Abstract
Objective
To identify EEG features that index pain-related cortical activity, and to identify factors that can mask the pain-related EEG features and/or produce features that can be misinterpreted as pain-specific.
Methods
The EEG was recorded during 3 conditions presented in counterbalanced order: a tonic cold pain condition, and pain anticipation and arithmetic control conditions. The EEG was also recorded while the subjects made a wincing facial expression to estimate the contribution of scalp EMG artifacts to the pain-related EEG features.
Results
Alpha amplitudes decreased over the contralateral temporal scalp and increased over the posterior scalp during the cold pain condition. There was an increase in gamma band activity during the cold pain condition at most electrode locations that was due to EMG artifacts.
Conclusions
The decrease in alpha over the contralateral temporal scalp during cold pain is consistent with pain-related activity in the primary somatosensory cortex and/or the somatosensory association areas located in the parietal operculum and/or insula. This study also identified factors that might mask the pain-related EEG features and/or generate EEG features that could be misinterpreted as being pain-specific. These include (but are not limited to) an increase in alpha generated in the visual cortex that results from attention being drawn towards the pain; the widespread increase in gamma band activity that results from scalp EMG generated by the facial expressions that often accompany pain; and the possibility that non-specific changes in the EEG over time mask the pain-related EEG features when the pain and control conditions are given in the same order across subjects.
Significance
This study identified several factors that need to be controlled and/or isolated in order to successfully record EEG features that index pain-related activity in the somatosensory cortices.
Keywords: EEG, pain, primary somatosensory cortex, second somatosensory cortex, insula
INTRODUCTION
Psychophysical and functional imaging studies have shown that activation of a cortical area is characterized by a decrease in the amplitude of electroencephalographic (EEG) oscillations in the alpha band (8–12 Hz) and an increase in the gamma band (25–100 Hz), and that suppression of cortical activity is associated with an increase in alpha amplitude (Edwards et al. 2005; Fan et al. 2007; Feige et al. 2005; Foxe et al. 1998; Fu et al. 2001; Kahana 2006; Kelly et al. 2006; Laufs et al. 2006; Martinez-Montes et al. 2004; Moosmann et al. 2003; Pfurtsceller 1992; Sauseng et al. 2005; Stancak et al. 2003; Thut et al. 2006; Worden et al. 2000). Kilner et al. (2005) presented a theoretical model that explains how an increase in cortical activity will produce both an increase in fMRI BOLD activity and a shift from low (alpha) to high frequency EEG activity (gamma). Hence there appears to be a close relationship between the alpha and gamma band activities and the functional state of the cortex (see also Lopes da Silva 1991; Steriade et al. 1990).
This relationship predicts that a tonic experimental pain stimulus will produce a decrease in alpha and an increase in gamma amplitudes in the cortical areas that have been shown in regional cerebral blood flow studies to be consistently activated by painful stimuli, namely the primary somatosensory cortex (SI), the anterior cingulate cortex, and the somatosensory association areas located in the parietal operculum and insula (see Bushnell & Apkarian 2006; Craig 2002, 2003; Peyron et al. 2000; Rainville 2002 for reviews). Given the volume conduction properties of the head, these pain-related EEG activities should be evident over the central scalp contralateral to the pain stimulus, the fronto-central scalp, and the temporal scalp regions, respectively (see Bromm & Lorenz 1998; Dowman & Darcey 1994; Dowman & Schell 1999a,b; Dowman et al. 2007; Garcia-Larrea et al. 2003; Lenz et al. 1998a,b). However, despite its strong theoretical basis, this hypothesis was not confirmed in 11 studies performed over the past two decades that included electrodes over the scalp regions of interest and used a tonic experimental pain stimulus (see Table 1). Three of the 11 studies reported a decrease in the fronto-central alpha during pain, but 7 reported no change and 1 reported an increase. Four of the 11 studies found a decrease in the alpha recorded from the scalp overlying SI during pain, but one reported an increase and the remaining 6 reported no change. Two of the 11 studies observed a pain-related decrease in alpha over the temporal scalp, but one reported an increase and the rest no change. None of the studies measured gamma band activity.
Table 1.
Changes in alpha band amplitude during tonic experimental pain vs. passive no task control.
| STUDY | Pain Stimulus | Fronto-central Scalp | Central Scalp | Temporal Scalp |
|---|---|---|---|---|
| Backonja et al. (1991) | CPT | ↑ | n.c. | n.c. |
| Chang et al. (2001a) | capsaicin sc | n.c. | n.c. | n.c. |
| Chang et al. (2001b) | capsaicin im | n.c. | ↓ | n.c. |
| Chang et al. (2002a) | CPT | n.c. | ↓ | n.c. |
| Chang et al. (2002b) | saline im | n.c. | ↓ | n.c. |
| Chen et al. (1989) | CPT | n.c. | n.c. | n.c. |
| Chen & Rappelsberger (1994) | CPT | ↓ | ↓ | ↓ |
| Chen et al. (1998) | CPT | n.c. | n.c. | n.c. |
| Ferracuti et al. (1994) | CPT | ↓ | n.c. | n.c. |
| Huber et al. (2006) | Heat | ↓ | n.c. | ↓ |
| LaPera et al. (2000) | saline im | n.c. | ↑ | ↑ |
Abbreviations: Fronto-central scalp (Fz, Fpz, Fp1, Fp2 electrodes of the 10–20 electrode system) estimating alpha generated in the anterior cingulate cortex; central scalp (C3, C3′, C4, C4′ of the 10–20 electrode system) estimating alpha generated in SI; temporal scalp (T7 and T8 of the 10–20 electrode system (Sharbrough et al. 1991)) estimating alpha generated in the parietal operculum/insula; CPT = cold pressor test (cold pain); im = intramuscular; sc = subcutaneous; ↑ pain > passive no task control; ↓ pain < passive no task control; n.c. pain = passive no task control.
These negative results are surprising given how consistently the SI, parietal operculum/insula, and anterior cingulate cortices are activated by experimental pain (see above), and the robust decrease in alpha amplitude over the SI and primary motor cortex during movement and innocuous somatosensory stimulation (Pfurtsceller 1992; Stancak et al. 2003) and over the visual cortex during visual stimulation (Feige et al. 2005; Foxe et al. 1998; Fu et al. 2001; Kahana 2006; Kelly et al. 2006; Sauseng et al. 2005; Thut et al. 2006; Worden et al. 2000). There are, however, a number of factors that might have masked the pain-related EEG features in these 11 studies. First, most used short recording times. All recorded EEG for less than 6 minutes, and 9 recorded EEG for between 1.0 – 3.3 minutes. Backonja et al. (1991) found instabilities in the EEG during the first minute of the recording period that evolved into a more stable pattern later in the recording. Hence, this initial unstable period may have dominated the EEG measurements in many of the studies. Second, all studies compared the tonic experimental pain condition, where the subjects were required to rate the intensity of the pain either during or after the pain condition, to a passive no-task control condition. Variability both between and within subjects related to the direction of attention, vigilance, and/or arousal levels during the passive no-task control condition may have inflated error variance and consequently masked the pain-related EEG features. Third, in many cases details of the criteria used to reject EEG artifacts were not given. In most studies visual inspection for obvious eye movement and/or scalp muscle artifacts were performed. It is possible, therefore, that scalp muscle and/or eye movement artifacts masked the pain-related EEG features. The scalp EMG artifacts are of particular concern, given wincing and related facial expressions often accompany pain (Craig & Patrick 1985). Fourth, all but 2 of the studies (Chen & Rappelsberger 1994; Huber et al. 2006) presented the experimental and passive no-task control conditions in the same order across subjects, with the control condition preceding the tonic experimental pain condition. This may have introduced non-specific changes over time that masked the pain-related EEG features.
