Abstract
Recent studies suggest that nuclear factor κB-inducing kinase (NIK) is suppressed through constitutive proteasome-mediated degradation regulated by TRAF2, TRAF3 and cIAP1 or cIAP2. Here, we demonstrated that the degradation of NIK occurred upon assembly of a regulatory complex through TRAF3 recruitment of NIK and TRAF2 recruitment of cIAP1 and cIAP2. In contrast to TRAF2 and TRAF3, cIAP1 and cIAP2 seem to play redundant roles in the degradation of NIK, as inhibition of both cIAPs was required for noncanonical NF-κB activation and increased survival and proliferation of primary B lymphocytes. Furthermore, the lethality of TRAF3-deficient mice could be rescued by a single NIK gene, highlighting the importance of tightly regulated NIK.
The Rel-NF-κB family of transcription factors has a tremendous impact on numerous biological processes, including bone homeostasis, cellular proliferation and apoptosis, the initiation and propagation of innate and adaptive immune responses, and the development of secondary lymphoid tissue architecture1–3. Research in recent years has defined two distinct NF-κB activation pathways, termed the canonical and noncanonical NF-κB pathways4. Briefly, the canonical NF-κB pathway, activated by most stress stimuli, results in IκB kinase (IKK) complex-mediated degradation of IκB and rapid nuclear accumulation of p50-RelA and p50-cRel NF-κB complexes5. In contrast, activation of the noncanonical NF-κB pathway by a select group of tumor necrosis factor (TNF) receptors such as CD406, Lymphotoxin β receptor (LTβR)7 and BAFF receptor (BAFF-R)7, results in slow processing of the C-terminus of p100 to produce p52 and kinetically slower nuclear translocation of the p52-RelB NF-κB complex8.
In contrast to receptor-mediated activation of the canonical NF-κB pathway, which occurs within minutes and does not require new protein synthesis, activation of the noncanonical NF-κB pathway takes several hours and requires new protein synthesis. Importantly, activation of the noncanonical NF-κB pathway requires NIK9–11. Evidence suggests that NIK protein is suppressed by direct interaction with TRAF312, a member of the tumor necrosis factor receptor associated factor (TRAF) family, including TRAFs 1–6. In support of this concept, TRAF3-deficient cells display constitutive p100 to p52 processing as a result of accumulated NIK13. In addition, a recent report demonstrated spontaneous p100 to p52 processing in mice with a conditional disruption of the gene for TRAF2 in B lymphocytes14, suggesting that TRAF2 also functions as an essential negative regulator of the noncanonical NF-κB pathway.
A recent characterization of primary multiple myelomas displaying high NIK levels and constitutive p100 to p52 processing, identified several classes of chromosomal alterations. These included NIK amplifications as well as TRAF2 or TRAF3 chromosomal deletions15,16. Interestingly, these analyses also identified a subset of patients exhibiting dual chromosomal loss of cellular inhibitor of apoptosis 1 and 2 (cIAP1 and cIAP2), suggesting that cIAP1 and cIAP2 are also involved in the negative regulation of NIK. Coincidently, development of pharmacological inhibitors of cIAPs has been a major goal in advancing cancer therapeutics as cIAPs are believed to antagonize activated caspases and are often amplified in human cancers17,18. Recent studies characterizing several newly developed inhibitors of cIAPs found that global inhibition of cIAPs activated the noncanonical NF-κB pathway in a NIK-dependent manner19,20 and that overexpression of cIAP1 could target NIK for ubiquitination and degradation. Importantly, neither cIAP1- or cIAP2-deficient mice present with the perinatal lethality of TRAF3-deficient mice, indicating that the absence of either cIAP1 or cIAP2 is insufficient to affect the regulation of NIK21,22. However, how TRAF2 and TRAF3 cooperate with cIAP1 and/or cIAP2 in the proteasomal targeting of NIK remains to be defined.
In this report, we provide evidence that TRAF2 and TRAF3 play distinct roles in recruiting cIAP1 and cIAP2 to a regulatory complex that promotes NIK degradation and suppression of the noncanonical NF-κB pathway. In contrast to previous reports showing cell death induced by pharmacological inhibitors of IAPs, we found that the same IAP inhibitors significantly enhanced B lymphocyte survival and proliferation through activation of the noncanonical NF-κB pathway. Collectively, these data provide insight into the assembly of the NIK regulatory complex and bring to light potential immunological hazards in the application of IAP inhibition for the treatment of cancer.
Results
TRAF2 and TRAF3 perform nonredundant functions
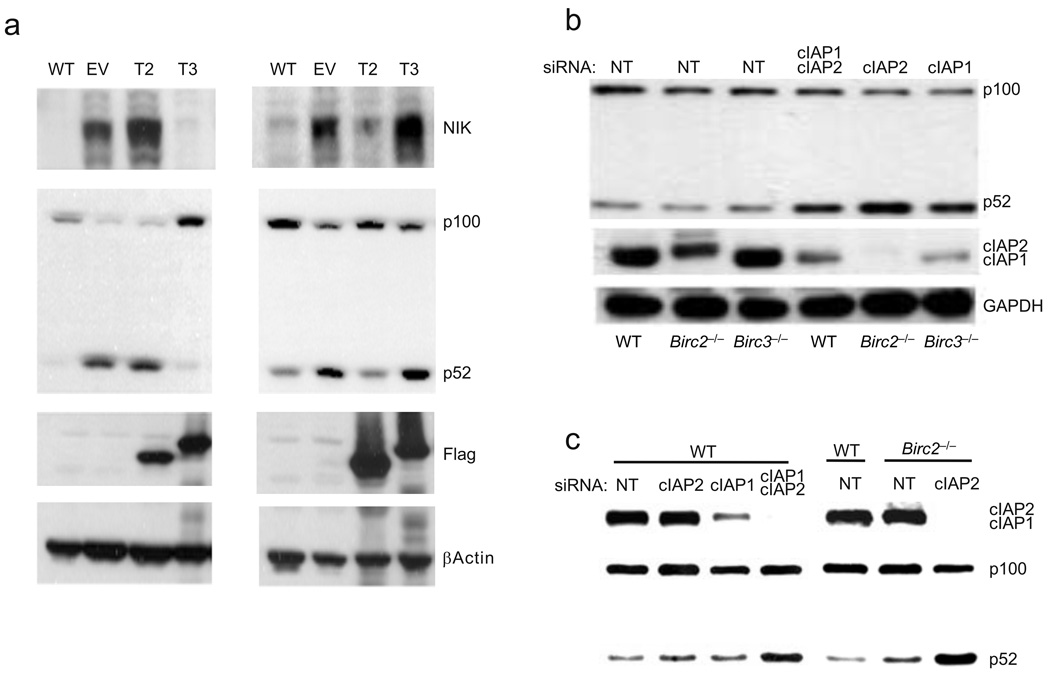
While recent studies indicate that TRAF2, TRAF3, cIAP1 and cIAP2 all function as negative regulators of noncanonical NF-κB activation, the molecular mechanisms responsible for these individual players in controlling basal NIK levels or NIK levels upon TNF receptor activation remain to be elucidated. In particular, little is known about the functional specificity between TRAF2 and TRAF3 despite the fact that mice lacking either TRAF2 or TRAF3 die within two weeks of birth with constitutively activated noncanonical NF-κB. In order to directly compare the abilities of TRAF2 and TRAF3 to negatively regulate noncanonical NF-κB, Flag-TRAF2 or Flag- TRAF3 was stably expressed in TRAF3-deficient (Traf3−/−) and TRAF2-deficient (Traf2−/−) murine embryonic fibroblasts (MEFs). Immunoblot analysis of NIK and p100 to p52 processing showed that even when expressed at equivalent levels, TRAF2 failed to compensate for the spontaneous accumulation of NIK and p100 to p52 processing resulting from the loss of TRAF3 (Fig. 1a, left panel). Likewise, TRAF3 failed to compensate for spontaneous activation of noncanonical NF-κB resulting from loss of TRAF2 (Fig. 1a, right panel), indicating that TRAF2 and TRAF3 perform highly specific functions in negatively regulating noncanonical NF-κB activation.
Figure 1. Non-redundancy of TRAF2 and TRAF3 and redundancy of cIAP1 and cIAP2 in suppression of the noncanonical NF-κB pathway.
