Abstract
Purpose
During cancer progression, the oncoprotein MUC1 binds β-catenin while simultaneously inhibiting the degradation of the epidermal growth factor receptor (EGFR), resulting in enhanced transformation and metastasis. The purpose of this study was to design a peptide-based therapy which would block these intracellular protein-protein interactions as a treatment for metastatic breast cancer.
Experimental Design
The amino acid residues responsible for these interactions lie in tandem in the cytoplasmic domain of MUC1, and we have targeted this sequence to produce a MUC1 peptide that blocks the pro-tumorigenic functions of MUC1. We designed the MUC1 inhibitory peptide (MIP) to block the intracellular interactions between MUC1/β-catenin and MUC1/EGFR. To allow for cellular uptake we synthesized MIP adjacent to the protein transduction domain, PTD4 (PMIP).
Results
We have found that PMIP acts in a dominant-negative fashion, blocking both MUC1/β-catenin and MUC1/EGFR interactions. In addition, PMIP induces ligand-dependent reduction of EGFR levels. These effects correspond to a significant reduction in proliferation, migration and invasion of metastatic breast cancer cells in vitro and inhibition of tumor growth and recurrence in an established MDA-MB-231 scid mouse model. Importantly, PMIP also inhibits genetically-driven breast cancer progression, as injection of tumor-bearing MMTV-pyV mT transgenic mice with PMIP results in tumor regression and a significant inhibition of tumor growth rate.
Conclusions
These data demonstrate that intracellular MUC1 peptides possess significant anti-tumor activity and has important clinical applications in the treatment of cancer.
Keywords: MUC1, peptide, EGFR, breast cancer, β-catenin
Statement of Translational Relevance
The peptide-based therapy presented in this research describes a novel approach of targeting cancer specific protein-protein interactions. PMIP’s targets, MUC1, EGFR, and β-catenin are all clinically relevant and act together to play an important role in the progression of breast cancer. We have demonstrated PMIP’s ability to slow proliferation and invasion of human breast cancer cells. Additionally, PMIP displayed efficacy in both transgenic and xenograft mouse models of breast cancer. In both of these mouse models PMIP was able to significantly slow the growth of primary tumors and reduce the amount of regrowth following tumor resection. All of these results indicate that PMIP has significant clinical implications in the treatment of breast cancer.
Introduction
MUC1 (DF3, CD227, episialin, PEM) is a heavily O-glycosylated heterodimeric protein of >300 kDa, normally expressed abundantly on the apical surface of glandular epithelia. In greater than 90% of human breast carcinomas and metastases, apical localization is lost and MUC1 is overexpressed (by greater than 10 fold) and underglycosylated (1, 2). Deregulated expression of MUC1 is found in many other types of adenocarcinomas as well, including cancers of the lung, pancreas, ovary and prostate, in addition to being highly expressed in leukemias, myelomas and lymphomas (3–5). Studies in both genetic mouse models and cell line models have demonstrated that MUC1 is an oncogene. A transgenic mouse model driving MUC1 (human) overexpression to the mouse mammary gland (MMTV-MUC1) results in the development of breast cancer and is accompanied by a failure of the mammary gland to undergo complete postlactational regression via apoptosis (6). Transfection of MUC1 into 3Y1 fibroblasts induces their transformation, and transfection of MUC1 into colon cancer cells demonstrates that MUC1 overexpression inhibits drug-induced apoptosis (7).
The cytoplasmic domain of MUC1 contains sites for multiple protein interactions, although these interactions go largely unformed in the polarized epithelium of the normal breast, as the binding partners of MUC1 are typically found on the basolateral membrane (Reviewed in (8) and (9, 10)). During cancer progression, when there is a loss of cellular polarization, MUC1 is overexpressed and functionally interacts with src, GSK3β, epidermal growth factor receptor (EGFR) and β-catenin, among others (9, 11, 12). The sites for interaction between MUC1 and these proteins have been mapped to distinct domains within the 72-amino acid cytoplasmic tail of MUC1 (11, 13–15). Both EGFR and src can phosphorylate MUC1 on a YEKV motif, and this phosphorylation results in increased binding of MUC1 to β-catenin through an SAGNGGSSLS domain (11). Recent evidence demonstrates that the interaction between MUC1 and EGFR can significantly modulate EGFR biology and effect EGFR-dependent transformation (9, 10).
It has been established in both human breast cancer cell lines and transgenic mice overexpressing MUC1 (MMTV-MUC1) that MUC1 and EGFR biochemically interact, resulting in the potentiation of EGF-dependent p42/44 ERK activation during lactation (11, 21). Recently, our laboratory has demonstrated that MUC1 expression inhibits the ligand-mediated ubiquitination and degradation of EGFR while enhancing its internalization and recycling (9). To evaluate the role of Muc1 in transformation, we further generated WAP-TGFα mice on a Muc1 null background which revealed that Muc1 expression has a dominant effect on TGFα-dependent transformation of the breast, promoting both onset and progression (10).
While it is now established that EGFR is potently regulated by MUC1 expression, transgenic mouse models have also implicated the cell adhesion protein, β-catenin, in EGFR and MUC1 signaling. In a study of the WAP-TGFα transgenic model, Wnt1 and Wnt3 were found to be selectively activated in the most aggressive breast tumors (22). The Wnts are secreted glycoproteins that bind the transmembrane frizzled receptor, resulting in a signaling cascade that inactivates the mechanism for β-catenin degradation and results in transformation (23–28). Additionally, in MMTV-Wnt1 transgenic mice, EGFR was found to interact with and phosphorylate β-catenin in a tumor-specific manner (29). These studies demonstrate that β-catenin and EGFR can affect their respective pathways to promote transformation. Finally, MUC1 is also implicated in β-catenin dependent transformation, indicating that these three proteins have the ability to cooperatively promote cancer progression. In MMTV-Wnt-1 transgenic mouse models crossed onto a Muc1-null background, loss of Muc1 corresponds to a significant reduction in tumor progression (12). In a subsequent study, interactions between MUC1 and β-catenin were found to be highly increased in samples from human metastatic breast tumors, indicating that these interactions are clinically relevant (12).
Together, these studies demonstrate the strong potential for MUC1, EGFR and β-catenin to affect each other during transformation, including their striking co-upregulation during transformation and metastasis. MUC1 can inhibit the downregulation of EGFR and promote the transforming ability of both EGFR and β-catenin. Additionally, genetically derived mouse models implicate MUC1 in both EGFR- and β-catenin-dependent transformation and metastasis. Interestingly, the interaction sites on MUC1 for both EGFR and β-catenin lie in tandem on the MUC1 cytoplasmic domain. This study demonstrates that targeting the interaction domain of MUC1 for both EGFR and β-catenin through the utilization of MUC1 dominant negative peptides can significantly affect breast cancer progression. Importantly, this dominant negative peptide can significantly inhibit tumor progression in a genetically-driven mouse model of breast cancer over a relatively short treatment time and may have important clinical applications.
Materials and Methods
Cell Culture
Metastatic breast cancer cell lines BT20 and MDA-MB-231 cells were obtained from the American Tissue Culture Collection. These cell lines were cultured with 10% Fetal Bovine Serum (Cellgro, Herndon, VA), 1% Penicillin-Streptomysin-Glutamine (Invitrogen, Eugene, OR) and EMEM (BT20, ATCC) media or RPMI 1640 (MDA-MB-231, Cellgro) at 37°C with 5% CO2 in a humidified incubator.