Five of the 11 studies addressed the attention, arousal, and vigilance issues noted above by comparing the experimental pain condition to either a non-painful (Backonja et al. 1991; Chang et al. 2001a,b; LaPera et al. 2000) or a pain threshold (Huber et al. 2006) somatosensory control stimulus applied to the same location as the tonic experimental pain. As in the pain condition, the subjects were required to attend to and rate the intensity of the non-painful or pain threshold control stimulus. Only one study reported a significant result, though its direction was the opposite of what was expected. That is, the alpha recorded from the contralateral central and temporal scalp during the experimental pain condition was larger than that for an innocuous vibration stimulus (LaPera et al. 2000). None of the other studies reported any differences in the alpha band activity recorded from the fronto-central, contralateral central, or temporal scalp locations between the experimental pain and the non-painful (Backonja et al. 1991; Chang et al. 2001a,b) or pain threshold (Huber et al. 2006) stimulus control conditions.
The failure to observe a decrease in alpha amplitude during the experimental pain condition compared to the non-painful and pain threshold stimulus control conditions in these studies can be explained by the organization of the pain-related cortical areas. In SI and the parietal operculum there is considerable spatial overlap between the neurons that respond to painful stimuli and those that respond to non-painful stimuli. Indeed, some neurons in these areas respond to both non-painful and painful stimuli (the wide dynamic range neurons; see Dong & Chudler 1995; Frot et al. 2001; Robinson & Burton 1980). Similarly, many nociresponsive neurons in the anterior cingulate cortex also respond to abrupt onset innocuous tactile stimuli (Sikes & Vogt 1992). Hence, both painful and non-painful somatosensory stimuli should produce decreases in alpha amplitude at fronto-central, central, and temporal scalp regions. The larger alpha recorded from the scalp regions overlying SI and the parietal operculum/insula during the pain vs. the innocuous vibration control condition in LaPera et al. (2000) may be due to the number of neurons in these areas that respond to innocuous vibration being considerably greater than those responding to noxious stimuli (Dong & Chudler 1995; Kenshalo & Willis 1991; Robinson & Burton 1980). Hence, the alpha recorded from over SI and the parietal operculum will be dominated by changes associated with the innocuous vibration, which will be larger in the pain condition (when not processing the vibratory stimulus) than the innocuous vibration condition.
The objective of this experiment was to identify EEG features indexing activity in the SI, anterior cingulate cortex, and/or parietal operculum/insula elicited by tonic experimental pain. Based on the evidence reviewed above we predict that the tonic experimental pain will be associated with a decrease in alpha and an increase in gamma amplitudes over the contralateral central, fronto-central, and temporal scalp regions, respectively. We employed a strong tonic pain stimulus in order to maximize the difference between the pain and no-pain control states. We also addressed the factors that may have prevented these pain-related EEG features from being observed in previous studies: 1). The pain and control conditions were presented in counterbalanced order across subjects to eliminate non-pain-related changes in the EEG that might occur over time. 2). We used 2 control conditions, an arithmetic task control that that kept the subjects’ attention, arousal, and vigilance states constant throughout the control condition, and a pain anticipation control that attempted to produce attention, arousal and vigilance states comparable to the pain condition. 3). A 10-minute recording block was used to ensure that the pain-related EEG features had stabilized. 4). A strict EEG artifact rejection criteria was used to minimize scalp EMG and eye movement artifacts. 5). We compared the pain-related EEG features to those produced by wincing to determine whether any of the EEG frequency bands were contaminated by scalp EMG activity generated by the facial expressions that often accompany pain (Craig & Patrick 1985).
METHODS
Subjects
Fifteen healthy young adults participated in the experiment (mean ± SD age= 20.1 ± 2.9 years, 9 males). Each participant was given a detailed explanation of the procedure and each signed an informed consent document prior to participating. At each stage of the study the participants were reminded that they could withdraw from the experiment at any time for any reason. The procedure was approved by the Clarkson University Institutional Review Board. The participants were comfortably positioned in a recliner chair located in an electrically shielded, sound attenuated, and temperature controlled (21 ± 1 °C) recording chamber.
Recording Parameters
The EEG was recorded from 29 electrodes arranged on the scalp in a grid centered on a location 2-cm posterior to the vertex position (Cz′) of the International 10–20 Electrode System (Sharbrough et al. 1991). The inter-electrode distance along the sagittal and coronal axes was 5 cm. The scalp electrodes were referenced to the non-cephalic sternovertebral electrode, which has been shown to have negligible pain-related cortical activity (Dowman & Goshko 1992). Eye movements were recorded from 2 electrodes positioned just lateral to the lateral canthus and over the inferior portion of the orbicularis oculi muscle of the left eye. The recording electrode impedances were less than 5000 Ohms. The EEG and eye movement potentials were filtered between 0.3 and 100 Hz (−6 dB points) and sampled at 200 Hz.
Procedure
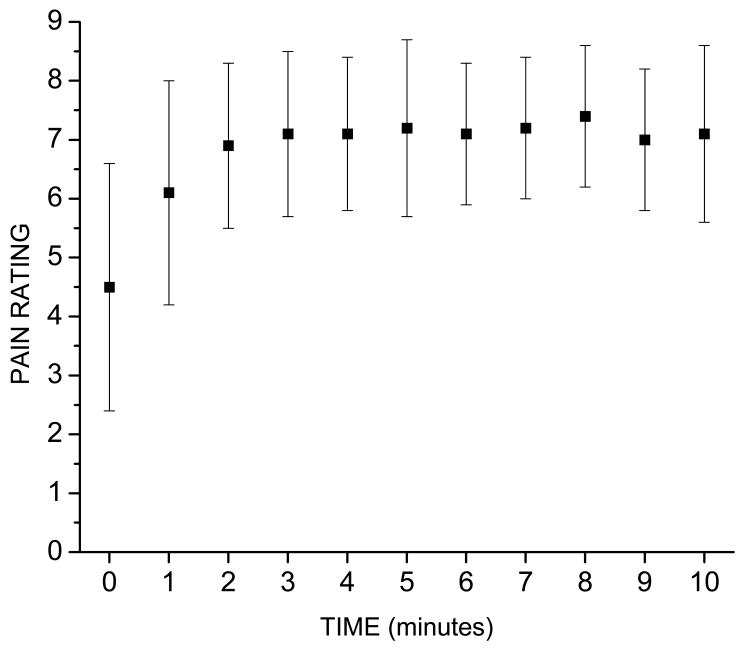
Each subject participated in three conditions presented in counterbalanced order within a single session. Each condition lasted 600 seconds and the subjects were given at least 5 minutes between each condition to rest. In the cold pain condition subjects placed their left hand in a bucket of ice water. The mean (± SD) temperatures at the beginning and end of the cold pain block were 4.3 ± 0.8 °C and 4.5 ± 2.1 °C, respectively. At one minute intervals the subjects were asked to verbally rate the perceived intensity of the cold pain on a 9-point rating scale, where 1= sensory threshold, 5= pain threshold, and 9= maximum pain tolerable. The subjects were told that if the pain became unbearable before the 600 second recording block was finished that they could take their hand out of the water. However, none did so. The mean (± SD) pain ratings recorded throughout the cold pain block are shown in Figure 1. The cold was near pain threshold immediately upon placing the hand in the water, was moderately painful at the end of the first minute, and was more or less constant for the remaining 9 minutes.