(a) Immunoblot of lysates of wild-type (WT) and Traf3−/− (left panel), WT and Traf2−/− (right panel) MEFs reconstituted with empty pBABE-Flag vector (EV), pBABE-Flag-TRAF2 (T2) and pBABE-Flag-TRAF3 (T3). Noncanonical NF-κB activation was assessed by immunoblot analysis of NIK and p100 to p52 processing. (b) Immunoblot of WT, Birc2−/− and Birc3−/− MEFs transiently transfected with non-targeting (NT) siRNA or cIAP1-specific and/or cIAP2-specific siRNA as indicated. Activation of the noncanonical NF-κB pathway was then assessed by analysis of p100 to p52 processing 24 hr post-transfection. (c) Immunoblot of WT and Birc2−/− primary hepatocytes transiently transfected with NT, cIAP1-specific and/or cIAP2-specific siRNA as indicated. Activation of the noncanonical NF-κB pathway was then assessed by analysis of p100 to p52 processing 24 hr post-transfection. Results are representative of at least three independent experiments.
cIAP1 and cIAP2 perform redundant functions
Understanding potential functional redundancy of cIAP1 and cIAP2 has been impeded because of their chromosomal proximity, which prevents the generation of mice with targeted disruption of both molecules. To determine if cIAP1 and cIAP2 perform redundant regulatory roles in the negative regulation of NIK, we introduced small interfering RNAs (siRNA) against cIAP1, cIAP2 or both into wild-type MEFs, cIAP2-specific siRNA into cIAP1-deficient (Birc2−/−) MEFs and cIAP1-specific siRNA into cIAP2-dificient (Birc3−/−) MEFs. Activation of NIK was then assessed by immunoblot analysis of p100 to p52 processing (Fig. 1b). We found that treatment of wild-type cells with siRNA specific for either cIAP1 or cIAP2 had a negligible effect on p100 to p52 processing; in contrast, treatment with both cIAP1-specific and cIAP2-specific siRNAs resulted in an increased in p52 levels. Moreover, where treatment with either cIAP2-specific or cIAP1-specific siRNA alone failed to induce p100 to 52 processing in wild-type cells, in cIAP1-deficient and cIAP2-deficient MEFs treatment with cIAP2-specific and cIAP1-siRNA alone, respectively, activated p100 to p52 processing, (similar results were also obtained in primary hepatocytes) (Fig. 1c). These data strongly suggested that cIAP1 and cIAP2 function redundantly in the degradation of NIK. We hypothesized that this function was via cIAP ubiquitination of NIK. To verify this hypothesis, HEK 293T cells were transfected with NIK and ubiquitin in the absence or presence of cIAP1 or cIAP2. Both cIAP1 and cIAP2 (cIAPs) promoted ubiquitination of NIK, while the RING domain deletion mutants, cIAP1Δ and cIAP2Δ lacking E3 ligase activity failed to do so (Supplementary Fig. 1a). Immunoprecipitation of ubiquitin followed by immunoblot analysis of NIK was also performed to definitively verify NIK ubiquitination by cIAPs (Supplementary Fig. 1b). We therefore conclude that cIAPs perform redundant roles as ubiquitin ligases in the negative regulation of the NIK and noncanonical NF-κB activation.
TRAF2 and TRAF3 promote cIAP association with NIK
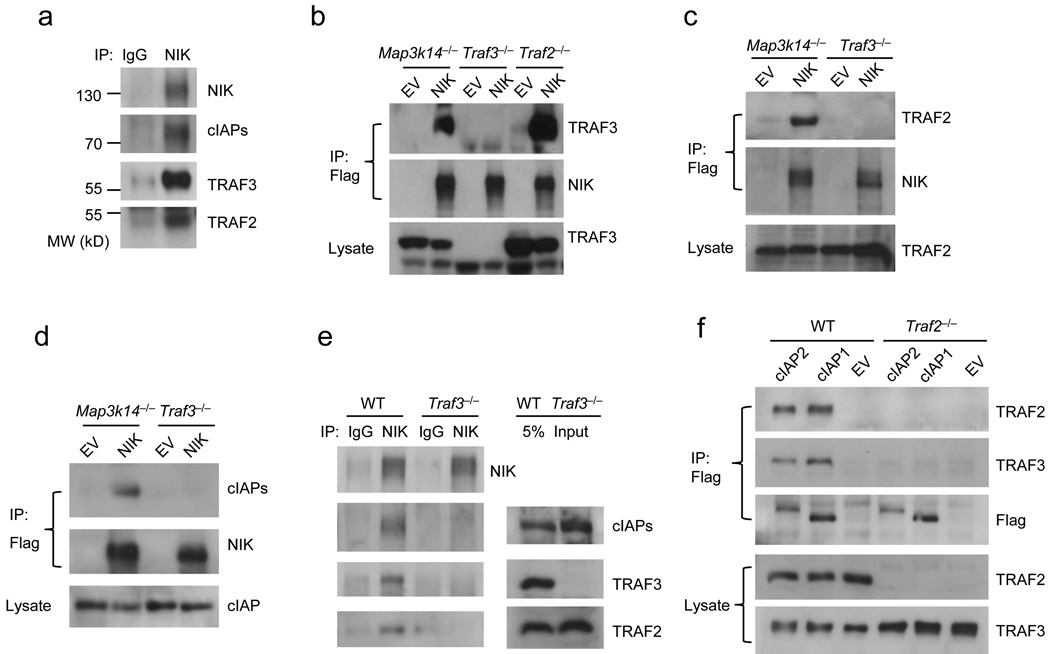
Genetic data provide strong evidence for a NIK regulatory complex consisting of TRAF2, TRAF3, cIAP1, cIAP2 and NIK. To test this model, we treated primary B lymphocytes with the proteasome inhibitor MG132 to allow for NIK accumulation. Here, immunoprecipitation of NIK resulted in the co-immunoprecipitation of TRAF2, TRAF3, and cIAPs (Fig. 2a; similar results were also obtained in MEFs; Supplementary Fig. 2). These analyses verify the proposed NIK regulatory complex. We next wanted to determine if TRAF2 and TRAF3 binding to NIK was independent of one another, cooperative, or coordinated. Here, pBABE-Flag-NIK was introduced into NIK-deficient (Map3k14−/−), Traf3−/−, and Traf2−/− MEFs. Flag immunoprecipitates were then analyzed by immunoblot. This analysis demonstrated that TRAF2 is not required for the association of NIK with TRAF3 (Fig. 2b). In contrast, TRAF2 binding to NIK required TRAF3 (Fig. 2c). Thus, TRAF3 binds NIK independently, serving to coordinate the recruitment of TRAF2 to the NIK regulatory complex. Interestingly, association of cIAPs with NIK was also lost in the absence of TRAF3 (Fig. 2d). Similar results were also obtained in BCR-Abl transformed wild-type and Traf3−/− B-cell lines (Fig. 2e). These results led to our speculation that TRAF2 might mediate the recruitment of cIAPs to NIK through TRAF3. To explore this we generated wild-type and Traf2−/− 3T3s lines expressing pBABE-Flag-cIAP1 or pBABE-Flag-cIAP2. Flag immunoprecipitates were then analyzed by immunoblot (Fig. 2f). Here, we detected association of both cIAPs with TRAF2 and TRAF3 in the wild-type cell lines. We also found that the interaction between TRAF3 and cIAPs was lost in the Traf2−/− cells indicating that TRAF2 is required for TRAF3-cIAPs interaction. We therefore suggest that cIAPs are recruited to the TRAF3-NIK complex via TRAF2.
Figure 2. Assembly of the NIK regulatory complex by TRAF2 and TRAF3.
(a) Immunoassays on lysates of primary murine B cells treated with the proteasomal inhibitor MG132 for 4 hr and then incubated with rabbit-IgG or anti-NIK. Protein complexes were then immunoprecipitated with Protein G beads followed by the indicated immunoblot analysis. (b) Immunoassays on lysates of Map3k14−/−, Traf3−/−, and Traf2−/− MEFs reconstituted with empty pBABE-Flag vector (EV) or pBABE-Flag-NIK (NIK) and complexes were immunoprecipitated with anti-Flag M2 beads followed by the indicated immunoblot. (c,d) Immunoassays on lysates from Map3k14−/− and Traf3−/− MEFs reconstituted with EV or NIK incubated with anti-Flag M2 beads and complexes were immunoprecipitated followed by the indicated immunoblot analysis. (e) Immunoassays on lysates from MG132 treated, BCR-Abl transformed WT and Traf3−/− B-cell lines incubated with control rabbit-IgG or anti-NIK followed by Protein G beads and complexes were immunoprecipitated followed by the indicated immunoblot analysis. (f) Immunoassays on lysates from WT and Traf2−/− 3T3s reconstituted with pBABE-Flag-cIAP1, -cIAP2 or empty vector (EV) control. Cell lysates were then immunoprecipitated with anti-Flag M2 beads and co-immunoprecipitation of TRAF2 and TRAF3 was detected by immunoblot. Data are representative of at least three experiments with similar results.
