Abstract
Objective
Gene transfer to hematopoietic stem cells has recently been demonstrated to benefit a small number of patients in whom a selective advantage is conferred upon genetically modified cells; however, in disorders where no such selective advantage is conferred, conditioning appears necessary to allow adequate engraftment. To decrease the toxicity profile, we sought to develop nonmyeloablative conditioning regimens and in this work, explored the use of intravenous busulfan in a large animal model.
Methods
Busulfan pharmacokinetics and toxicity were monitored in young rhesus macaques at two dosing levels (4 and 6 mg/kg). These doses were then employed to condition two animals at each dose level prior to autologous transplantation of genetically modified cells using our standard methods.
Results
Busulfan pharmacokinetic (PK) data showed the area under the curve (AUC), drug half-life, and drug clearance were consistent within each dose group and similar to those reported in children. Single doses of busulfan were well tolerated and produced dose-dependent myelosuppression, most notably in the neutrophil and platelet counts. Although marking levels reached over 1% early in one animal, the long-term marking was low but detectable at 0.01 to 0.001%.
Conclusions
We conclude that low-dose intravenous bolus infusion of busulfan is well tolerated, has dose-dependent effects on peripheral blood counts, and allows long-term engraftment of genetically modified cells, but at levels too low for most clinical disorders.
Introduction
In early clinical transplantation studies, the infusion of retrovirally transduced autologous CD34+ cells without host conditioning resulted in engraftment levels too low to provide clinical benefit [1–3]. Later, significant hematopoietic reconstitution and clinical success were obtained in a clinical trial in children with X-linked severe combined immune deficiency (X-SCID) without conditioning [4]. Several factors may have contributed to sustained correction of the immunodeficiency [5], including improved transduction methods, high transplant cell doses, the young age of the patients, a host environment uniquely permissive to engraftment, and/or a proliferative advantage imbued upon the transduced cells. Certainly, the fact that nearly all T and NK cells but only few myeloid cells harbored the γc cDNA [5] supports the long held notion that restoration of γc expression confers a strong proliferative advantage, permitting expansion of lymphoid progenitors despite modest engraftment of genetically modified stem cells [6].
These results suggest that in the absence of a strong proliferative advantage at the stem or progenitor cell level, some form of conditioning will be required to enhance engraftment at clinically relevant levels. Indeed, stable moderate-level gene transfer to long-term repopulating cells at 10% or higher can now be reliably achieved with optimized transduction methods, but after myeloablative conditioning in large animals [7–12]. Such levels are not, however, achievable with low-dose irradiation, even when doses are escalated to those approaching myeloablative [13,14]. Furthermore, sensitivity to irradiation follows a steep dose-response curve [15].
Based upon these observations, we sought to explore busulfan as an alternative to irradiation. Busulfan is an alkylating chemotherapeutic agent that was initially developed for use in chronic myelogenous leukemia because of its suppressive effect on peripheral granulocyte counts [16,17]. Busulfan was subsequently found to exert broad myelosuppressive effects [18]; high doses produce myeloablation and repeated doses deplete bone marrow precursors [19,20]. Busulfan soon found application as an alternative to total-body irradiation to “create space” in conjunction with cyclophosphamide for allogeneic bone marrow transplantation [21]. Though widely employed, erratic absorption of the oral busulfan formulation necessitated close pharmacokinetic monitoring to achieve predictable myelosuppression [22]. More recently, lower doses of busulfan have been employed in a number of nonmyeloablative conditioning regimens, yet the relevancy of these approaches to autologous stem cell gene therapy is more difficult to infer as other agents are often included in these regimens and graft-vs-host along with host-vs-graft mechanisms significantly affect the degree of donor engraftment achieved.
We reasoned that the recent availability of an intravenous formulation of busulfan would overcome the limitations of the oral formulation. The nonhuman primate model is useful for the optimization of gene transfer techniques, yet little is known regarding the effects of busulfan in this model. We therefore tested 4 and 6 mg/kg of busulfan in the rhesus macaques, to determine the safety of intravenous bolus infusion, the pharmacokinetics, and the hematologic toxicity. Additionally, we explored low-dose busulfan as a sole conditioning agent for the transplantation of genetically modified cells in the rhesus competitive repopulation model.
Materials and methods
Rhesus handling
For all experiments, young rhesus macaques (2- to 4-year-old) were housed and handled in accordance with the guidelines set by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council. The protocol was approved by the Animal Care and Use Committee of the National Heart, Lung, and Blood Institute.
Busulfan pharmacokinetics
Busulfan liquid (Orphan Medical, Minneapolis, Minnesota, USA), supplied in vials of 60 mg at 10 mg/mL, was resuspended at a concentration of 6 mg/mL in 0.9% NaCl for a total dose of 4 mg/kg in the animals 1 and 2 (RQ 2633, RC 902) and 6 mg/kg in animals 3 and 4 (RQ 2929, RQ 2937) and infused over 5 minutes. Blood samples were obtained just prior to and 1/2, 1, 2, 3, 4, and 6 hours after the infusion, centrifuged at 2000 RPM, and plasma collected at room temperature. Plasma busulfan levels were assayed using an HPLC method in the laboratory of Dr. James Ritchie, Emory University. The pharmacokinetic parameters were calculated using the WINNONLIN program (which uses a linear trapezoidal rule). To assess for hematopoietic toxicity, peripheral blood counts were obtained at regular time points pre and post busulfan infusion.
