Abstract
Purpose
Clinicians frequently administer sedation to facilitate mechanical ventilation. The purpose of this study was to examine the relationship between sedation level and patient-ventilator asynchrony.
Materials and Methods
Airway pressure and airflow were recorded for 15 minutes. Patient-ventilator asynchrony was assessed by determining the number of breaths demonstrating ineffective triggering, double triggering, short cycling, and prolonged cycling. Ineffective triggering index (ITI) was calculated by dividing the number of ineffectively triggered breaths by the total number of breaths (triggered and ineffectively triggered). Sedation level was assessed by 3 methods: Richmond Agitation-Sedation Scale (RASS), awake (yes or no), and delirium (CAM-ICU).
Results
Twenty medical intensive care unit patients underwent 35 observations. Ineffective triggering was seen in 17 of 20 patients and was the most frequent asynchrony (88% of all asynchronous breaths), being observed in 9 ± 12% of breaths. Deeper levels of sedation were associated with increasing ITI (Awake: yes 2% versus no 11% p = 0.05; CAM-ICU: coma 15% versus delirium 5% versus no delirium 2%, p < 0.05; RASS: 0, 0% versus −5, 15%, p < 0.05). Diagnosis of chronic obstructive pulmonary disease, sedative type or dose, mechanical ventilation mode, trigger method had no effect on ITI.
Conclusions
Asynchrony is common, and deeper sedation level is a predictor of ineffective triggering.
Keywords: Sedation, mechanical ventilation, asynchrony, patient-ventilator interaction, delirium, Richmond Agitation-Sedation Scale, Confusion Assessement Method for the ICU
Introduction
Patient-ventilator asynchrony is a frequently encountered problem in mechanically ventilated patients. Thille et al. found that 24% of patients experienced asynchrony in at least 10% of their breaths, with ineffective triggering and double triggering being the most common asynchronies.(1) Indeed, ineffective triggering is especially common in patients with chronic obstructive pulmonary disease (COPD) occurring in up to 80% of patients.(2-4) Patient-ventilator asynchrony has also been found to be associated with longer duration of mechanical ventilation and lower rates of successful weaning.(1,5)
A number of mechanisms for poor patient-ventilator interactions have been identified including abnormal respiratory mechanics and ventilator factors.(1,5-7) Clinicians cite facilitation of mechanical ventilation and promotion of patient-ventilator synchrony as among the most common reasons for sedation administration during the course of mechanical ventilation.(8,9) However, little evidence exists to support this practice. In a study of 8 patients undergoing mechanical ventilation, Grasso et al. found increasing sedation depth resulted in incremental decreases in inspiratory muscle effort.(10) Extending these findings, it is possible that increasing sedation depth results in progressively lower maximal inspiratory flow and weaker muscle effort, clinically manifesting as ineffective triggering. However, because these patients appear calm, the asynchrony may not be diagnosed by clinicians and may remain unrecognized. Paradoxically, patient agitation due to asynchrony is often treated with medications that cause respiratory depression which may then lead to ineffective triggering. To our knowledge other investigators have not accounted for sedation when analyzing the patient-ventilator interaction.
We conducted a pilot study to determine the frequency of asynchrony in medical intensive care unit patients and to evaluate the relationship between asynchrony and sedation level. We postulated that deeper levels of sedation were associated with more frequent asynchrony, particularly ineffective triggering.
Methods
Inclusion and Exclusion Criteria
All patients undergoing invasive mechanical ventilation in the medical intensive care unit at Virginia Commonwealth University Medical Center were eligible for study participation unless they met exclusion criteria. Exclusion criteria were age < 18 years, positive end expiratory pressure (PEEP) ≥ 9 cm H2O, partial pressure of oxygen divided by fraction of inspired oxygen < 150, ventilation through a tracheotomy, and inability to initiate breaths (including that due to neuromuscular blocking agents). Presence or absence of asynchronies was not a criteria for study participation, and pressure-time and flow-time waveforms were not reviewed prior to study enrollment. The institution's Human Investigation Review Committee approved the study and written consent was obtained from legally authorized representative. The study was conducted in accordance with the ethical standards of the Virginia Commonwealth University's Office of Research Subject Protection, the Declaration of Helsinki of 1975, as revised in 1983.