TRAF2 and TRAF3 affinities for cIAPs and NIK
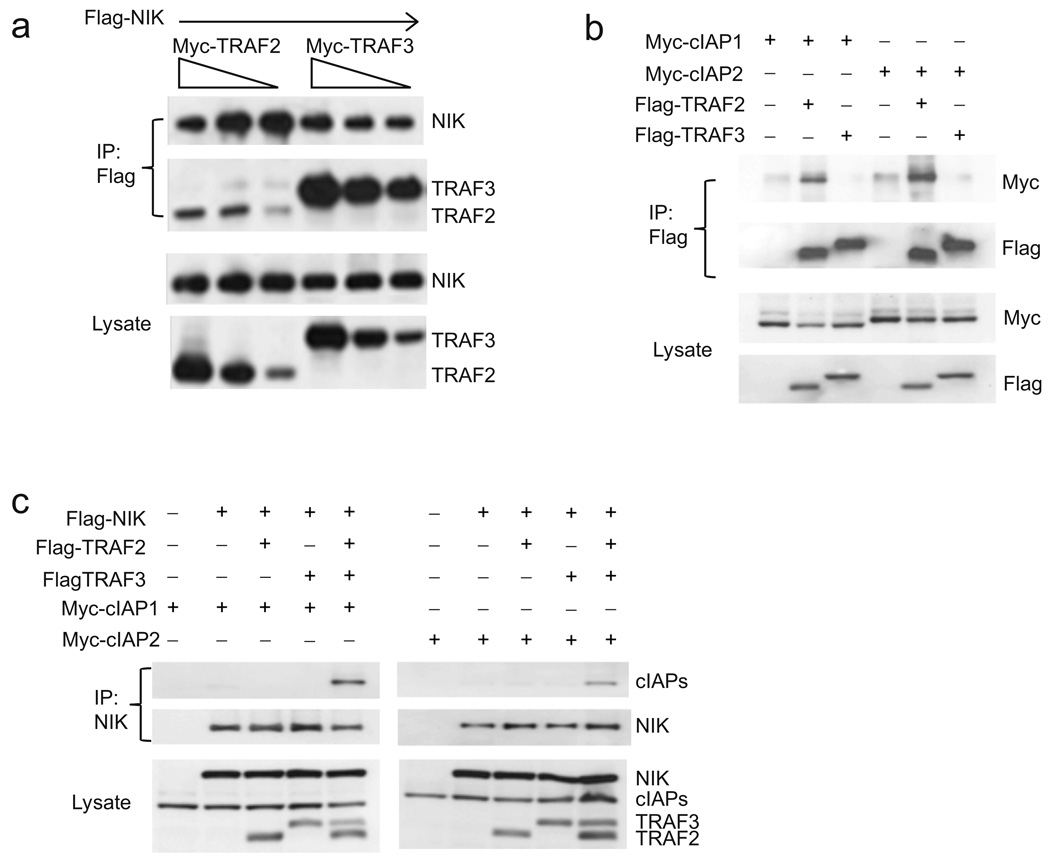
The finding that TRAF2 interaction with NIK required TRAF3 was surprising as NIK was first identified as a TRAF2 interacting protein by yeast-two hybrid assay23. To gain further insight, we directly compared the binding affinity of TRAF2 and TRAF3 for NIK in HEK 293T cells. This analysis revealed that TRAF3 bound NIK with much higher affinity than TRAF2 (Fig. 3a). In addition, siRNA-mediated knockdown of endogenous TRAF3 in HEK 293T cells markedly reduced TRAF2 interaction with NIK (Supplementary Fig. 3). These data strongly suggest that TRAF2 is unable to bind NIK under endogenous conditions, explaining the requirement of TRAF3 for TRAF2-NIK interaction (Fig. 2c). In contrast, TRAF2 has long been known to functionally associate with cIAP1 and cIAP224. No data regarding TRAF3 interaction with cIAP1 or cIAP2 has yet been reported. Therefore, we next compared the binding affinities of TRAF2 and TRAF3 for cIAP1 and cIAP2 in HEK 293T cells. Here, when TRAF2 and TRAF3 were expressed at low levels, we only observed TRAF2 but not TRAF3 in association with both cIAP1 and cIAP2 (Fig. 3b). When the levels of TRAF3 were increased in similar titration studies, we were able to detect TRAF3 association with cIAP2 but not cIAP1. However, the cIAP2 interaction with TRAF3 is always weaker than with TRAF2 (Supplementary Fig. 4a,b). These data strongly suggest that TRAF3 is unable to bind to either cIAP1 or cIAP2 under endogenous conditions, explaining the requirement of TRAF2 for TRAF3-cIAPs interaction (Fig. 2e). Taken together, we conclude that the non-redundant functions of TRAF2 and TRAF3 in the negative regulation of the noncanonical NF-κB pathway result from their differential high affinity associations with the cIAPs and NIK, respectively.
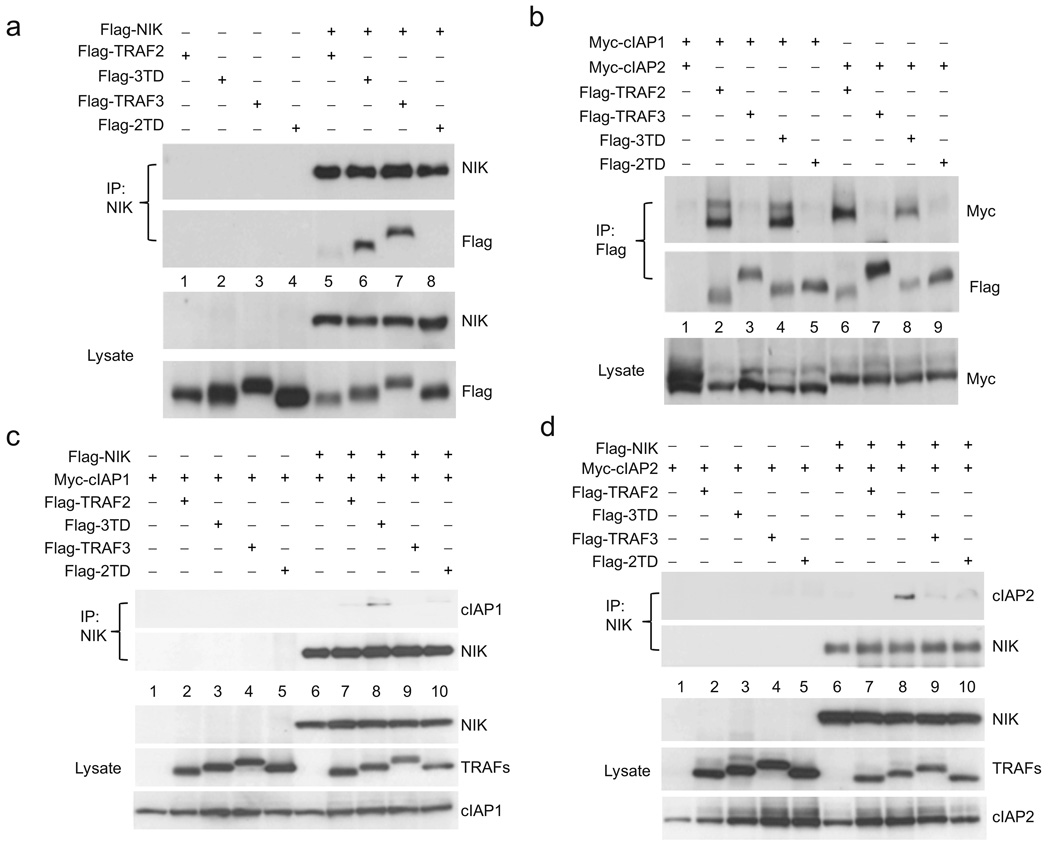
Figure 3. Differential affinities of TRAF2 and TRAF3 for cIAP1/2 and NIK.
(a) Immunoblot of lysates of HEK 293T cells co-expressing Flag-NIK and titrations of Myc-TRAF2 and TRAF3. Lysates were incubated with anti-Flag M2 beads and NIK-TRAF complexes were detected by immunoblot analysis against Myc. (b) Immunoblot of lysates of HEK 293T cells co-expressing Myc-tagged cIAP1 and cIAP2 and Flag-TRAF2 and Flag-TRAF3. Lysates were incubated with anti-Flag M2 beads and cIAP-TRAF complexes were detected by immunoblot analysis against Myc. (c) Immunoassays on lysates from HEK 293T cell co-expressing NIK and Myc-cIAP1 or Myc-cIAP2 in the presence or absence of TRAF2, TRAF3 or both TRAF2 and TRAF3. NIK was then immunoprecipitated by NIK-specific antibody and NIK-cIAPs complexes were detected by immunoblot against Myc. All experiments were done at least three times with similar results.