Peripheral blood progenitor cell harvesting, transduction, and transplantation
Animals 5, 6, 7, and 8 were treated with recombinant human stem cell factor (rhuSCF, 200 ug/kg, Amgen, Thousand Oaks, CA, USA) and colony-stimulating factor (rhuG-CSF, 10 ug/kg, Amgen) as daily subcutaneous injections for 5 days to collect cells to be infused back into the same animals. The mobilized hematopoietic stem cells were harvested by apheresis on day 5. Mononuclear cells were isolated by density-gradient centrifugation with lymphocyte separation media (LSM, Organon Teknika, Durham, NC, USA). CD34 enrichment was performed using Miltenyi magnetic bead selection (Miltenyi, Auburn, CA, USA). For comparison within each animal, CD34+cells were split equally for transduction with either G1NA, a vector for which expression of the neomycin phosphotransferase is driven by the long terminal repeat (LTR), or PLII, a vector designed to carry but not express the neomycin phosphotransferase by mutation of the ATG start codon to CTG. The use of the nonexpressing PLII vector therefore serves as a negative control in the assessment of potential immune rejection of cells expressing foreign transgenes as previously described [13,23].
Retroviral supernatant was harvested from producer cells G1Na and PLII cultured in DMEM (Mediatech, Herndon, VA, USA) supplemented with 10% fetal calf serum (HyClone, Logan, UT, USA), 4 mM L-glutamine, penicillin (50 mg/mL), and streptomycin (50 mg/mL) at 37°C in 5% CO2. Fresh vector supernatant was passed through a 0.45-um filter (Nalgene Penfield, NY) to remove cellular contamination before transduction. All transductions were carried out on culture flasks coated with fibronectin (Retronectin, Takara, Otsu, Japan), prepared as directed by the manufacturer. CD34+ cells were transduced for 96 hours at a starting concentration of 1 × 105 cells/mL with daily replacement of vector supernatant. The transduction cultures were supplemented with 100 ng/mL of MDGF, 100 ng/mL rhuSCF, and 100 ng/mL rhuFlt-3 ligand (RDI, Flanders, NJ, USA). Busulfan was administered one day prior to the day of transplant as a single intravenous bolus delivered over 5 minutes. Animals 5 and 6 (RQ 2814, RC 907) received 4 mg/kg and animals 7 and 8 (RQ 2915, CF 84) received 6 mg/kg. At the end of transduction, all cells were collected from both transduction cultures using trypsin-EDTA (Life Technologies, GIBCO BRL Grand Island, NY), washed, resuspended in heat-inactivated autologous serum, and infused via peripheral vein into the same animals from which they were originally mobilized and collected.
Colony-forming unit assays and gene marking analyses by real-time PCR
An aliquot of cells pre and post CD34 selection and an aliquot posttransduction from G1Na or PLII (as applicable) were plated in MethoCult H4230 (Stem Cell Technologies, Vancouver, BC, Canada) supplemented with 5 U/mL rhuerythropoietin (Amgen), 10 ng/ml IL-3, 10 ng/mL GM-CSF (Sandoz E. Hanover, NJ), and 100 ng/mL SCF at 37°C in 5% CO2. Twenty-four colonies from each of the posttransduction samples plated were plucked and then analyzed for the presence of the transferred gene (either G1Na or PL-II) by polymerase chain reaction (PCR). Colony DNA was prepared as previously described [24].
Peripheral blood and bone marrow samples were obtained at regular time points postinfusion. Granulocytes and mononuclear cells were isolated by density-gradient centrifugation as previously described [7]. DNA was extracted for PCR analysis using a phenol-chloroform extraction method as previously described [12]. Quantitation of the contribution to engraftment by genetically modified cells was performed by nested PCR for proviral DNA using vector-specific primers. Briefly, an outer round of PCR was performed by conventional PCR with inner PCR performed by real-time PCR with a syber green reporter [13]. Concurrently obtained blood and marrow from nontransplanted controls as well as copy number controls diluted in normal rhesus peripheral blood DNA were run with each PCR reaction.
Results
Busulfan pharmacokinetics
Pharmacokinetic (PK) data were collected after a single bolus infusion of either 4 mg/kg or 6 mg/kg in two monkeys per cohort (animals #1–4). These animals did not receive genetically modified cells. Table 1 shows the achieved maximum concentration (Cmax), the total area under the curve (AUC), drug half-life (t 1/2), and drug clearance along with those reported in children and adults for comparison. The PK data are concordant between the two animals within each dose group. The animals that received 4 mg/kg had a lower Cmax and AUC, shorter half-life, and higher drug clearance compared to animals that received 6 mg/kg.
Table 1.
