Abstract
Objective
Myeloablative total body irradiation (TBI) in the setting of autologous transplantation of genetically modified hematopoietic stem cells (HSC) is associated with substantial toxicity. Nonmyeloablative doses of TBI are less toxic, but result in low-level engraftment of genetically modified HSCs. As an alternative to TBI, escalating doses of parenteral busulfan were tested for their hematologic toxicity, their ability to promote donor leukocyte engraftment, and the time window for such engraftment.
Materials and Methods
Hematologic toxicity of busulfan was assessed in C57BL6 mice after single nonmyeloablative doses of intraperitoneal busulfan ranging from 1 to 40 mg/kg by serial complete blood counts monitored up to 40 days. The level of donor engraftment attainable after nonmyeloablative busulfan was determined by infusion of 20 million congenic murine bone marrow nucleated cells (BMNC) following 5 to 40 mg/kg of busulfan. To determine the effects of delayed HSC infusions, BMNCs were infused 1, 10, 15, and 20 days after a single dose of 10 mg/kg of busulfan.
Results
Busulfan doses from 1 to 40 mg/kg produced hematologic toxicity that was most pronounced in the 2nd to 3rd week. In transplantation experiments, dose-dependent donor leukocyte engraftment was attained with levels >70% after only 20 mg/kg of busulfan. Similar levels of engraftment were achieved even when infusion of BMNCs was delayed up to 20 days after busulfan injection.
Conclusion
Nonmyeloablative parenteral busulfan produced transient myelosuppressive effects, clinically relevant levels of engraftment, and an extended time window for HSC infusion in murine hosts.
The therapeutic potential of hematopoietic stem cell (HSC) transplantation has been proven in a number of disease settings, providing a rationale for further study in genetic disorders affecting the hematopoietic compartment. Indeed, transfer of the β-globin gene through the HSC compartment by allogeneic bone marrow transplantation has already proven curative in a select group of pediatric patients with sickle cell disease [1], and provides the proof of concept that genetic manipulation of autologous HSCs might be equally therapeutic. Conditioning strategies for allogeneic transplantation have traditionally dually employed myelosuppression for creation of “space” and immunosuppression to prevent graft rejection [2,3]. Procedural toxicities and the relative requirement of a human leukocyte antigen-matched sibling donor limit this approach to a fraction of affected individuals. For those lacking a human leukocyte antigen-matched donor, gene transfer to autologous HSCs is a viable option that is currently in active pre-clinical and clinical development. Several recent gene therapy clinical trials have demonstrated benefit in the immunodeficiencies [4–6], and these successes have resulted in part from improved viral vector design, improved transduction methods, and efficient marrow engraftment, even in the absence of any form of conditioning or immunosuppression and the selective advantage conferred upon the progeny of genetically modified HSCs in the immunodeficient setting. In diseases where no selective advantage is conferred, myelosuppression appears necessary for engraftment of genetically modified HSCs [5,6]. In contrast to allogeneic HSC transplantation (HSCT), our own work suggests that immunosuppression is not required when autologous HSCs carrying a foreign transgene are introduced after myeloablative irradiation [7].
Toxicity associated with myeloablative irradiation is substantial and unacceptable for nonmalignant hematologic disorders, hence nonmyeloablative conditioning strategies remain a goal for gene-therapy applications. Prior murine studies have demonstrated that TBI as low as 100 rads are well-tolerated and sufficient to allow moderate engraftment of genetically modified HSCs [8–10]. However, when applied in primates, TBI at doses ranging from 100 to 500 rads did not produce clinically relevant long-term in vivo engraftment, though long-term persistence at low levels suggests no limitation by a host immune response [11,12]. We therefore sought to identify an alternative to TBI that would allow dose-dependent engraftment along a less-steep dose-response curve.