Equipment Utilized
All patients underwent mechanical ventilation using a Puritan Bennett 840 (Puritan Bennett, Pleasanton, CA). Patients underwent recording of pressure-time and flow-time waveforms for a period of 15 minutes, and sedation levels were measured to coincide with the end of the monitoring period. Ventilator settings were set by clinicians caring for the patient and were not modified during the 15-minute observation period. Pressure-time and flow-time waveforms were acquired using Cosmo Plus (Novametrix, Wallingford, CT). A sensor was placed between the endotracheal tube and y-tubing of the ventilator circuit. No esophageal balloons were placed as part of this pilot study. The waveforms were downloaded to a laptop computer and analyzed at a later time point. Immediately after recording the waveforms, patients underwent a sedation evaluation.
Assessment of Sedation
Study investigators assessed sedation levels at the end of the observation period. Assessment of sedation level consisted of obtaining a clinical sedation and agitation score (Richmond Agitation-Sedation Scale, RASS), a delirium evaluation (Confusion Assessment Method for the Intensive Care Unit, CAM-ICU) and determination of awake (yes or no, as defined by Kress et al in a study to evaluate the effectiveness of daily interruption of sedation).(11-13) CAM-ICU classifications were absence of delirium, presence of delirium, or coma if patient was too sedated to be able to make a determination of presence or absence of delirium.(12) Awake was defined as a patient being able to perform 3 out of the following 4 commands: [a] open eyes, [b] visually track the investigator, [c] stick out tongue, and [d] squeeze hand.(13)
Patients could undergo multiple 15-minute observations (up to a maximum of 3), and each period was separated by at least 24 hours. Times of study were based on availability of patients and investigators' schedules. Patients could be studied at any time point during the course of their critical illness or weaning. However, patients could not be on a spontaneous breathing trial during an observation period.
Definition of Asynchrony
Criteria for asynchrony were predefined. We used the definitions set forth by Thille et al.(1) Inspiratory time was determined by measuring the time duration from breath initiation to cessation of ventilator flow. Mean inspiratory time was calculated by measuring the inspiratory time of 30 randomly selected machine delivered breaths. Figure 1 details examples of all the types of asynchronies. Ineffective triggering (also called untriggered breath) was defined as a simultaneous decrease in airway pressure and an increase in airflow without assisted cycle. Double triggering was present when 2 cycles were separated by an expiratory time less than half the mean inspiratory time. Short cycle is a cycle in which the inspiratory time is less than half the mean inspiratory time. Prolonged cycle is a cycle in which the inspiratory time is more than twice the mean inspiratory time. The asynchrony index (ASI) is the total number of asynchronies (ineffectively triggered breaths plus double triggered breaths plus short cycled breaths plus prolonged cycled breaths) divided by the number of triggered and ineffectively triggered breaths. The ineffective triggering index (ITI) is the total number of ineffectively triggered breaths divided by the number of triggered and ineffectively triggered breaths.
Figure 1.
