 10.1016/s0022-3476(05)82898-7. [DOI] [PubMed] [Google Scholar]