Previous work has shown dynamic interactions between TRAF2 and TRAF3 in living cells25. Given this, and the striking differential affinities of TRAF2 and TRAF3 for the cIAPs and NIK, we propose an uncomplicated model to explain the nonredundant roles of TRAF2 and TRAF3 in the negative regulation of NIK: specifically, direct interaction between TRAF2 and TRAF3 functions as a bridge between the cIAPs and NIK (Supplementary Fig. 5). To verify this model, we sought to reproduce the NIK regulatory complex in HEK 293T cells. Here, co-expression of NIK with either cIAP1 or cIAP2 alone resulted in no interaction between NIK and cIAPs. Likewise, the additional expression of TRAF2 or TRAF3 alone failed to mediate any interaction between the cIAPs and NIK. In contrast, simultaneous expression of TRAF2 and TRAF3 resulted in the assembly of a NIK-cIAPs complex (Fig. 3c). These data provide strong support for a model in which TRAF2 and TRAF3 cooperate in the negative regulation of NIK by bridging the gap between the cIAPs and NIK.
A chimera TRAF2-TRAF3 protein
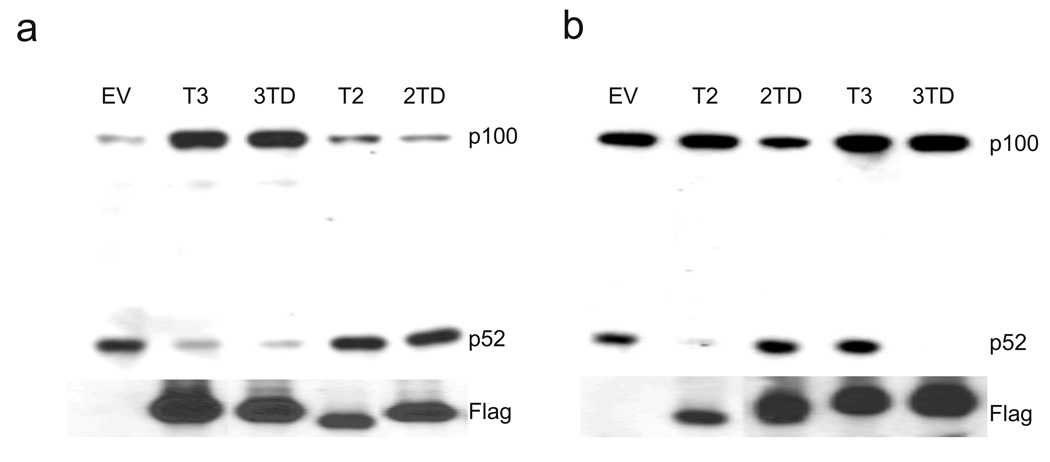
The study of TRAF family members has been enhanced by chimeric strategies that elucidate the sequence specificity of TRAF family members with biochemical pathway activation potential26–28. Given the prior success of this strategy, we generated a number of TRAF2-TRAF3 chimeras, two of which are shown in Supplementary Figure 6. Using the pBABE-Flag retroviral system, we introduced TRAF3, 3TD, TRAF2 and 2TD into Traf3−/− MEFs and then analyzed p100 to p52 processing by immunoblot (Fig. 4a). As expected, expression of TRAF3, but not TRAF2, in Traf3−/− MEFs restored p100 to p52 processing to normal basal levels. In addition, 3TD but not 2TD restored p100 to p52 processing to normal basal levels. These data demonstrate that the TRAF domain (TD) of TRAF3 provides a unique function in the negative regulation of NIK. Importantly, the ability of 3TD to prevent constitutive processing of p100 to p52 in Traf3−/− cells was not affected by siRNA-mediated knockdown of TRAF2 (Supplementary Fig. 7). Next, identical experiments were performed in Traf2−/− MEFs (Fig. 4b). As expected, expression of TRAF2, but not TRAF3, in Traf2−/− MEFs restored p100 to p52 processing to normal levels. Intriguingly, as seen in Traf3−/− cells, 3TD but not 2TD also restored p100 to p52 processing to normal basal levels. Thus, the RING domain (RD) and Zinc fingers (ZFs) of TRAF2 also provide a unique regulatory function in the negative regulation of NIK. Taken together, these data suggest that the 3TD chimera unites the nonredundant roles of TRAF2 and TRAF3 into a single molecule.
Figure 4. Generation of a TRAF2-TRAF3 chimera.
(a,b) Traf3−/− (a) and Traf2−/− (b) MEFs were reconstituted with pBABE-Flag-TRAF2 (T2), pBABE-Flag-TRAF3 (T3), pBABE-Flag-3TD, and pBABE-Flag-2TD. Rescue of constitutive noncanonical NF-κB activation was assessed by immunoblot analysis of p100 to p52 processing. Data are representative of at least three independent experiments.
To further characterize the 3TD chimera, we compared the binding capacity of these TRAF constructs for NIK in HEK 293T cells. As expected, TRAF2 exhibited a much weaker interaction with NIK as compared to TRAF3 (Fig. 5a, compare lanes 5 and 7). 3TD but not 2TD (lane 6 versus 8) interacted with NIK as strongly as TRAF3. Thus, the TD of TRAF3 mediates high affinity binding to NIK. We next compared the binding capacities of the TRAF constructs for cIAP1 and cIAP2. As expected, TRAF2, but not TRAF3, interacted with cIAP1 (Fig. 5b lane 2 versus 3) and cIAP2 (Fig. 5b lane 6 versus 7). 3TD, but not 2TD, interacted with both cIAP1 (Fig. 5b lane 4 versus 5) and cIAP2 (Fig. 5b lane 8 versus 9). These findings suggest that the ability of the 3TD chimera to rescue deregulated NIK in both Traf2−/− and Traf3−/− cells results from uniting the individual binding capacities of TRAF2 and TRAF3 for the cIAPs and NIK into a single molecule.
Figure 5. The TRAF2-TRAF3 Chimera, 3TD.
(a) Flag-NIK was co-expressed with Flag-tagged TRAF2, 3TD, TRAF3, and 2TD in HEK 293T cells. Lysates were incubated with NIK-specific antibody and NIK-TRAF complexes were detected by immunoblot analysis against Flag. (b) Myc-tagged cIAP1 and cIAP2 were co-expressed with Flag-tagged TRAF2, TRAF3, 3TD, and 2TD. Lysates were incubated with anti-Flag M2 beads and cIAP-TRAF complexes were detected by immunoblot analysis against Myc. (c,d) Myc-cIAP1 (c) or Myc-cIAP2 (d) together with Flag-NIK were co-expressed alone or in the presence of Flag-tagged TRAF2, 3TD, TRAF3, or 2TD in HEK 293T cells. Lysates were incubated with anti-NIK antibody and NIK-cIAPs complexes were detected by immunoblot analysis against Myc. All data are representative of at least three independent experiments.
Given the ability of the 3TD chimera to bind both cIAPs and NIK with high affinity, we would predict that this molecule should obviate the requirement for both TRAF2 and TRAF3 in promoting a NIK-cIAPs complex. To test this model, NIK and Myc-cIAP1 together were expressed alone or in the presence of the TRAF constructs in HEK 293T cells. Here, no interaction between cIAP1 and NIK was detected in the absence of exogenous TRAF expression (Fig. 5c, lane 6). In addition, while TRAF2, TRAF3 and 2TD did not lead to a significant interaction between cIAP1 and NIK, 3TD (Fig. 5c, lane 8) promoted a strong association. Identical experiments were also performed with cIAP2. Here, only 3TD (Fig. 5d, lane 8) was able to promote robust interaction of cIAP2 with NIK over the background control (Fig. 5d, lane 6). Collectively, these data demonstrate that the 3TD chimera is more efficient at promoting a NIK-cIAPs complex than either TRAF2 or TRAF3 alone.