Pharmacokinetic data of single bolus dose of intravenous busulfan is rhesus macaques
| Animal | Dose | C max (ug/mL) | AUC (uM * min) | t 1/2 (h) | Clearance (mL/min/kg) |
|---|---|---|---|---|---|
| #1 (RQ 2633) | 4 mg/kg | 3.01 | 1182 | 1.47 | 3.85 |
| #2 (RC 902) | 4 mg/kg | 2.70 | 997.8 | 1.18 | 5.40 |
| Average | 2.84 | 1090 | 1.33 | 4.63 | |
| #3 (RQ 2929) | 6 mg/kg | 5.80 | 1992 | 1.6 | 2.85 |
| #4 (RQ 2937) | 6 mg/kg | 4.55 | 2039 | 1.9 | 2.52 |
| Average | 5.18 | 2016 | 1.75 | 2.69 | |
| Prior studies | |||||
| Adults | 3.2 mg/kg (QD)* | 3.92 | 4867 | 2.6 | 2.49 |
| 3.2 mg/kg (QD)** | 3.75 | 5561 | 2.6 | 2.37 | |
| Children | 3.2–4 mg/kg (QID)+ | 883 | 2.2 | 5.0 | |
| 3.2 mg/kg (QID)++ | 1070 | 1.98 | 3.92 | ||
Hematologic effects of busulfan
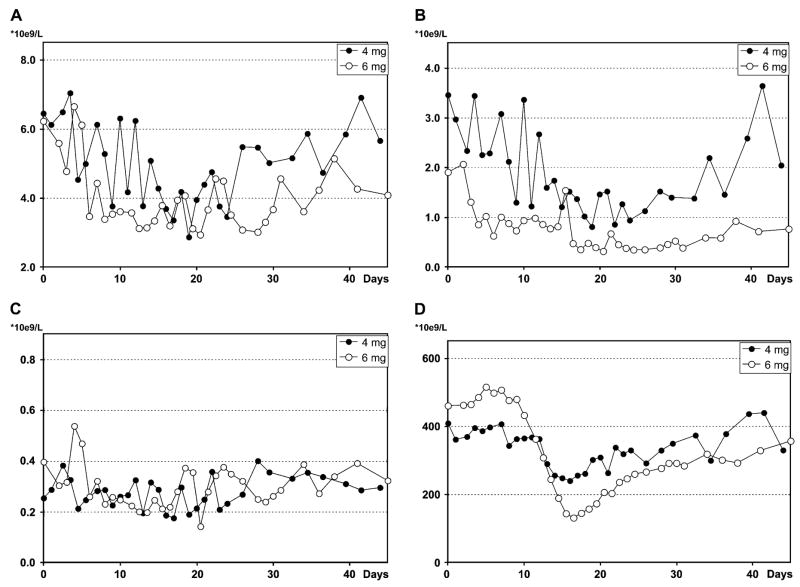
The effects of single-dose intravenous busulfan on blood counts are shown in Figure 1 (A–D) and summarized in Table 2. All animals (#1–4) tolerated the infusions with emesis in only one animal treated at the higher dose. No animal developed mucositis, neurologic symptoms, fevers, or infections, nor did any animal require antibiotics, transfusions, or cytokines. At both dose levels, there was modest suppression of all three cell lineages with notable decreases in the neutrophil and platelet counts. The duration of white blood cell and platelet suppression was significantly longer in the 6 mg/kg group. The total white blood cell counts nadired between days 19 and 22 for both dosing groups, and recovered to baseline by day 40 for the 4 mg/kg group (Fig. 1A) and later than 55 days for the 6 mg/kg group (data not shown). Neutrophil counts for the 4 mg/kg group remained within the normal range with an average nadir of 0.809 × 109/L on day 19 (Fig. 1B). On the other hand, neutrophil counts for the 6 mg/kg group were below 0.76 × 109/L (the lower limit of normal for our animals) for more than 25 days, below 0.5 × 109/L for 13 days with a nadir of 0.317 × 109/L (Fig. 1B), and required more than 55 days to recover to the normal range (data not shown). The lymphocyte counts were largely unaffected at either dose level (Fig. 1C). Total hemoglobin decreased during the first 10 days, and slowly recovered to near normal by day 40 for both dose groups (data not shown). For the 4 and 6 mg/kg groups, platelet counts were below 268 × 109/L (the lower limit of normal for our animals) for 6 and 14 days, and recovered by day 22 and 28, respectively (Fig. 1D).
Figure 1.
Effect of busulfan on total white blood cell count (A), neutrophil count (B), lymphocyte count (C), and platelet count (D). Each data point represents an averaged value from the two animals at each dose (animals 1 and 2 at 4 mg/kg; animals 3 and 4 at 6 mg/kg). These animals did not receive genetically modified cells.
Table 2.
Summary of hematologic effects of busulfan
| Busulfan 4 mg/kg | Busulfan 6 mg/kg | |
|---|---|---|
| Total white blood cells (normal 3.8–12.2 * 109/L) | ||
| Nadir | 2.87 (day 20) | 2.94 (day 20) |
| Total days ≤3.8 | 4 | 16 |
| Days needed to reach >3.8 | 23 | 31 |
| Neutrophils (normal 0.76–6.8 * 109/L) | ||
| Nadir | 0.809 (day 19) | 0.317 (day 21) |
| Total days ≤0.76 | 0 | 25+ |
| Days needed to reach >0.76 | 0 | more than 55 |
| Platelets (normal 268–507 * 109/L) | ||
| Nadir | 240 (day 16) | 132 (day 16) |
| Total days ≤268 | 6 | 14 |
| Days needed to reach >268 | 22 | 28 |
Data are composite from two animals at each dose group. Normal ranges in parentheses are determined from other healthy rhesus macaques at our facility.