First used in the treatment of chronic myelogenous leukemia because of its effects on peripheral blood granulocyte counts [13,14], busulfan is an alkylating agent that has been employed as an alternative agent to TBI in hematopoietic stem cell transplantation [15–17]. However, the sole availability of an oral formulation required frequent dosing and was limited by unpredictable absorption that necessitated close pharmacokinetic monitoring. Based upon the clinical availability of an intravenous formulation from which reliable pharmacokinetics can be achieved with once daily rapid infusion with fewer side effects [18–21], we initiated parallel studies in both murine and nonhuman primate models to determine the suitability of this agent for gene therapy applications. In rhesus macaques, pharmacokinetics were similar to that of humans and moderate levels of myelosuppression were seen at two nonmyeloablative dosing levels. Long-term persistence of genetically modified cells at low levels (similar to control cells carrying but not expressing the transgene) suggested no immune clearance of genetically modified cells in recipients pairs receiving low dose (4 and 6 mg/kg) intravenous busulfan as the sole conditioning agent. However, the high cost of the primate model does not permit extensive dose range or timing testing [22,23]. We thus employed the murine congenic transplantation model to test a wide range of doses well below the myeloablative dose of 150 mg/kg in mice [16]. Additionally, as busulfan is primarily toxic against metabolically quiescent cells, resulting in delayed suppression of peripheral blood counts [24,25], we reasoned that heightened engraftment might be achieved at the nadir of blood counts, and performed a delayed infusion time course study to test this hypothesis.
Materials and methods
To assess hematopoietic toxicity, busulfan liquid (ESP Pharma, Edison, NJ, USA) at 1, 5, 10, 15, 20, and 40 mg/kg was injected into 10- to 12-week-old C57BL6 mice (Jackson Laboratory, Bar Harbor, ME, USA) intraperitoneally in cohorts of five mice. Tail-vein blood samples were obtained at 1, 5, 13, 18, 25, 28, and 40 days after busulfan administration. Complete blood counts were obtained from diluted venous blood samples on CellDyne 3500 Analyzer (Abbott Laboratories, Chicago, IL, USA).
To determine the level of engraftment attainable with parenterally administered busulfan, bone marrow were collected from 6- to 8-week-old B6.SJL-Ptprca-Pep3b-/BoyJ donors (CD45.1, Jackson Laboratory) as described previously [10] and 20 million bone marrow nucleated cells (BMNCs) were injected via tail vein into 10- to 12-week-old recipient C57BL6 mice (CD45.2), 1 day after 5 (n = 5), 10 (n = 20), 20 (n = 20), and 40 (n = 5) mg/kg of busulfan. The 40 mg/kg busulfan doses were given in two 20 mg/kg injections 1 day apart. Donor chimerism was assessed by the percentage of CD45.1 lymphocytes and granulocytes in peripheral blood at 4, 8, and 13 weeks posttransplantation after staining with CD45.1 fluorescein isothiocyanate and CD45.2 phycoerythrin-conjugated antibodies (PharMingen, BD Biosciences, San Jose, CA, USA). Stained leukocytes were analyzed on a FACS Calibur using CellQuest software (BD Biosciences).
For time-course studies, bone marrow cells from 6- to 8-week-old donor C57BL6 mice were collected as described above. Twenty million BMNCs were injected at 1, 10, 15, and 20 days after a single intraperitoneal injection of 10 mg/kg busulfan into 10- to 12-week-old recipient mice, B6.SJL-Ptprca-Pep3b-/BoyJ. There were 5 mice per cohort, and the experiment was performed in triplicate (15 mice per group, total 60 mice). Donor chimerism (CD45.2 lymphocytes and granulocytes) was determined as described above. Student’s t-test was used to compare the percentage of donor engraftment at 12 to 13 weeks. There was no viral exposure to murine BMNCs in all the transplantation experiments.