Invasion Assay
Collagen matrix (0.9 mg/ml Type I Rat tail collagen (BD Bioscicences, Billerica, MA), 83.0% (v/v) M-199 medium (Life Technologies), and 0.18% NaHCO3 (Fisher, Hampton, NH)) was poured into a 96 well plate. Prior to collagen polymerization a 0.8µm pore transwell insert (Corning Inc., Corning, NY) was placed on top of the matrix. The polymerized collagen was rehydrated using a chemoattractant (20% FBS/EMEM). BT-20 cells were treated with 10µM of hPMIP, CP, PTD4 peptides or water in serum free EMEM media overnight. Prior to loading the cells onto the transwell inserts they were fluorescently labeled with Calcein-AM (Invitrogen) for 30 minutes and then washed with PBS. The cells were then resuspended in a 10µM peptide/EMEM serum free media and loaded onto the inserts in each well (15,000 cells/well). The cells were allowed to invade into the matrix for 10hrs at 37°C with 5% CO2 in a humidified incubator. After 10hrs, the collagen matrix was treated with a 0.25% Collagenase in 40% FBS/PBS and the inserts were removed (Calbiochem, San Diego, CA). The number of fluorescently labeled cells that had invaded into the collagen were measured using a Molecular Devices spectrophotometer (Ex:485, Em:538, and Cutoff:530). For each treatment group, a set of cells were placed directly into the bottom chamber and used as a reference for 100% of cell invasion. Invasion is then indicated as percent invasion (invaded cells/total cells).
MTT Proliferation Assay
BT20 cells were plated into a 96 well plate (5 × 103 cells per well). The cells were allowed to adhere and then they were treated daily with 10µM of hPMIP, CP, PTD4 peptides or water (treatments diluted in 10% FBS/EMEM) for seven days. After treatment the Vybrant MTT Cell Proliferation Assay (Molecular Probes) was performed following the manufacturers’ protocol.
Immunoprecipitation and Immunoblotting
Protein was treated as described in (9).
Densitometry
Immunoblotting analysis was performed as in (9).
Antibodies and growth factors
Anti-MUC1 (CT-2) and anti-EGFR (Ab-13) antibodies were purchased from Neomarkers Inc. (Fremont, CA). Anti-MUC1 (H295) was purchased from Santa Cruz Biotechnology (H295). The total Erk (9102) and phoso-EGFR (1173) were purchased from Cell Signaling (Danvers, MA).Antibodies against phosphorylated tyrosine (PY99), and EGFR/erbB1 (1005), β-catenin (C-18) were all purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). The β-catenin antibody used for immunofluorescence was from BD Scientific. The β-actin (AC-15) and dual-phosphorylated Erk (M-8159) antibodies were purchased from Sigma Chemical Company (St. Louis, MO). Secondary antibodies conjugated to HRP were acquired from Molecular Probes (Invitrogen) and the anti-Hamster HRP conjugated antibody was purchased from Jackson Labs (West Grover, PA). The secondary antibodies, donkey anti-mouse 488 and donkey anti-rabbit 594 were purchased from Molecular Probes (Invitrogen). Epidermal growth factor (EGF) was stored at −20°C at a concentration of 100ng/µl (Invitrogen).
Immunofluorescence
Peptide uptake: BT20 cells were treated for 4 hours with 10µM hPMIP tagged with Biotin and fixed in 1:1 Acetone:Methanol. Fixed cells were treated with an anti-Streptavidin-488 antibody and washed with 0.02% NaN3/PBS and incubated with Slowfade Gold antifade reagent with DAPI (Invitrogen). Cells were visualized with a fluorescence DMLB Leica compound microscope. β-catenin/MUC1: BT-20 cells were incubated with 10 µM of hPMIP, CP, PTD4 or water. The immunostaining of the cells was performed as in (9). Cells were visualized with a fluorescence Zeiss confocal microscope.
Peptide Synthesis
hPMIP, Biotin-hPMIP, msPMIP, FITC-msPMIP, CP and PTD4 polypeptides were synthesized by GenScript (Scotch Plains, NJ) and delivered lyophilized. The peptides were resuspended at a concentration of 500µM in water and stored at −80°C in single-use aliquots. Peptide sequences are shown in figure 1a.
Figure 1. A MUC1 peptide (PMIP) can efficiently enter cells.
a) MUC1’s cytoplasmic amino acid sequence (MUC1CT), which includes an EGFR phosphorylation site (underline) and β-catenin binding site (double underline). Human and mouse sequence for the PMIP peptide (human PMIP, hPMIP and mouse PMIP, msPMIP), the PTD4 protein transduction domain and the control peptide (CP) containing the last 12 amino acids of the human MUC1 cytoplasmic domain are shown. b) BT-20 cells were treated with 10µM Biotin-hPMIP for 4hrs (Green=Biotin-PMIP and Blue=DAPI (nucleus), 400× and 630×)
Human Breast Tumor Xenografts
Immunocompromised (scid) mice (Taconic, Rockville, MD) were tested for the presence of serum IgG and found to be <20 µg/ml IgG. Female mice (four to six weeks old) were injected with 1×107 cells embedded in Matri-gel (BD Biosciences) into the mammary fat pad and allowed to grow to either 100mm3 or 500mm3, based on the formula a2 × b/2 where a is the smaller diameter and b is the larger diameter. Mice were injected intraperitoneally (i.p.) with 50 µg/g body weight of either PMIP or PTD4 control peptide for 21 days and measured with calipers every two days. Either at the end of 21 days, or after the tumor had reached 800mm3, tumors were resected by injecting the mice with buprenorphine (2.5 mg/kg body weight, Infusion Solutions, Totowa, NJ) at least one hour prior to resection and anesthetizing the mice with isoflurane (Abbott, Abbott Park, Il). Following surgery, mice were treated with buprenorphine (2.5mg/kg body weight) 8 and 16hrs post-surgery. Animals were then followed for 10 days to examine regrowth at the primary tumor site or at secondary mammary glands, then sacrificed.
Transgenic Mice
MMTV- pyV mT mice on an FVB background (W. Muller (30), obtained from Jackson Laboratories) entered into study upon the development of tumors that measured ≥ 0.5cm in at least one diameter. Only mice greater than seven weeks of age were included in the study and mice that developed fluid cysts were excluded. Animals were injected i.p. with 50µg/g body weight of msPMIP or PTD4 once daily for 21 days. Each of ten mammary glands were measured using calipers every two days, and measurements were used to determine tumor volume based on the formula a2 × b/2, with a being the smaller diameter and b being the larger diameter. After treatment for 21 days, mice were sacrificed by CO2 inhalation and tissues resected. Several msPMIP treated mice were injected with FITC-msPMIP (50µg/g body weight) one or four hours prior to sacrifice and intact tissues were visualized using a fluorescence MZFLIII Leica dissection microscope.
Both the xenograft and transgenic mouse studies were performed by the Experimental Mouse Shared Service (University of Arizona, Tucson) under protocols approved by the Institutional Animal Care and Use Committee of the University of Arizona.
Tissue Analysis
Tumors tissues from the xenograft and transgenic mouse models were fixed in 10% buffered formalin and stored in 70% ethanol. Tissues were paraffin embedded, sectioned and subsequently used for hematoxylin-eosin staining and caspase-3 immunohistochemistry. Tissues embedding, sectioning, hematoxylin-eosin staining, and cleaved caspase-3 immunohistochemistry were all performed by the Tissue Acquisition and Cellular Molecular Analysis Shared Service at the Arizona Cancer Center (Tucson, AZ).