Degradation of TRAF2 and TRAF3 prevents cIAPs-NIK association
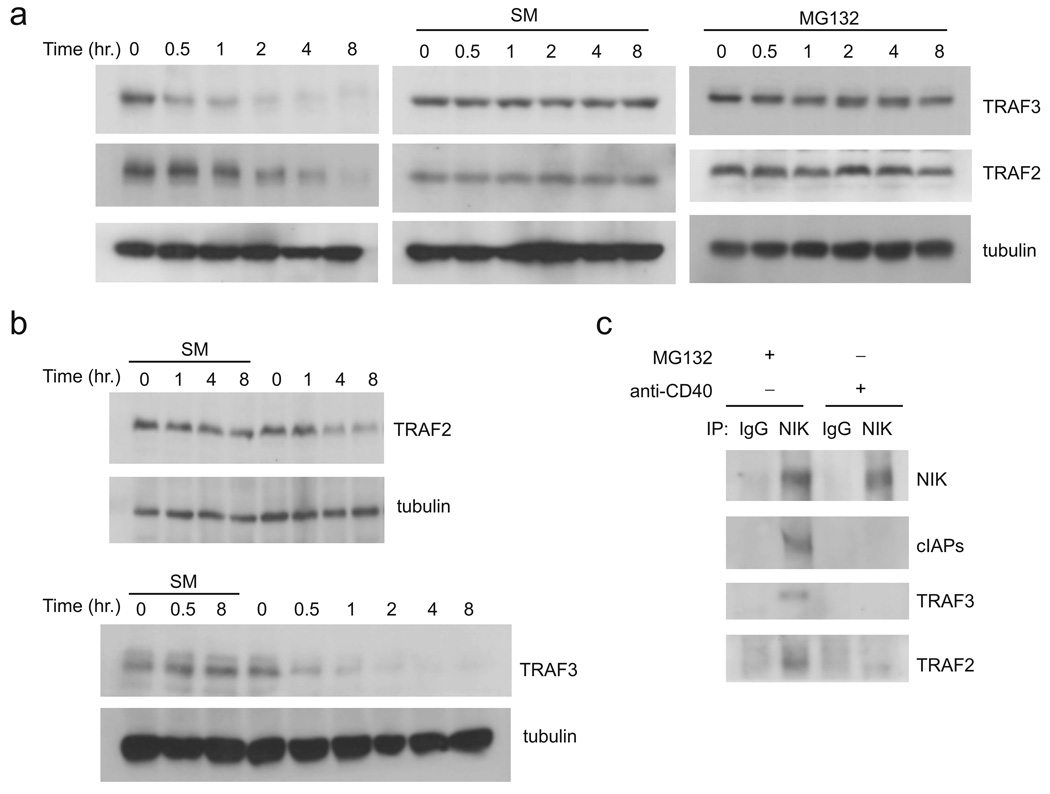
At present, a model explaining the mechanism of receptor induced activation of the noncanonical NF-κB activation pathway has not been provided. Various groups have shown receptor induced degradation of TRAF2 upon CD40 engagement. Interestingly, we found that treatment of A20 and primary murine B lymphocytes with agonistic anti-CD40 (FgK45) induced both TRAF2 and TRAF3 degradation, with kinetics similar to activation of the noncanonical NF-κB pathway (Fig. 6a and b). In both cell types, TRAF3 degradation preceded more rapidly that degradation of TRAF2, suggesting that degradation of TRAF3 initiates NIK-cIAPs disassociation. Additionally, CD40 induced degradation of TRAF2 and TRAF3 was inhibited by the small-molecule cIAP inhibitor (SMAC mimetic; SM)29. These data suggest that TRAF3-binding receptors stabilize NIK by activating cIAP-dependent degradation of TRAF2 and TRAF3. To test this model further we first introduced CD40L and CD40 to the NIK regulatory complex assay in HEK 293T cells (see Fig. 3d). We found that engagement of CD40 prevented the ability of TRAF2 and TRAF3 to coordinate assembly of the NIK-cIAPs complex (Supplementary Fig. 8). In addition, we also analyzed the NIK regulatory complex in the A20 B cell line before and after ligation of CD40 (Fig. 6c). Unstimulated cells treated with MG132 for 4 hr to stabilize NIK to levels resulting from CD40 engagement, and ligation of CD40 also prevented the association between cIAPs and NIK. These data demonstrate that stabilization of NIK by TRAF3-binding receptors results from degradation of TRAF2 and TRAF3, thereby preventing cIAP-mediated ubiquitination and degradation of NIK.
Figure 6. Receptor-induced stabilization of NIK after TRAF2 and TRAF3 degradation.
(a) A20 cells were treated with 5 µg/ml of agonistic anti-CD40 antibody (FgK-45) in media alone (left panel), and in the presence of the cIAP inhibitor, SM (100nM, middle panel) or the proteasome inhibitor MG132 (10 µM, right panel), for the indicated times. SDS Lysates were then subjected to immunoblot analysis of the indicated proteins. (b) Primary murine B-cells were treated with 5 µg/ml of FgK in media alone and in the presence SM (100nM). SDS Lysates were then subjected to immunoblot analysis of the indicated proteins. (c) A20 cells were treated with MG132 or FgK for 4 hr as indicated. Cell Lysates were then incubated with either rabbit-IgG or anti-NIK antibody followed by immunoprecipitation with anti-rabbit-IgG beads. Immunoprecipitates were then subjected to immunoblot detection of the indicated proteins. Data are representative of at least three independent experiments.
Smac mimetic compounds mimic BAFF stimulation
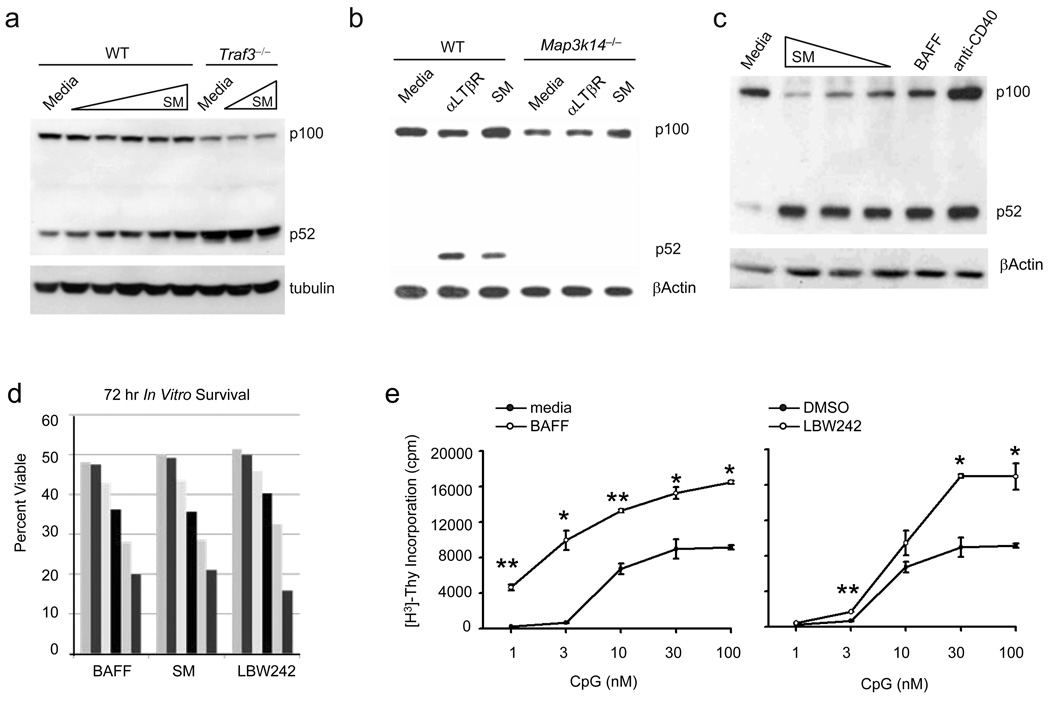
Recently, two reports used two different second generation SMAC mimetic compounds to show that inhibition of cIAPs can activate noncanonical NF-κB19,20. Taking advantage of these compounds, we treated wild-type, Traf3−/− and Map3k14−/− MEF cells with the cIAP inhibitor SM. As expected, SM induced p100 processing in wild-type but not Map3k14−/− cells, whereas it did not further enhance the constitutively activated p100 processing in Traf3−/− MEFs (Fig. 7a,b). To examine the effects of cIAP inhibition on B cells, primary B-lymphocytes were treated with either SM or known activators of the noncanonical NF-κB pathway (BAFF or anti-CD40) for 12 hours. Consistent with the results in the MEF cells, SM-treatment of primary B cells induced p100 to p52 processing, to a degree similar to that elicited by BAFF or anti-CD40 (Fig. 7c). We next compared the abilities of BAFF, SM, and the second generation Smac mimetic LBW242, to protect B cells from undergoing spontaneous apoptosis during in vitro culture (Fig. 7d). We found that either SM-or LBW242-treatment elicited a similar level of B-lymphocyte survival as compared to BAFF, indicating, in contrast to their ability to potentiate cell death in numerous cancer lines, a unique proapoptotic function for cIAPs in B lymphocytes.
Figure 7. SMAC mimetic compounds mimic co-stimulatory properties of BAFF in B lymphocyte activation.