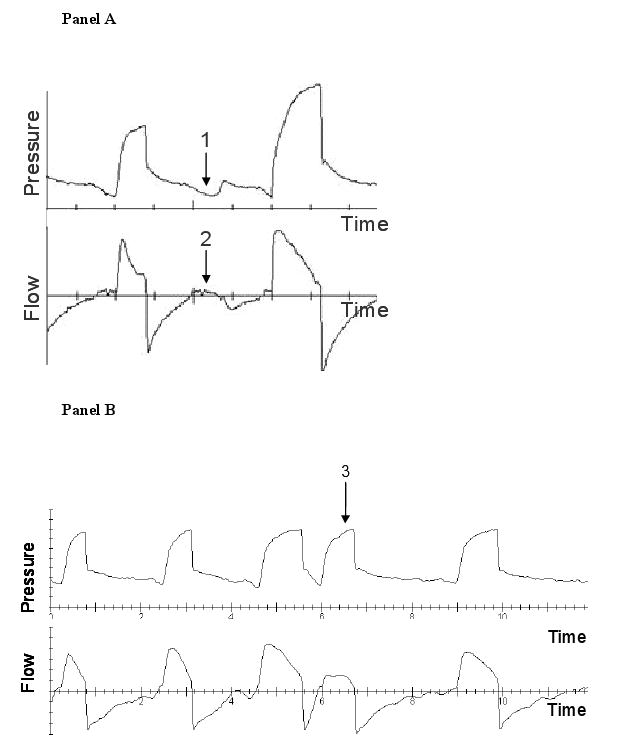
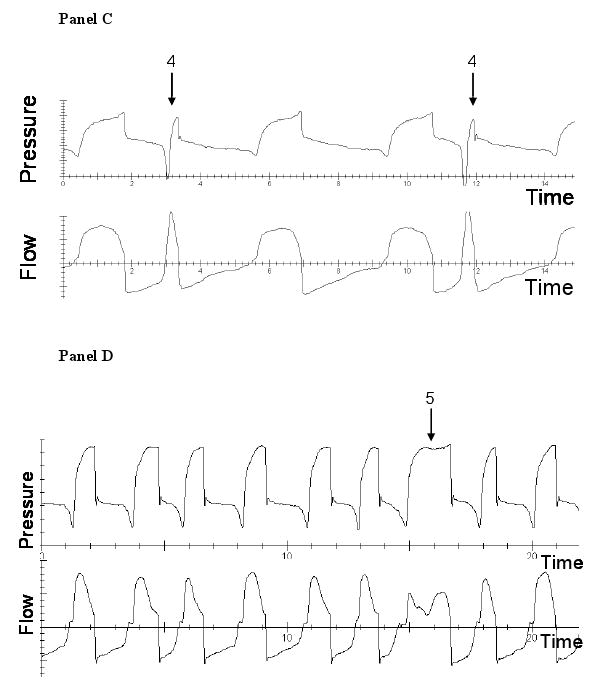
Examples of asynchronies. Panel A demonstrates ineffective triggering which is marked by a decrease in airway pressure (arrow 1) and simultaneous increase in airflow (arrow 2). Panel B demonstrates an example of double triggering (arrow 3). Panel C shows an example of short cycling (arrow 4). Panel D shows an example of prolonged cycling (arrow 5).
Other Variables
Baseline characteristics of age, gender, race, ethnicity, reason for mechanical ventilation, severity of illness as measured by Acute Physiology and Chronic Health Evaluation II (APACHE II) were recorded.(14) History of COPD was ascertained by interviewing the attending physician caring for the patient and reviewing pulmonary function tests, when available. Severity of illness using the Sequential Organ Failure Assessment (SOFA) was ascertained for the 24 hour period prior to asynchrony determination.(15) Total amount of sedation administered in the 24 hour period prior to sedation evaluation was recorded, as was the duration of continuous intravenous infusion. Benzodiazepines were converted to lorazepam equivalents, and narcotics to morphine equivalents using standard conversion formulas.(16) The medications were adjusted for body weight. Ventilator settings of mode, set rate, pressure and/or tidal volume, maximal inspiratory flow rate, cycling sensitivity for pressure support breaths and inspiratory time for pressure control breaths, trigger method and sensitivity, and PEEP were recorded. Peak inspiratory and plateau pressures were recorded. Plateau pressure was measured at the end of 0.5 second inspiratory hold maneuver.