Figure 1.
Mean (± SD) pain ratings obtained immediately after putting the hand in the cold water (time 0) and at 1 minute intervals throughout the cold pain condition. Pain was rated on a 9-point scale, where 1= sensory threshold, 5= pain threshold, and 9= maximum pain tolerable.
In the arithmetic control condition the subjects placed their left hand in a bucket of luke-warm water. The mean (± SD) water temperatures at the beginning and end of the block were 36.9 ± 0.4 °C and 35.4 ± 0.5 °C, respectively. The subjects were given a randomly chosen 4-digit number at the beginning of the block and were asked to count backwards by 7’s throughout the 600 second recording block. The arithmetic control condition attempted to maintain constant levels of vigilance and focused attention throughout the control recording epoch, and it controlled for any innocuous pressure sensation associated with placing the hand in the water.
In the pain anticipation condition 11 electrical stimuli were presented to the right sural nerve at the ankle. The electrical stimuli consisted of a 5-pulse train, with a 1 ms pulse duration and a 250 Hz pulse frequency. The subjects were told that most of the electrical stimuli would be non-painful, but that some would be very painful. They were also told that the longer they went without receiving the very painful stimulus the more likely it was to occur. Immediately prior to the pain anticipation condition the subjects were given a single ascending series of electrical stimuli in order to estimate their pain threshold and pain tolerance levels. One strong painful stimulus was given at the very beginning of the recording block and another after the end of the 600 second pain anticipation recording block. The remaining 9 stimuli were non-painful. The interval between electrical stimuli was random, with a mean of 56.0 seconds and a range of 10–107 seconds. The subjects rated the intensity of each stimulus verbally, using the 9-point scale describe above. The mean (± SD) stimulus currents for the non-painful and painful stimuli were 1.7 ± 1.0 mA and 8.3 ± 3.1 mA, respectively. The mean (± SD) ratings for the non-painful and painful electrical stimuli were 3.2 ± 1.0 and 6.8 ± 1.4 respectively. The pain anticipation control attempted to provide the same level of vigilance, arousal, and attention as the cold pain condition.
Data Analysis
Each digitized 600 second recording block was separated into 128 data point segments, each accounting for 0.64 seconds. We chose this short analysis epoch to maximize the duration of the artifact-free EEG. Analysis epochs whose eye potentials were greater than 40 μV and/or whose EEG amplitudes were greater than 75 μV were rejected off-line in order to reduce eye movement and/or scalp muscle EMG artifacts. In the pain anticipation condition, the 128 data point analysis epochs that immediately followed each electrical stimulus were also rejected to eliminate somatosensory evoked potentials from the analysis. The mean (± SD) duration of the artifact-free analysis epochs for the cold pain, arithmetic control, and pain anticipation control conditions were 402 ± 101 seconds, 411 ± 108 seconds, and 399 ± 114 seconds, respectively. There were no significant differences in the duration of the analysis epochs across the 3 conditions (F(2,28) = 0.13 p>.10 ε = .82). Six subjects were given an additional condition at the end of the experiment where they were asked to make a wincing facial expression for 600 seconds. This helped determine whether the EEG was contaminated by scalp EMG activity related to the wincing facial expression that often accompanies pain (Craig & Patrick 1985). The same artifact rejection criteria were applied to the EEG recorded during the wincing condition as the other 3 conditions.
The amplitudes of the EEG frequency bands were computed using a Fast Fourier Transform (FFT), and were averaged across the analysis epochs. The FFT was applied to the Laplacian of the artifact-free epochs. The Laplacian is the second spatial derivative of the spherical spline polynomials used to interpolate the electric potential across the scalp. The Laplacian has been shown to improve the signal-to-noise ratio, especially noise originating outside the electrode grid such as noise originating at or near the reference electrode (Klein & Carney 1995; Nunez et al. 1994). The Laplacian is also thought to reduce the smearing of the scalp topography produced by the high impedance skull (Gevins & Cutillo 1995). Indeed, the scalp topographic patterns of the Laplacian EEG were similar to but more sharply defined than the raw EEG topographies. Likewise, the results of the statistical analyses were essentially the same for the raw and Laplacian EEG data, including EEG recorded from the edges of the electrode grid. The frequency component amplitudes were averaged across the delta (1.6–3.1 Hz), theta (4.7–6.3 Hz), alpha1 (7.8–9.4 Hz), alpha2 (10.9–12.5 Hz), beta1 (14.1–17.2 Hz), beta2 (18.8–25.0 Hz), gamma1 (26.6–56.3 Hz), and gamma2 (62.5–98.4 Hz) frequency bands.
Pain-related changes in the EEG were evaluated using a 2-way repeated measures analysis of variance (ANOVA: condition (cold pain, arithmetic control, pain anticipation) x recording electrode location). When appropriate (i.e., numerator degrees of freedom > 1) Greenhouse Geisser-corrections were applied to the repeated measures ANOVA to correct for violations of the sphericity assumption. In reporting significance levels, the uncorrected degrees of freedom are given along with the epsilon (ε) values used to adjust the degrees of freedom in determining the significance level. We compared the amplitudes of the cold pain and the control conditions at each electrode location when there was a significant condition x electrode location interaction (see below). These comparisons were performed using the Newman-Keuls test to correct for multiple comparisons.
RESULTS
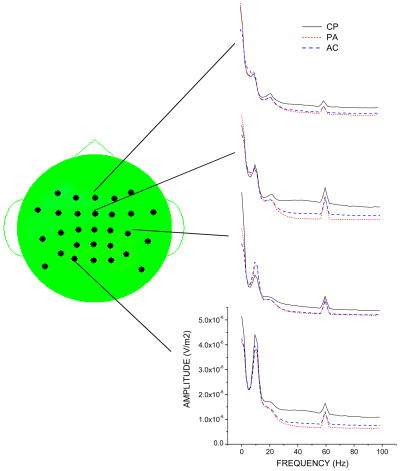
In most cases the dominant topographic features of the EEG frequency bands recorded during each condition did not correspond to pain-related activity. For example, in the cold pain and control conditions the maxima for the alpha band activity were located at the posterior electrodes overlying the visual cortices (data not shown). The pain-related EEG features appear as subtle changes in the EEG topography across the cold pain and control conditions. These subtle changes are best revealed by computing difference topographies between the pain and control conditions, as shown in Figure 2. (Note that the difference topographies are shown for illustrative purposes only. The statistical analyses reported below involve comparisons between the means of the pain and control conditions.) The EEG spectra obtained during the cold pain and control conditions at the scalp locations showing the largest differences between the pain and control conditions are shown in Figure 3.