Statistical Analysis
All statistics were performed in Excel (Microsoft).
Results
PMIP efficiently enters the cells, reduces EGFR protein levels and inhibits MUC1 colocalization with β-catenin
The MUC1 cytoplasmic domain is composed of 72 amino acids, within which lies a 15 amino acid domain containing sites of EGFR phosphorylation (human=YEKV and mouse=YEEV) and β-catenin binding (human=SAGNGGSSLS and mouse=SAGNGSSSLS, Figure 1a) (11, 31). We synthesized a 15 amino acid peptide to determine if it could act in a dominant negative fashion to block interactions between endogenous MUC1 and EGFR/β-catenin. To allow this peptide to gain entrance to the cell, we synthesized it in tandem with a protein transduction domain [PTD4, Figure 1a (32), Reviewed in (33)]. To demonstrate that PMIP was entering the cells, we pulsed cells in vitro with a biotin-labeled hPMIP (10µM) peptide (biotin-hPMIP). We found that the peptide was taken up and is retained in BT20 breast cancer cells (Figure 1b).
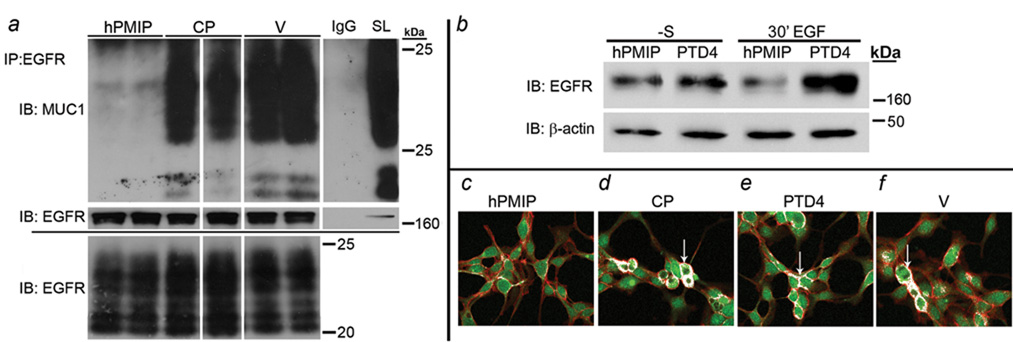
To determine if the hPMIP peptide was able to block the interaction between EGFR and MUC1, we treated BT20 cells overnight with either 10 µM hPMIP, 10 µM control peptide (CP) or peptide vehicle (V), immunoprecipitated EGFR and immunoblotted for MUC1 (Figure 2a). While we were able to detect coimmunoprecipitation between MUC1 and EGFR in control treated cells, interaction was significantly reduced in hPMIP treated cells (Figure 2a, top panel). In addition to protein interaction, we had previously demonstrated that MUC1 expression inhibits the EGF-dependent degradation of EGFR (9). To determine if PMIP was able to block the MUC1-mediated effects on EGFR degradation, we treated BT20 and MDA-MB-231 cells with hPMIP or PTD4 control, and induced EGFR endocytosis by treatment with EGF for 30 minutes. Examination of EGFR protein levels by immunoblotting demonstrated that hPMIP treatment resulted in a reduction of EGFR levels following EGF treatment (Figure 2b and data not shown). Note that in the absence of EGF treatment, hPMIP treatment does not affect EGFR levels.
Figure 2. PMIP blocks MUC1/β-catenin and MUC1/EGFR interactions.
a) BT-20 cells were treated overnight (-serum) with either 10µM hPMIP, 10µM control peptide (CP) or vehicle control (water; V), and protein lysates generated. Lysates (500µg) were immunoprecipitated (IP) with either anti-EGFR (Ab-13) (lanes 1–6, top and middle panel) or IgG (lane 7, top and middle panel) and immunoblotted (IB) with anti-MUC1 (top panel; CT2) or anti-EGFR (middle panel; 1005). Total levels of MUC1 (35 µg) are shown in the bottom panel and in right column of top panel (straight lysate, SL). White lines through blots indicate same gel and exposure but were non-contiguous. b) BT-20 cells were treated for 18hrs (-S) with hPMIP (10µM) or PTD4 (10µM). The cells were treated with EGF (30’ EGF, 10ng/ml) to induce endocytosis or left serum free (-S) and protein lysates were generated. The lysates were immunoblotted (IB) for EGFR (1005) and β-actin (AC-15) c–f) BT-20 cells were treated (-serum) overnight with (c) hPMIP (10µM), (d) control peptide (10µM), (e) PTD4 (10µM) or (f) water (V) and pulsed with the same peptides for an additional 30’ prior to fixation. The cells were probed for MUC1 (primary; H295: red) and β-catenin (primary; C-14: green). Co-localization (arrows) is designated with white pixels. Magnification=400×.
To determine if PMIP could block those interactions between MUC1 and β-catenin that occur during transformation, we examined the effects of hPMIP treatment on protein colocalization in the BT-20 breast cancer cell line (12, 29). Cells were serum starved and treated with either 10 µM hPMIP, 10 µM control peptide (CP), 10 µM PTD4, or vehicle control (V) overnight, fixed and immunofluorescence was performed. While β-catenin and MUC1 colocalization at the cell cortex could be identified in control conditions (Figure 2c–f, arrows), their interactions were significantly inhibited by hPMIP treatment (Figure 2c).
These results demonstrate that PMIP treatment inhibits both MUC1/β-catenin and MUC1/EGFR interactions. In addition, PMIP inhibits MUC1-mediated inhibition of EGF-dependent degradation of EGFR.