Results
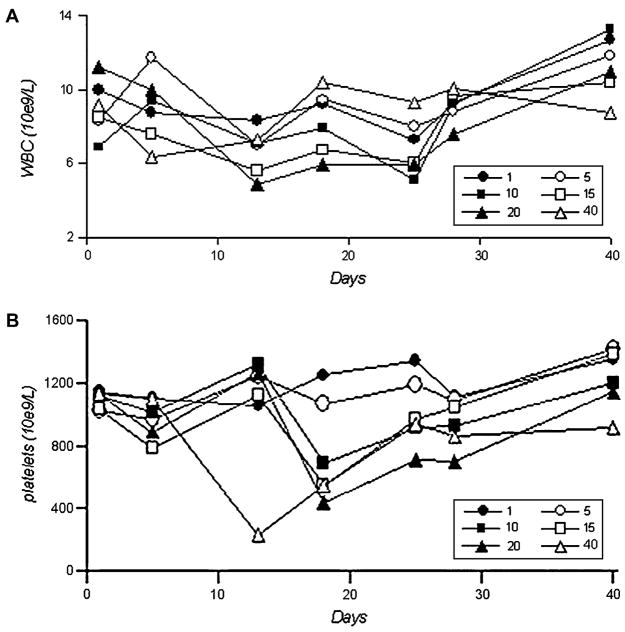
Parenterally administered busulfan produced a transient myelosuppressive effect (Fig. 1A and B). White blood cell (WBC) and platelet counts were the lowest between the 2nd and 3rd week, and recovered by the 4th and 5th week. WBC counts were reduced following all busulfan doses, and rebounded higher than baseline values at recovery. The WBC nadir in the 10 mg/kg cohort was 5.15 k/uL on day 25; in 15 mg/kg cohort, 5.62 k/uL on day 13; in 20 mg/kg cohort, 4.90 k/uL on day 13; and 6.31 k/uL in 40 mg/kg cohort, on day 5. Platelet counts showed little change in the 1 mg/kg and 5 mg/kg groups. The platelet nadir in the 10 mg/kg group was 693 k/uL on day 18; in the 15 mg/kg group, 556 k/uL on day 18; in the 20 mg/kg group, 431 k/uL on day 18; and in the 40 mg/kg group, 233 k/uL on day 13. WBC and platelet count percentages with respect to baseline values are summarized in Table 1.
Figure 1.
Total white blood cell (WBC) (A) and platelet (B) counts after one dose of busulfan at indicated doses (mg/kg). Each data point represents an average of five mice in the same dose cohort.
Table 1.
Percentage of lowest white blood cell and platelet counts with respect to baseline values after one dose of busulfan
| Busulfan dose (mg/kg) | % baseline WBC count | % of baseline platelet count |
|---|---|---|
| 1 | 73.0 | 92.3 |
| 5 | 84.0 | 94.9 |
| 10 | 75.0 | 61.4 |
| 15 | 66.0 | 53.7 |
| 20 | 43.9 | 38.1 |
| 40 | 69.0 | 20.5 |
WBC = white blood cell.
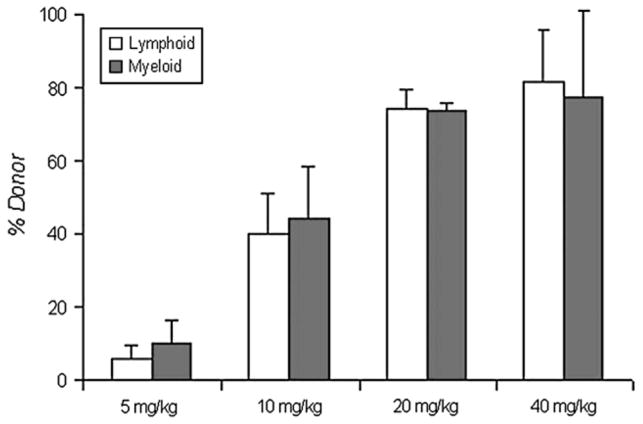
Because busulfan at 1 and 5 mg/kg produced little hematologic effect, doses of 10, 20, and 40 mg/kg (given 1 day prior to stem cell infusion) were used to assess dose-dependent engraftment of donor leukocytes (Fig. 2). At 12 to 13 weeks posttransplantation, respective donor and myeloid engraftments were 5.8% and 9.8% (busulfan 5 mg/kg), 40.1% and 44.3% (10 mg/kg), 74.3% and 73.8% (20 mg/kg), and 81.3% and 77.2% (40 mg/kg). The percent of donor leukocytes was most variable at the 10 mg/kg, ranging from 29.2% to 56.4% in lymphoid cells, and from 32.7% to 80.2% in myeloid cells.
Figure 2.
Percent donor leukocyte engraftment at 12 to 13 weeks post-transplantation. Single doses of 5, 10, 20, and 40 mg/kg of busulfan were used to condition the mice 1 day prior to bone marrow nucleated cell infusions. Numbers of animals were 5, 20, 20, and 5, respectively.