The results of this study were presented in abstract form at the 2005 Annual Congress of the European Society of Intensive Care Medicine.(17)
Statistical Analysis
Mixed model repeated measures ANOVA were used to test the relationship between sedation level (RASS, CAM-ICU and wakefulness) and the proportion of asynchronous breaths. Mixed model repeated measures ANOVA allows for multiple observations per patient. Other variables entered in the model were diagnosis of COPD, arterial partial pressure of carbon dioxide, trigger method (pressure versus flow trigger), mode of mechanical ventilation, peak inspiratory pressure, plateau pressure, amount of pressure support, sedative type (benzodiazepine, narcotic, propofol), dose of sedation, duration of continuous infusion in previous 24 hours, reason for mechanical ventilation, and SOFA.
Normally distributed data are reported as mean ± standard deviation or mean and 95% confidence interval (CI). Non normally distributed data are reported as median and interquartile range (IQR). Alpha was set at 0.05.
Sample Size Calculation
We undertook sample size calculation. Assuming the proportion of asynchrony decreased from 67% at the deepest level of sedation to zero in the awake and cooperative patient, and assuming an odds-ratio of 0.45, at least 35 observations were required to obtain an 80% power.
Results
Twenty medical patients underwent 35 observations. Table 1 lists the demographics of the patients. Five patients had COPD and underwent 9 observations. Mechanical ventilation modes were synchronized intermittent mandatory ventilation with pressure support (19 observations), pressure support alone (15 observations) and pressure control (1 observation). Trigger method was flow triggering on 22 occasions (2.6 ± 0.7 liters/minute) and pressure triggering on 13 occasions (2.1 ± 0.2 cm H2O). PEEP was 5.0 cm H2O, IQR [5.0 – 5.0]. Plateau pressure was 20 ± 7cm H2O. Partial pressure of oxygen divided by fraction of inspired oxygen was 302 ± 103.
Table 1.
Patient Characteristics
| n = 20 | |
|---|---|
| Age (years) (mean ± SD) | 58 ± 16.9 |
| Gender (n men/women) | 12 / 8 |
| Race (n African American/White/Hispanic) | 14 / 6 / 0 |
| COPD (yes) | 5 |
| APACHE II (mean ± SD) | 25 ± 8.8 |
| SOFA (mean ± SD) | 7 ± 3.4 |
| Reason for mechanical ventilation | |
| Pneumonia | 6 |
| Upper airway obstruction | 4 |
| Asthma | 2 |
| Sepsis | 2 |
| Neurologic | 2 |
| Delirium | 1 |
| Pulmonary edema | 1 |
| Hemorrhagic shock | 1 |
| Unclear cause | 1 |
SD: standard deviation
COPD: chronic obstructive pulmonary disease
APACHE II: Acute Physiology and Chronic Health Evaluation II
SOFA: Sequential Organ Failure Assessment
All 20 patients were observed to have at least one asynchronous breath during their evaluation periods, and 31 out of 35 observations had evidence of asynchrony. Asynchrony occurred in 11 ± 14% of breaths. The most common asynchrony was ineffective triggering, accounting for 88.3% of events and occurred in 9 ± 12% of breaths. A total of 17 patients had ineffective triggering, and these patients underwent 26 observations. In 7 of these observations, ITI was 20% or greater.
Short cycling and double triggering were the next most common asynchronies, accounting for 5.8% and 5.6% of events respectively. Short cycling was noted in 12 patients who underwent 13 observations and occurred with a median frequency of 1 breath, IQR [1 – 7]. Similarly, double triggering was noted in 10 patients who underwent 11 observations and occurred in a median of 2 breaths, IQR [1 – 6]. Prolonged cycling accounted for 0.3% of asynchronies and was noted in 5 breaths of 1 patient. Because ineffective triggering occurred with such high frequency compared to the other asynchronies, we limited further analysis to this asynchrony.