(a) WT and Traf3−/− MEFs were cultured in media alone or a 5-fold dilution series (high dose 500 nM) of SM for 12 hr. Activation of the noncanonical NF-κB pathway was then assessed by immunoblot analysis of p100 to p52 processing. (b) WT and Map3k14−/− 3T3s were cultured in media alone, in the presence of 2 µg/ml agonistic αLTβR antibody, or 50 nM SM for 12 hr. Activation of the noncanonical NF-κB pathway was then assessed by immunoblot analysis of p100 to p52 processing. (c) Primary B-lymphocytes were cultured in media alone, in the presence of a 5-fold dilution series (high dose 500 nM) of SM, 100 ng/ml of BAFF, or 5 µg/ml of anti-CD40 for 12 hr. Activation of the noncanonical NF-κB pathway was then assessed by immunoblot analysis of p100 to p52 processing. (d) Primary B-lymphocytes were cultured in media alone or in the presence of 3 fold titrations of BAFF, SM, or LBW242 (high dose 300 ng/ml, 300 nM, 300 nM respectively) for 72 hr. Cell viability was then determined by propidium iodide exclusion assay and analyzed by FACS. Stimulations were performed in triplicate. (e) Primary B-lymphocytes were cultured in the presence of the indicated titrations of CpG alone, or in the presence of the indicated concentrations of BAFF or LBW242 for 72 hr. Cellular Proliferation was then assessed by measurement of incorporated [H3]-Thymidine. Stimulations were performed in triplicate with bars indicating ± s.e.m. *, P < 0.05, **, P < 0.01. Data are representative of at least three independent experiments.
Co-stimulation of B-lymphocytes with Toll-Like Receptor (TLR) ligands and BAFF results in robust augmentation of B-lymphocyte proliferation. To explore the role of cIAPs in B-lymphocyte proliferation, we examined whether SM compounds could ‘mimic’ BAFF-mediated enhancement of B-cell proliferation in response to TLR ligation. B cells were treated with incremental concentrations of the TLR9 ligand, CpG, in the presence or absence of BAFF or LBW242 and then proliferation was assessed (Fig. 7e). As expected, co-stimulation of B-lymphocytes with BAFF significantly augmented proliferative responses elicited by CpG (left panel); similarly, LBW242 also markedly augmented proliferative responses elicited by CpG (right panel). These data demonstrate that cIAPs have an anti-proliferative function in B lymphocytes.
Rescue of TRAF3-deficiency by a single NIK gene
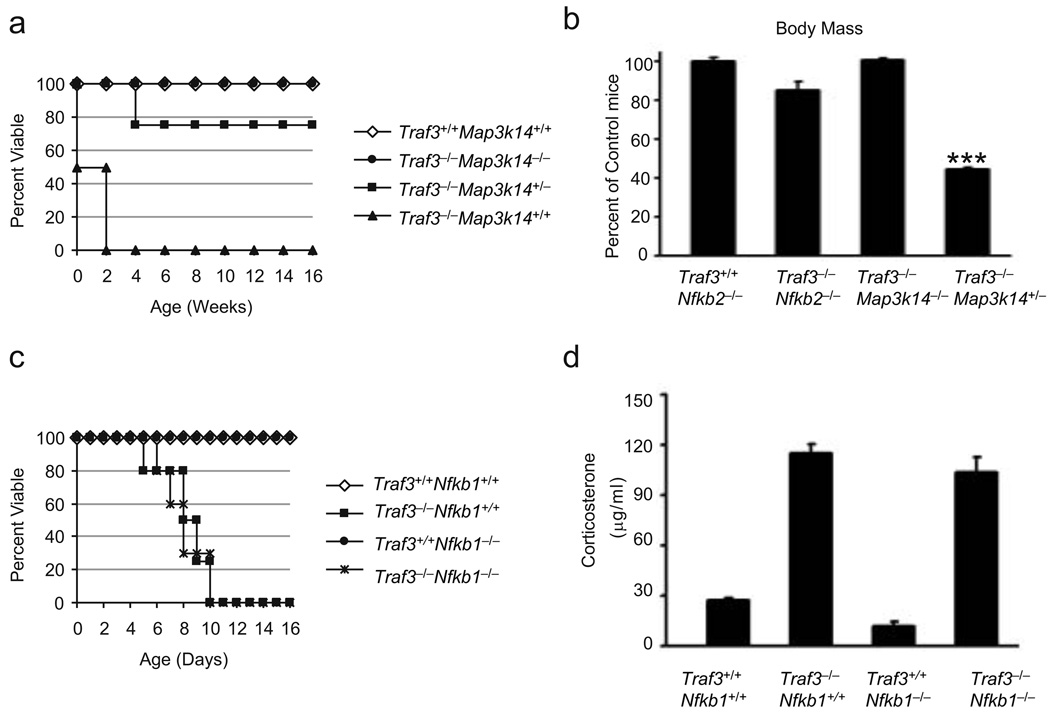
As a critical kinase involved in noncanonical NF-κB activation, NIK is not detectable in the basal state of most cell types and can only be induced by a select group of receptors such as BAFF-R, CD40 and LTβR. Elevated NIK expression resulting from Map3k14 gene amplification or mutations of the NIK-binding site on TRAF3 has recently been implicated in the development of human multiple myeloma. These data indicate the importance for tight control of NIK levels. This necessity is of course problematic for the potential use of SMAC mimetic compounds in cancer therapy regimes due to potential undesirable effects resulting from NIK activation. We have previously shown that all Traf3−/− mice die within two weeks of birth and that Traf3−/− cells have elevated NIK expression and constitutive noncanonical NF-κB activation. In addition, we have also shown that deletion the NF-κB p100 gene (Nfkb2) rescued the TRAF3-null phenotype. We have now generated Traf3−/−Map3k14−/− mice, which, as expected, rescued the TRAF3-null phenotype (Fig. 8a). In addition, where Traf3−/− mice exhibit severe depletion of all lymphocyte lineages, Traf3−/−Map3k14−/− mice produce lymphocyte populations similar to that observed in Map3k14−/− mice (Supplementary Fig. 9a). Surprisingly however, whereas Traf3−/− mice heterozygous at the p100 gene survive only a few days longer than Traf3−/− mice, Traf3−/−Map3k14+/− mice can survive for months after birth, though these mice do display a significant reduction in body mass (Fig. 8b). It was recently reported that endogenous accumulation of NIK markedly amplifies activation of the canonical NF-κB pathway and gene induction through the IKK complex30. Indeed we found that the inflammatory gene target Cxcl13 (gene for IP-10), which is elevated in Traf3−/− tissues, is normally expressed in the tissues of Traf3−/−Map3k14−/− mice (Supplementary Fig. 9b). One potential difference between the Traf3−/−Nfkb2+/− and Traf3−/−Map3k14+/− survival curves could be the level of canonical NF-κB amplification resulting from accumulated NIK as the Traf3−/− Nfkb2+/− still have two alleles of NIK. To determine the contribution of the canonical NF-κB pathway to the TRAF3-null phenotype, we crossed Traf3+/− mice to mice deficient for the p105-p50 gene (Nfkb1). Deletion of p50 is very effective in disrupting pathological responses associated with hyperactive canonical NF-κB activity as evidenced by the partial rescue of IκBα-deficient (Nfkbib−/−) phenotype by simultaneous deletion of Nfkb131. Analysis of Traf3−/−Nfkb1−/− mice, however, revealed no impact on the post-natal lethality or elevated serum corticosterone resulting from loss of TRAF3 (Fig. 8c,d). This finding suggests that NIK levels may be insufficient to induce robust p100 to p52 processing in Traf3−/−Map3k14+/− mice.
Figure 8. Rescue of the TRAF3-null phenotype by deletion of a single allele of NIK.
(a) Survival curve of mice of the indicated genotypes (n = 5 per strain). (b) Body Mass of mice of the indicated genotypes (n = 3 per strain), ***, P < 0.001. (c,d) Survival curve (n = 6 per strain) (c) and serum corticosterone levels (n = 3 per strain) (d) of mice of the indicated genotypes.
Discussion
Activation of the noncanonical NF-κB pathway by NIK is essential for the organization of secondary lymphoid tissue, whereas deregulation of this pathway results in multiple human diseases including cancers. Although genetic studies indicate that TRAF2, TRAF3, cIAP1 and cIAP2 are all involved in the negative regulation the noncanonical NF-κB pathway, little is known about the biochemical mechanisms responsible for regulation of NIK levels before and after receptor activation. In this study, we elucidate a simple model showing the distinct roles of TRAF2 and TRAF3 in bridging the gap between the cIAPs and NIK to form a complex to promote NIK degradation. In addition, we explored the potential of SMAC mimetic compounds to mimic BAFF-like co-stimulation of B lymphocytes. Strikingly, we found that such IAP antagonists greatly augmented B lymphocyte survival and proliferation. Collectively these data advance our mechanistic understanding of the NIK regulatory complex and reveal potential complications in therapeutic application of SMAC mimetic compounds.