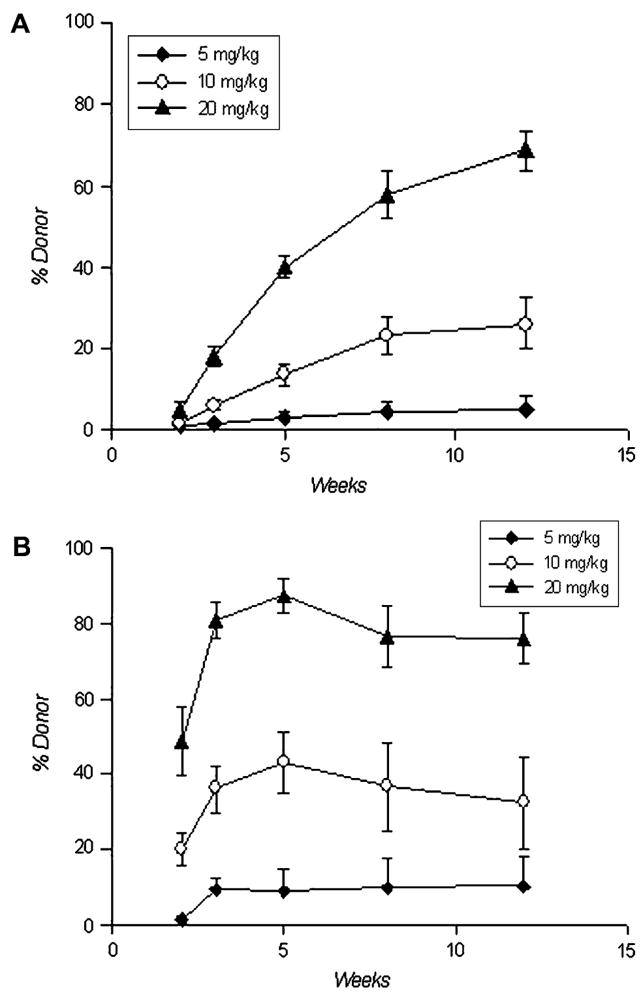
Kinetics of donor leukocyte engraftment were also examined. Lymphoid engraftment was gradual, slowly increased over the monitoring period, and plateaued by 13 weeks (Fig. 3A). In contrast, myeloid engraftment was rapid, reached maximal levels at 3 to 4 weeks, and remained stable through the duration of the monitoring period (Fig. 3B).
Figure 3.
Pattern of lymphoid (A) and myeloid (B) engraftment over time. Five, 10, and 20 mg/kg of busulfan were given 1 day prior to bone marrow nucleated cell infusions. There were 5, 20, and 10 mice in each cohort, respectively.
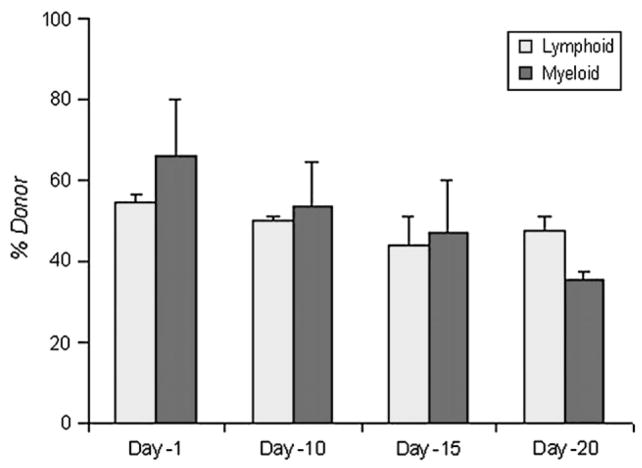
As busulfan produced delayed decreases in the blood counts and, at 10 mg/kg, led to moderate level of donor leukocyte engraftment, this dose was chosen to test whether delaying infusion of HSCs would allow heightened donor leukocyte engraftment. When BMNCs were infused after 1, 10, 15, and 20 days after busulfan was administered, lymphoid engraftment was 54.5%, 49.6%, 44.0%, and 47.5%; myeloid engraftment was 65.8%, 53.5%, 46.9%, 35.6% (Fig. 4, p > 0.05, Student’s t-test comparing day −1 to other time points).
Figure 4.
Percent donor leukocyte chimerism at 13 weeks after transplantation; 10 mg/kg of busulfan were administered at 1, 10, 15, and 20 days before infusion of bone marrow nucleated cells. There were 15 mice per each time cohort. Comparing day −1 to other time points, p > 0.05.