In vivo marking using busulfan
Two additional pairs of animals (animals #5–8) were conditioned with either 4 or 6 mg/kg intravenous busulfan followed by transplantation of autologous peripheral blood CD34+ cells split for transduction with two retroviral vectors (G1Na and PLII) carrying a neomycin resistance gene. The characteristics of the transplant grafts are summarized in Table 3. Gene marking levels as measured by real-time PCR in the two groups of animals are shown in Figure 2 (A and B). The apheresis product from each animal was sufficient, ranging from 0.86 to 2.27 × 109 nucleated cells per kilogram. After CD34 selection, CFU were enriched by 143-fold to 400-fold. Transduction efficiency, determined by analyses of individual CFU posttransduction, ranged from 65 to 83% for the 4 mg/kg group and 10 to 50% for 6 mg/kg group. One animal conditioned with busulfan 4 mg/kg (animal 5, RQ 2814) received only cells transduced with G1Na due to technical difficulties with the PLII producer cell line at the time of transduction. The other 3 animals received cells transduced with both G1Na and PL II. The total number of nucleated cells infused was adequate, ranging from 1.62 to 10.2 × 106 cells per kilogram per animal. No animal developed mucositis or neurologic symptoms, or required transfusions or antibiotics.
Table 3.
Graft characteristics
| Transduction efficiencyd |
Posttransductione |
|||||||
|---|---|---|---|---|---|---|---|---|
| Animal | Busulfan dose | Apheresis producta | Column yieldb | Total cells infusedc | G1 Na | PL II | G1 Na | PL II |
| #5 (RQ 2814) | 4 mg/kg | 0.86 | 2.3 | 1.62 | 83% | n/a | 1.62 | n/a |
| #6 (RC 907) | 4 mg/kg | 0.27 | 11.4 | 7.17 | 65% | 71% | 3.86 | 3.41 |
| #7 (RQ 2915) | 6 mg/kg | 1.09 | 7.2 | 9.92 | 20% | 50% | 3.72 | 6.20 |
| #8 (CF 84) | 6 mg/kg | 0.92 | 6.0 | 10.2 | 50% | 10% | 4.86 | 5.33 |
n/a, not applicable.
total number of nucleated cells × 109 per kg.
number of CD34+ cells × 106 per kg obtained after selection.
total number of nucleated cells × 106 per kg infused.
transduction efficiency determined by colony-forming units analyses.
total number of nucleated cells × 106 per kg after 4 days of transduction.
Figure 2.
Percent gene marking in peripheral blood granulocytes in animals conditioned with busulfan at 4 mg/kg (A), in animals 5 and 6; and 6 mg/kg (B), in animals 7 and 8. Each data point represents an averaged value from the two animals for each vector. The lowest level of detection by quantitative real-time PCR is 10−3.
The average level of marking from the G1Na-transduced fraction in the 4 mg/kg group (animals 5 and 6) at 8 to 20 weeks posttransplant reached greater than 3% of peripheral blood granulocytes and mononuclear cells, but subsequently declined to 0.01 to 0.001% by 28 weeks. The average PL-II marking in the 4 mg/kg group was 0.001% for the first 12 weeks, increased to 0.1%, and decreased to 0.001% by 28 weeks. For the 6 mg/kg group (animals 7 and 8), both G1Na and PL II marking approached 1% at 2 weeks, but quickly decreased to and remained near 0.01 to 0.02% by 40 weeks. There was no difference between the contributions of cells transduced with G1Na or PL-II in the 6 mg/kg group.
Discussion
To develop an alternative to irradiation for conditioning in the context of transplantation of genetically modified autologous stem cells, we initiated a pharmacokinetic and toxicity study to evaluate intravenous busulfan in the nonhuman primate model. We first demonstrated that a single intravenous bolus (over 5 minutes) infusion of busulfan at 4 or 6 mg/kg is well tolerated and produces reliable pharmacokinetic (PK) data. In animals treated at the 4 mg/kg level, the mean AUC, drug half-life, and drug clearance are similar to those reported in children receiving busulfan as a part of their conditioning regimen for malignant diseases (summarized in Table 1) [25,26]. While the dosing schedule in our animals (4 mg/kg once daily) differs from that reported in children (0.8 mg/kg every 6 hours for 12–16 doses), similarity in pharmacokinetic data indicates concordance between the young nonhuman primates employed in our study and children. Similar PK data, with the exception of peak plasma concentration, and a low side effect profile was recently documented in children receiving once-daily oral busulfan (4 mg/kg) as part of the preparative regimen for autologous or allogeneic transplantation for acute leukemia [27]. This report and our PK data in rhesus macaques suggest that once-daily intravenous busulfan may be a safe alternative to the current dosing schedule and validate the rhesus macaque as an attractive model for further studies of busulfan conditioning.
On the other hand, adults treated with intravenous busulfan (3.2 mg/kg once daily) [28,29] achieve a higher AUC, lower drug clearance, and longer t 1/2 compared to children or our animals. While AUC greater than 1500 uM/min was initially reported to correlate oral busulfan dosing with the development of hepatic veno-occlusive disease (HVOD) [30], recent reports have demonstrated low incidence of HVOD [31] with intravenous busulfan despite AUC greater than 3000 uM/min [28,29,32]. These findings indicate busulfan given intravenously once daily has low toxicity, and reinforce that the etiology of HVOD is multifactorial, including existing abnormal hepatic function, previous chemotherapy for malignant disease, and concurrent administration of cyclophosphamide or progesterone [32].