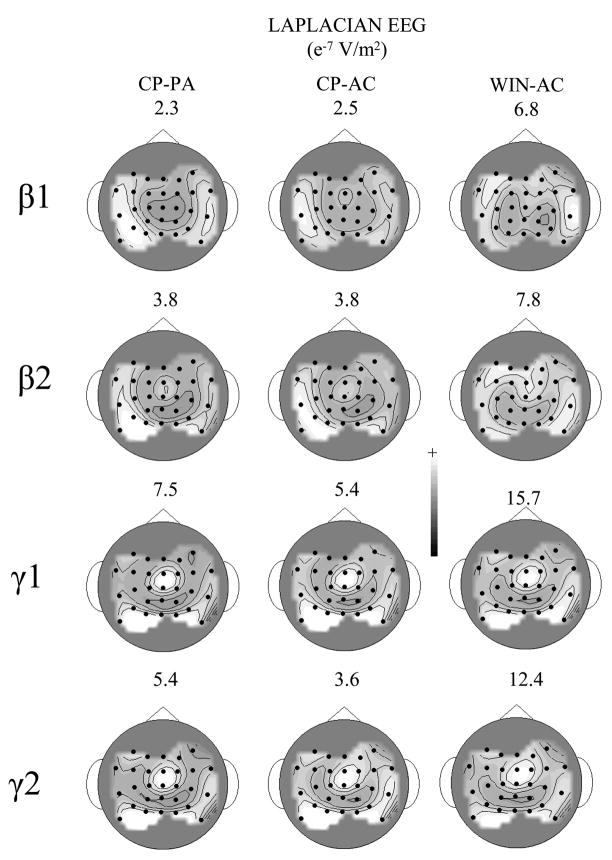
Figure 2.
Grand average Laplacian scalp topographic patterns of the differences between the cold pain (CP) and the pain anticipation (PA) conditions (left most column), between the cold pain and the arithmetic control (AC) conditions (middle column), and between the wincing (WIN) and the arithmetic control conditions (right most column) at each EEG frequency band. The solid circles identify recording electrode locations, where Cz′ (2-cm posterior to the Cz location of the 10–20 system; Sharbrough et al. 1991) is the third electrode from the bottom along the sagittal midline. The inter-electrode distance along the sagittal and coronal axes is 5 cm. The lines are isovoltage contours. The scaling was adjusted in each topography to best depict its topographic pattern. The scaling (in e−7 V/m2) is given at the top of each topography.
Figure 3.
EEG spectra obtained from scalp locations showing the largest differences between the cold pain and the control conditions. Abbreviations: CP = cold pain condition; PA = pain anticipation control condition; AC= arithmetic control condition.
The differences between the grand average topographies obtained during the wince condition and the arithmetic control condition at each frequency band are also shown in Figure 2. Note that there is little or no similarity between the cold pain-related changes in the EEG and the wince patterns within the delta, theta, alpha and beta frequency bands (r2 (the proportion of variance one topographic pattern accounts for another) ranged from 0.00 – 0.30). The cold pain and wince topographic patterns for the gamma bands on the other hand were very similar (r2 ranged from 0.75–0.87). (Note that the scalp topographic patterns were essentially the same at all frequencies within the gamma bands.) Hence, we can assume that the gamma1 and gamma2 bands were heavily contaminated by scalp EMG artifacts associated with wincing and, consequently, they will not be analyzed further.
Our working hypothesis states that there will be pain-related changes in the EEG at specific electrode locations, namely the contralateral central, fronto-central and the temporal scalp regions. These changes will be indexed by a significant condition x electrode interaction term in the ANOVA. Only the cold pain vs. pain anticipation comparison for the beta1 band had a significant condition main effect in the absence of a significant condition x electrode interaction (Table 2). This demonstrates that the cold pain-related increase in beta1 amplitude was the same at all electrodes. Given our understanding of the cortical representation of pain and the relationship between cortical activation and the EEG reviewed in the Introduction, it is unlikely that this non-specific change reflects a pain-related EEG feature. It will not, therefore, be dealt with further.
Table 2.
ANOVA Summary for the Condition x Electrode Interaction (df = 28,392)
| delta |
theta |
alpha1 |
alpha2 |
beta1 |
beta2 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | F | ε | F | ε | F | ε | F | ε | F | ε | F | ε |
| CP vs PA | 1.54 | .11 | 4.86** | .16 | 7.00*** | .11 | 6.46*** | .14 | 2.59 | .09 | 3.81* | .11 |
| CP vs AC | 1.48 | .08 | 6.90** | .13 | 4.90** | .09 | 3.78* | .12 | 1.63 | .10 | 2.81* | .12 |
| PA vs AC | 0.95 | .15 | 4.19** | .12 | 1.00 | .11 | 0.42 | .11 | 1.37 | .14 | 1.02 | .08 |
All of the electrode main effects were significant (Fs ≥ 9.44, p<.05, ε ranged from .06– .18), and all the condition main effect terms were non-significant (Fs ≤ 3.17, p≥ .10), with the exception of the beta1 CP vs. PA (F(1,14) = 17.39 p<.0001).
Abbreviations: CP = cold pain; PA = pain anticipation control; AC = arithmetic control.
= p<.001,
= p<.01,
= p<.05
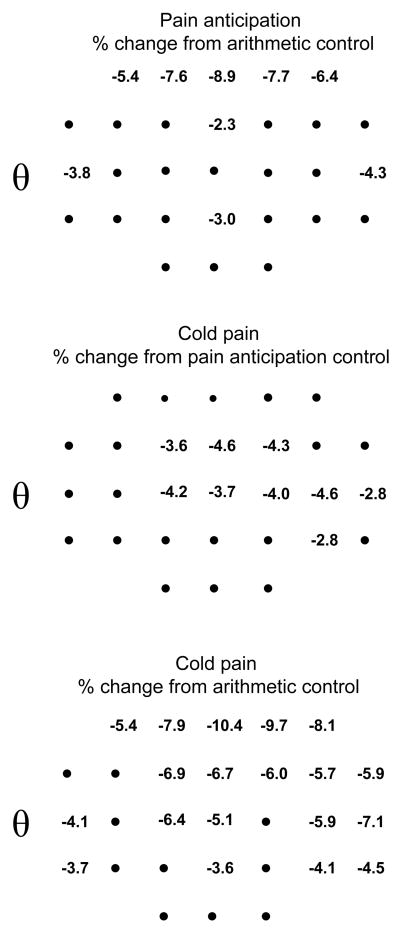
The ANOVA condition × electrode interaction terms for the cold pain vs. pain anticipation, the cold pain vs. arithmetic control, and the pain anticipation vs. arithmetic control comparisons are given in Table 2. The only significant difference between the pain anticipation and arithmetic control conditions occurred at the theta frequency band, where the amplitudes at the frontal, fronto-central, bilateral temporal, and parietal midline locations were smaller during the pain anticipation control than during the arithmetic control (Figure 4).
Figure 4.
Percent change in theta amplitude between the pain anticipation (PA) and arithmetic control conditions (AC) (computed as ([PA−AC]/AC)*100), between the cold pain and pain anticipation conditions (computed as ([CP−PA]/PA)*100), and between the cold pain and arithmetic control conditions (computed as ([CP−AC]/AC)*100). The numbers are the percent change at electrode locations showing a statistically significant change in amplitude between conditions (Newman-Keuls test, p<.05). The closed circles correspond to electrode locations that did not show a statistically significant change in amplitude. The nose is located towards the top of each panel, and the left ear towards the left of each panel. Cz′ (2-cm posterior to the Cz location of the 10–20 system; Sharbrough et al. 1991) is the third location from the bottom along the sagittal midline. The inter-electrode distance along the sagittal and coronal axes is 5 cm.
