Figure 9.
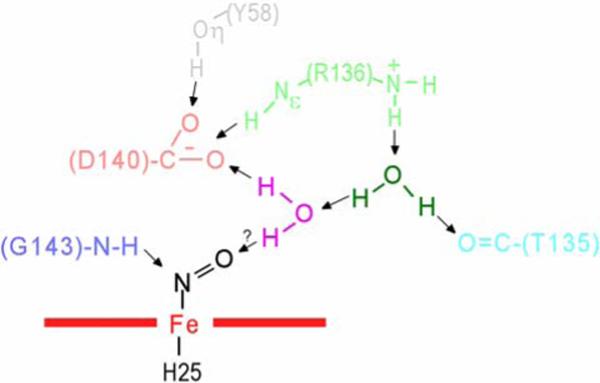
Schematic of active site structural features for the crystal structures (13) of WT hHO-PH-NO, illustrating the relative positions of four key residues in the distal H-bonding network, Tyr58 (grey), Thr135(aqua), Arg136(light green), Asp140(pink), Gly143(light blue), as well as two non-ligated, ordered water molecules, water#1(magenta) and water#2(dark green). (13) The most significant structural difference in the D140A-hHO mutant is the replacement of the Asp140 carboxylate with a new water molecule. H-bonds are shown by arrows.