PMIP inhibits cellular invasion and proliferation in vitro
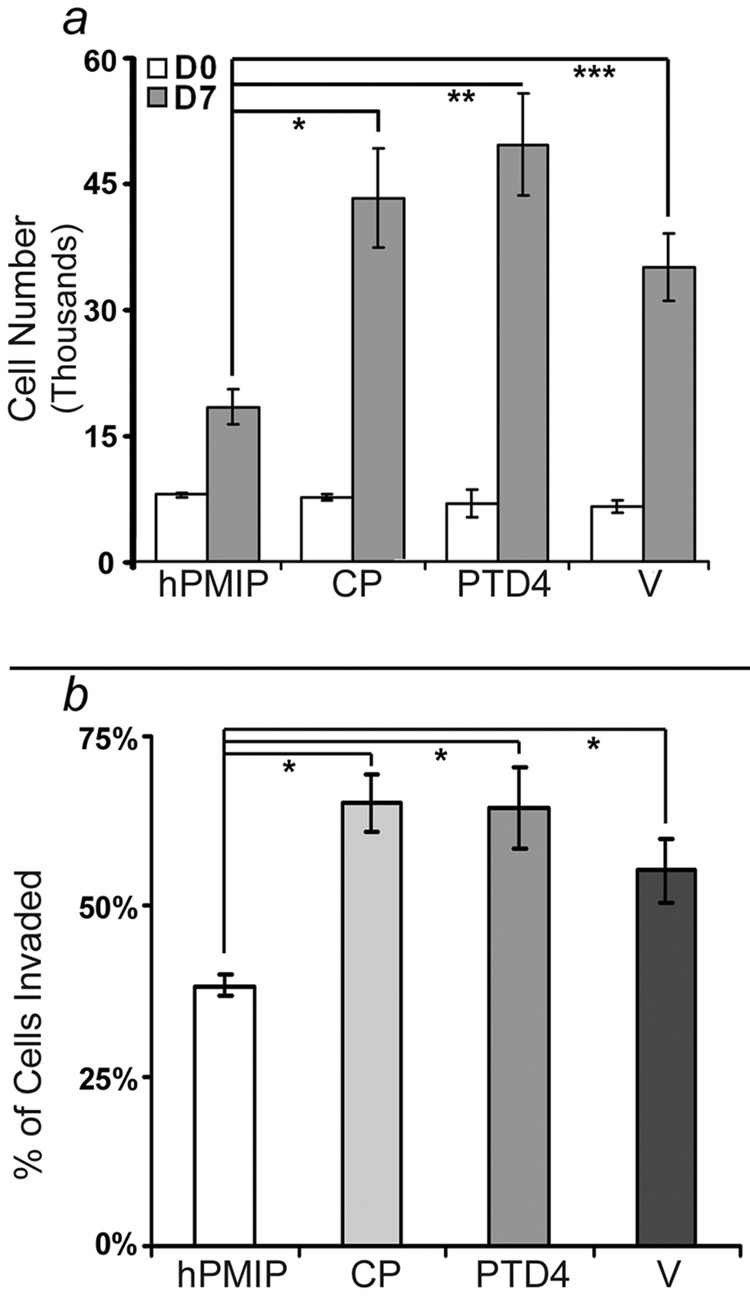
As hPMIP treatment demonstrated a strong biochemical inhibition of MUC1 interactions with both β-catenin and EGFR, we next examined potential effects of treatment on cell growth and invasion. To determine the effects of PMIP treatment on cell proliferation, BT20 cells were treated with either 10 µM hPMIP, 10 µM control peptide (CP), 10 µM PTD4 or peptide vehicle (V) for 7 days continually, then the number of cells was evaluated by quantifying the differences in formazan conversion through a colormetric MTT assay (34). Following seven days of hPMIP treatment we observed that the growth of BT20 cells was significantly inhibited compared to the control peptide (CP), PTD4 and water (V) (*, p<0.001, **, p<0.0002, ***, p<0.002, Figure 3a).
Figure 3. PMIP inhibits cell proliferation and invasion of breast cancer cells in vitro.
a) BT20 cells were cultured in a 96-well dish (5 × 103 cells/well) and treated daily for 7 days with either hPMIP (10µM), CP (10µM), PTD4 (10µM) or water (V) in EMEM 10% FBS. An MTT assay was performed to quantify cell number at the start of treatment (Day 0, DO) and after treatment was complete (Day 7, D7) (*, p<0.001, **, p<0.0002, ***, p<0.002, ANOVA). Error bars represent standard error. b) BT-20 cell lines were treated with either hPMIP (10µM), CP (10µM), PTD4 (10µM) or water (V) overnight, labeled with Calcein AM, allowed to invade through a transwell (8.0µM) insert into a Type I collagen gel, and the invaded cells were fluorescently measured. (#p<0.0001, ANOVA). The data from the invasion and proliferation assays represents four independent experiments with at least seven replicates per experiment. Error bars represent standard deviation.
We next evaluated the effect of PMIP on cellular invasion. BT20 or MDA-MB-231 breast cancer cells were treated overnight with either hPMIP or PTD4 control peptide (10µM), then induced to invade through an 8.0µM filter into Type I collagen using 20% fetal bovine serum (FBS) as a chemoattractant. Cells that were able to migrate through the filter and invade into the gels were fluorescently labeled and quantified. We found that PMIP treatment significantly inhibited the ability of cells to invade compared to cells treated with controls in both BT-20 cells and MDA-MB-231 (#p<0.0001 and data not shown, Figure 3b).
PMIP inhibits tumor growth and inhibits recurrence in a xenograft breast cancer model
As hPMIP strongly inhibited cellular growth and invasion in vitro, we next evaluated the ability of hPMIP to suppress tumor growth and metastasis in vivo. In these experiments, highly metastatic MDA-MB-231 breast cancer cells were implanted into the mammary fat pad of severe combined immune deficiency (scid) mice and tumor growth was evaluated.
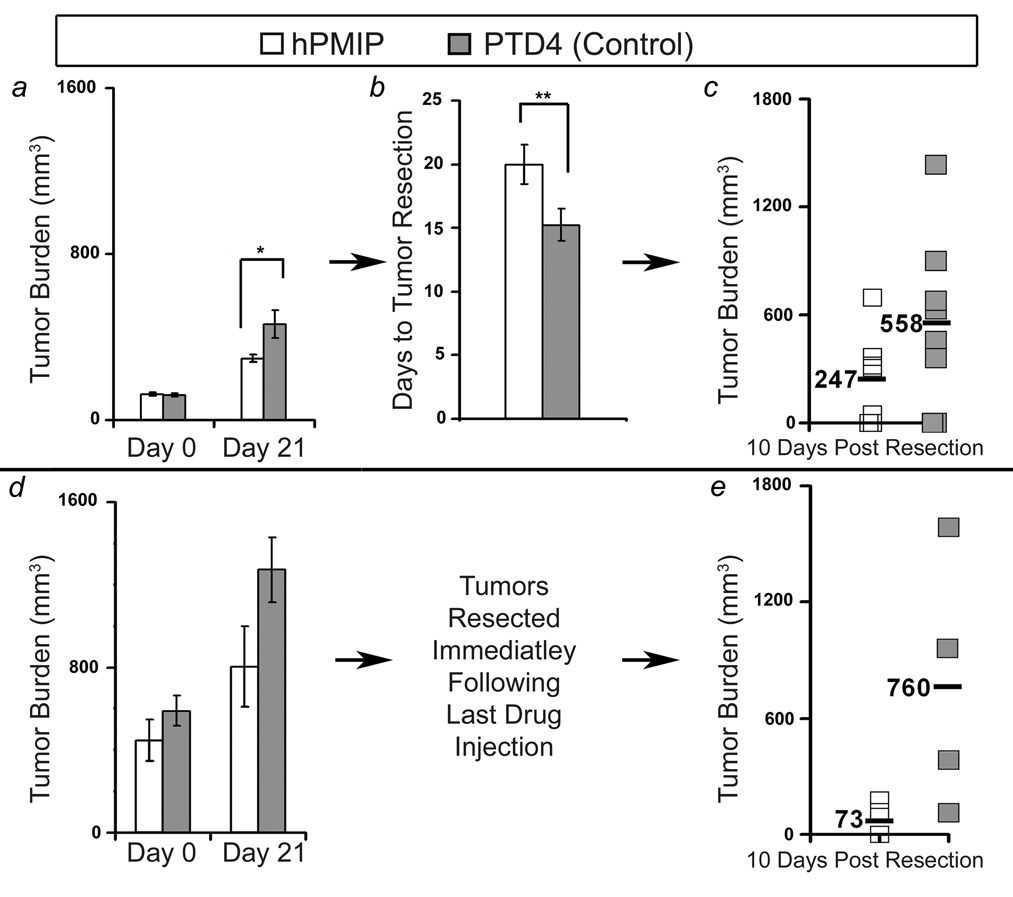
To determine potential effects of hPMIP on primary tumor growth, scid mice bearing MDA-MB-231 tumors (100mm3) were treated for 21 days with either hPMIP or PTD4 control peptide (i.p. injections) (Figure 4a–c). Upon removal of drug, tumors were allowed to continue to grow until they reached a volume of 1000mm3, then surgical resection of primary tumors was performed (Figure 4b). Treatment with hPMIP resulted in a significant decrease in tumor size compared to control treated animals at 21 days when drug administration was stopped (*p=0.028, Figure 4a). Additionally, this corresponded to a significant increase in the length of time required for hPMIP treated mice to reach resection size of 1000mm3 (**p=0.03, Figure 4b). Although treatment ended approximately 20 days prior to resection, we observed that hPMIP treatment substantially decreased the amount of tumor regrowth and spread 10 days after resection (Figure 4c). We also noted a decrease in the size of metastatic tumors in the hPMIP-treated animals compared to control, and next designed an experiment that would allow us to determine if hPMIP was affecting tumor spread following tumor resection.