In our animals, busulfan at 4 or 6 mg/kg produced transient myelosuppression, most notably in the granulocyte lineage. While there were no fevers or infections, a prolonged period of neutropenia in the 6 mg/kg group, 13 days of less than 0.5 k/uL and more than 55 days of less than 0.76 k/uL (our lower normal range), was observed and is a potential concern when considering dosing greater than 6 mg/kg/day, especially in the context of transplantation for nonmalignant diseases. Additionally, differing degrees of myelosuppression have been reported after treatment with similar doses of intravenous busulfan in preclinical animal models. The lethal dose for mice is 150 mg/kg [33], while in rats it is 35 mg/kg [20]. In dogs, 20 mg/kg delivered as 4 equally divided doses produced myeloablation [34]. These differences emphasize the need for both PK and toxicity monitoring in studies using intravenous busulfan conditioning to allow the comparison of results obtained with respect to drug exposure. Furthermore, while our animals had no profound granulocytopenia or lymphopenia, 4 mg/kg busulfan was recently reported to reduce hematopoietic clones contributing to granulocytes and lymphocytes by as much as 60 to 80% in the first 3 to 4 months [35]. This observation supports that busulfan, even at 4 mg/kg intravenously, produces significant myelosuppression.
When animals were transplanted with genetically modified cells after conditioning with the 4 or 6 mg/kg dosing level, long-term marking, albeit low-level, was observed. PL-II, a neomycin nonexpression vector, and G1Na, a standard neomycin expression vector, were used to compare the in vivo marking after conditioning with low-dose busulfan as an indirect measure of the potential immune rejection of neo-expressing cells. Marking was similar from the neo-expression and nonexpression vector–transduced fractions, suggesting that immune rejection did not play a role in the low-level marking observed following low-intensity conditioning. The low-level engraftment seen is similar to our prior report after low-dose irradiation [13], and contrasts with those obtained after myeloablative conditioning using nearly identical cell doses, conditions, and vectors [8,36–38]. Additionally, we previously demonstrated only transient marking (weeks) in nonconditioned recipients of cells transduced with the neomycin expressing vector, G1Na [23]. These collective observations suggest that the degree of conditioning is an important factor in determining the level of engraftment attainable. While transduction efficiency assessed at the level of CFU pretransplantation was variable among animals, CFU transduction efficiency does not correlate with engraftment, even after myeloablation. Indeed, terminal differentiation of hematopoietic stem cells during the ex vivo culture may have impacted upon their ability to engraft. However, our prior work demonstrates that the current culture conditions employed have proven reliable in attaining high-level, long-term engraftment [8,12,37,39], even after more prolonged ex vivo culture [40].
One alternative strategy to improve engraftment is to use very high doses of hematopoietic stem cells. Indeed, engraftment in murine hosts can be achieved without conditioning [41–44], yet levels approaching those expected to yield clinical benefit require bone marrow doses that are not yet clinically feasible [45,46]. The doses of CD34+ cells collected in the animals in this study ranged from 2.3 to 11.4 × 106 CD34+ cells/kg, and are well within the currently achievable range for human transplantation studies using mobilized peripheral blood [47]. Attempts to expand hematopoietic stem cells have sought to address this limitation, but have not yet produced reliable results. Our own experience with ex vivo–expanded peripheral blood CD34+ cells in the rhesus macaque competitive repopulation model demonstrates at best only maintenance of repopulating ability during prolonged ex vivo expansion [7,48]. Additionally, increasing the stem cell dose to very high levels could theoretically increase the risk of insertional oncogenesis [49].
In children with adenosine deaminase–deficient severe combined immunodeficiency (ADA-SCID), 4 mg/kg of busulfan was administered intravenously in divided doses prior to the infusion of retrovirally transduced hematopoietic stem cells [50]. Though a selective advantage conferred upon T cells likely contributed to the correction of the immunodeficiency, more than 5% of circulating granulocytes also contained the therapeutic vector over the first year in one of the two patients. These results suggest that the marrow of children with ADA-SCID may be more sensitive to the effects of busulfan, or alternatively, that the divided dosing schedule produced greater myelosuppression and allowed the higher level of engraftment. Indeed, a higher degree of myelosuppression was reported, with a neutrophil nadir of 0.150 ×109/L and 0.400 ×109/L reported in the two treated children. Additionally, the high-level contribution to myeloid cells was reported in the patient with the higher degree of myelosuppression. In disorders such as thalassemia and sickle cell anemia that have long been the target of hematopoietic stem cell gene transfer techniques, recent progress in vector development has reinvigorated interest in clinical application [51–55]. Certainly, less than full engraftment by genetically modified cells might be sufficient to correct the phenotype as recipients of allogeneic bone marrow from unaffected siblings have experienced disease amelioration with white blood cell chimerism levels as low as 11% [56]. However, transplantation studies in murine models of sickle cell disease suggest that this apparent advantage favoring erythroid correction reflects enhanced survival of normal red blood cells rather than enhanced engraftment or proliferation of erythroid progenitors [57,58]. Interestingly, red cell chimerism was markedly improved in one study by the administration of busulfan at nonmyeloablative doses [57]. These results again reinforce the concept that some form of conditioning will be required for the severe hemoglobinopathies as for all disorders in which there is no proliferative advantage in the genetically corrected cells.