At present, it is clear that basal activity of the noncanonical NF-κB pathway is suppressed by constitutive suppression of NIK protein levels through direct interaction with TRAF312,13,15. Recent genetic and pharmacological studies further suggest that TRAF2 and the cIAPs cooperate with TRAF3 in the proteasomal targeting of NIK, but a potential mechanism has not been described 12,13,15,16,19,20,32. We find that overexpression of either TRAF2 or TRAF3 in Traf3−/− and Traf2−/− cells, respectively, cannot compensate for the loss of the other. These results strongly suggest that both TRAF2 and TRAF3 possess highly specific and unique functions in the basal suppression of NIK. A comparison of the binding of TRAF2 and TRAF3 to the cIAPs and NIK revealed strikingly different abilities, with TRAF2 binding both cIAP1 and cIAP2 strongly and TRAF3 binding NIK strongly. With this finding, and the knowledge that TRAF2 and TRAF3 can heterodimerize in living cells25, we reasoned that TRAF2 and TRAF3 might function as a bridge to couple cIAPs with NIK. We have further identified a chimera 3TD, containing the zinc ring and zinc finger domains of TRAF2 and the TRAF domain of TRAF3, that promotes cIAPs interaction with NIK more efficiently than either TRAF2 or TRAF3 alone. Interestingly, this 3TD chimera is able to suppress the high basal noncanonical NF-κB activities of both Traf3−/− and Traf2−/− cells, which strongly supports our proposed model.
In addition to similar results in previous reports that TRAF2 and TRAF3 are degraded after receptor activation, our studies have further demonstrated that SMAC mimetic compounds can strongly inhibit receptor-induced TRAF2 and TRAF3 degradation, suggesting cIAPs are required for their degradation. Based upon our model, degradation of either TRAF2 or TRAF3 is sufficient to cause the dissociation of the NIK-cIAPs complex, resulting in accumulation of NIK through new protein synthesis. This also agrees with previous reports showing that BAFF-R, CD40 or LTβR-mediated noncanonical NF-κB requires new protein synthesis.
While TRAF2 and TRAF3 play distinct roles, our studies indicate that cIAP1 and cIAP2 are redundant in the negative regulation of noncanonical NF-κB activation. We have shown that either cIAP1 or cIAP2 can be recruited to the NIK complex through TRAF2 and TRAF3. More importantly, knockdown of cIAP1 in Birc3−/− cells, knockdown of cIAP2 in Birc2−/− cells or knockdown of both in wild-type cells results in NIK accumulation and noncanonical NF-κB activation. Consistent with recently studies, SMAC mimetic compounds, which strongly inhibit both cIAP1 and cIAP2, leads to NIK accumulation and noncanonical NF-κB activation. However, while those studies showed that SMAC mimetic compounds can potentiate cell death in certain cancer cell lines20,33, our study has shown that the same SMAC mimetic compounds can actually promote the survival and proliferation of primary B lymphocytes due to the induction of the noncanonical NF-κB pathway.
The present work further demonstrates that disruption of a single allele of the NIK gene is sufficient to rescue the TRAF3-null lethal phenotype, suggesting that NIK levels need to be tightly and precisely regulated. Elegant work recently15,16 found that a subgroup of multiple myeloma cells expresses elevated noncanonical NF-κB activity due to Map3k14 amplification, Traf3 mutation or loss of expression of both Birc2 and Birc3 genes. These results fit well with our proposed model in which non-redundant TRAFs cooperate with redundant cIAPs in negative regulation of NIK and noncanonical NF-κB activity. Finally, Given the known dangers of deregulated noncanonical NF-κB activity in B lymphocytes, SM compounds, which show great promise for the treatment of cancers, could inadvertently sensitize patients towards autoimmune disease or lymphomas34. Consequently, the pursuit of this very promising new treatment strategy should proceed cautiously, in order to better define the therapeutic window and to avoid potential negative immunological side effects.
Methods
Mice
Traf3−/−Nfkb2−/− mice were described previously13. To generate Traf3−/−Map3k14−/− and Traf3−/−Nfkb1−/− mice, Traf3+/− mice were crossed with either Map3k14−/− (obtained from Amgen) or Nfkb1−/− mice (Jackson Laboratories). Targeted disruption of the Traf3, Map3k14, and Nfκb1 alleles has been previously described11,35,36. All mice were maintained and bred under specific pathogen-free conditions in the University of California, Los Angeles Life Sciences mouse facility, and experiments were conducted within the parameters of our approved protocol by the Animal Research Committee.
cDNA constructs and reagents
Agonistic anti-CD40 antibody was generated by hybridoma, FgK-45. Antibodies against TRAF2 (H249), TRAF3 (M20), and Myc (9E10) were obtained from Santa Cruz Biotechnology. Antibodies against NIK and p100 (p52) were obtained from Cell Signaling Technology. Anti-rat IAP1 was used to detect endogenous cIAP1 and cIAP2, and has been described previously (Holcik, BMC Genomics). M2 (anti-Flag) agarose beads and anti-β-actin were obtained from Sigma. Rabbit TrueBlot Horseradish Peroxidase (HRP) anti-rabbit IgG was obtained from eBioscience. The protein G sepharose beads were obtained from Amersham.
Cell culture and transfections
Human embryonic kidney (HEK) 293T cells and murine embryonic fibroblasts (MEFs) were cultured in Dulbecco’s modified Eagle’s medium (Mediatech Inc) supplemented with 5% fetal bovine serum, penicillin (100 units/ml), and streptomycin (100 µg/ml). B cells were cultured in RPMI medium 1640 supplemented with 10% FBS, 50 µM β-mercaptoethanol, 100 U/ml penicillin and 100 µg/ml streptomycin (Life Technologies) at 37 °C under 10% CO2. BCR-Abl transformed wild-type and Traf3−/− B cell lines were generated as described and maintained in RPMI medium supplemented as above37. All reconstituted cell lines were generated as described previously27. Briefly, HEK 293T cells were transfected with Moloney Murine Leukemia virus-ψA helper construct plus either pBABEpuro alone or the indicated pBABEpuro construct. Traf3−/− and Traf2−/− MEFs were then infected with the filtered 293T cell supernatants followed by selection with 2.5 µg/ml of puromycin. Transient transfection of HEK 293T cells were performed using the standard calcium phosphate method. For all transfection experiments CMV-β-gal was used as a normalization control.
Immunoblotting
Whole cells were lysed in 1 × SDS loading buffer (62.5 mM Tris-Cl, pH 6.8, 5% β-mercaptoethanol, 2% (wt/vol) SDS, 10% (vol/vol) glycerol, and 0.1% (wt/vol) bromophenol blue). SDS extracts were sonicated for 10 seconds using the Misonix Sonicator 3000 and then boiled for 5 minutes. Equal amounts of whole cell lysates were fractionated by 8.5% SDS-PAGE, transferred to polyvinylidene fluoride membranes (Immobilon-P™) and immunoblotted with antibodies according to the manufacturer’s recommended instructions. Primary antibodies were detected with either anti-rabbit or anti-mouse antisera conjugated to horseradish peroxidase (Santa Cruz) and were visualized with electrochemiluminescence.
Immunoprecipitation
Cells were homogenized for 30 min at 4 °C in a modified radioimmune precipitation (mRIPA) buffer, containing 0.5% (vol/vol) NP-40, 0.1% (wt/vol) Na-Deoxycholate, and no SDS. Protease inhibitor cocktail (Sigma) was included in all lysates. For pBABE-Flag reconstituted cell lines, 2–5 mg of cell lysates were then incubated with anti-Flag M2 affinity gel for 2 hr, followed by treatment with 10 µl of protein G-Sepharose beads (Amersham Biosciences) for an additional 1 hr. For endogenous immunoprecipitation of NIK in primary B cells, A20 cells, and BCR-Abl transformed B cells, 5 × 107 cells were incubated with rabbit-IgG (Sigma) or anti-NIK for 4 hr, followed by treatment with 10 µl of protein G beads for an additional hour. The immunoprecipitated complexes were separated by SDS-PAGE and blotted with the indicated antibodies. Rabbit TrueBlot HRP anti-IgG was used as secondary antibody.
siRNA-mediated knockdown
Chemically synthesized siRNA duplexes were purchased from Invitrogen, and the following sequences (see Supplementary Table) were used for all experiments. For each experiment, 1 × 105 cells MEFs or primary hepatocytes derived from 3 month old C57BL/6 mice, were seeded onto 35 mm wells, and the following morning, 500 ng of siRNA duplex was transfected using Lipofectamine RNAiMAX Reagent (Invitrogen). Total cell lysates were collected 24 hours later for analysis.