Figure 4. PMIP significantly inhibits tumor growth and recurrence in vivo.
a) MDA-MB-231 cells in Matrigel were injected into the mammary fat pad of scid mice, and daily peptide treatment (50µg/g body weight of hPMIP or PTD4) began when tumors reached 100mm3 (hPMIP and PTD4 n=8 mice) and primary tumor growth was assessed (*, p=0.028, ANOVA). Tumor burden represents the average volume (v= a2 × b/2) of all tumors at the stage indicated. b) After the end of treatment, the amount of time the tumors took to progress to 1000mm3 was measured (**, p=0.03, ANOVA). c) After resection, mice were observed for tumor regrowth at the primary site or secondary mammary glands (hPMIP n=7 mice and PTD4 n=8 mice). d) MDA-MB-231 cells in Matrigel were injected into the mammary fat pad of scid mice, and daily peptide treatment (50µg/g of hPMIP or PTD4) began when tumors reached 500mm3 (hPMIP n=6 mice and PTD4 n=4 mice) and primary tumor growth was assessed. e) Following 21 days of treatment the tumors were resected immediately and tumor regrowth at the primary site and spread to secondary mammary glands was monitored (hPMIP n=4 mice and PTD4 n=4 mice). Error bars represent standard error.
To evaluate the effects of PMIP on tumor spread (Figure 4a and b), MDA-MB-231 breast cancer cells were allowed to establish a large tumor mass (500mm3) and mice were injected (i.p.) for 21 days with hPMIP or control peptide (PTD4) (Figure 4d). Immediately after the end of treatment, primary breast tumors were resected, and animals were followed to examine rates of tumor regrowth and/or metastasis to secondary mammary glands. While the presence of secondary mammary gland tumors were found in equal number for both treatment groups, the tumor volume for the control treated animals averaged 760mm3 while the PMIP treated averaged only 73mm3 (Figure 4e). Note that mice were not treated with drug during the 10 days in which regrowth was followed. These xenograft models revealed that PMIP treatment could have long lasting anti-growth effect, which was observed in tumor regrowth following resection.
PMIP inhibits tumor growth and induces regression in genetically-driven breast cancer
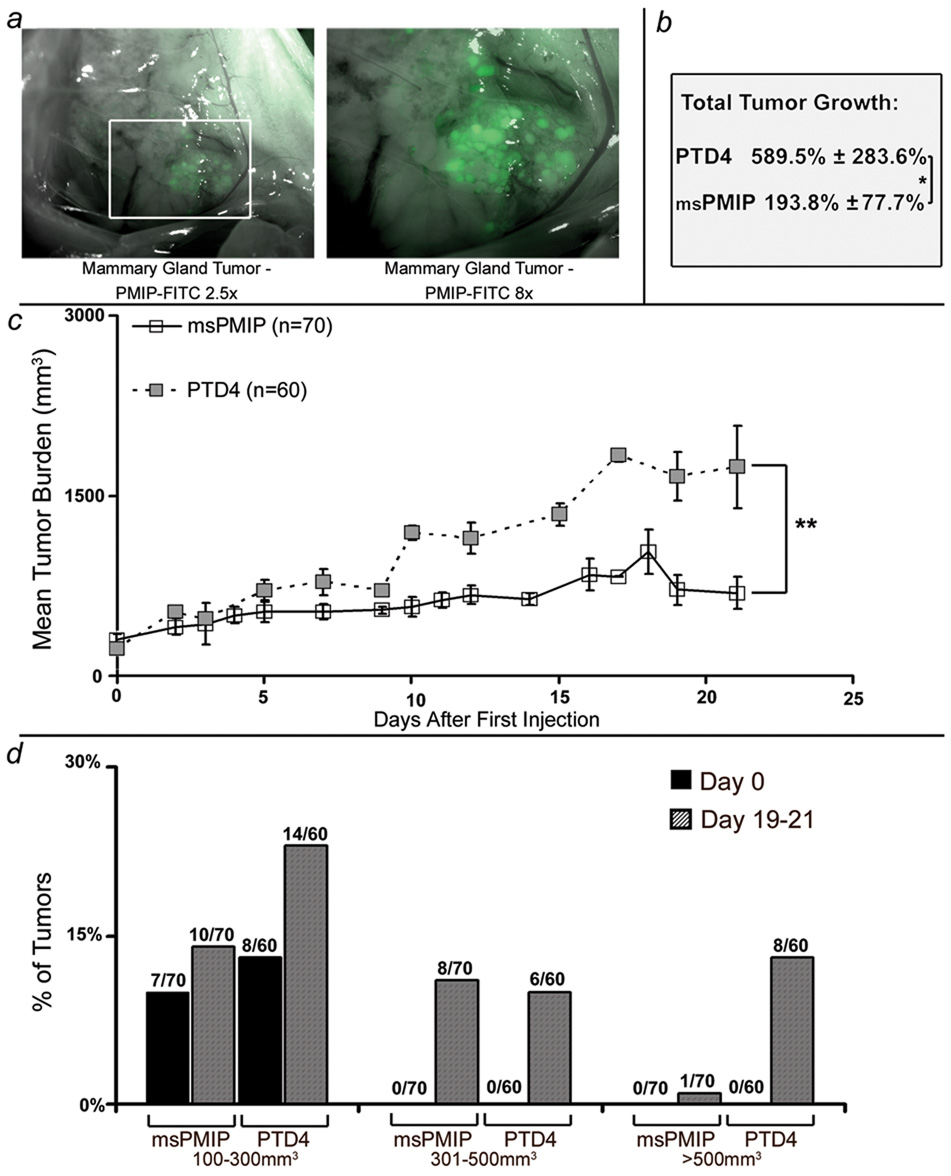
While the xenograft model demonstrated the effect of hPMIP treatment on growth and progression of established cell lines, we wanted to determine how msPMIP would affect tumor initiation and progression in a mouse model which better recapitulates human breast cancer. The MMTV-pyV mT transgenic mouse model of breast cancer strongly resembles human breast cancer by activating multiple signaling pathways, including AKT, src and shc (30, 35). Studies have demonstrated that the resulting breast cancer pathologically and molecularly mimics the full progression of hyperplasia, ductal carcinoma in situ and adenocarcinomas observed in human disease (36, 37). To first determine if peptide could be delivered to the mammary glands and tumors of these animals, we injected FITC-labeled msPMIP and analyzed peptide retention (Figure 5a). At two hours post-injection, FITC was detected throughout the animal’s body cavity, including all organs (data not shown). After four hours, FITC-msPMIP was found to be retained selectively in the mammary gland, mammary gland tumors and in the colon and skin (Figure 5a and data not shown).