We therefore conclude that single-bolus infusion of low-dose busulfan is well tolerated in the rhesus macaque model, produces modest myelosuppression, and allows low-level engraftment of genetically modified cells. Importantly, our results demonstrate a high degree of correlation between the rhesus macaque model and humans with respect to both busulfan pharmacokinetics and hematologic toxicity. Busulfan doses of greater than 6 mg/kg/day thus warrant exploration and further dose escalation studies in the nonhuman primate are ongoing in order to determine a drug exposure level that will allow sufficient engraftment of genetically modified cells at an acceptable level of toxicity with the eventual goal of application to transplantation of genetically modified cells in the context of nonmalignant disorders.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Kohn DB, Weinberg KI, Nolta JA, et al. Engraftment of gene-modified umbilical cord blood cells in neonates with adenosine deaminase deficiency. Nat Med. 1995;1:1017. doi: 10.1038/nm1095-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bordignon C, Notarangelo LD, Nobili N, et al. Gene therapy in peripheral blood lymphocytes and bone marrow for ADA-immunodeficient patients. Science. 1995;270:470. doi: 10.1126/science.270.5235.470. [DOI] [PubMed] [Google Scholar]
- 3.Malech HL, Maples PB, Whiting-Theobald N, et al. Prolonged production of NADPH oxidase-corrected granulocytes after gene therapy of chronic granulomatous disease. Proc Natl Acad Sci U S A. 1997;94:12133. doi: 10.1073/pnas.94.22.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease [see comments] Science. 2000;288:669. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 5.Hacein-Bey-Abina S, Le Deist F, Carlier F, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346:1185. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- 6.Kohn DB, Sadelain M, Glorioso JC. Occurrence of leukaemia following gene therapy of X-linked SCID. Nat Rev Cancer. 2003;3:477. doi: 10.1038/nrc1122. [DOI] [PubMed] [Google Scholar]
- 7.Tisdale JF, Hanazono Y, Sellers SE, et al. Ex vivo expansion of genetically marked rhesus peripheral blood progenitor cells results in diminished long-term repopulating ability. Blood. 1998;92:1131. [PubMed] [Google Scholar]
- 8.Wu T, Kim HJ, Sellers S, et al. Prolonged high-level detection of retrovirally marked hematopoietic cells in nonhuman primates after transduction of CD34+ progenitors using clinically feasible methods. Mol Ther. 2000;1:285. doi: 10.1006/mthe.2000.0034. [DOI] [PubMed] [Google Scholar]
- 9.Kiem HP, Andrews RG, Morris J, et al. Improved gene transfer into baboon marrow repopulating cells using recombinant human fibronectin fragment CH-296 in combination with interleukin-6, stem cell factor, FLT-3 ligand, and megakaryocyte growth and development factor. Blood. 1998;92:1878. [PubMed] [Google Scholar]
- 10.Kiem HP, McSweeney PA, Bruno B, et al. Improved gene transfer into canine hematopoietic repopulating cells using CD34-enriched marrow cells in combination with a gibbon ape leukemia virus–pseudotype retroviral vector. Gene Ther. 1999;6:966. doi: 10.1038/sj.gt.3300925. [DOI] [PubMed] [Google Scholar]
- 11.Goerner M, Bruno B, McSweeney PA, Buron G, Storb R, Kiem HP. The use of granulocyte colony-stimulating factor during retroviral transduction on fibronectin fragment CH-296 enhances gene transfer into hematopoietic repopulating cells in dogs. Blood. 1999;94:2287. [PubMed] [Google Scholar]
- 12.Kim HJ, Tisdale JF, Wu T, et al. Many multipotential gene-marked progenitor or stem cell clones contribute to hematopoiesis in nonhuman primates. Blood. 2000;96:1. [PubMed] [Google Scholar]
- 13.Kang EM, Hanazano Y, Frare P, et al. Persistent low-level engraftment of rhesus peripheral blood progenitor cells transduced with the fanconi anemia c gene after conditioning with low-dose irradiation. Mol Ther. 2001;3:911. doi: 10.1006/mthe.2001.0337. [DOI] [PubMed] [Google Scholar]
- 14.Rosenzweig M, MacVittie TJ, Harper D, et al. Efficient and durable gene marking of hematopoietic progenitor cells in nonhuman primates after nonablative conditioning. Blood. 1999;94:2271. [PubMed] [Google Scholar]
- 15.Giri N, Kaushiva A, Wu T, Sellers SE, Tisdale JF. The effects of SCF/G-CSF prestimulation on radiation sensitivity and engraftment in nonmyeloablated murine hosts. Exp Hematol. 2001;29:779. doi: 10.1016/s0301-472x(01)00646-4. [DOI] [PubMed] [Google Scholar]
- 16.Haddow A, Timmis GM. Myleran in chronic myeloid leukaemia; chemical constitution and biological action. Lancet. 1953;264:207. doi: 10.1016/s0140-6736(53)90884-8. [DOI] [PubMed] [Google Scholar]
- 17.Galton DA. Myleran in chronic myeloid leukaemia; results of treatment. Lancet. 1953;264:208. doi: 10.1016/s0140-6736(53)90885-x. [DOI] [PubMed] [Google Scholar]
- 18.Elson LA, Galton DA, Till M. The action of chlorambucil (CB. 1348) and busulphan (myleran) on the haemopoietic organs of the rat. Br J Haematol. 1958;4:355. doi: 10.1111/j.1365-2141.1958.tb06038.x. [DOI] [PubMed] [Google Scholar]