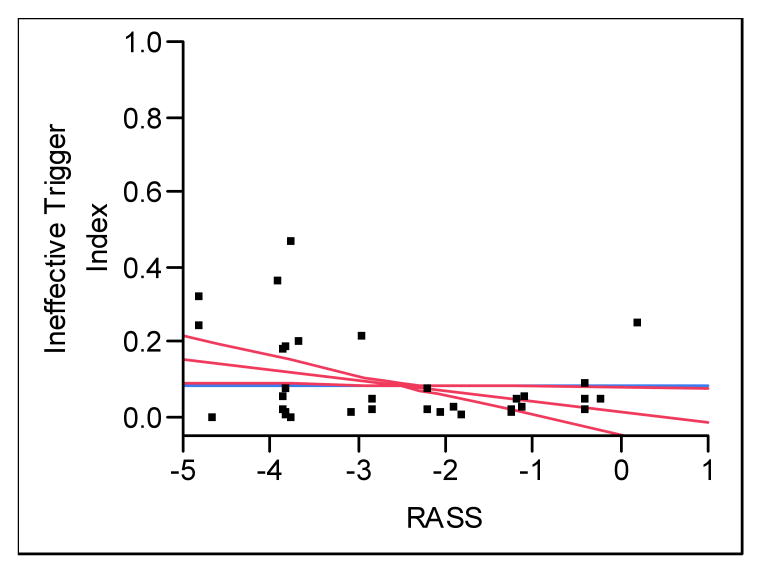
Sedation levels for the patient observations are outlined in Table 2 and are representative of a range of sedation levels observed in our mechanically ventilated patients. The ITI increased with deeper levels of sedation as measured by RASS (p = 0.04); lower RASS scores were associated with more frequent ineffective triggering (Figure 2). On occasions when patients were alert and calm (RASS = 0), ineffective triggering did not occur, but it increased linearly up to 15% of breaths when RASS = -5. For every 1 unit decrease in RASS, ITI increased by 2.7%, 95% CI [0.21 - 5.20]. The proportion of ineffective triggering was significantly higher in patients who were comatose using the CAM-ICU (Table 3, p = 0.03). Among non comatose patients, we found no difference in ITI when comparing delirious and non-delirious states. Occasions when patients were awake had approximately one fifth the number of ineffectively triggered breaths compared to observations where patients were not awake (Table 3, p = 0.04).
Table 2.
Sedation Levels
| n = 35 | |
|---|---|
| Awake (yes/no) | 11 / 24 |
| RASS (mean ± SD) | -2.5 ± 1.7 |
| CAM-ICU (delirium absent/ present/coma | 8 / 14 / 13 |
RASS: Richmond Agitation-Sedation Scale
SD: standard deviation
CAM-ICU: Confusion Assessment Method for the Intensive Care Unit
Figure 2.
The relationship between Richmond Agitation-Sedation Scale (RASS) and ineffective triggering index (ITI). For one unit decrease in RASS, ITI increased by 2.7%, p = 0.04.
Table 3.
Relationship between ineffective triggering index and sedation levels
| ITI | ||||
|---|---|---|---|---|
| Mean | 95% CI | p | ||
| Awake | Yes (n = 11) | 2% | 0-10% | 0.04 |
| No (n = 24) | 11% | 6-17% | ||
| CAM-ICU | Delirium absent (n = 8) | 2% | 0-11% | 0.03 |
| Delirium present (n = 14) | 5% | 1-12% | ||
| Coma (n = 13) | 15% | 8-22% | ||
| RASS | For one unit decrease in RASS, ITI increases by 2.7% | 0.04 | ||
CI: confidence interval
ITI: Ineffective Triggering Index
CAM-ICU: Confusion Assessment Method for the Intensive Care Unit
RASS: Richmond Agitation-Sedation Scale
Diagnosis of COPD, arterial partial pressure of carbon dioxide, trigger method (pressure versus flow trigger), mode of mechanical ventilation, peak inspiratory pressure, plateau pressure, and amount of pressure support did not impact ITI (Table 4). Additionally, sedative dose, duration of continuous infusion had no effect of ITI (Table 5). On 7 occasions (representing 5 patients) no sedatives and no opioids were administered in the 24 hours prior to observation. On 6 occasions (representing 5 patients) only boluses of sedatives or opioids were administered in the 24 hours prior to observation. On 22 occasions (13 patients) continuous infusions of sedatives or opioids were administered in the 24 hours prior to observation. Sedation depth did not predict respiratory rate or mode of mechanical ventilation mode set by clinicians caring for the patients. The reason for mechanical ventilation and SOFA also had no effect on ITI (Table 5).
Table 4.
Relationship between ineffective triggering index and parameters typically associated with ineffective triggering
| F Ratio | p | |
|---|---|---|
| pH | 0.78 | 0.38 |
| COPD | 0.32 | 0.58 |
| MV mode | 1.16 | 0.29 |
| Peak inspiratory pressure | 0.25 | 0.62 |
| Plateau pressure | 0.03 | 0.87 |
| Trigger method | 1.42 | 0.25 |
| Pressure support | 0.00 | 0.98 |
COPD: chronic obstructive pulmonary disease
MV: mechanical ventilation
Table 5.
Relationship between ineffective triggering index and sedatives, opioids, route of administration, severity of illness and diagnosis. The doses and duration of sedative and opioid administration represent the values collected for the 24 hours prior to observations
| F Ratio | p | |
|---|---|---|
| Lorazepam equivalents (weight adjusted) | 3.21 | 0.16 |
| Propofol (weight adjusted) | 2.76 | 0.12 |
| Morphine equivalents (weight adjusted) | 2.12 | 0.18 |
| Duration of continuous infusion | 2.43 | 0.17 |
| SOFA | 0.05 | 0.83 |
| Reason for MV | 1.92 | 0.27 |
SOFA: Sequential Organ Failure Assessment
MV: mechanical ventilation
Discussion
The main finding of our pilot study is that (i) asynchrony is common, with ineffective triggering being the most common asynchrony, and that (ii) sedation level affects patient-ventilator interactions, with deeper sedation level associated with more ineffective triggering. In particular, comatose patients, patients who are not awake and those who are more deeply sedated have significantly higher rates of ineffective triggering compared to non-comatose patients who are awake, alert, and interactive.
Previous studies clearly demonstrate that abnormal pulmonary mechanics and ventilator settings contribute to patient-ventilator asynchrony. Patients with COPD and those with intrinsic PEEP resulting from hyperinflation are more likely to demonstrate ineffective triggering.(1,2,3,4,5,7,18) Although we did not measure intrinsic PEEP in our pilot study, we found no correlation between plateau pressure and ineffective triggering. Our study had relatively few patients with COPD (5 out of 20 patients) while in the study by Chao et al., 44% of 174 patients had COPD and in the study by Leung et al. 8 out of 11 patients had COPD.(5,7) Therefore, we may not have had the power to detect COPD as a marker of ineffective triggering. Ineffective triggering can also result from improper trigger sensitivity. We think this is unlikely in our study because the mean trigger pressure and flow trigger were 2.1 cm H2O and 2.6 liters/minute, respectively. In addition, no correlation between trigger sensitivity and ineffective triggering was detected. Other investigators found that adjusting the ventilator to improve trigger sensitivity did not eliminate ineffective triggering.(5) Finally, it is possible that mode of ventilation may influence asynchrony. Patients having higher set mandatory rates may have less asynchrony because these higher rates may results in less frequent attempts by patients to trigger the ventilator. However, we did not find that asynchrony was dependent on mode of ventilation. It is possible that we did not find a difference because of our small sample size.
We found a high frequency of asynchrony in our medical intensive care unit patients, with ineffective triggering being the most common asynchrony, accounting for 88% of events. Additionally, on 20% of occasions, ineffective triggering occurred in at least 20% of breaths. We found that ineffective triggering was more frequent in more deeply sedated and comatose patients. A stepwise increment in ineffective triggering was noted for successively deeper sedation levels as measured using the RASS. Additionally, non-awake patients had five times as frequent ineffective triggering compared with those who were awake. Using the CAM-ICU, comatose patients had 7.5 times as many ineffective efforts as those without delirium.
Our study has several limitations. First, we studied patients at different time points of critical illness or weaning. It is possible that patients who were ill for a longer time period were more likely to be included in the study compared to patients who underwent mechanical ventilation for a shorter duration. This would possibly introduce a bias toward finding a higher rate of asynchrony. Patients could be studied on multiple occasions (up to a total of 3 occasions). It is conceivable that patients who had more asynchrony underwent more evaluations, and that bias was introduced. We accounted for some patients undergoing multiple observations while other underwent one observation by adjusting our analyses for repeated measures. We also did not measure respiratory drive, and cannot conclude about its relationship to asynchrony. Additionally, we did not measure intrinsic PEEP; instead, we used plateau pressure as a marker of intrinsic PEEP.
The effect of sedation on patient-ventilator asynchrony has not been extensively studied. The studies by Chao et al. and Leung et al. examined patients who were markedly less sedated than our cohort, while Thille et al. did not report sedation depth.(1,5,7) Leung et al.'s patients were “alert, communicative, cooperative and had not received sedation for ≥ 8 hours” while the patients in Chao et al.'s study were alert enough to provide informed consent for study participation.(5,7) The narrow spectrum of sedation ranges precluded these investigators from assessing the relationship between sedation depth and ineffective triggering. In our study, patients were more deeply sedated. Patients were awake during 11 out of 35 observations, did not meet criteria for delirium in 8 out of 35 observations, and had RASS = 0 during 6 out of 35 observations (Table 2).
Ineffective triggering may result from inappropriate ventilator settings (e.g, insufficient trigger sensitivity) or the effect of imposed resistance from the artificial airway. Patient factors also may cause ineffective triggering by two distinct pathophysiologic mechanisms. Ineffective triggering may result from an inability to overcome the effects of intrinsic PEEP: an inability to reduce airway pressure measured extrinsic to the patient or an ability to reverse expiratory recoil and create measurable inspiratory flow.(7) Alternatively, ineffective triggering may result from decreased respiratory drive associated with deeper sedation level, as supported by our study and that of Grasso et al.(10) In our study, dose of sedation administered did not predict ineffective triggering, which may be explained by a wide fluctuation in patient sensitivity to sedatives.(19)
Ineffective triggering represents wasted respiratory work and could contribute to respiratory muscle injury (eccentric or pliometric contractions) or fatigue.(20,21) A failure to appreciate the presence of ineffective respiratory efforts will lead to false conclusions about a patient's respiratory rate and underestimation of rapid shallow breathing index, if this parameter is being used.(5,18) It may also lead to false conclusions about patient tolerance for weaning. For example, upon discontinuation of ventilator support the ventilator recorded rate may appear to rise substantially, falsely suggesting weaning intolerance, as ineffective (unrecorded) efforts become effective efforts.(18)
In summary, we found that ineffective triggering was associated with deeper levels of sedation, which may be the result of lower maximal inspiratory flow. Appreciation of this association will improve the capacity to evaluate the patient-ventilator interaction.
Acknowledgments
Funding: NIH K23 GM068842, NIH M01 RR00065; provided equipment and supplies at no cost.
Footnotes
Conflict of interest: No author has a conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Thille AW, Rodriguez P, Cabello B, et al. Patient-ventilator asynchrony during mechanical ventilation: Prevalence and risk factors. Intensive Care Med. 2006;32:1515–1522. doi: 10.1007/s00134-006-0301-8. [DOI] [PubMed] [Google Scholar]
- 2.Fabry B, Guttmann J, Eberhard L, et al. An analysis of desynchronization between the spontaneously breathing patient and ventilator during inspiratory pressure support. Chest. 1995;107:1387–1394. doi: 10.1378/chest.107.5.1387. [DOI] [PubMed] [Google Scholar]
- 3.Nava S, Bruschi C, Fracchia C, et al. Patient-ventilator interaction and inspiratory effort during pressure support ventilation in patients with different pathologies. Eur Respir J. 1997;10:177–183. doi: 10.1183/09031936.97.10010177. [DOI] [PubMed] [Google Scholar]
- 4.Purro A, Appendini L, De Gaetano A, et al. Physiologic determinants of ventilator dependence in long-term mechanically ventilated patients. Am J Respir Crit Care Med. 2000;161:1115–1123. doi: 10.1164/ajrccm.161.4.9812160. [DOI] [PubMed] [Google Scholar]
- 5.Chao DC, Scheinhorn DJ, Stearn-Hassenpflug M. Patient-ventilator trigger asynchrony in prolonged mechanical ventilation. Chest. 1997;112:1592–1599. doi: 10.1378/chest.112.6.1592. [DOI] [PubMed] [Google Scholar]
- 6.Epstein SK. Optimizing patient-ventilator synchrony. Semin Respir Crit Care Med. 2001;22:137–152. doi: 10.1055/s-2001-13828. [DOI] [PubMed] [Google Scholar]
- 7.Leung P, Jubran A, Tobin MJ. Comparison of assisted ventilator modes on triggering, patient effort, and dyspnea. Am J Respir Crit Care Med. 1997;155:1940–1948. doi: 10.1164/ajrccm.155.6.9196100. [DOI] [PubMed] [Google Scholar]
- 8.Hansen-Flaschen JH, Brazinsky S, Basile C, et al. Use of sedating drugs and neuromuscular blocking agents in patients requiring mechanical ventilation for respiratory failure. A national survey. JAMA. 1991;266:2870–2875. [PubMed] [Google Scholar]
- 9.Rhoney DH, Murry KR. National survey of the use of sedating drugs, neuromuscular blocking agents, and reversal agents in the intensive care unit. J Intensive Care Med. 2003;18:139–145. doi: 10.1177/0885066603251200. [DOI] [PubMed] [Google Scholar]
- 10.Grasso S, Fanelli V, Cafarelli A, et al. Patient-ventilator interactions during psv at different levels of sedation in ALI patients. Intensive Care Med. 2004;30:A36. abstr. [Google Scholar]
- 11.Sessler CN, Gosnell MS, Grap MJ, et al. The richmond agitation-sedation scale: Validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 12.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 13.Kress JP, Pohlman AS, O'Connor MF, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 14.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 15.Vincent JL, Moreno R, Takala J, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure on behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 16.Lacy CF, Armstrong LL, Goldman MP, Lance LL. Lexi-Comp's Drug Information Handbook. Hudson, Ohio: Lexi-Comp; 2004. [Google Scholar]
- 17.de Wit M, Pedram S, Epstein SK. Relationship between patient-ventilator dyssynchrony and sedation level. Intensive Care Med. 2005;31:A752. abstr. [Google Scholar]
- 18.Appendini L, Purro A, Patessio A, et al. Partitioning of inspiratory muscle workload and pressure assistance in ventilator-dependent COPD patients. Am J Respir Crit Care Med. 1996;154:1301–1309. doi: 10.1164/ajrccm.154.5.8912740. [DOI] [PubMed] [Google Scholar]
- 19.Greenblatt DJ, Gan L, Harmatz JS, et al. Pharmocokinetics and pharmacodynamics of single-dose triazolam: Electroencephalography compared with the digit-symbol substitution test. Br J Clin Pharmacol. 2005;60:244–248. doi: 10.1111/j.1365-2125.2005.02409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunter KD, Faulkner JA. Pliometric contraction-induced injury of mouse skeletal muscle: Effect of initial length. J Appl Physiol. 1997;82:278–283. doi: 10.1152/jappl.1997.82.1.278. [DOI] [PubMed] [Google Scholar]
- 21.McCully KK, Faulkner JA. Characteristics of lengthening contractions associated with injury to skeletal muscle fibers. J Appl Physiol. 1986;61:293–299. doi: 10.1152/jappl.1986.61.1.293. [DOI] [PubMed] [Google Scholar]