Discussion
TBI has been successfully employed as the core conditioning agent in allogeneic HSCT, providing robust engraftment in the majority of humans, even after nonmyeloablative dosing [26–28]. When employed in preclinical studies testing engraftment of autologous genetically modified HSCs, however, gene marking remained low and a steep dose-response curve complicates nonmyeloablative dosing strategies [10–12]. In addition, a component of the robust engraftment seen in human nonmyeloablative allogeneic HSCT trials derives from a donor alloimmune response not active in the autologous HSCT setting. Busulfan represents an attractive alternative to TBI as a conditioning agent for autologous HSCT applications, and the availability of an intravenous formulation with predictable pharmacokinetics could allow more precise dosing strategies for the desired level of engraftment if a dose-dependent relationship could be established. Parenteral nonablative busulfan proved easy to administer, was well-tolerated, and produced modest transient effects on peripheral blood counts with a nadir in blood counts during the 2nd to 3rd week. Mice treated at doses up to 40 mg/kg did not require hematopoietic support for survival. These results, while similar to those reported previously with oral dosing [24,29], represent a significant advance, given the practical difficulties associated with the enteral formulation in clinical use.
Importantly, busulfan allowed dose-dependent donor leukocyte engraftment in a congenic murine transplantation model. At 5 mg/kg, donor engraftment was <10%; at 10, 20, and 40 mg/kg, donor engraftment increased linearly and was similar to that achieved with the oral formulation [17,30]. The percentage of donor leukocytes varied at 10 mg/kg, suggesting a threshold of adequate myeloid suppression was achieved in some cohorts but not others. Pretreating mice with granulocyte colony-stimulating factor for 1 or 4 days before 10 mg/kg busulfan administration did not lead to higher percentages of donor cells (data not shown). The lack of improvement by granulocyte colony-stimulating factor and the nadir of blood counts occurring at 2 to 3 weeks after dosing support that busulfan primarily targets metabolically quiescent marrow cells, a desirable property for transplantation of genetically modified HSCs. Compared to lower doses, busulfan at 20 or 40 mg/kg led to more consistent engraftment, and appeared to plateau at these two doses as a sole conditioning agent. Busulfan, therefore, provides dose-dependent engraftment with clinically relevant levels achievable at doses well below myeloablative.
The distinct pattern of lymphoid and myeloid donor engraftment is noteworthy and consistent with recent work in murine transplantation studies [31]. These findings reinforce busulfan’s dominant myelosuppressive effect, which is desirable in some disorders as prompt and robust myeloid engraftment of genetically modified autologous HSCs would be of benefit to patients with leukocyte disorders [6,32,33]. Indeed, the predominance of myeloid cells carrying the corrective transgene in a recent clinical gene therapy trial in chronic granulomatous disease attributed to the influence of vector integration sites could also be due in part to the choice of busulfan as the sole conditioning agent [6].
With conventional myeloablative conditioning, HSCs are infused as early as possible to minimize duration of cytopenias in order to reduce transplant-related toxicities. However, with nonmyeloablative conditioning, the degree of cytopenias can be comparatively mild, and with regimens that do not produce significant cytopenias, immediate infusion is not a necessary safety measure. Thus, delaying infusion of HSCs to optimize donor leukocyte engraftment is a reasonable approach, yet no preclinical studies to date have assessed the effect of timing of the conditioning with respect to the infusion. One early study with myeloablative oral busulfan in rats showed improved survival when the infusion was delayed from 4 hours to 24 to 72 hours, but longer delays were not assessed [29].
Our experiments with delayed infusion of BMNCs demonstrated that moderate donor engraftment can be achieved after nonmyeloablative busulfan conditioning over a prolonged window of up to 3 weeks. Although lymphoid engraftment did not differ significantly, myeloid engraftment appeared to decline. Low-dose (10 mg/kg) busulfan led to 50% to 60% myeloid engraftment when given 1 day prior, and decreased to 35% to 40% when given 20 days prior (not statistically significant). These results demonstrate that in disorders where less than complete engraftment is sufficient for disease amelioration [34,35], there is a prolonged window of time for transplantation of HSCs.
Our current work in the congenic murine transplant model, along with our recent studies in nonhuman primates [22], demonstrate that intravenous busulfan exerts predictable pharmacokinetic and hematologic effects, produces dose-dependent engraftment at nonmyeloablative doses, provides an extended window for reliable engraftment of HSCs, and is thus a reasonable alternative to TBI for HSC transplantation.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH at the National Institute of Diabetes, Digestive and Kidney Diseases.