There were no significant differences between the cold pain and either the pain anticipation or arithmetic control conditions for the delta frequency band (Table 2). There were, however, significant differences between the cold pain and the pain anticipation and arithmetic control conditions for theta band amplitude (Table 2, Figure 4). Both comparisons involved smaller theta amplitudes in the fronto-central and contralateral temporal scalp regions in the cold pain condition (Figure 4). The cold pain condition also demonstrated smaller delta amplitudes at frontal scalp regions than the arithmetic control.
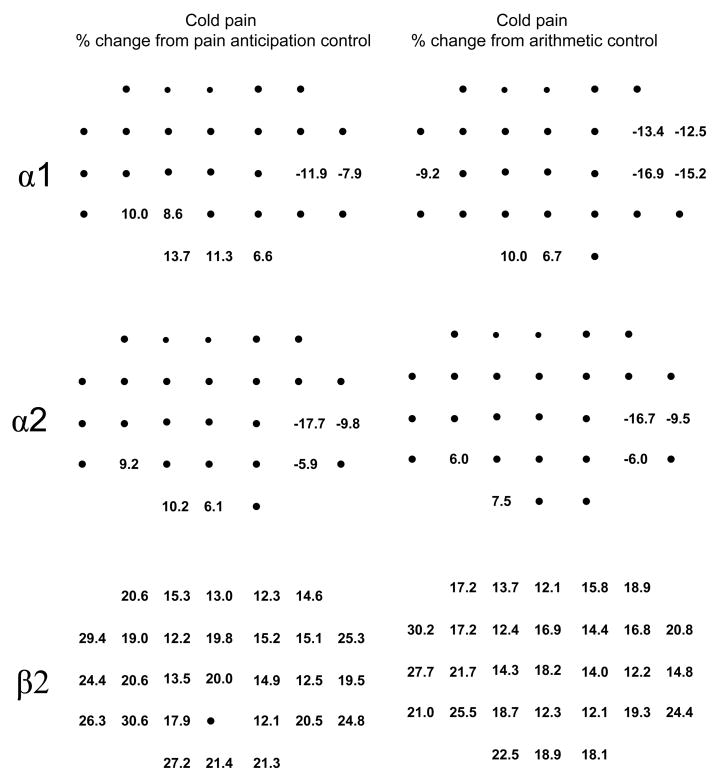
Cold pain exhibited similar differences with the two control conditions at the alpha1 and alpha2 bands, where the cold pain condition had smaller alpha amplitudes at the contralateral temporal scalp sites and larger alpha amplitudes at posterior scalp sites (Table 2, Figure 5). The alpha1 recorded during the cold pain was also smaller over the ipsilateral temporal scalp, but only when compared to the arithmetic control (Figures 2 & 5). Cold pain had larger beta2 amplitudes than both control conditions at essentially all scalp recording locations though, as indicated by the significant condition x electrode location interaction term, some locations exhibited larger increases than others (Table 2, Figure 5). However, the pattern of these changes is not consistent with our understanding of the cortical representation of pain and is suspiciously close to that for the wince pattern obtained in the gamma bands (see Figure 2). It seems unlikely, therefore, that the beta2 pattern constitutes a pain-related EEG feature and, consequently, it will not be dealt with further.
Figure 5.
Percent change in amplitude between the cold pain (CP) and pain anticipation (PA) control (left column) (computed as ([CP−PA]/PA)*100) and between the cold pain and arithmetic control (AC) (right column) (computed as ([CP−AC]/AC)*100) for the alpha1 (top row), alpha2 (middle row) and beta2 frequency bands (bottom row). The numbers are the percent change at electrode locations showing a statistically significant change in amplitude between conditions (Newman-Keuls test, p<.05). The closed circles correspond to electrode locations that did not show a statistically significant change in amplitude. The nose is located towards the top of each panel, and the left ear towards the left of each panel. Cz′ (2-cm posterior to the Cz location of the 10–20 system; Sharbrough et al. 1991) is the third location from the bottom along the sagittal midline. The inter-electrode distance along the sagittal and coronal axes is 5 cm.
DISCUSSION
In this study we observed EEG features that are consistent with pain-evoked activity in the somatosensory cortices. We also identified a number of factors that could mask these pain-related EEG features and/or result in features that could be misinterpreted as pain-specific. These include changes in the EEG caused by a shift in attention away from some other sensory modality and towards the pain; differences in working memory load that might occur between the pain and some control conditions; the possibility that there are non-specific changes in the EEG over time that could mask the pain-related EEG features if the order of the pain and control conditions are not counterbalanced; and the possibility that the no-task control condition results in large between and within subject variability in arousal, attention, vigilance, etc., that makes it more difficult to identify and isolate the pain-related EEG features.
EEG Indices of Pain-Related Cortical Activity
The decrease in alpha over the contralateral temporal scalp during the cold pain condition relative to the pain anticipation and arithmetic control conditions is consistent with the pain-related activation of the somatosensory cortices reviewed in the Introduction. The low spatial resolution of the scalp potentials and the close proximity of the SI hand area and the somatosensory association areas in the parietal operculum make it impossible to determine which of these somatosensory areas is responsible for the pain-related decrease in the temporal scalp alpha. Unfortunately, the contralateral localization of the alpha decrease does not help discriminate between these two cortical areas. The SI response to noxious inputs is well known to largely involve the side contralateral to the stimulus (Coghill et al. 2001; Peyron et al. 2000). Although the somatosensory association areas in the parietal operculum and insula receive bilateral nociceptive inputs, electrophysiological and regional cerebral blood flow studies have shown that their response is largest and most consistent on the side contralateral to the painful stimulus (Coghill et al. 2001; Peyron et al. 2000; Robinson & Burton 1980). Hence, the decrease in the temporal scalp alpha could reflect pain-related activity in the parietal operculum/insula, the SI hand area (Coghill et al. 2001; Peyron et al. 2000), or a combination of both. Further studies comparing the alpha scalp topographies recorded while painful stimuli are applied to the foot and hand will be necessary to address this question. That is, if the pain-related decrease in alpha is generated in SI then it will be located over the central midline scalp for pain applied to the foot, and if is generated in the parietal operculum/insula then the foot pain-related decrease in alpha will be over the temporal scalp. This question should also be addressed using intracranial recordings.
We did not observe a decrease in alpha over the fronto-central scalp indicative of pain-evoked activation of the anterior cingulate cortex. There are two possible reasons for this. First, there are some cortical areas for which a decrease in alpha cannot be reliably detected from the scalp. For example, voluntary hand movements decrease the alpha recorded from the scalp overlying the primary sensorimotor cortex hand area, whereas foot movements do not result in a reliable decrease in alpha recorded from the central midline scalp overlying the primary sensorimotor cortex foot area (see Pfurtscheller & Lopes da Silva 1999 for review). Second, the anterior cingulate cortex has multiple, functionally distinct subregions located in close proximity, including subregions involved in pain affect (Davis et al. 1997; Rainville 2002; Vogt et al. 1993), attentional control (Botvinick et al. 2001, Dowman 2004, 2007a,b; Dowman et al. 2007; Yeung et al. 2004), and response selection (Devinsky et al. 1995; Vogt & Sikes 2000). It may be that activation of the pain-related subregion of the anterior cingulate was masked by activities in the other subregions.