B cell purification and stimulation
To obtain pure naive B cells, total splenocytes from 6–8 weeks old C57BL/6 mice were harvested and stained with a biotin-conjugated anti-CD43 (BD Biosciences), followed by streptavidin-conjugated magnetic microbeads (Miltenyi Biotec), and passed through a depletion type magnetic sorting column (Miltenyi Biotec). Unbound cells were collected as the purified naive B cell sample and analyzed by FACS for B220 and IgM markers. For the B cell survival assay, 2 × 105 B cells were stimulated for 72 hr in a flat-bottom 96-well plate, and cell death was measured after staining with propidium iodide and analyzed using a flow cytometer (FACScan; Becton Dickinson). For the B cell proliferation assay, 0.5 × 105 B cells were stimulated for 72 hr in a flat-bottom 96-well plate, and proliferation measured by addition of 0.5 µCi/well [H3]-Thymidine for the final 24 hr. Cellular DNA was then transferred to 96-well Filter Mat by automated cell harvester and analyzed by scintillation count.
Measurement of serum corticosterone levels
The corticosterone amount from mouse sera was determined as described previously13.
Supplementary Material
Acknowledgements
We would like to thank Dr. Xiadong Wang (University of Texas Southwestern Medical Center) for kindly providing the Smac mimetic (SM) compound, Dr. Leigh Zawel and Novartis Pharmaceuticals (Switzerland) for providing LBW242, and Amgen (Thousand Oaks, CA) for providing BAFF and Map3k14−/− mice. Part of this work was supported by the National Institutes of Health research grants R01GM078607 and R01GM57559 (G.C.) and part of this work was supported by funds from the Canadian Institutes of Health Research and the Howard Hughes Medical Institute (R.G.K).
References
- 1.Baeuerle PA, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 2.Iotsova V, et al. Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat Med. 1997;3:1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- 3.Kopp EB, Ghosh S. NF-κB and rel proteins in innate immunity. Adv Immunol. 1995;58:1–27. doi: 10.1016/s0065-2776(08)60618-5. [DOI] [PubMed] [Google Scholar]
- 4.Bonizzi G, Karin M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Zandi E, Karin M. Bridging the gap: composition, regulation, and physiological function of the IκB kinase complex. Mol Cell Biol. 1999;19:4547–4551. doi: 10.1128/mcb.19.7.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coope HJ, et al. CD40 regulates the processing of NF-κB2 p100 to p52. EMBO J. 2002;21:5375–5385. doi: 10.1093/emboj/cdf542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claudio E, Brown K, Park S, Wang H, Siebenlist U. BAFF-induced NEMO-independent processing of NF-κB2 in maturing B cells. Nat Immunol. 2002;3:958–965. doi: 10.1038/ni842. [DOI] [PubMed] [Google Scholar]
- 8.Dejardin E. The alternative NF-κB pathway from biochemistry to biology: pitfalls and promises for future drug development. Biochem Pharmacol. 2006;72:1161–1179. doi: 10.1016/j.bcp.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Matsushima A, et al. Essential role of nuclear factor (NF)-κB-inducing kinase and inhibitor of κB (IκB) kinase α in NF-κB activation through lymphotoxin β receptor, but not through tumor necrosis factor receptor I. J Exp Med. 2001;193:631–636. doi: 10.1084/jem.193.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shinkura R, et al. Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-κb-inducing kinase. Nat Genet. 1999;22:74–77. doi: 10.1038/8780. [DOI] [PubMed] [Google Scholar]
- 11.Yin L, et al. Defective lymphotoxin-β receptor-induced NF-κB transcriptional activity in NIK-deficient mice. Science. 2001;291:2162–2165. doi: 10.1126/science.1058453. [DOI] [PubMed] [Google Scholar]
- 12.Liao G, Zhang M, Harhaj EW, Sun SC. Regulation of the NF-κB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J Biol Chem. 2004;279:26243–26250. doi: 10.1074/jbc.M403286200. [DOI] [PubMed] [Google Scholar]
- 13.He JQ, et al. Rescue of TRAF3-null mice by p100 NF-κB deficiency. J Exp Med. 2006;203:2413–2418. doi: 10.1084/jem.20061166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grech AP, et al. TRAF2 differentially regulates the canonical and noncanonical pathways of NF-κB activation in mature B cells. Immunity. 2004;21:629–642. doi: 10.1016/j.immuni.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Annunziata CM, et al. Frequent engagement of the classical and alternative NF-κB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–130. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keats JJ, et al. Promiscuous mutations activate the noncanonical NF-κB pathway in multiple myeloma. Cancer Cell. 2007;12:131–144. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonizzi G, et al. Activation of IKKα target genes depends on recognition of specific κB binding sites by RelB:p52 dimers. EMBO J. 2004;23:4202–4210. doi: 10.1038/sj.emboj.7600391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter AM, LaCasse EC, Korneluk RG. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis. 2007;12:1543–1568. doi: 10.1007/s10495-007-0087-3. [DOI] [PubMed] [Google Scholar]
- 19.Varfolomeev E, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-κB activation, and TNFα-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 20.Vince JE, et al. IAP antagonists target cIAP1 to induce TNFα-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 21.Conte D, et al. Inhibitor of apoptosis protein cIAP2 is essential for lipopolysaccharide-induced macrophage survival. Mol Cell Biol. 2006;26:699–708. doi: 10.1128/MCB.26.2.699-708.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conze DB, et al. Posttranscriptional downregulation of c-IAP2 by the ubiquitin protein ligase c-IAP1 in vivo. Mol Cell Biol. 2005;25:3348–3356. doi: 10.1128/MCB.25.8.3348-3356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malinin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 24.Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 25.He L, Grammer AC, Wu X, Lipsky PE. TRAF3 forms heterotrimers with TRAF2 and modulates its ability to mediate NF-κB activation. J Biol Chem. 2004;279:55855–55865. doi: 10.1074/jbc.M407284200. [DOI] [PubMed] [Google Scholar]
- 26.Dadgostar H, Cheng G. An intact zinc ring finger is required for tumor necrosis factor receptor-associated factor-mediated nuclear factor-κB activation but is dispensable for c-Jun N-terminal kinase signaling. J Biol Chem. 1998;273:24775–24780. doi: 10.1074/jbc.273.38.24775. [DOI] [PubMed] [Google Scholar]
- 27.He JQ, Saha SK, Kang JR, Zarnegar B, Cheng G. Specificity of TRAF3 in its negative regulation of the noncanonical NF-κB pathway. J Biol Chem. 2007;282:3688–3694. doi: 10.1074/jbc.M610271200. [DOI] [PubMed] [Google Scholar]
- 28.Saha SK, et al. Regulation of antiviral responses by a direct and specific interaction between TRAF3 and Cardif. EMBO J. 2006;25:3257–3263. doi: 10.1038/sj.emboj.7601220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L, et al. A small molecule Smac mimic potentiates TRAIL- and TNFα-mediated cell death. Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 30.Zarnegar B, Yamazaki S, He JQ, Cheng G. Control of canonical NF-κB activation through the NIK-IKK complex pathway. Proc Natl Acad Sci U S A. 2008;105:3503–3508. doi: 10.1073/pnas.0707959105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beg AA, Sha WC, Bronson RT, Baltimore D. Constitutive NF-vB activation, enhanced granulopoiesis, and neonatal lethality in IκBα-deficient mice. Genes Dev. 1995;9:2736–2746. doi: 10.1101/gad.9.22.2736. [DOI] [PubMed] [Google Scholar]
- 32.Gardam S, Sierro F, Basten A, Mackay F, Brink R. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity. 2008;28:391–401. doi: 10.1016/j.immuni.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Gaither A, et al. A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-α signaling. Cancer Res. 2007;67:11493–11498. doi: 10.1158/0008-5472.CAN-07-5173. [DOI] [PubMed] [Google Scholar]
- 34.Fischer U, Janssen K, Schulze-Osthoff K. Cutting-edge apoptosis-based therapeutics: a panacea for cancer? BioDrugs. 2007;21:273–297. doi: 10.2165/00063030-200721050-00001. [DOI] [PubMed] [Google Scholar]
- 35.Xu Y, Cheng G, Baltimore D. Targeted disruption of TRAF3 leads to postnatal lethality and defective T-dependent immune responses. Immunity. 1996;5:407–415. doi: 10.1016/s1074-7613(00)80497-5. [DOI] [PubMed] [Google Scholar]
- 36.Sha WC, Liou HC, Tuomanen EI, Baltimore D. Targeted disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 37.Scherle PA, Dorshkind K, Witte ON. Clonal lymphoid progenitor cell lines expressing the BCR/ABL oncogene retain full differentiative function. Proc Natl Acad Sci U S A. 1990;87:1908–1912. doi: 10.1073/pnas.87.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.