Figure 5. PMIP significantly slows progression of MMTV-pyV mT induced mammary gland tumors.
a) MMTV-pyV mT transgenic mice were injected with FITC-msPMIP (50µg/g body weight), sacrificed four hours later and various tissues visualized using fluorescence microscopy. FITC-msPMIP localization in the mouse mammary gland tumors is shown (2.5× and 8×). b) Mammary gland tumors (>0.5 cm in diameter) were allowed to develop and mice were injected daily (50µg/g body weight, 21 day treatment, i.p. injection, 1× per day) with either msPMIP (7 mice) or PTD4 (6 mice). At the end of treatment, animals were sacrificed and proteins lysates made of the tumors for later analysis. In the course of treatment, total tumor growth for all tumor sites (msPMIP n=70, PTD4 n=60) was significantly lower in the msPMIP treated mice than in the PTD4 mice (193.8% ± 77.7% vs. 589.5% ± 283.6%, *, p=0.039, ANOVA). c) Mammary gland tumors of msPMIP treated mice grew at a significantly slower rate than PTD4 treated tumors (p=0.0076, ANOVA). d) Tumor size distribution for the msPMIP or PTD4 treated transgenic mice revealed that 47% (28 out of 60 possible tumor sites) of the PTD4 treated tumors were larger than 100mm3 compared to 27% (19 out of 70 possible tumor sites) of the msPMIP treated tumors. Numbers above data are numbers of tumors that meet the size criteria over the total potential tumor sites.
To determine the effects of msPMIP on genetically-driven breast cancer progression, MMTV-pyV mT mice bearing at least one mammary gland tumor of ≥0.5cm in diameter (in MMTV-pyV mT mice there are ten potential tumor sites) were treated for 21 days with either msPMIP or PTD4 control peptide. Treatment had a dramatic effect on tumor growth, as msPMIP significantly slowed the total tumor growth from ∼590% to ∼194% over the 21 days of treatment (*p=0.039; Figure 5b). Additionally, msPMIP treatment significantly decreased the tumor growth rate compared to control (PTD4) treated tumors (**p=0.007; Figure 5c). Note that treatment of MMTV-pyV mT mice with hPMIP (as opposed to msPMIP) had no effect on tumor growth, emphasizing the amino acid specificity of PMIP.
We next analyzed the overall size of tumors that arose throughout the study. This analysis demonstrates that while 13% of the tumors in the control (PTD4) group grew larger than 500mm3 by the end of the study, only 1% of the tumors in the msPMIP treated group reached that size (Figure 5d). As this transgenic model had continual expression of the polyoma middle T transgene driving tumorigenesis throughout the study, we next examined the effects of drug treatment on the formation of new tumors. Although both msPMIP and control (PTD4) groups had a similar number of tumors sized 100–300mm3 at the beginning of treatment, this number nearly doubled by the end of treatment in the control group, but remained the similar in the msPMIP group (Figure 5d). These data indicated that msPMIP treatment inhibits tumor initiation in this model (Initiation equals percent of tumor transitions from 0mm3 to 100mm3). To analyze tumor initiation further, we evaluated the percent of tumors that were initiated during drug treatment. This analysis demonstrates that in the msPMIP group there was a significant decrease (12 vs. 20 initiated tumors, data not shown, p=0.0045) of tumor initiation during the study.
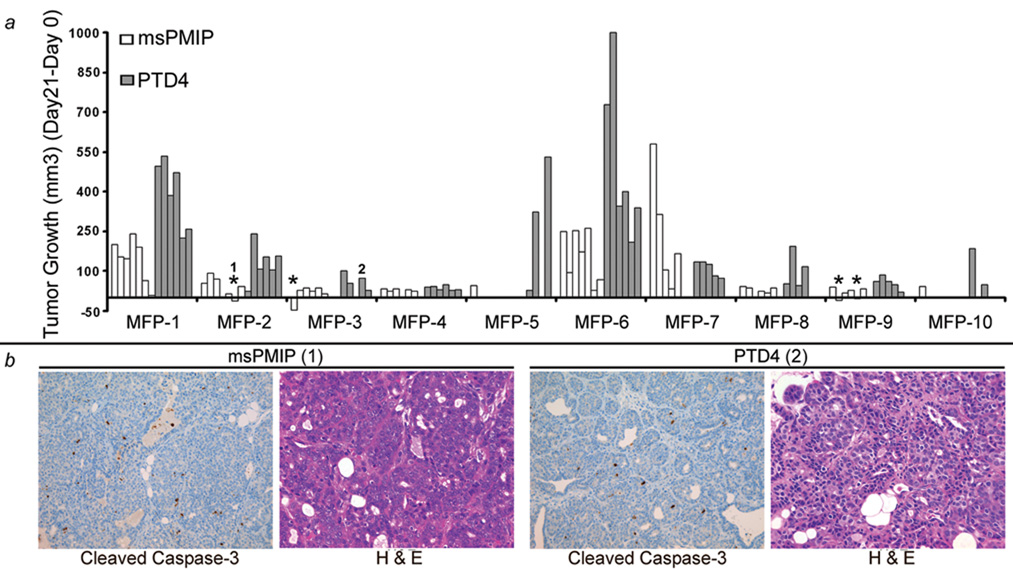
Although highly significant decreases in tumor formation and growth were observed from treatment of tumor-bearing MMTV-pyV mT mice with msPMIP, not all tumors in the study responded to treatment (Figure 6a). Analysis of each mammary gland demonstrates that while most tumor growth rates slowed substantially in response to msPMIP (white bars), a number of tumors continued to grow, indicative of the stochastic pathway activation in this model. Importantly, a subset of established tumors treated with msPMIP (four tumors) regressed completely under treatment, although none of the control treated tumors did so (Figure 6a, *). In addition, there was no detectable toxicity (no weight loss, organ failure or disorientation) following treatment with msPMIP or PTD4. Further investigation of tumor tissue sections by cleaved caspase-3 immuno-histochemistry and hemotoxylin-eosin staining of tumors that regressed or responded to msPMIP treatment did not reveal a difference in apoptotic cells compared to PTD4 control peptide treated tumors (Figure 6b).
Figure 6. MMTV-pyV mT tumors have differential response to msPMIP.
a) Individual growth of each tumor site (each bar is an individual tumor site) from animals described in figure 3 were treated daily with msPMIP (open bars) or PTD4 (grey bars) at 50µg/g body weight for 21 days. Tumor progression was observed every three days during the 21 day treatment (PTD4, n=60 tumor sites and PMIP, n=70 tumor sites). In four instances (*), msPMIP treated tumors completely regressed, however none of the control (PTD4) treated tumors regressed. Individual tumor sites are grouped by mammary fat pads (1–10) to allow for the comparison of similar anatomical sites. MFP=mammary fat pad b) Tumors from msPMIP (1, left 2 panels) and PTD4 (2, right 2 panels) treated mice were sectioned (3µm) and subsequently used for hemotoxylin-eosin staining and cleaved caspase-3 immunohistochemistry (200×).
msPMIP treatment results in a reduction of EGFR and Muc1 levels
To determine if msPMIP was affecting EGF-dependent degradation of EGFR in vivo, we generated protein lysates from tumors of MMTV- pyV mT animals injected with EGF and peptide 30 minutes prior to animal sacrifice (after a standard 21 day drug treatment). We observed a striking reduction in the expression of EGFR and corresponding phosphotyrosine in the msPMIP treated mouse compared to the control treated animal (Figure 7a). Note that in this mouse, there were no frank tumors remaining after the 21 day msPMIP treatment (remaining mammary fat pads were analyzed), while we obtained six tumors of greater than 400mm3 from control treated animals. Analysis of EGFR expression and activation of the MDA-MB-231 xenograft tumors was restricted to tumors that were not treated with EGF. Nonetheless, we did observe a reduction in the levels of phospho-EGFR (Y1173) and activated MAP kinase (dpERK) in PMIP treated versus control treated tumors (Supplementary Figure 1).