- 19.Morley A, Blake J. An animal model of chronic aplastic marrow failure. I. Late marrow failure after busulfan. Blood. 1974;44:49. [PubMed] [Google Scholar]
- 20.Santos GW, Tutschka PJ. Marrow transplantation in the busulfan-treated rat: preclinical model of aplastic anemia. J Natl Cancer Inst. 1974;53:1781. [PubMed] [Google Scholar]
- 21.Santos GW, Tutschka PJ, Brookmeyer R, et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med. 1983;309:1347. doi: 10.1056/NEJM198312013092202. [DOI] [PubMed] [Google Scholar]
- 22.Santos GW, Tutschka PJ, Brookmeyer R, et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med. 1983;309:1347. doi: 10.1056/NEJM198312013092202. [DOI] [PubMed] [Google Scholar]
- 23.Heim D, Hanazono Y, Giri N, et al. Introduction of a xenogeneic gene via hematopoietic stem cell leads to specific tolerance in a rhesus monkey model. Mol Ther. 2000;1:533. doi: 10.1006/mthe.2000.0072. [DOI] [PubMed] [Google Scholar]
- 24.Cassel A, Cottler-Fox M, Doren S, Dunbar CE. Retroviral-mediated gene transfer into CD34-enriched human peripheral blood stem cells. Exp Hematol. 1993;21:585. [PubMed] [Google Scholar]
- 25.Tran H, Petropoulos D, Worth L, et al. Pharmacokinetics and individualized dose adjustment of intravenous busulfan in children with advanced hematologic malignancies undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2004;10:805. doi: 10.1016/j.bbmt.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Zwaveling J, Bredius RG, Cremers SC, et al. Intravenous busulfan in children prior to stem cell transplantation: study of pharmacokinetics in association with early clinical outcome and toxicity. Bone Marrow Transplant. 2005;35:17. doi: 10.1038/sj.bmt.1704707. [DOI] [PubMed] [Google Scholar]
- 27.Shaw PJ, Scharping CE, Brian RJ, Earl JW. Busulfan pharmacokinetics using a single daily high-dose regimen in children with acute leukemia. Blood. 1994;84:2357. [PubMed] [Google Scholar]
- 28.Russell JA, Tran HT, Quinlan D, et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002;8:468. doi: 10.1053/bbmt.2002.v8.pm12374451. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez HF, Tran HT, Albrecht F, Lennon S, Caldera H, Goodman MS. Evaluation of safety and pharmacokinetics of administering intravenous busulfan in a twice-daily or daily schedule to patients with advanced hematologic malignant disease undergoing stem cell transplantation. Biol Blood Marrow Transplant. 2002;8:486. doi: 10.1053/bbmt.2002.v8.pm12374453. [DOI] [PubMed] [Google Scholar]
- 30.Grochow LB, Jones RJ, Brundrett RB, et al. Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol. 1989;25:55. doi: 10.1007/BF00694339. [DOI] [PubMed] [Google Scholar]
- 31.Kashyap A, Wingard J, Cagnoni P, et al. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant. 2002;8:493. doi: 10.1053/bbmt.2002.v8.pm12374454. [DOI] [PubMed] [Google Scholar]
- 32.Williams CB, Day SD, Reed MD, et al. Dose modification protocol using intravenous busulfan (Busulfex) and cyclophosphamide followed by autologous or allogeneic peripheral blood stem cell transplantation in patients with hematologic malignancies. Biol Blood Marrow Transplant. 2004;10:614. doi: 10.1016/j.bbmt.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Mauch P, Down JD, Warhol M, Hellman S. Recipient preparation for bone marrow transplantation. I. Efficacy of total-body irradiation and busulfan. Transplantation. 1988;46:205. doi: 10.1097/00007890-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Deeg HJ, Schuler US, Shulman H, et al. Myeloablation by intravenous busulfan and hematopoietic reconstitution with autologous marrow in a canine model. Biol Blood Marrow Transplant. 1999;5:316. doi: 10.1016/s1083-8791(99)70007-8. [DOI] [PubMed] [Google Scholar]
- 35.Kuramoto K, Follman D, Hematti P, et al. The impact of low-dose busulfan on clonal dynamics in nonhuman primates. Blood. 2004;104:1273. doi: 10.1182/blood-2003-08-2935. [DOI] [PubMed] [Google Scholar]
- 36.Hematti P, Sellers SE, Agricola BA, Metzger ME, Donahue RE, Dunbar CE. Retroviral transduction efficiency of G-CSF+SCF-mobilized peripheral blood CD34+ cells is superior to G-CSF or G-CSF+Flt3-L-mobilized cells in nonhuman primates. Blood. 2003;101:2199. doi: 10.1182/blood-2002-08-2663. [DOI] [PubMed] [Google Scholar]
- 37.Hematti P, Tuchman S, Larochelle A, Metzger ME, Donahue RE, Tisdale JF. Comparison of retroviral transduction efficiency in CD34+ cells derived from bone marrow versus G-CSF-mobilized or G-CSF plus stem cell factor–mobilized peripheral blood in nonhuman primates. Stem Cells. 2004;22:1062. doi: 10.1634/stemcells.22-6-1062. [DOI] [PubMed] [Google Scholar]