The close similarity between the gamma band scalp topographic patterns obtained during the cold pain and wincing conditions implies that the gamma band was heavily contaminated by scalp EMG associated with the wincing facial expression that often accompanies pain (Craig & Patrick 1985), even with the strict artifact rejection criteria employed here. Techniques that eliminate these EMG artifacts must be developed before the gamma band activity can be used to measure pain-related cortical activity. Importantly, the wincing data also demonstrate that scalp EMG artifacts do not appear to contaminate the pain-related changes in the alpha band, at least when strict artifact rejection criteria are used.
Of the 11 EEG studies reviewed in the Introduction, only the two that counterbalanced the control and pain conditions (Chen & Rappelsberger 1994; Huber et al. 2006) reported the same pain-related decrease in alpha over the temporal scalp that was observed here. This raises the possibility that there are non-specific changes in the EEG over time that masked the pain-related EEG features in the other studies. It is important, therefore, to counterbalance the order of presentation of the pain and control conditions across subjects.
Our study also differs from the previous EEG studies of pain in that we did not use a passive no-task control condition. As explained in the Introduction, variability both between and within subjects in vigilance, attention, arousal, etc. during the no-task control could result in large error variance, which will make it much more difficult to identify and isolate the pain-related EEG features. In the pain anticipation and arithmetic control conditions used in our study attention and vigilance would have been much less variable within their respective recording epochs.
Effects of Attention on Pain- and Non-Pain-Related EEG Features
There was an increase in alpha at the posterior scalp during the cold pain condition that was probably generated by the visual cortices. Several authors have reported an increase in alpha over the posterior scalp when attention is directed away from the visual modality and towards an auditory target stimulus (Feige et al. 2005; Foxe et al. 1998; Fu et al. 2001) or towards a voluntary movement (see Pfurtscheller & Lopes da Silva 1999 for review). This change in attention results in a decrease in the activation of the visual cortex and an accompanying increase in its alpha amplitude. The larger alpha amplitude at the posterior scalp regions during the cold pain condition observed here implies that there was a greater reduction in visual cortex activity during the cold pain condition than during the pain anticipation and arithmetic control conditions. It is well known that pain demands attention (Eccleston & Crombez 1999), and it might be the case that the strong cold pain stimulus drew more attentional resources from the visual modality than the subtraction task in the arithmetic control condition or waiting for the next sural nerve electrical stimulus in the pain anticipation condition.
We should expect, therefore, that directing attention towards pain will affect the alpha generated by the visual cortices. Whether or not the subject directs their attention away from the visual modality and towards the pain will depend on factors that are not directly related to the pain or easy to predict. For example, subjects who attempt to cope with the pain by visualizing themselves on a tropical beach should exhibit a decrease in posterior scalp alpha. Subjects who focus their attention on the pain, whether because of its task relevance as in this study or because of its inherent threat value, should exhibit an increase in posterior scalp alpha. These different attentional strategies might explain why our study and Backonja et al. (1991) reported an increase in posterior scalp alpha amplitude during the tonic experimental pain condition whereas Ferracuti et al. (1994), Chang et al. (2001a,b) and Chang et al. (2002a,b) observed a decrease.
Fortunately, the attention-related changes in alpha generated by the visual cortex can be easily separated from the pain-related changes based on their different scalp distributions. Distinguishing a pain-related decrease in alpha from attention-related changes in alpha generated by the auditory cortex might be more problematic, given the close proximity of the auditory cortex to the parietal/operculum and SI hand area. The alpha generated by the auditory cortex is not reliably detected from the scalp (Foxe et al. 1998; Klimesch 1999), and hence it might not interfere with the pain-related decreases in alpha recorded from the temporal scalp. More work is needed to verify this.
Interestingly, there were no differences in alpha between the pain anticipation and arithmetic control conditions even though the subjects were attending to a painful stimulus expected at the ankle during the pain anticipation condition. Directing attention to a location in sensory space has been shown to decrease alpha in the cortical area representing that sensory space even when a stimulus is not presented (Babiloni et al. 2003, 2004, 2005, 2006; Del Perico et al. 2006; Kelly et al. 2006; Sauseng et al. 2005; Thut et al. 2006; Worden et al. 2000; Yamagishi et al. 2003). The decrease in alpha amplitude in this situation is consistent with regional cerebral blood flow and electrophysiological studies showing that merely directing attention towards an expected target stimulus increases the baseline activity of the sensory cortices involved in processing that stimulus (see Kanwisher & Wojciulik 2000 and Kastner & Ungerleider 2000 for reviews). It is surprising, therefore, that attending to the ankle in the pain anticipation condition did not result in a decrease in alpha at the central midline scalp overlying the SI foot area and/or at the temporal scalp overlying the parietal operculum/insula. Indeed, there were no differences in alpha amplitude between the pain anticipation and arithmetic control conditions at any scalp location.
Studies that have reported attention-related decreases in alpha amplitude over sensory cortex in the absence of a target stimulus employed a cue stimulus that immediately preceded the target. The cue stimulus predicted exactly when the target was going to occur, and the decrease in alpha band activity occurred during the cue – target stimulus interval (Foxe et al. 1998; Sauseng et al. 2005; Thut et al. 2006; Worden et al. 2000). This paradigm differs considerably from our experiment, where the sural nerve electrical stimuli were not cued, were given at long, random inter-stimulus intervals and were, therefore, unpredictable. Hence, attention-related decreases in alpha in the absence of a stimulus may not occur under these conditions. This implies that merely thinking about a painful stimulus will not elicit the same changes in alpha as presenting an actual painful stimulus. However, it is also possible that sustained attention only affects the SI cortex and that alpha generated by the SI foot area is not reliably recorded from the scalp. Indeed, as noted above, decreases in alpha associated with voluntary movements can be detected from over the primary sensorimotor cortex hand area but not from over the foot area (Pfurtscheller & Lopes da Silva 1999). Further work will be necessary delineating the effects of sustained attention on alpha amplitude and whether it could confound any decreases in alpha amplitude due to pain-evoked activity.
Effects of Changing Working Memory Load on the Pain-Related EEG Features
We unexpectedly found that the frontal theta amplitude was smaller during the pain anticipation than the arithmetic control condition. This difference is probably related to the increase in frontal theta amplitude that has been shown to occur with an increase in working memory load (Gevins et al. 1997; Gomarus et al. 2006; Jacobs et al. 2006; Kahana 2006), given working memory load was greater when performing the subtraction task than merely waiting for the next sural nerve electrical stimulus. Note, however, that the lack of difference between these control conditions for the alpha bands implies that changes in working memory load should not interfere with the pain-related changes in alpha.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babiloni C, Brancucci A, Arendt-Nielsen L, Babiloni F, Capotosto P, Carducci F, Cincotti F, Del Percio C, Petrini L, Rossini PM, Chen ACN. Attentional processes and cognitive performance during expectancy of painful galvanic stimulations: a high-resolution EEG study. Behav Brain Res. 2004;152:137–147. doi: 10.1016/j.bbr.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Brancucci A, Capotosto P, Arendt-Nielsen L, Chen ACN, Rossini PM. Expectancy of pain is influenced by motor preparation: A high resolution EEG study of cortical alpha rhythms. Behavioral Neurosci. 2005;119:503–511. doi: 10.1037/0735-7044.119.2.503. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Brancucci F, Capotosto P, Carducci F, Cincotti F, Arendt-Nielse L, Chen ACN, Rossini PM. Anticipatory cortical responses during the expectancy of a predictable painful stimulation. A high-resolution electroencephalography study. Eur J Neurosci. 2003;18:1692–1700. doi: 10.1046/j.1460-9568.2003.02851.x. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Brancucci A, Del Percio C, Capotosto P, Arendt-Nielsen L, Chen ACN, Rossini PM. Anticipatory electroencephalography alpha rhythm predicts subjective perception of pain intensity. J Pain. 2006;7:709–717. doi: 10.1016/j.jpain.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Backonja M, Howland EW, Want J, Smith J, Salinsky M, Cleeland CS. Tonic changes in alpha power during immersion of the hand in cold water. Electroencephalogr clinical Neurophysiol. 1991;79:192–203. doi: 10.1016/0013-4694(91)90137-s. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psych Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bromm B, Lorenz J. Neurophysiological evaluation of pain. Electroencephalogr clinical Neurophysiol. 1998;107:227–253. doi: 10.1016/s0013-4694(98)00075-3. [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Apkarian AV. Representation of pain in the brain. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. Edinburgh: Elsevier Churchill Livingston; 2006. pp. 107–124. [Google Scholar]
- Chang PF, Arendt-Nielsen L, Chen ACN. Dynamic changes and spatial correlation of EEG activities during cold pressor test in man. Brain Res Bull. 2002a;57:667–675. doi: 10.1016/s0361-9230(01)00763-8. [DOI] [PubMed] [Google Scholar]
- Chang PF, Arendt-Nielsen L, Chen ACN. Differential cerebral responses to aversive auditory arousal versus muscle pain: Specific EEG patterns area associated with human pain processing. Exp Brain Res. 2002b;147:387–393. doi: 10.1007/s00221-002-1272-9. [DOI] [PubMed] [Google Scholar]
- Chang PF, Arendt-Nielsen L, Graven-Nielsen T, Svensson P, Chen ACN. Topographic effects of tonic cutaneous nociceptive stimulation on human electroencephalograph. Neurosci Lett. 2001a;305:49–52. doi: 10.1016/s0304-3940(01)01802-x. [DOI] [PubMed] [Google Scholar]
- Chang PF, Arendt-Nielsen L, Graven-Nielsen T, Svensson P, Chen ACN. Different EEG topographic effects of painful and non-painful intramuscular stimulation in man. Exp Brain Res. 2001b;141:195–203. doi: 10.1007/s002210100864. [DOI] [PubMed] [Google Scholar]
- Chen ACN, Dworkin SF, Haug J, Gehrig J. Topographic brain measures of human pain and pain responsivity. Pain. 1989;37:129–141. doi: 10.1016/0304-3959(89)90125-5. [DOI] [PubMed] [Google Scholar]
- Chen ACN, Rappelsberger P. Brain and human pain: Topographic EEG amplitude and coherence mapping. Brain Topogr. 1994;7:129–140. doi: 10.1007/BF01186771. [DOI] [PubMed] [Google Scholar]
- Chen ACN, Rappelsberger P, Filz O. Topology of EEG coherence changes may reflect differential neural network activation in cold and pain perception. Brain Topogr. 1998;11:125–132. doi: 10.1023/a:1022254505510. [DOI] [PubMed] [Google Scholar]
- Coghill RJ, Gilron I, Iadarola MJ. Hemispheric lateralization of somatosensory processing. J Neurophysiol. 2001;85:2602–2612. doi: 10.1152/jn.2001.85.6.2602. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of physiological condition of the body. Nature Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig KD, Patrick CJ. Facial expressions induced by pain. J Pers Soc Psych. 1985:1080–1091. doi: 10.1037/0022-3514.48.4.1089. [DOI] [PubMed] [Google Scholar]
- Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis MJ. Functional MRI of pain- and attention-related activations in the human cingulate cortex. J Physiol. 1997;77:3370–3380. doi: 10.1152/jn.1997.77.6.3370. [DOI] [PubMed] [Google Scholar]
- Del Percio C, Le Pera D, Arendt-Nielsen L, Babiloni C, Brancucci A, Chen ACN, De Armas L, Miliucci R, Restuccia D, Valeriani M, Rossini PM. Distraction affects frontal alpha rhythms related to expectancy of pain: An EEG study. NeuroImage. 2006;31:1268–1277. doi: 10.1016/j.neuroimage.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behavior. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dong WK, Chudler EH. Cortical nociceptive mechanisms. A review of neurophysiological and behavioral evidence in the primate. In: Besson JM, Guilbaud G, Ollat H, editors. Forebrain Areas Involved in Pain Processing. Paris: John Libbey Eurotext; 1995. pp. 183–195. [Google Scholar]
- Dowman R. Electrophysiological indices of orienting attention towards pain. Psychophysiol. 2004;41:749–761. doi: 10.1111/j.1469-8986.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- Dowman R. Neural Mechanisms Of Detecting and Orienting Attention Towards Unattended Threatening Somatosensory Target Stimuli. I. Inter-modal effects. Psychophysiol. 2007a;44:407–419. doi: 10.1111/j.1469-8986.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- Dowman R. Neural Mechanisms Of Detecting and Orienting Attention Towards Unattended Threatening Somatosensory Target Stimuli. II. Intensity Effects. Psychophysiol. 2007b;44:420–430. doi: 10.1111/j.1469-8986.2006.00491.x. [DOI] [PubMed] [Google Scholar]
- Dowman R, Darcey TM. SEP topographies elicited by innocuous and noxious sural nerve stimulation. III. Dipole source analysis. Electroencephalogr clinical Neurophysiol. 1994;92:373–391. doi: 10.1016/0168-5597(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Dowman R, Darcey TM, Barkan H, Thadani V, Roberts D. Human intracranially-recorded cortical responses evoked by painful electrical stimulation of the sural nerve. NeuroImage. 2007;34:743–763. doi: 10.1016/j.neuroimage.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Dowman R, Goshko L. Evaluation of reference sites for scalp potentials elicited by painful and non-painful sural nerve stimulation. Electroencephalogr clinical Neurophysiol. 1992;84:477–485. doi: 10.1016/0168-5597(92)90036-b. [DOI] [PubMed] [Google Scholar]
- Dowman R, Schell S. Innocuous-related sural nerve-evoked and finger-evoked scalp potentials generated in the primary somatosensory and supplementary motor cortices. Clinical Neurophysiol. 1999a;110:2104–2116. doi: 10.1016/s1388-2457(99)00203-5. [DOI] [PubMed] [Google Scholar]
- Dowman R, Schell S. Evidence that the anterior cingulate and supplementary somatosensory cortices generate the pain-related negative difference potential. Clinical Neurophysiol. 1999b;110:2117–2126. doi: 10.1016/s1388-2457(99)00196-0. [DOI] [PubMed] [Google Scholar]