References
- 1.Walters MC, Patience M, Leisenring W, et al. Bone marrow transplantation for sickle cell disease. N Engl J Med. 1996;335:369–376. doi: 10.1056/NEJM199608083350601. [DOI] [PubMed] [Google Scholar]
- 2.Woolfrey AE, Pulsipher MA, Storb R. Non-myeloablative hematopoietic cell transplant for treatment of nonmalignant disorders in children. Int J Hematol. 2002;76(Suppl 2):271–277. doi: 10.1007/BF03165129. [DOI] [PubMed] [Google Scholar]
- 3.Storb RF, Lucarelli G, McSweeney PA, Childs RW. Hematopoietic cell transplantation for benign hematological disorders and solid tumors. Hematology Am Soc Hematol Educ Prog. 2003:372. doi: 10.1182/asheducation-2003.1.372. [DOI] [PubMed] [Google Scholar]
- 4.Hacein-Bey-Abina S, Le Deist F, Carlier F, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346:1185–1193. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- 5.Aiuti A, Slavin S, Aker M, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- 6.Ott MG, Schmidt M, Schwarzwaelder K, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12:401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- 7.Heim DA, Hanazono Y, Giri N, et al. Introduction of a xenogeneic gene via hematopoietic stem cells leads to specific tolerance in a rhesus monkey model. Mol Ther. 2000;1:533–544. doi: 10.1006/mthe.2000.0072. [DOI] [PubMed] [Google Scholar]
- 8.Mardiney M, 3rd, Malech HL. Enhanced engraftment of hematopoietic progenitor cells in mice treated with granulocyte colony-stimulating factor before low-dose irradiation: implications for gene therapy. Blood. 1996;87:4049–4056. [PubMed] [Google Scholar]
- 9.Giri N, Kaushiva A, Wu T, Sellers SE, Tisdale JF. The effects of SCF/G-CSF prestimulation on radiation sensitivity and engraftment in nonmyeloablated murine hosts. Exp Hematol. 2001;29:779–785. doi: 10.1016/s0301-472x(01)00646-4. [DOI] [PubMed] [Google Scholar]
- 10.Kang E, Giri N, Wu T, et al. In vivo persistence of retrovirally transduced murine long-term repopulating cells is not limited by expression of foreign gene products in the fully or minimally myeloablated setting. Hum Gene Ther. 2001;12:1663–1672. doi: 10.1089/10430340152528156. [DOI] [PubMed] [Google Scholar]
- 11.Huhn RD, Tisdale JF, Agricola B, Metzger ME, Donahue RE, Dunbar CE. Retroviral marking and transplantation of rhesus hematopoietic cells by nonmyeloablative conditioning. Hum Gene Ther. 1999;10:1783–1790. doi: 10.1089/10430349950017464. [DOI] [PubMed] [Google Scholar]
- 12.Kang EM, Hanazano Y, Frare P, et al. Persistent low-level engraftment of rhesus peripheral blood progenitor cells transduced with the fanconi anemia C gene after conditioning with low-dose irradiation. Mol Ther. 2001;3:911–919. doi: 10.1006/mthe.2001.0337. [DOI] [PubMed] [Google Scholar]
- 13.Haddow A, Timmis GM. Myleran in chronic myeloid leukaemia; chemical constitution and biological action. Lancet. 1953;264:207–208. doi: 10.1016/s0140-6736(53)90884-8. [DOI] [PubMed] [Google Scholar]
- 14.Galton DA. Myleran in chronic myeloid leukaemia; results of treatment. Lancet. 1953;264:208–213. doi: 10.1016/s0140-6736(53)90885-x. [DOI] [PubMed] [Google Scholar]
- 15.Santos GW, Tutschka PJ, Brookmeyer R, et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med. 1983;309:1347–1353. doi: 10.1056/NEJM198312013092202. [DOI] [PubMed] [Google Scholar]
- 16.Mauch P, Down JD, Warhol M, Hellman S. Recipient preparation for bone marrow transplantation. I. Efficacy of total-body irradiation and busulfan. Transplantation. 1988;46:205–210. doi: 10.1097/00007890-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Yeager AM, Shinn C, Pardoll DM. Lymphoid reconstitution after transplantation of congenic hematopoietic cells in busulfan-treated mice. Blood. 1991;78:3312–3316. [PubMed] [Google Scholar]