- 38.Kiem HP, Sellers S, Thomasson B, et al. Long-term clinical and molecular follow-up of large animals receiving retrovirally transduced stem and progenitor cells: no progression to clonal hematopoiesis or leukemia. Mol Ther. 2004;9:389. doi: 10.1016/j.ymthe.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Kiem HP, Sellers S, Thomasson B, et al. Long-term clinical and molecular follow-up of large animals receiving retrovirally transduced stem and progenitor cells: no progression to clonal hematopoiesis or leukemia. Mol Ther. 2004;9:389. doi: 10.1016/j.ymthe.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Sellers S, Tisdale J, Agricola B, Kato I, Donahue R, Dunbar C. Genetically-marked rhesus peripheral blood progenitor cells (PBPC) ex vivo expanded in the presence of fibronectin 296 contribute to both short and long term engraftment. Blood. 2000;96:589a. [Google Scholar]
- 41.Micklem HS, Clarke CM, Evans EP, Ford CE. Fate of chromosome-marked mouse bone marrow cells tranfused into normal syngeneic recipients. Transplantation. 1968;6:299. [PubMed] [Google Scholar]
- 42.Brecher G, Ansell JD, Micklem HS, Tjio JH, Cronkite EP. Special proliferative sites are not needed for seeding and proliferation of transfused bone marrow cells in normal syngeneic mice. Proc Natl Acad Sci U S A. 1982;79:5085. doi: 10.1073/pnas.79.16.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saxe DF, Boggs SS, Boggs DR. Transplantation of chromosomally marked syngeneic marrow cells into mice not subjected to hematopoietic stem cell depletion. Exp Hematol. 1984;12:277. [PubMed] [Google Scholar]
- 44.Voralia M, Semeluk A, Wegmann TG. Facilitation of syngeneic stem cell engraftment by anti-class I monoclonal antibody pretreatment of unirradiated recipients. Transplantation. 1987;44:487. doi: 10.1097/00007890-198710000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Brecher G, Ansell JD, Micklem HS, Tjio JH, Cronkite EP. Special proliferative sites are not needed for seeding and proliferation of transfused bone marrow cells in normal syngeneic mice. Proc Natl Acad Sci U S A. 1982;79:5085. doi: 10.1073/pnas.79.16.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stewart FM, Crittenden RB, Lowry PA, Pearson-White S, Quesenberry PJ. Long-term engraftment of normal and post–5-fluorouracil murine marrow into normal nonmyeloablated mice. Blood. 1993;81:2566. [PubMed] [Google Scholar]
- 47.Urbano-Ispizua A, Carreras E, Marin P, et al. Allogeneic transplantation of CD34+ selected cells from peripheral blood from human leukocyte antigen-identical siblings: detrimental effect of a high number of donor CD34+ cells? Blood. 2001;98:2352. doi: 10.1182/blood.v98.8.2352. [DOI] [PubMed] [Google Scholar]
- 48.Sellers SE, Tisdale JF, Agricola BA, Donahue RE, Dunbar CE. The presence of the carboxy-terminal fragment of fibronectin allows maintenance of non-human primate long-term hematopoietic repopulating cells during extended ex vivo culture and transduction. Exp Hematol. 2004;32:163. doi: 10.1016/j.exphem.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 50.Aiuti A, Ficara F, Cattaneo F, Bordignon C, Roncarolo MG. Gene therapy for adenosine deaminase deficiency. Curr Opin Allergy Clin Immunol. 2003;3:461. doi: 10.1097/00130832-200312000-00007. [DOI] [PubMed] [Google Scholar]
- 51.May C, Rivella S, Callegari J, et al. Therapeutic haemoglobin synthesis in β-thalassaemic mice expressing lentivirus-encoded human β-globin. Nature. 2000;406:82. doi: 10.1038/35017565. [DOI] [PubMed] [Google Scholar]
- 52.Pawliuk R, Westerman KA, Fabry ME, et al. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science. 2001;294:2368. doi: 10.1126/science.1065806. [DOI] [PubMed] [Google Scholar]
- 53.Rivella S, May C, Chadburn A, Riviere I, Sadelain M. A novel murine model of Cooley’s anemia and its rescue by lentiviral mediated human β-globin gene transfer. Blood. 2003;101:2932. doi: 10.1182/blood-2002-10-3305. [DOI] [PubMed] [Google Scholar]
- 54.Levasseur DN, Ryan TM, Pawlik KM, Townes TM. Correction of a mouse model of sickle cell disease: lentiviral/antisickling β-globin gene transduction of unmobilized, purified hematopoietic stem cells. Blood. 2003;102:4312. doi: 10.1182/blood-2003-04-1251. [DOI] [PubMed] [Google Scholar]
- 55.Persons DA, Hargrove PW, Allay ER, Hanawa H, Nienhuis AW. The degree of phenotypic correction of murine β-thalassemia intermedia following lentiviral-mediated transfer of a human γ-globin gene is influenced by chromosomal position effects and vector copy number. Blood. 2003;101:2175. doi: 10.1182/blood-2002-07-2211. [DOI] [PubMed] [Google Scholar]
- 56.Walters MC, Patience M, Leisenring W, et al. Stable mixed hematopoietic chimerism after bone marrow transplantation for sickle cell anemia. Biol Blood Marrow Transplant. 2001;7:665. doi: 10.1053/bbmt.2001.v7.pm11787529. [DOI] [PubMed] [Google Scholar]
- 57.Kean LS, Manci EA, Perry J, et al. Chimerism and cure: hematologic and pathologic correction of murine sickle cell disease. Blood. 2003;102:4582. doi: 10.1182/blood-2003-03-0712. [DOI] [PubMed] [Google Scholar]
- 58.Hayashi S, Abdulmalik O, Peranteau WH, et al. Mixed chimerism following in utero hematopoietic stem cell transplantation in murine models of hemoglobinopathy. Exp Hematol. 2003;31:176. doi: 10.1016/s0301-472x(02)01024-x. [DOI] [PubMed] [Google Scholar]