Figure 7. PMIP is associated with reduced Muc1 expression.
a) A representative MMTV- pyV mT msPMIP (P) treated mouse and a PTD4 mouse (C) were each injected 30 minutes prior to sacrifice with epidermal growth factor (1µg/g body weight) and with peptide. Following sacrifice the tumors were collected and protein lysates were generated. Protein (50µg) was separated by SDS-PAGE, transferred and immunoblotted for expression of phosphotyrosine (PY99), EGFR (1005), Muc1 (CT2), and β-actin (AC-15). b) Lysates from MDA-MD-231 xenograft tumors (not treated with EGF; described in Figure 2) were similarly analyzed to determine levels of EGFR and MUC1 protein expression. Relative protein levels of Muc1 were measured by densitometry and graphed (Muc1/β-actin, *, p=0.014, ANOVA). Molecular weights are shown on the right. IB = immunoblot. White lines through blots indicate same gel and exposure but were non- contiguous.
To determine if msPMIP blocked interaction between Muc1 and β-catenin, we began by establishing levels of Muc1 protein expression in the tumor lysates. Interestingly, we found that msPMIP treatment induced a loss of Muc1 protein in both the MMTV- pyV mT model (Figure 7a and b) and in the MDA-MB-231 xenograft model (Figure 7c and d). Total levels of β-catenin and its target genes cyclin D1 and Twist are unchanged between PMIP and control (Data not shown).
Discussion
We report here that a MUC1 mimetic peptide (MIP) linked to a protein transduction domain (PTD4) can freely enter transformed cells and inhibit their invasion in vitro. This same peptide can inhibit primary tumor growth, tumor spread, and recurrence of tumors after resection in an orthotopically implanted breast cancer model. PMIP can survive in circulation with tissue specific retention and no detectable toxicity. Importantly, PMIP can significantly inhibit tumor growth in a genetically-driven mouse model that mimics human breast cancer. Mechanistically, this tumor-inhibitory effect is closely associated with a decrease in EGFR and MUC1 expression. Together these data demonstrate that this peptide-based intracellular drug shows strong efficacy as a non-toxic treatment for breast cancer.
The over-expression of human MUC1 in mouse mammary glands promotes transformation and the loss of MUC1 in several transgenic models can significantly delay tumor onset (6, 10, 12). This may be due to the number of oncogenic partners MUC1 has been demonstrated to interact with, namely β-catenin, src, and EGFR [Reviewed in (8)]. This mimetic peptide is designed to block interactions between MUC1, β-catenin and EGFR, and we have demonstrated that PMIP treatment does block MUC1 interactions with these proteins. In addition, we observed a loss of MUC1 expression in response to PMIP treatment under certain conditions, which may be the result of downregulation of EGFR. While the mechanism of MUC1 loss is unknown, it would certainly result in a loss of Muc1-dependent oncogenic signaling. It is tempting to speculate that in the presence of PMIP, MUC1 and EGFR are alternatively trafficked and enter the lysosomal degradation pathway, leading to their enhanced degradation. PMIP treatment of MDA-MB-231 cells overexpressing MUC1 under a CMV promoter for only 3 hours in culture induces a loss of MUC1 overexpression, indicating that the effect is not transcriptionally regulated (data not shown). Future studies will focus on determining the precise mechanism by which PMIP suppresses protein expression and tumor progression.
One important observation is the lack of toxicity associated with PMIP treatment (no weight loss, signs of distress or organ failure). While this is not an unexpected result with the use of an endogenous peptide, it points to a potentially low level of toxicity in patients. We have also examined the possibility that PMIP has activated an immune response in the immune-intact MMTV- pyV mT transgenic animals. Examining leukocytic infiltrates using protein levels of CD45 as a marker, we found no increase in those tumors treated with PMIP versus control ((38); data not shown). Additionally, studies by groups investigating the potential adjuvant activity of the protein transduction domain have found that the TAT protein transduction domain is not immunogenic (39). One potential reason for PMIP’s lack of toxicity may be due to the relatively fast clearance of small peptides from the body REVIEW by Torchilin at al. Importantly, while PMIP-FITC appeared to be cleared overall from the body 4 hours after injection, it was retained in specific organ sites, including the mammary gland and colon. This site specific retention may be due to an increase in peptide binding partners in these particular tissues.
We have previously demonstrated that MUC1 inhibits the ligand-dependent degradation of EGFR, resulting in enhanced receptor stability (9). Furthermore, we have demonstrated that this interaction promotes the oncogenic properties of EGFR (9). Neither of the mouse models used in these experiments were previously shown to be dependent upon EGFR for progression, and yet, PMIP is significantly effective in each model. This indicates that PMIP may have broad applications against tumors overexpressing MUC1, which encompasses most epithelial neoplasias (40). In addition, PMIP may serve as an important adjuvant therapy with anti-EGFR treatments [Reviewed in (41)]. Our data indicate that MUC1 induces the internalization, altered trafficking and enhanced signaling of EGFR (9). Therefore, if PMIP blocks these interactions, anti-EGFR therapy that relies on surface-localization of EGFR could by enhanced by PMIP co-delivery.
This study demonstrates the efficacy of PTD4-linked peptide-based drugs and the value of MUC1-directed targets in breast cancer. Importantly, these data indicate that PMIP is a potent drug that is active at all stages of tumor progression; inhibiting growth, inducing regression and inhibiting metastatic spread.
Supplementary Material
Acknowledgments
This study was funded in part by the Arizona Biomedical Research Corporation (JAS), the National Cancer Institute (JAS, BGB and IM) and the BIO5 Institute (JAS). We are thankful to Jamie Bitler, Jeanne Louderbough, Rachid el Bejjani and Jose Lopez for editing of the manuscript.