- 18.Andersson BS, Madden T, Tran HT, et al. Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclophosphamide as pretransplantation conditioning therapy: a phase I study. Biol Blood Marrow Transplant. 2000;6:548–554. doi: 10.1016/s1083-8791(00)70064-4. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez HF, Tran HT, Albrecht F, Lennon S, Caldera H, Goodman MS. Evaluation of safety and pharmacokinetics of administering intravenous busulfan in a twice-daily or daily schedule to patients with advanced hematologic malignant disease undergoing stem cell transplantation. Biol Blood Marrow Transplant. 2002;8:486–492. doi: 10.1053/bbmt.2002.v8.pm12374453. [DOI] [PubMed] [Google Scholar]
- 20.de Lima M, Couriel D, Thall PF, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 21.Madden T, de Lima M, Thapar N, et al. Pharmacokinetics of once-daily IV busulfan as part of pretransplantation preparative regimens: a comparison with an every 6-hour dosing schedule. Biol Blood Marrow Transplant. 2007;13:56–64. doi: 10.1016/j.bbmt.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 22.Kang EM, Hsieh MM, Metzger M, et al. Busulfan pharmacokinetics, toxicity, and low-dose conditioning for autologous transplantation of genetically modified hematopoietic stem cells in the rhesus macaque model. Exp Hematol. 2006;34:132–139. doi: 10.1016/j.exphem.2005.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahl CA, Tarantal AF, Lee CI, et al. Effects of busulfan dose escalation on engraftment of infant rhesus monkey hematopoietic stem cells after gene marking by a lentiviral vector. Exp Hematol. 2006;34:369–381. doi: 10.1016/j.exphem.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Elson LA, Galton DA, Till M. The action of chlorambucil (CB. 1348) and busulphan (myleran) on the haemopoietic organs of the rat. Br J Haematol. 1958;4:355–374. doi: 10.1111/j.1365-2141.1958.tb06038.x. [DOI] [PubMed] [Google Scholar]
- 25.Dunn CD. The chemical and biological properties of busulphan (“Myleran”) Exp Hematol. 1974;2:101–117. [PubMed] [Google Scholar]
- 26.Mielcarek M, Sandmaier BM, Maloney DG, et al. Nonmyeloablative hematopoietic cell transplantation: status quo and future perspectives. J Clin Immunol. 2002;22:70–74. doi: 10.1023/a:1014532401666. [DOI] [PubMed] [Google Scholar]
- 27.Georges GE, Storb R. Review of “minitransplantation”: nonmyeloablative allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2003;77:3–14. doi: 10.1007/BF02982597. [DOI] [PubMed] [Google Scholar]
- 28.Baron F, Storb R. Allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning as treatment for hematologic malignancies and inherited blood disorders. Mol Ther. 2006;13:26–41. doi: 10.1016/j.ymthe.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Santos GW, Tutschka PJ. Marrow transplantation in the busulfan-treated rat: preclinical model of aplastic anemia. J Natl Cancer Inst. 1974;53:1781–1785. [PubMed] [Google Scholar]
- 30.Adams AB, Durham MM, Kean L, et al. Costimulation blockade, busulfan, and bone marrow promote titratable macrochimerism, induce transplantation tolerance, and correct genetic hemoglobinopathies with minimal myelosuppression. J Immunol. 2001;167:1103–1111. doi: 10.4049/jimmunol.167.2.1103. [DOI] [PubMed] [Google Scholar]
- 31.Anam K, Black AT, Hale DA. Low dose busulfan facilitates chimerism and tolerance in a murine model. Transpl Immunol. 2006;15:199–204. doi: 10.1016/j.trim.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Bauer TR, Jr, Hai M, Tuschong LM, et al. Correction of the disease phenotype in canine leukocyte adhesion deficiency using ex vivo hematopoietic stem cell gene therapy. Blood. 2006;108:3313–3320. doi: 10.1182/blood-2006-03-006908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malech HL, Hickstein DD. Genetics, biology and clinical management of myeloid cell primary immune deficiencies: chronic granulomatous disease and leukocyte adhesion deficiency. Curr Opin Hematol. 2007;14:29–36. doi: 10.1097/00062752-200701000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Persons DA, Tisdale JF. Gene therapy for the hemoglobin disorders. Semin Hematol. 2004;41:279–286. doi: 10.1053/j.seminhematol.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Sadelain M, Boulad F, Galanello R, et al. Therapeutic options for patients with severe beta-thalassemia: the need for globin gene therapy. Hum Gene Ther. 2007;18:1–9. doi: 10.1089/hum.2006.151. [DOI] [PubMed] [Google Scholar]