- Eccleston C, Crombez G. Pain demands attention: A cognitive-affective model of the interruptive function of pain. Psych Bull. 1999;125:356–366. doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]
- Edwards E, Soltani M, Deoueli LY, Berger MS, Knight RT. High gamma activity in response to deviant auditory stimuli recorded directly from human cortex. J Neurophysiol. 2005;94:4269–4280. doi: 10.1152/jn.00324.2005. [DOI] [PubMed] [Google Scholar]
- Fan J, Kolster R, Ghajar J, Suh M, Knight RT, Sarkar R, McCandliss BD. Response anticipation and response conflict: An event-related potential and functional magnetic resonance imaging study. J Neurosci. 2007;27:2272–2282. doi: 10.1523/JNEUROSCI.3470-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige B, Scheffler K, Esposito F, Di Salle FD, Hennig J, Seifritz E. Cortical and subcortical correlates of electroencephalographic alpha rhythm modulation. J Neurophysiol. 2005;93:2864–2872. doi: 10.1152/jn.00721.2004. [DOI] [PubMed] [Google Scholar]
- Ferracuti S, Seri S, Mattia D, Cruccu G. Quantitative EEG modifications during the cold water pressor test: hemispheric and hand differences. Int J Psychophysiol. 1994;17:261–268. doi: 10.1016/0167-8760(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV, Ahlfors SP. Parieto-occipital ~10 Hz activity reflects anticipatory state of visual attention mechanisms. NeuroReport. 1998;9:3929–3933. doi: 10.1097/00001756-199812010-00030. [DOI] [PubMed] [Google Scholar]
- Frot M, Garcia-Larrea L, Guenot M, Mauguiere F. Response of the supra-sylvian (SII) cortex in humans to painful and innocuous stimuli. A study using intra-cerebral recordings. Pain. 2001;94:65–73. doi: 10.1016/S0304-3959(01)00342-6. [DOI] [PubMed] [Google Scholar]
- Fu KMG, Foxe JJ, Murray MM, Higgins BA, Javitt DC, Schroeder CE. Attention dependent suppression of distracter visual input can be cross-modally cued as indexed by anticipatory parieto-occipital alpha band oscillations. Cog Brain Res. 2001;21:145–152. doi: 10.1016/s0926-6410(01)00034-9. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Frot M, Valeriani M. Brain generators of laser-evoked potentials: From dipoles to functional significance. Neurophysiologie Clinique. 2003;33:279–292. doi: 10.1016/j.neucli.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Gevins AS, Cutillo BA. Neuroelectric measures of mind. In: Nunez PL, editor. Neocortical Dynamics and Human EEG Rhythms. New York: Oxford University Press; 1995. pp. 304–338. [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D. High-resolution EEG mapping of cortical activation related to working memory: Effects of task difficulty, type of processing, and practice. Cerebral Cortex. 1997;7:374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Gomarus HK, Althaus M, Wijers AA, Minderaa RB. The effects of memory load and stimulus relevance on the EEG during a visual selective memory search task: An ERP, ERD/ERS study. Clinical Neurophysiol. 2006;117:871–884. doi: 10.1016/j.clinph.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Huber M, Bartling J, Pachur D, Woikowsky-Biedau Sv, Lautenbacher S. EEG responses to tonic heat pain. Exp Brain Res. 2006;173:14–24. doi: 10.1007/s00221-006-0366-1. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Hwang G, Curran T, Kahana MJ. EEG oscillations and recognition memory: Theta correlates of memory retrieval and decision making. NeuroImage. 2006;32:978–987. doi: 10.1016/j.neuroimage.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Kahana MJ. The cognitive correlates of human brain oscillations. J Neurosci. 2006;26:1669–1672. doi: 10.1523/JNEUROSCI.3737-05c.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwhisher N, Wojciulik E. Visual attention: insights from brain imaging. Nature Rev Neurosci. 2000;1:91–100. doi: 10.1038/35039043. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Ann Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kelly S, Lalor EC, Reilly RB, Foxe JJ. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distractor suppression during sustained visuospatial attention. J Neurophysiol. 2006;95:3844–3851. doi: 10.1152/jn.01234.2005. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR, jr, Willis WD. The role of the cerebral cortex in pain sensation. In: Petersen A, Jones EG, editors. The Cerebral Cortex. Vol. 9. New York: Plenium Press; 1991. pp. 153–212. [Google Scholar]
- Kilner JM, Mattout J, Henson R, Friston KJ. Hemodynamic correlates of EEG: A heuristic. NeuroImage. 2005;28:280–286. doi: 10.1016/j.neuroimage.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Klein SA, Carney T. The usefulness of the Laplacian in principal component analysis and dipole source localization. Brain Topogr. 1995;8:91–108. doi: 10.1007/BF01199773. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- LePera D, Svensson P, Valeriani M, Watanabe I, Arendt-Nielsen L, Chen ACN. Long-lasting effect evoked by tonic muscle pain on parietal EEG activity in humans. Clinical Neurophysiol. 2000;111:2130–2137. doi: 10.1016/s1388-2457(00)00474-0. [DOI] [PubMed] [Google Scholar]
- Laufs H, Holt JL, Elfont R, Krams M, Paul JS, Krakow K, Kleinschmidt A. Where the BOLD signal goes when alpha EEG leaves. NeuroImage. 2006;31:1408–1418. doi: 10.1016/j.neuroimage.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Rios MD, Chau, Krauss GL, Zirh TA, Lesser RP. Painful stimuli evoked potentials recorded from the parasylvian cortex in humans. J Neurophysiol. 1998a;80:2077–2088. doi: 10.1152/jn.1998.80.4.2077. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Rios M, Zirh A, Chau D, Krauss G, Lesser RP. Painful stimuli evoke potentials recorded over the human anterior cingulate gyrus. J Neurophysiol. 1998b;79:2231–2234. doi: 10.1152/jn.1998.79.4.2231. [DOI] [PubMed] [Google Scholar]
- Lopes da Silva F. Neural mechanisms underlying brain waves: from neural membranes to networks. Electroencephalogr clinical Neurophysiol. 1991;79:81–93. doi: 10.1016/0013-4694(91)90044-5. [DOI] [PubMed] [Google Scholar]
- Martinez-Montes E, Valdes-Sosa PA, Miwakeichi F, Goldman RI, Cohen MS. Concurrent EEG/fMRI anlaysis by multiway partial least squares. NeuroImage. 2004;22:1023–1034. doi: 10.1016/j.neuroimage.2004.03.038. [DOI] [PubMed] [Google Scholar]
- Moosmann M, Ritter P, Krastel I, Brink A, Thees S, Blankenburg F, Taskin B, Obrig H, Villringer A. Correlates of alpha rhythm in functional magnetic resonance imaging and near infrared spectroscopy. NeuroImage. 2003;20:145–158. doi: 10.1016/s1053-8119(03)00344-6. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Silberstein RB, Cadusch PJ, Wijesinghe RS, Westdorp AF, Srinivasan R. A theoretical and experimental study of high resolution EEG based on surface Laplacians and cortical imaging. Electroencephalogr clinical Neurophysiol. 1994;90:40–57. doi: 10.1016/0013-4694(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiologie Clinique. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G. Event-related synchronization (ERS): an electrophysiological correlate of cortical areas at rest. Electroencephalogr clinical Neurophysiol. 1992;83:62–69. doi: 10.1016/0013-4694(92)90133-3. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clinical Neurophysiology. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Rainville P. Brain mechanisms of pain affect and pain modulation. Current Opinions in Neurobiology. 2002;12:195–204. doi: 10.1016/s0959-4388(02)00313-6. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Burton H. Somatic submodality distribution within the second somatosensory (SII), 7b, retroinsular, postauditory, and granular insular cortical areas. J Comp Neurol. 1980;192:93–108. doi: 10.1002/cne.901920106. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimech W, Stadler W, