Abstract
The non-heme iron enzyme phenylalanine hydroxylase, tyrosine hydroxylase, and tryptophan hydroxylase catalyze the hydroxylation of their aromatic amino acid substrates using a tetrahydropterin as the source of electrons. The hydroxylating intermediate is proposed to be an Fe(IV)O species. We report here that all three enzymes will catalyze hydroxylation reactions using H2O2 in place of tetrahydropterin and oxygen, forming tyrosine and 3-hydroxyphenylalanine from phenylalanine, 4-HOCH2-phenylalanine from 4-CH3-phenylalanine, and hydroxycyclohexylalanine from 3-cyclohexylalanine. No peroxide-dependent reaction is seen with active site mutants of TyrH and PheH in which the stability or reactivity of the iron center is compromised. These results provide further support for an Fe(IV)O hydroxylating intermediate.
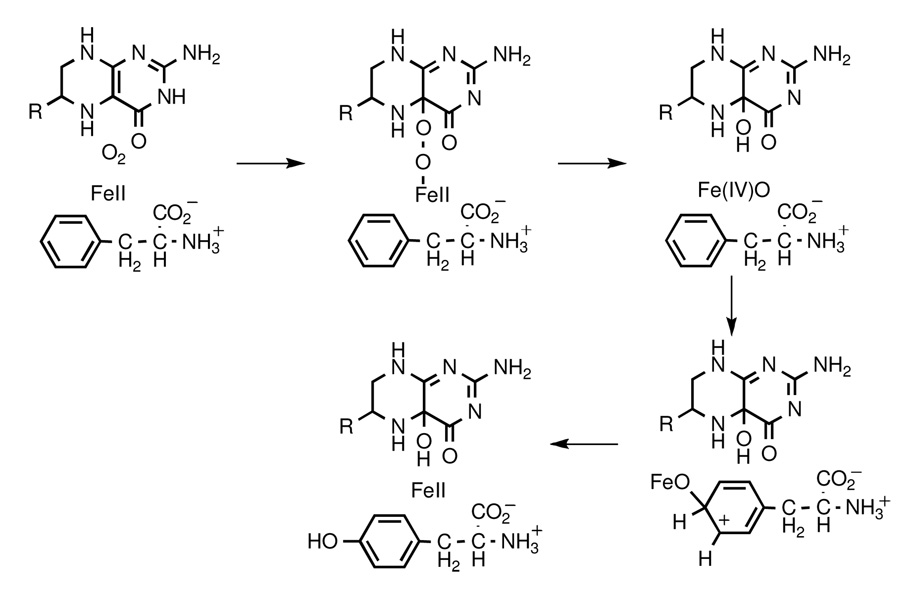
Phenylalanine hydroxylase (PheH), tyrosine hydroxylase (TyrH) and tryptophan hydroxylase (TrpH) are non-heme iron monooxygenases that catalyze the insertion of an oxygen atom from O2 into the aromatic side chain of their corresponding substrates using a tetrahydropterin (PH4) substrate as the reductant.1–3 Their active sites each contain a mononuclear iron coordinated by two histidines and a glutamate,4–6 an arrangement that has been termed a 2-his-1-carboxylate facial triad.7,8 Scheme 1 shows the chemical mechanism proposed for PheH, TyrH, and TrpH.3 The hydroxylating intermediate is an Fe(IV)O capable of aromatic, benzylic, and aliphatic hydroxylation.9,10 This species has recently been detected in TyrH by freeze-quench Mössbauer spectroscopy.11 The spectra and reactivity of the Fe(IV)O intermediate resemble those in members of the α-ketoglutarate-dependent hydroxylase family, which also contain a mononuclear iron coordinated by a 2-his-1-carboxylate facial triad.12,13
Scheme 1.
In Scheme 1 the PH4 supplies two electrons to reduce one atom of O2 to the level of water, but it plays no role in the actual oxygen transfer to the amino acid substrate. This suggests that it could be possible to bypass the PH4 and generate the Fe(IV)O intermediate directly with an alternative oxygen donor. Such a shunt has been possible in the cases of the heme-based cytochrome P450,14 the binuclear non-heme methane monooxygenase,15 and mononuclear non-heme dioxygenases,16,17 but not with a mononuclear non-heme monooxygenase.
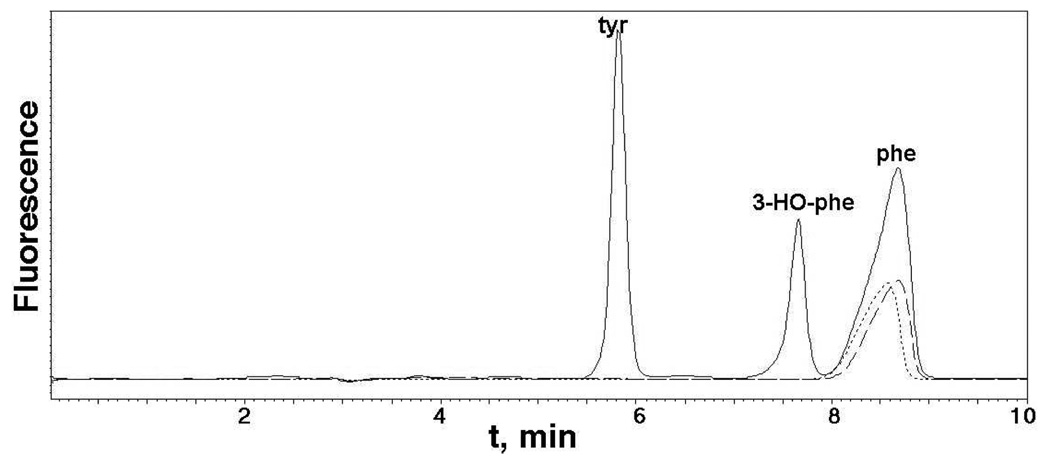
We now report that H2O2 can replace PH4 and O2 to support amino acid hydroxylation by the aromatic amino acid hydroxylases. Incubation of PheH,18,19 TyrH,20 or TrpH19,21 with phenylalanine and H2O2 results in the formation of tyrosine and 3-HO-phenylalanine (Figure 1). No hydroxylated amino acids are detectable if apoenzyme is used. The rate of hydroxylation is unchanged when the reaction is carried out in the absence of O2. The ratio of tyrosine to 3-HO-phenylalanine produced is different for the three enzymes, with ratios of 1.5, 1.2, and 1.6 for PheH, TyrH and TrpH, respectively. Controls showed that the PheH does not lose activity under these conditions. No amino acid products could be detected with sodium periodate, cumene hydroperoxide, peracetic acid, or tbutyl hydroperoxide instead of H2O2. The yield of hydroxylated amino acids was not affected by the radical quenchers mannitol or benzoate (10 mM) or by 2 mM 5-deaza-6-methyltetrahydropterin.
Figure 1.
Peroxide-dependent hydroxylation of phenylalanine by tyrosine hydroxylase. Solid line: TyrH (25 µM) was incubated for 15 min at 30 °C with 10 mM H2O2, 400 µM ferrous ammonium sulfate, 20 min phenylalanine, and 100 mM NaCl in 150 mM Hepes buffer, pH 7.0. Dashed line: reaction with 50 µM apo-TyrH. Dotted line: Reaction with 400 µM ferrous ammonium sulfate but no enzyme. The products of the reaction were analyzed by reverse-phase HPLC, using a mobile phase of 15 mM sodium phosphate, pH 7.0, 1% tetrahydrofuran, with excitation at 270 nm and emission at 310 nm.
TyrH has previously been shown to produce tyrosine and 3-HO-phenylalanine from phenylalanine in PH4-dependent turnover,22 but PheH and TrpH only produce tyrosine.18,21 Thus, the lack of the PH4 has an effect on the substrate specificity of these enzymes. Tyrosine and tryptophan were also examined as substrates in the peroxide-dependent reactions for TyrH and TrpH, but the expected products 3,4-dihydroxyphenylalanine and 5-HO-tryptophan were not detected. Control reactions showed that these two compounds are not stable to the reaction conditions.
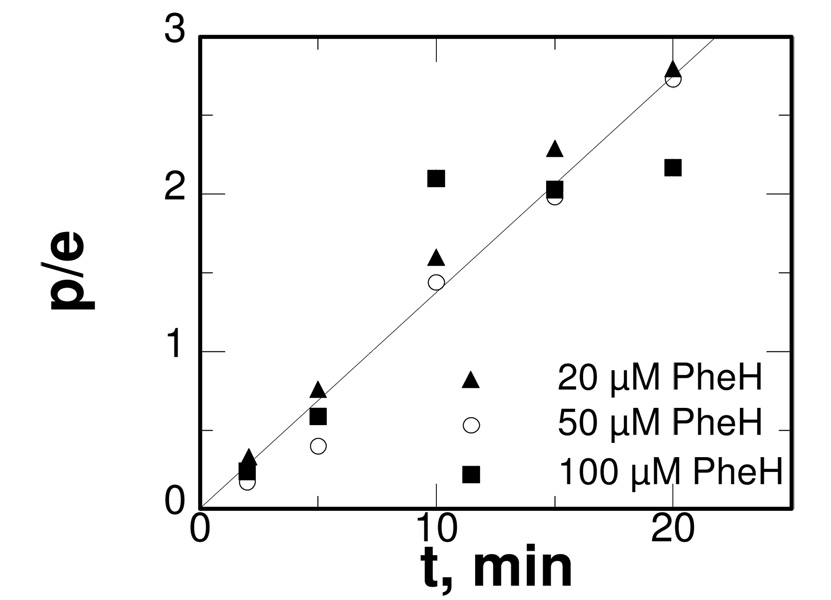
The kinetics of the H2O2-dependent reactions were examined for comparison with PH4-dependent turnover. The initial rate of phenylalanine hydroxylation was directly dependent on the concentration of enzyme (Figure 2). As also shown in Figure 2, the reaction continued for multiple turnovers. When the concentration of H2O2 was varied, the initial rate of the reaction for all three enzymes showed saturation kinetics, with Km values of ~20 mM for each enzyme. In contrast, the initial rate of the reaction did not show evidence for saturation with phenylalanine at concentrations as high as 50 mM for any of the enzymes. The linear dependence of the rate on the concentration of phenylalanine yields kcat/Kphe values of 6.4, 5.3, and 4.3 M−1 min−1 for the H2O2-dependent reaction for PheH, TyrH and TrpH, respectively. These values are 5–6 orders of magnitude smaller than the corresponding values for PH4-dependent turnover.18,21 As shown in Scheme 1, formation of the Fe(IV)O species is proposed to require heterolytic cleavage of the O-O bond in an iron-peroxo-pterin intermediate, with a HO-pterin as the leaving group. Formation of the Fe(IV)O in the H2O2-dependent reaction would be expected to result from loss of water from an iron-peroxide intermediate. The much slower reaction with H2O2 is consistent with the different pKa values of the leaving groups in the H2O2 and PH4-dependent reactions. In addition, the similar kinetic parameters for all three enzymes in the H2O2-dependent reactions are consistent with the hydroxylating intermediates having similar reactivity for all three enzymes. The reactivities of the Fe(IV)O intermediates in PH4-dependent turnover have previously been shown to be similar.23
Figure 2.
Dependence of the amount of hydroxylated phenylalanine on the concentration of PheH for the peroxide-dependent reaction; p/e, moles of hydroxylated amino acid per mole of enzyme. Conditions as for Figure 1.
Active site mutants of TyrH and PheH that affect PH4-dependent turnover were examined in the H2O2-dependent reaction. With E332A TyrH, only 2.5% of the reducing equivalents from 6MePH4 are used for productive turnover.20 S395A TyrH forms the 4a-HO-pterin at a normal rate, but the hydroxylating intermediate breaks down unproductively so that only 1% is used to hydroxylate tyrosine.24 V379D and F263A PheH have low turnover due to uncoupling of PH4 oxidation and amino acid hydroxylation.19,25 With all four mutant enzymes no tyrosine or 3-HO-phenylalanine could be detected in the H2O2-dependent reactions. Thus, amino acid residues required for proper reactivity of the Fe(IV)O intermediate in PH4-dependent turnover are also required for H2O2-dependent turnover.
The aromatic amino acid hydroxylases have previously been shown to catalyze benzylic9,23 and aliphatic hydroxylation.26 To determine if the H2O2-dependent reaction is also capable of supporting these nonphysiological reactions, 4-CH3-phenylalanine and cyclohexylalanine were examined as substrates. In our hands TyrH and PheH catalyze the PH4-dependent hydroxylation of cyclohexylalanine to form 4-HO-cyclohexylalanine with kcat values of 10 and 5 min−1, respectively, at 30 °C. Both enzymes also catalyze the same reaction using H2O2, with second order rate constants of 0.17 M−1 min−1 and 0.28 M−1 min−1. With 4-CH3-phenylalanine as substrate for PH4-dependent turnover, all three enzymes produce a combination of 4-CH2OH-, 3-HO,4-CH3-, and 4-HO,3-CH3-phenylalanine.23 In the H2O2-dependent reactions, 4-CH2OH-phenylalanine is produced but the other two products could not be detected.
The present results establish that H2O2 can replace PH4 and O2 to form the hydroxylating intermediate in the aromatic amino acid hydroxylases. The similar kinetic parameters for all three enzymes in the H2O2-dependent reactions are consistent with the hydroxylating intermediates having similar reactivity for all three enzymes. These results provide support for a hydroxylating intermediate such as Fe(IV)O that does not involve the pterin.
ACKNOWLEDGMENT
This work was supported in part by NIH grants R01 GM047291 (PFF) and F31 GM077092 (JAP) and Welch Foundation Grant A1245 (PFF). We thank Michaela Hyunh for technical assistance.
REFERENCES
- 1.Fitzpatrick PF. In: Advances in Enzymology and Related Areas of Molecular Biology. Purich DL, editor. Vol. 74. John Wiley & Sons, Inc.; 2000. pp. 235–294. [Google Scholar]
- 2.Kappock TJ, Caradonna JP. Chem.Rev. 1996;96:2659–2756. doi: 10.1021/cr9402034. [DOI] [PubMed] [Google Scholar]
- 3.Fitzpatrick PF. Biochemistry. 2003;42:14083–14091. doi: 10.1021/bi035656u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L, Erlandsen H, Haavik J, Knappskog PM, Stevens RC. Biochemistry. 2002;41:12569–12574. doi: 10.1021/bi026561f. [DOI] [PubMed] [Google Scholar]
- 5.Erlandsen H, Fusetti F, Martinez A, Hough E, Flatmark T, Stevens RC. Nature Struct.Biol. 1997;4:995–1000. doi: 10.1038/nsb1297-995. [DOI] [PubMed] [Google Scholar]
- 6.Goodwill KE, Sabatier C, Marks C, Raag R, Fitzpatrick PF, Stevens RC. Nature Struct. Biol. 1997;4:578–585. doi: 10.1038/nsb0797-578. [DOI] [PubMed] [Google Scholar]
- 7.Hegg EL, Que L. Eur. J. Biochem. 1997;250:625–629. doi: 10.1111/j.1432-1033.1997.t01-1-00625.x. [DOI] [PubMed] [Google Scholar]
- 8.Que L., Jr Nature Struct. Biol. 2000;7:182–184. doi: 10.1038/73270. [DOI] [PubMed] [Google Scholar]
- 9.Hillas PJ, Fitzpatrick PF. Biochemistry. 1996;35:6969–6975. doi: 10.1021/bi9606861. [DOI] [PubMed] [Google Scholar]
- 10.Moran GR, Derecskei-Kovacs A, Hillas PJ, Fitzpatrick PF. J. Am. Chem.Soc. 2000;122:4535–4541. [Google Scholar]
- 11.Eser BE, Barr EW, Frantom PA, Saleh L, Bollinger J, Martin, Krebs C, Fitzpatrick PF. J. Am. Chem. Soc. 2007;129:11334–11335. doi: 10.1021/ja074446s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bollinger JM, Jr, Krebs C. J. Inorg. Biochem. 2006;100:586–605. doi: 10.1016/j.jinorgbio.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Krebs C, Galonic Fujimori D, Walsh CT, Bollinger JM., Jr Acc. Chem. Res. 2007;40:484–492. doi: 10.1021/ar700066p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hrycay EG, Gustafsson JA, Ingelman-Sundberg M, Ernster L. Biochem. Biophys. Res. Commun. 1975;66:209–216. doi: 10.1016/s0006-291x(75)80315-9. [DOI] [PubMed] [Google Scholar]
- 15.Froland WA, Andersson KK, Lee SK, Liu Y, Lipscomb JD. J. Biol. Chem. 1992;267:17588–17597. [PubMed] [Google Scholar]
- 16.Neibergall MB, Stubna A, Mekmouche Y, Munck E, Lipscomb JD. Biochemistry. 2007;46:8004–8016. doi: 10.1021/bi700120j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolfe MD, Lipscomb JD. J. Biol. Chem. 2003;278:829–835. doi: 10.1074/jbc.M209604200. [DOI] [PubMed] [Google Scholar]
- 18.Daubner SC, Hillas PJ, Fitzpatrick PF. Biochemistry. 1997;36:11574–11582. doi: 10.1021/bi9711137. [DOI] [PubMed] [Google Scholar]
- 19.Pavon JA, Fitzpatrick PF. Biochemistry. 2006;45:11030–11037. doi: 10.1021/bi0607554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daubner SC, Fitzpatrick PF. Biochemistry. 1999;38:4448–4454. doi: 10.1021/bi983012u. [DOI] [PubMed] [Google Scholar]
- 21.Moran GR, Daubner SC, Fitzpatrick PF. J. Biol. Chem. 1998;273:12259–12266. doi: 10.1074/jbc.273.20.12259. [DOI] [PubMed] [Google Scholar]
- 22.Fitzpatrick PF. J. Am. Chem. Soc. 1994;116:1133–1134. [Google Scholar]
- 23.Pavon JA, Fitzpatrick PF. J. Am. Chem. Soc. 2005;127:16414–16415. doi: 10.1021/ja0562651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellis HR, Daubner SC, Fitzpatrick PF. Biochemistry. 2000;39:4174–4181. doi: 10.1021/bi9928546. [DOI] [PubMed] [Google Scholar]
- 25.Daubner SC, Melendez J, Fitzpatrick PF. Biochemistry. 2000;39:9652–9661. doi: 10.1021/bi000493k. [DOI] [PubMed] [Google Scholar]
- 26.Carr RT, Balasubramanian S, Hawkins PCD, Benkovic SJ. Biochemistry. 1995;34:7525–7532. doi: 10.1021/bi00022a028. [DOI] [PubMed] [Google Scholar]