References
- 1.Hilkens J, Vos HL, Wesseling J, et al. Is episialin/MUC1 involved in breast cancer progression? Cancer Lett. 1995;90:27–33. doi: 10.1016/0304-3835(94)03674-8. [DOI] [PubMed] [Google Scholar]
- 2.Zotter S, Hageman PC, Lossnitzer A, Mooi WJ, Hilgers J. Tissue and tumor distribution of human polymorphic epithelial mucin. Cancer Reviews. 1988;11–12:55–101. [Google Scholar]
- 3.Brossart P, Schneider A, Dill P, et al. The epithelial tumor antigen MUC1 is expressed in hematological malignancies and is recognized by MUC1-specific cytotoxic T-lymphocytes. Cancer Res. 2001;61:6846–6850. [PubMed] [Google Scholar]
- 4.Takahashi T, Makiguchi Y, Hinoda Y, et al. Expression of MUC1 on myeloma cells and induction of HLA-unrestricted CTL against MUC1 from a multiple myeloma patient. J Immunol. 1994;153:2102–2109. [PubMed] [Google Scholar]
- 5.Teruya-Feldstein J, Donnelly GB, Goy A, et al. MUC-1 mucin protein expression in B-cell lymphomas. Appl Immunohistochem Mol Morphol. 2003;11:28–32. doi: 10.1097/00129039-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder JA, Masri AA, Adriance MC, et al. MUC1 overexpression results in mammary gland tumorigenesis and prolonged alveolar differentiation. Oncogene. 2004;23:5739–5747. doi: 10.1038/sj.onc.1207713. [DOI] [PubMed] [Google Scholar]
- 7.Ren J, Agata N, Chen D, et al. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents. Cancer Cell. 2004;5:163–175. doi: 10.1016/s1535-6108(04)00020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 9.Pochampalli MR, el Bejjani RM, Schroeder JA. MUC1 is a novel regulator of ErbB1 receptor trafficking. Oncogene. 2007;26:1693–1701. doi: 10.1038/sj.onc.1209976. [DOI] [PubMed] [Google Scholar]
- 10.Pochampalli MR, Bitler BG, Schroeder JA. Transforming growth factor alpha dependent cancer progression is modulated by Muc1. Cancer Res. 2007;67:6591–6598. doi: 10.1158/0008-5472.CAN-06-4518. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Ren J, Yu W, et al. The epidermal growth factor receptor regulates interaction of the human DF3/MUC1 carcinoma antigen with c-Src and beta-catenin. J Biol Chem. 2001;276:35239–35242. doi: 10.1074/jbc.C100359200. [DOI] [PubMed] [Google Scholar]
- 12.Schroeder JA, Adriance MC, Thompson MC, Camenisch TD, Gendler SJ. MUC1 alters β-catenin-dependent tumor formation and promotes cellular invasion. Oncogene. 2003;22:1324–1332. doi: 10.1038/sj.onc.1206291. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto M, Bharti A, Li Y, Kufe D. Interaction of the DF3/MUC1 breast carcinoma-associated antigen and beta-catenin in cell adhesion. J Biol Chem. 1997;272:12492–12494. doi: 10.1074/jbc.272.19.12492. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Bharti A, Chen D, Gong J, Kufe D. Interaction of glycogen synthase kinase 3beta with the DF3/MUC1 carcinoma-associated antigen and beta-catenin. Mol Cell Biol. 1998;18:7216–7224. doi: 10.1128/mcb.18.12.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Kuwahara H, Ren J, Wen G, Kufe D. The c-Src tyrosine kinase regulates signaling of the human DF3/MUC1 carcinoma-associated antigen with GSK3 beta and beta-catenin. J Biol Chem. 2001;276:6061–6064. doi: 10.1074/jbc.C000754200. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder JA, Lee DC. Transgenic mice reveal roles for TGFalpha and EGF receptor in mammary gland development and neoplasia. J Mammary Gland Biol Neoplasia. 1997;2:119–129. doi: 10.1023/a:1026347629876. [DOI] [PubMed] [Google Scholar]
- 17.Alroy I, Yarden Y. The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997;410:83–86. doi: 10.1016/s0014-5793(97)00412-2. [DOI] [PubMed] [Google Scholar]
- 18.Olayioye MA, Graus-Porta D, Beerli RR, Rohrer J, Gay B, Hynes NE. ErbB-1 and ErbB-2 acquire distinct signaling properties dependent upon their dimerization partner. Mol Cell Biol. 1998;18:5042–5051. doi: 10.1128/mcb.18.9.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olayioye MA, Beuvink I, Horsch K, Daly JM, Hynes NE. ErbB receptor-induced activation of stat transcription factors is mediated by Src tyrosine kinases. J Biol Chem. 1999;274:17209–17218. doi: 10.1074/jbc.274.24.17209. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter G. The EGF receptor: a nexus for trafficking and signaling. Bioessays. 2000;22:697–707. doi: 10.1002/1521-1878(200008)22:8<697::AID-BIES3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder JA, Thompson MC, Gardner MM, Gendler SJ. Transgenic MUC1 interacts with epidermal growth factor receptor and correlates with mitogen-activated protein kinase activation in the mouse mammary gland. J Biol Chem. 2001;276:13057–13064. doi: 10.1074/jbc.M011248200. [DOI] [PubMed] [Google Scholar]
- 22.Schroeder JA, Troyer KL, Lee DC. Cooperative induction of mammary tumorigenesis by TGFalpha and Wnts. Oncogene. 2000;19:3193–3199. doi: 10.1038/sj.onc.1203652. [DOI] [PubMed] [Google Scholar]
- 23.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 24.He TC, Sparks AB, Rago C, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 25.Shtutman M, Zhurinsky J, Simcha I, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 27.Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55:619–625. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- 28.Michaelson JS, Leder P. beta-catenin is a downstream effector of Wnt-mediated tumorigenesis in the mammary gland. Oncogene. 2001;20:5093–5099. doi: 10.1038/sj.onc.1204586. [DOI] [PubMed] [Google Scholar]
- 29.Schroeder JA, Adriance MC, McConnell EJ, Thompson MC, Pockaj BA, Gendler SJ. ErbB/beta -catenin complexes are associated with human infiltrating ductal breast and MMTV-Wnt-1 and MMTV-c-neu transgenic carcinomas. J Biol Chem. 2002;277:22692–22698. doi: 10.1074/jbc.M201975200. [DOI] [PubMed] [Google Scholar]
- 30.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spicer AP, Duhig T, Chilton BS, Gendler SJ. Analysis of mammalian MUC1 genes reveals potential functionally important domains. Mamm Genome. 1995;6:885–888. doi: 10.1007/BF00292441. [DOI] [PubMed] [Google Scholar]
- 32.Ho A, Schwarze SR, Mermelstein SJ, Waksman G, Dowdy SF. Synthetic protein transduction domains: enhanced transduction potential in vitro and in vivo. Cancer Res. 2001;61:474–477. [PubMed] [Google Scholar]
- 33.Wadia JS, Dowdy SF. Transmembrane delivery of protein and peptide drugs by TAT-mediated transduction in the treatment of cancer. Adv Drug Deliv Rev. 2005;57:579–596. doi: 10.1016/j.addr.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 35.Webster MA, Hutchinson JN, Rauh MJ, et al. Requirement for both Shc and phosphatidylinositol 3' kinase signaling pathways in polyomavirus middle T-mediated mammary tumorigenesis. Mol Cell Biol. 1998;18:2344–2359. doi: 10.1128/mcb.18.4.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maglione JE, Moghanaki D, Young LJ, et al. Transgenic Polyoma middle-T mice model premalignant mammary disease. Cancer Res. 2001;61:8298–8305. [PubMed] [Google Scholar]
- 37.Lin EY, Jones JG, Li P, et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–2126. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trivedi P, Cuomo L, Christensson B, et al. Augmentation of leukocyte infiltration in murine tumors expressing B-cell derived but not nasopharyngeal carcinoma derived EBV membrane protein LMP1. J Med Virol. 2000;60:417–424. [PubMed] [Google Scholar]
- 39.Kittiworakarn J, Lecoq A, Moine G, et al. HIV-1 Tat Raises an Adjuvant-free Humoral Immune Response Controlled by its Core Region and its Ability to Form Cysteine-mediated Oligomers. The Journal of Biological Chemistry. 2005;281:3105–3115. doi: 10.1074/jbc.M509899200. [DOI] [PubMed] [Google Scholar]
- 40.Packer LM, Williams SJ, Callaghan S, Gotley DC, McGuckin MA. Expression of the cell surface mucin gene family in adenocarcinomas. Int J Oncol. 2004;25:1119–1126. [PubMed] [Google Scholar]
- 41.Dassonville O, Bozec A, Fischel JL, Milano G. EGFR targeting therapies: monoclonal antibodies versus tyrosine kinase inhibitors. Similarities and differences. Crit Rev Oncol Hematol. 2007;62:53–61. doi: 10.1016/j.critrevonc.2006.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.