Abstract
The chemical constituents and antiproliferative effects on SW480 human colorectal cancer cells of different plant parts of P. notoginseng were evaluated. The contents of saponins in extracts from root, rhizome, flower and berry of P. notoginseng were determined using high performance liquid chromatography. The contents and proportions of saponins were different among the four plant parts. Using the cell counting method, the antiproliferative effects were evaluated and the results indicated all four extracts, at 0.05–1.0 mg/mL, showed concentration-related antiproliferative effects on the cancer cells. The flower extract had stronger effects compared with the other three extracts; at 1.0 mg/mL, it inhibited the cell growth by 93.1% (p < 0.01). The antiproliferative effects of major saponins in notoginseng, notoginsenoside R1, ginsenosides Rb1, Rb3 and Rg1, were also evaluated, and the observed effects of major constituents support the pharmacological activities of extracts. The effects of notoginseng extracts on cell cycle and apoptosis of SW480 cells were determined using flow cytometry. Notoginseng extract can arrest the cells in S and G2/M phases. Remarkably apoptosis induction activities of notoginseng extracts were observed with the flower extract possessing the most potent effect, supporting the antiproliferative effect.
Keywords: Panax notoginseng, HPLC analysis, anticancer, cell cycle, apoptosis, SW480 human colorectal cancer cells
INTRODUCTION
Panax notoginseng (Burk.) F. H. Chen is a commonly used herbal medicine in many East Asian countries including China, Japan and Korea. Notoginseng is commercially cultivated throughout the southwest of China. The commonly used part is the root, which has a long history of use as a remedy in traditional medicine (Ng, 2006). Notoginseng reportedly exerts beneficial effects in treating trauma and bleeding caused by internal and external injury, promoting blood circulation and reducing blood clotting. Data from chemical analysis demonstrated that the main constituents in notoginseng are saponins that are responsible for the herb’s multifaceted pharmacological activities (Ng, 2006; Wang et al., 2006a). To date, over 50 saponins, including ginsenosides, notoginsenosides, and gypenosides, have been identified in notoginseng. Ginsenosides Rg1, Rb1 and Rd, and notoginsenoside R1 are considered to be the major components (Wang et al., 2006a). Besides the root, the pharmacological effects of other plant parts of notoginseng have also been studied (Chen et al., 1983). Recently, several reports found evidence that notoginseng root extract may possess anticancer activity (Chen et al., 2001; Ng, 2006).
Colorectal cancer is the second leading cause of death from cancer in the United States. It is third leading cause of cancer death in men behind lung and prostate cancer respectively, and behind lung and breast in cancer deaths among women (Jemal et al., 2004). Most colorectal cancers take approximately 8–10 years to develop from an adenomatous polyp into an invasive cancer (Thiis-Evensen et al., 1999). Although early stage colorectal cancer could be cured by surgical resection, most often surgery is combined with adjuvant radiotherapy and chemotherapy. Radiotherapy is often combined with one or more chemotherapeutic agents. While advances continue to be made in developing effective strategies for treating colorectal cancer, chemotherapy is still often limited by severe side effects and dose-limiting toxicity. The drug-related adverse events not only worsen the patients’ quality of life, but can also lead to their refusal to continue the potentially curative chemotherapy (Martin et al., 2003; Schnell, 2003; Wang et al., 2005). It seems that identifying non-toxic chemoadjuvants from herbal medicines is an essential step in advancing the treatment of the cancer.
It is known that botanicals have been a significant resource to several of the currently used efficacious chemotherapeutic agents. Cancer treatment and care by botanicals, including notoginseng, has received increasing attention in the past decade (Konoshima et al., 1999; Wang et al., 2006a). However, effects of notoginseng on colorectal cancer have not been tested. In addition, the mechanism of antiproliferative activities of notoginseng was not clear. Very few studies have been conducted involving the mechanisms of notoginseng in connection with cancer (Yang et al., 2006). In this study, in addition to notoginseng root, other portions of notoginseng plant, such as rhizome, flower and berry were analysed, and the major constituents in these plant portions compared. Then, the analytical data of the different notoginseng portions were investigated for correlation of their effects on SW480 human colorectal cancer cells. Finally, using flow cytometry, the effects of notoginseng on the cell cycle and cell apoptosis on SW480 cells were assayed.
MATERIALS AND METHODS
Chemicals
The Panax notoginseng samples were obtained from Wenshan, Yunnan province, China. The plant materials were identified by Dr Chong-Zhi Wang according to the Chinese Pharmacopoeia, Panax notoginseng (Burk.) F. H. Chen. The voucher specimens of notoginseng root (Pn2004001r), rhizome (Pn2004004r), flower (Pn2004008f) and berry (Pn2004011b) were stored in our laboratory. For the extraction of ginsenosides, each sample was ground to powder and extracted with 75% ethanol. The solvent of extract solution was evaporated under vacuum. The dried extract was dissolved in water then extracted with water-saturated n-butanol. The n-butanol phase was evaporated under vacuum, and then lyophilized. The ratios of starting materials to finished extracts were 6:1 to 15:1 for the four extracts. Trypsin, Leibovitz’s L-15 medium and PBS were obtained from Mediatech (Herndon, VA). Penicillin/streptomycin, propidium iodide (PI) and RNase were obtained from Sigma (St Louis, MO). The annexin V-FITC apoptosis detection kit was obtained from BD Biosciences (San Diego, CA). Ginsenosides Rb1, Rb3, Rc, Rd, Re, Rg1 and notoginsenoside R1 were obtained from the Delta Information Center for Natural Organic Compounds (Xuancheng, China). HPLC grade methanol, n-butanol, acetonitrile and ethanol were obtained from Fisher Scientific (Pittsburgh, PA).
High performance liquid chromatography (HPLC) analysis
The HPLC system was a Waters 2960 instrument (Milford, MA), with a quaternary pump, automatic injector, a photodiode array detector (Model 996) and Waters Millennium32 software for peak identification and integration. The separation was carried out on an Alltech Ultrasphere C18 column (5 μm, 250 × 3.2 mm i.d.) (Deerfield, IL) with an Alltech Ultrasphere C18 guard column (5 μm, 7.5 × 3.2 mm i.d.). For HPLC analysis, a 20 μL sample was injected into the column and eluted at room temperature with a constant flow rate of 1.0 mL/min. Acetonitrile (solvent A) and water (solvent B) were used as the mobile phase. Gradient elution started with 17.5% solvent A and 82.5% solvent B, changed to 21% A for 20 min, then changed to 26% A for 3 min and held for 19 min; changed to 36% A for 13 min; changed to 50% A for 9 min; changed to 95% A for 2 min and held for 3 min; changed to 17.5% A for 3 min and held for 8 min. The detection wavelength was set to 202 nm.
Cell culture
The SW480 cells (ATCC, Manassas, VA) were routinely maintained in Leibovitz’s L-15 medium, supplemented with 10% fetal bovine serum and 50 IU penicillin/streptomycin. Cancer cells were grown in a tissue culture dish (100 mm in diameter) and kept in a humidified incubator (5% CO2 and at 37 °C) with a medium change every 2–3 days. When the cells reached >80% confluence, they were trypsinized, harvested and seeded into a new tissue culture dish.
Cell proliferation analysis
The SW480 cell proliferative assay was done as previously described (Xie et al., 2006). Briefly, cell viability was measured after 72 h incubation with test extract/compound. The cell monolayer was washed twice with phosphate buffered saline (PBS). Cultures were harvested and their cell counts were monitored by using a Coulter cell counter (Coulter Electronics, Hialeah, FL). The cell proliferative assay was performed at least three times. The percentage of cell proliferation was calculated as follows: cell proliferation (%) = (cell number in each experimental well ÷ average of cell number in all control wells) × 100. Ethanol concentrations in the cell culture experiments did not exceed 1% and the controls were always treated with the same amount of ethanol as that used in the corresponding experiments.
3H-thymidine incorporation assay
The 3H-thymidine incorporation assay using SW480 cells was performed for exploring the possible mechanisms of action of notoginseng. As described above, SW480 cells were seeded in 24-well plate and adhered for 24 h, the cells were incubated with test compounds at various concentrations and 300 μL media containing 1 λ/mL 3H-thymidine in each well for 72 h. After washing the cells with PBS and 10% trichloroacetic acid, 0.5 mL NaOH (0.2 m) was added to each well and agitated for 5 min. Finally, the cells with solution were transferred into vials and 30% liquid scintillation 5 mL was added and radioactivity counts were measured using a liquid scintillation analyser (TRI-CARB 1500, Packard). The results are presented as a percentage of control values (Gieni et al., 1995).
Cell cycle assay
The cells were seeded in 24-well tissue culture plates. On day 2, the medium was changed and the cells were treated with extracts. The cells were incubated for 48 h before the cells were harvested. The cells were fixed gently by adding 80% ethanol and placing them in a −20 °C freezer for 2 h. They were then treated with 0.25% Triton X-100 for 5 min in an ice bath. The cells were resuspended in 300 μL of PBS containing 40 μg/mL propidium iodide and 0.1 mg/mL RNase. Then the cells were incubated in a dark room for 20 min at room temperature, and analysed with a FACScan flow cytometer (Becton Dickinson, Mountain View, CA) and FlowJo 7.1.0 software (Tree Star, Ashland, OR). For each measurement, at least 20 000 cells were counted.
Apoptosis assay
The cells were seeded in 24-well tissue culture plates. After culturing for 1 day, the medium was changed and extracts/compounds were added. After being treated for 48 h, cells floating in the medium were collected. The adherent cells were detached with 0.05% trypsin. Then culture medium containing 10% FBS (and floating cells) was added to inactivate trypsin. After being pipetted gently, the cells were centrifuged for 5 min at 1500 × g. The supernatant was removed and the cells were stained with annexin V-FITC and propidium iodide according to the manufacturer’s instructions. Untreated cells were used as a control for double staining. The cells were analysed immediately after staining using a FACScan flow cytometer. For each measurement, at least 20 000 cells were counted.
Statistics
The results are presented as mean ± standard error (SE). Data were analysed using Student’s t-test and analysis of variance (ANOVA) for repeated measures. The level of statistical significance was set at p < 0.05.
RESULTS
HPLC analysis of notoginseng root, rhizome, flower and berry extracts
Figure 1 shows the chemical structures of seven major saponins in the different plant parts of notoginseng. Notoginseng saponins in the extracts of root (NGRE), rhizome (NGZE), flower (NGFE) and berry (NGBE) were identified by observing the retention times and UV spectrum of authentic ginsenoside Rb1, Rb3, Rc, Rd, Re, Rg1 and notoginsenoside R1 standards obtained from the mixed standards’ chromatograms.
Figure 1.
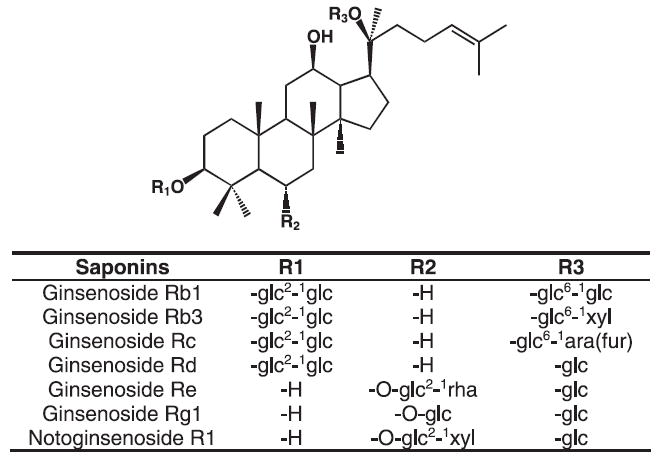
Chemical structures of major saponins in notoginseng, ginsenosides Rb1, Rb3, Rc, Rd, Re, Rg1 and notoginsenoside R1.
The HPLC analysis data of the four different extracts are shown in Fig. 2A. In NGRE, the major saponins were ginsenoside Rg1 (29.8%), Rb1 (27.0%) and notoginsenoside R1 (6.0%), while ginsenoside Rb3 was in trace amount (0.06%). For NGFE, although the total saponin was relatively low, the saponin profile was different compared with the others. For example, ginsenoside Rb3 was present in the highest concentration (15.2%) while notoginsenoside R1 was detected as a trace saponin in the NGFE (0.07%). Figure 2B shows representative HPLC chromatograms of the extracts from root, rhizome, flower and berry.
Figure 2.
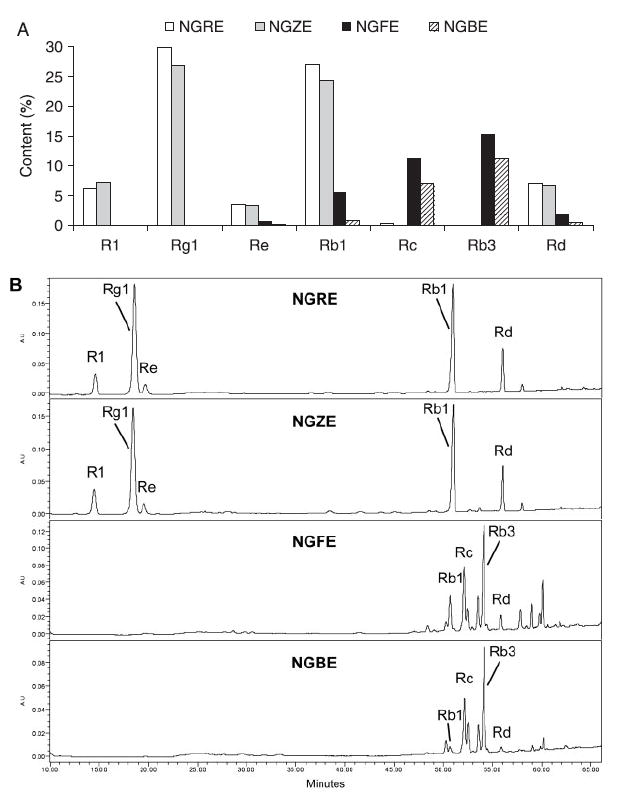
HPLC analysis of the four notoginseng extracts, notoginseng root extract (NGRE), rhizome extract (NGZE), flower extract (NGFE) and berry extract (NGBE). (A) Contents of major saponins in NGRE, NGZE, NGFE and NGBE. (B) Representative HPLC chromatograms of the four notoginseng extracts.
Effects of notoginseng extracts and major constituents on SW480 cell proliferation
The antiproliferative effect of the notoginseng root extract was observed at different time points. NGRE 1.0 mg/mL decreased the cell proliferation by 26.0 ±0.8% at 24 h, 51.0 ± 2.0% at 48 h and 76.0 ± 2.2% at 72 h. Since further prolonging treatment time often induced unstable results based on pilot observations of this experiment, 72 h treatment time was selected in this study.
Antiproliferative effects of four notoginseng extracts, NGRE, NGZE, NGFE and NGBE, on SW480 human colorectal cancer cells are shown in Fig. 3. The results indicated that, after treatment for 72 h, all four notoginseng extracts significantly inhibited cell proliferation in a concentration dependent manner. Compared with the control (normalized to 100%), NGRE reduced cell growth by 14.1 ± 1.5% (p < 0.05), 39.6 ± 1.0% (p < 0.01) and 75.8 ± 2.2% (p < 0.01) at 0.05, 0.5 and 1.0 mg/mL, respectively (Fig. 3A).
Figure 3.
Antiproliferative effects of notoginseng extracts on the growth of SW480 human colorectal cancer cells after 72 h of treatment. NGRE, notoginseng root extract; NGZE, notoginseng rhizome extract; NGFE, notoginseng flower extract; NGBE, notoginseng berry extract. * p < 0.05; ** p < 0.01 vs control.
At lower concentrations (0.05 mg/mg) of NGZE, NGFE and NGBE, no antiproliferative effects were observed in the SW480 cells (Fig. 3B–D). NGFE had a stronger effect compared with the other three extracts; at 0.5 and 1.0 mg/mL, it inhibited the cell growth by 65.5 ± 2.4% (p < 0.01) and 93.1 ± 0.4% (p < 0.01), respectively (Fig. 3C).
To explore the inhibitory activities of single chemical constituents in notoginseng, the effects of notoginsenoside R1 (a unique constituent found in the root and rhizome), ginsenosides Rg1 and Rb1 (relatively high contents in the root and rhizome) and ginsenosides Rb3 (relatively high content in the flower and berry) were determined in SW480 cells. As shown in Fig. 4, compared with the control, notoginsenoside R1 at concentrations of 30, 100 and 300 μm inhibited SW480 cell growth by 17.3 ± 2.5%, 22.9 ± 2.9%, and 22.5 ± 2.0%, respectively (all p < 0.05). Similar effects were observed with ginsenosides Rg1 and Rb1, while at the same concentration, Rb3 had the most significant effect.
Figure 4.
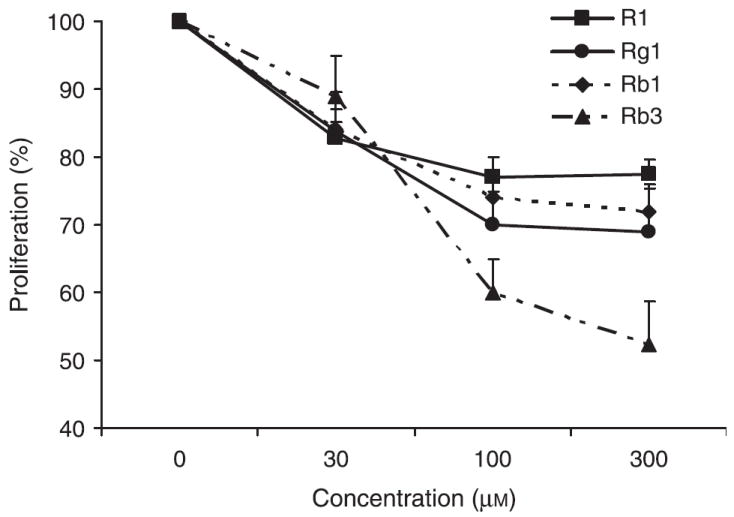
Antiproliferative effects of notoginsenoside R1, ginsenosides Rg1, Rb1 and Rb3 on SW480 human colorectal cancer cells after 72 h treatment.
Effects of notoginseng extracts on DNA synthesis by measuring 3H-thymidine incorporation
The effects of NGRE, NGZE and NGFE on the incorporation of SW480 cells were measured using 3H-thymidine assay. The results showed that at concentrations of 0.2 mg/mL, NGRE, NGZE and NGFE suppressed cellular incorporation of 3H-thymidine by 27.0 ± 4.4% (p < 0.01), 17.8 ± 3.0% (p < 0.05), 35.8 ± 4.1% (p < 0.01) (Fig. 5). These data showed the extracts were endowed with inhibiting the cellular incorporation of 3H-thymidine.
Figure 5.

Effects of notoginseng extracts on DNA synthesis. 3H-thymidine incorporation in SW480 human colorectal cancer cells was measured after 72 h of treatment (0.2 mg/mL). NGRE, notoginseng root extract; NGZE, notoginseng rhizome extract; NGFE, notoginseng flower extract. * p < 0.05; ** p < 0.01 vs control.
Effects of notoginseng extracts on cell cycle
As shown in Fig. 6, after being treated with notoginseng extracts, the cells were arrested in S phase and/or G2/M phase. Compared with the control (S phase, 31.5%; G2/M phase, 14.4%), after being treated with 0.5 mg/mL NGRE for 48 h, the fractions of the cells in the S phase and the G2/M phase increased to 51.2% and 21.2%, respectively. After being treated with 0.5 mg/mL of NGZE and NGBE, 29.1% and 31.9% cells were in G2/M phase. NGRE, NGZE and NGBE notably increased the fraction of SW480 cells in the G2/M phase. After treatment with NGFE at 0.5 mg/mL for 48 h, not enough living cells were obtained. Thus, in this particular observation, the fractions of cells in different cell cycle phases were not available (Fig. 6). Subsequently the treatment concentration of NGFE was decreased to 0.2 mg/mL. After 48 h treatment, the cell cycle assay result was similar to that of NGBE at 0.5 mg/mL for 48 h. This result suggested that notoginseng extract influences SW480 cell cycle significantly.
Figure 6.
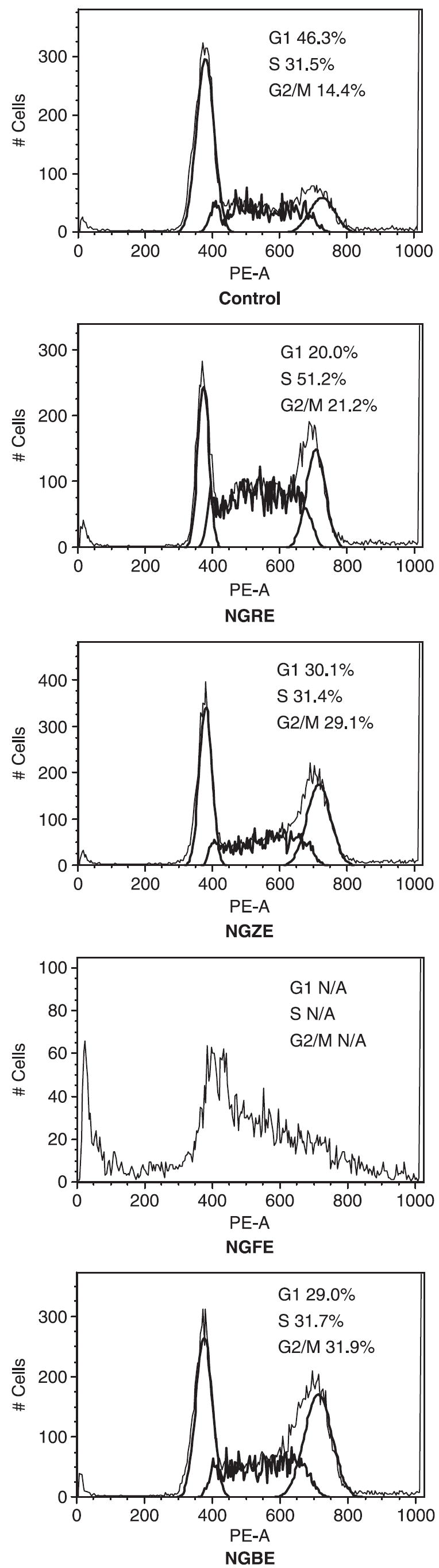
Cell cycle analysis of SW480 cells using flow cytometry after propidium iodide staining. SW480 cells were treated with 0.5 mg/mL notoginseng extracts for 48 h. The percentage of cells in G1, S and G2/M were indicated.
Effect of notoginseng extracts and saponins on cell apoptosis
After staining with annexin V/PI, the cells were analysed with flow cytometry. Figure 7 show cytograms with bivariate annexin V/PI analysis of SW480 cells after treatment. Viable cells were negative for both PI and annexin V (lower left quadrant); early apoptotic cells, which are real apoptotic cells, were positive for annexin V and negative for PI (lower right quadrant); late apoptotic/necrotic cells displayed both positive for annexin V and PI (upper right quadrant); Non-viable cells which underwent necrosis were positive for PI and negative for annexin V (upper left quadrant). In the four extracts, the notoginseng flower extract has the most potent effect on the induction of cell apoptosis. The percentage of early apoptotic cells induced by 0.5 mg/mL NGFE was increased remarkably after 48 h treatment (40.0%), while the control was 4.2%. Treated with 1 mg/mL of NGRE, the apoptotic cells were increased to 53.0%. Data from this study suggested that the antiproliferative effect of notoginseng extract was mediated by the induction of cell apoptosis.
Figure 7.
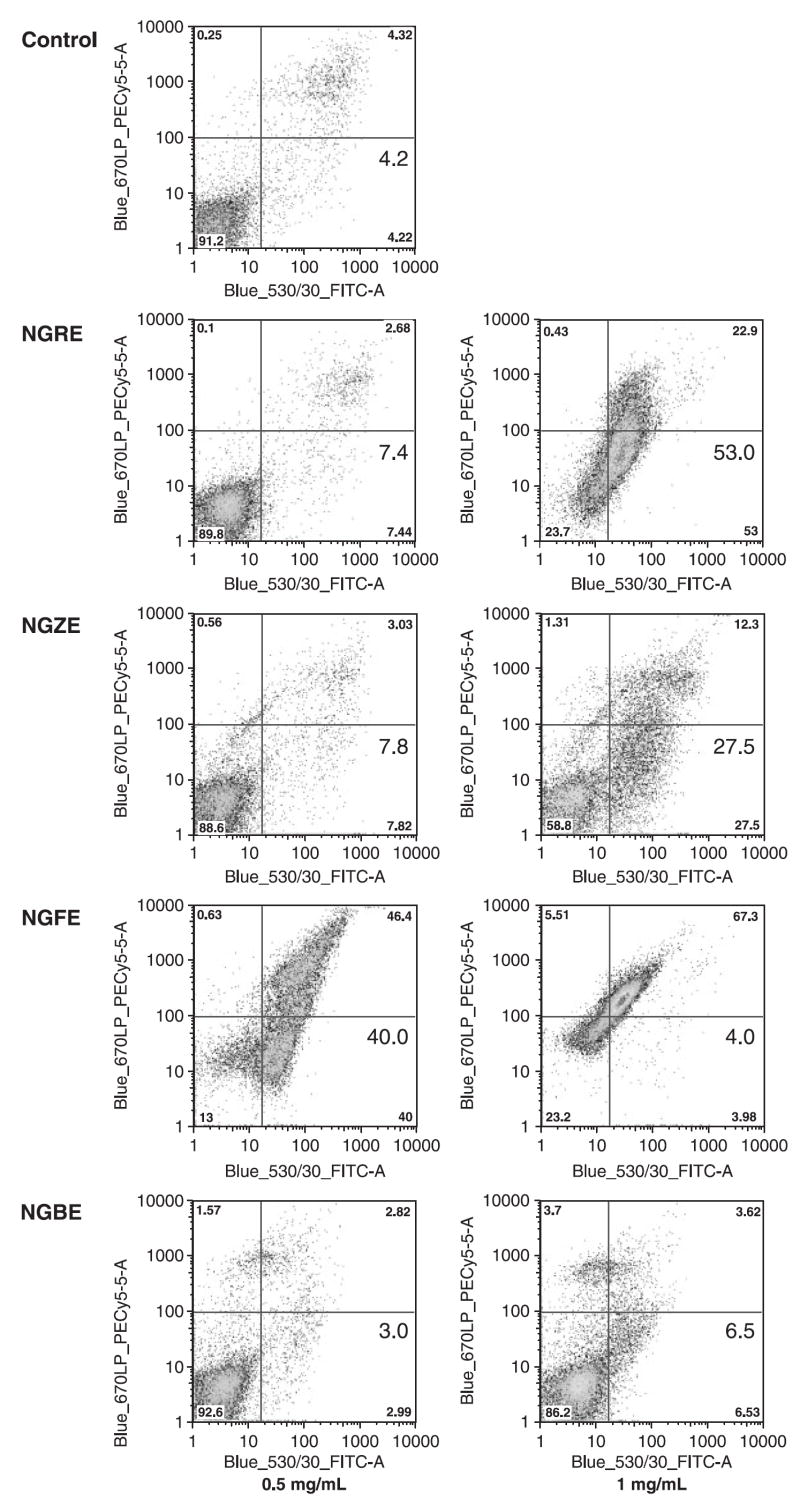
Apoptosis assay using flow cytometry after annexin V-FITC/propidium iodide staining. SW480 cells were treated with 0.5 and 1 mg/mL of NGRE, NGZE, NGFE and NGBE for 48 h, while untreated cells were as the control. Early apoptotic cells were positive for annexin V and negative for PI (lower right quadrant). The percentage of early apoptotic cells was shown.
The main constituents in notoginseng are ginsenosides, in which ginsenosides Rb1, Rb3 and Rg1 are the major compounds in different plant parts of notoginseng. The three ginsenosides were evaluated for the induction of apoptosis in the SW480 cells. Cells were treated with 300 μm of ginsenosides for 48 h, the proportion of early apoptotic cells was 7.97% for Rb1, 6.41% for Rb3 and 5.33% for Rg1, and 3.64% for control. Although late apoptotic cells did not increase after being treated with ginsenosides, the proportion of early apoptotic cells, which are representative of real apoptosis, increased significantly.
DISCUSSION
Panax notoginseng belongs to the genus Panax L., which is a small genus of the family Araliaceae. Nearly all species in this genus are important herbal medicines, such as Asian ginseng and American ginseng. Up to now, anticancer studies of ginseng plants were focused on Asian ginseng and American ginseng (Helms, 2004; Wang et al., 2006b), while only few reports involved the anticancer activities of notoginseng (Wang et al., 2007). Although the main constituents in notoginseng are dammarane saponins, similar to those in Asian ginseng and American ginseng, the content and proportion of saponins in notoginseng are distinct (Washida and Kitanaka, 2003). Quantitative determination of herbal medicines is important to determine their safety and efficacy (Khan, 2006). Some analytical studies have been conducted for the determination of the saponins of notoginseng (Wang et al., 2006a). However, almost all studies have focused on the root extract. In addition to notoginseng root extract, this study also analysed the saponin composition of notoginseng rhizome, flower and berry extracts. Compared with the root extract, the chemical composition of notoginseng flower and berry extracts have special aspects. In notoginseng root, the main constituents were notoginsenoside R1, ginsenoside Rb1, Re and Rg1. However, for the flower and berry extract, the main constituents were ginsenoside Rb3 and Rc, while notoginsenoside R1, ginsenoside Re and Rg1 were present only in limited amounts. For the notoginseng rhizome extract, the saponin composition was similar to that of notoginseng root extract and the content of major ginsenosides was lower than in the root extract. The content of notoginsenoside R1 was higher in the rhizome extract than in the root extract (Fig. 2).
Recently, antitumor effects of notoginseng were observed in sarcoma and prostate cancer cells, but the responsible compounds have not been identified (Chen et al., 2001; Chung et al., 2004). This study observed the tumoricidal activity of different portions of notoginseng and their active compounds on SW480 human colorectal cancer cells. Interestingly, a very significant antiproliferative effect from the notoginseng flower extract was shown in the cells. Since the antiproliferative activity of Rb3 was not strong enough, the potent activity of NGFE may be related to some undetermined compounds or metabolites. The study also evaluated the effect of the extracts on the incorporation of 3H-thymidine in SW480 cells by using a 3H-thymidine labeling assay. 3H-thymidine incorporation is related but not equal to cell proliferation. Thymidine assay reflects the DNA synthesis in these cancer cells. The data suggest that the notoginseng extracts directly inhibit the synthesis of DNA in the SW480 cells.
Although the anticancer mechanisms of ginseng have been studied, to date, no mechanistic studies have been reported using notoginseng on the inhibition of cancer cell growth. Other studies found that the selected single compounds from notoginseng, (S)-25-methoxyl-dammarane-3beta, 12beta, 20-triol [20(S)-25-OCH3-PPD] and ginsenoside Rd, can arrest cancer cells in the G1 phase (Zhao et al., 2007) or the G2/M phase (Yang et al., 2006). However, the concentrations of the two compounds in notoginseng extract are relatively low. As suggested by our data from cell cycle analysis, notoginseng extracts arrested SW480 cells in S phase and G2/M phase.
Cell cycle arrest may trigger the DNA repair machine, leading to apoptosis. Induction of apoptosis in tumor cells is considered an important approach for treatment of cancer (Reed and Pellecchia, 2005; Wu et al., 2006). In this study, notoginseng extracts and the main constituents in notoginseng inhibited the proliferation of SW480 human colorectal cancer cells by inducing apoptosis. The ratio of apoptotic cells detected by flow cytometry after annexin V/PI staining were observed to have increased significantly by the treatment of notoginseng root and flower extracts and ginsenosides Rb1, Rb3 and Rg1, suggesting that apoptosis plays an important role in the chemoprevention of notoginseng extracts on SW480 cells.
Colon cancer is a significant public health problem in the Western world (Hawk and Levin, 2005). Botanicals have recently gained more attention for colon cancer management. Herbal medicines could potentially have several anticancer compounds that may be used alone or as adjuncts to existing chemotherapy to improve efficacy and reduce the drug-induced toxicity (Sadeghi and Yazdanparast, 2005; Zhang et al., 2007). In this study, the chemical composition of notoginseng extracts were analysed by HPLC and the analytical results can be a reference for the future studies. Using a cell counting method, the antiproliferative effects of the extracts from different plant parts of notoginseng were evaluated. It was found that the flower extract had the most potent activity. Data from this preclinical study suggested that the antiproliferative activity of notoginseng extract is most probably linked to cell cycle arrest and the induction of cell apoptosis.
Acknowledgments
This work was supported in part by grants from the NIH/NCCAM AT004418 and AT003255 (to C.S.Y.), from the American Cancer Society and Leukemia Society Scholar and a Fletcher Scholar of the Cancer Research Foundation (to W.D.) and from the NIH/NCI CA106569 and American Cancer Society RSG-05-254-01DDC (to T.C.H.).
Contract/grant sponsor: NIH/NCCAM; contract/grant number: AT004418; AT003255.
Contract/grant sponsor: Leukemia Society; Cancer Research Foundation; NIH/NCI; contract/grant number: CA106569.
Contract/grant sponsor: American Cancer Society; contract/grant number: RSG-05-254-01DDC.
References
- Chen FD, Wu MC, Wang HE, et al. Sensitization of a tumor, but not normal tissue, to the cytotoxic effect of ionizing radiation using Panax notoginseng extract. Am J Chin Med. 2001;29:517–524. doi: 10.1142/S0192415X0100054X. [DOI] [PubMed] [Google Scholar]
- Chen ZH, Wang DC, Li HL, Wei JX, Wang JF, Du YC. Hemodynamic effects of san chi (Panax notoginseng) root, leaf, flower and saponins on anesthetized dogs. Yao Xue Xue Bao. 1983;18:818–822. [PubMed] [Google Scholar]
- Chung VQ, Tattersall M, Cheung HT. Interactions of a herbal combination that inhibits growth of prostate cancer cells. Cancer Chemother Pharmacol. 2004;53:384–390. doi: 10.1007/s00280-003-0746-1. [DOI] [PubMed] [Google Scholar]
- Gieni RS, Li Y, HayGlass KT. Comparison of [3H]thymidine incorporation with MTT-and MTS-based bioassays for human and murine IL-2 and IL-4 analysis. Tetrazolium assays provide markedly enhanced sensitivity. J Immunol Methods. 1995;187:85–93. doi: 10.1016/0022-1759(95)00170-f. [DOI] [PubMed] [Google Scholar]
- Hawk ET, Levin B. Colorectal cancer prevention. J Clin Oncol. 2005;23:378–391. doi: 10.1200/JCO.2005.08.097. [DOI] [PubMed] [Google Scholar]
- Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004;9:259–274. [PubMed] [Google Scholar]
- Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- Khan IA. Issues related to botanicals. Life Sci. 2006;78:2033–2038. doi: 10.1016/j.lfs.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Konoshima T, Takasaki M, Tokuda H. Anti-carcinogenic activity of the roots of Panax notoginseng. II. Biol Pharm Bull. 1999;22:1150–1152. doi: 10.1248/bpb.22.1150. [DOI] [PubMed] [Google Scholar]
- Martin AR, Carides AD, Pearson JD, et al. Functional relevance of antiemetic control. Experience using the FLIE questionnaire in a randomised study of the NK-1 antagonist aprepitant. Eur J Cancer. 2003;39:1395–1401. doi: 10.1016/s0959-8049(03)00299-5. [DOI] [PubMed] [Google Scholar]
- Ng TB. Pharmacological activity of sanchi ginseng (Panax notoginseng) J Pharm Pharmacol. 2006;58:1007–1019. doi: 10.1211/jpp.58.8.0001. [DOI] [PubMed] [Google Scholar]
- Reed JC, Pellecchia M. Apoptosis-based therapies for hematologic malignancies. Blood. 2005;106:408–418. doi: 10.1182/blood-2004-07-2761. [DOI] [PubMed] [Google Scholar]
- Sadeghi H, Yazdanparast R. Isolation and structure elucidation of a new potent anti-neoplastic diterpene from Dendrostellera lessertii. Am J Chin Med. 2005;33:831–837. doi: 10.1142/S0192415X05003387. [DOI] [PubMed] [Google Scholar]
- Schnell FM. Chemotherapy-induced nausea and vomiting: the importance of acute antiemetic control. Oncologist. 2003;8:187–198. doi: 10.1634/theoncologist.8-2-187. [DOI] [PubMed] [Google Scholar]
- Thiis-Evensen E, Hoff GS, Sauar J, Langmark F, Majak BM, Vatn MH. Population-based surveillance by colonoscopy: effect on the incidence of colorectal cancer. Telemark Polyp Study I. Scand J Gastroenterol. 1999;34:414–420. doi: 10.1080/003655299750026443. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Basila D, Aung HH, et al. Effects of Ganoderma lucidum extract on chemotherapy-induced nausea and vomiting in a rat model. Am J Chin Med. 2005;33:807–815. doi: 10.1142/S0192415X05003429. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Luo X, Zhang B, et al. Notoginseng enhances anti-cancer effect of 5-fluorouracil on human colorectal cancer cells. Cancer Chemother Pharmacol. 2007;60:69–79. doi: 10.1007/s00280-006-0350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, McEntee E, Wicks S, Wu JA, Yuan CS. Phytochemical and analytical studies of Panax notoginseng (Burk.) FH Chen. J Nat Med. 2006a;60:97–106. [Google Scholar]
- Wang CZ, Zhang B, Song WX, et al. Steamed American ginseng berry: ginsenoside analyses and anticancer activities. J Agric Food Chem. 2006b;54:9936–9942. doi: 10.1021/jf062467k. [DOI] [PubMed] [Google Scholar]
- Washida D, Kitanaka S. Determination of polyacetylenes and ginsenosides in Panax species using high performance liquid chromatography. Chem Pharm Bull (Tokyo) 2003;51:1314–1317. doi: 10.1248/cpb.51.1314. [DOI] [PubMed] [Google Scholar]
- Wei F, Zou S, Young A, Dubner R, Ren K. Effects of four herbal extracts on adjuvant-induced inflammation and hyperalgesia in rats. J Altern Complement Med. 1999;5:429–436. doi: 10.1089/acm.1999.5.429. [DOI] [PubMed] [Google Scholar]
- Wu WY, Guo HZ, Qu GQ, Han J, Guo DA. Mechanisms of pseudolaric acid B-induced apoptosis in bel-7402 cell lines. Am J Chin Med. 2006;34:887–899. doi: 10.1142/S0192415X06004363. [DOI] [PubMed] [Google Scholar]
- Xie JT, Wang CZ, Wicks S, et al. Ganoderma lucidum extract inhibits proliferation of SW 480 human colorectal cancer cells. Exp Oncol. 2006;28:25–29. [PubMed] [Google Scholar]
- Yang ZG, Sun HX, Ye YP. Ginsenoside Rd from Panax notoginseng is cytotoxic towards HeLa cancer cells and induces apoptosis. Chem Biodivers. 2006;3:187–197. doi: 10.1002/cbdv.200690022. [DOI] [PubMed] [Google Scholar]
- Zhang M, Liu X, Li J, He L, Tripathy D. Chinese medicinal herbs to treat the side-effects of chemotherapy in breast cancer patients. Cochrane Database Syst Rev. 2007:CD004921. doi: 10.1002/14651858.CD004921.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Wang W, Han L, et al. Isolation, structural determination, and evaluation of the biological activity of 20(S)-25-methoxyl-dammarane-3beta, 12beta, 20-triol [20(S)-25-OCH3-PPD], a novel natural product from Panax notoginseng. Med Chem. 2007;3:51–60. doi: 10.2174/157340607779317508. [DOI] [PubMed] [Google Scholar]



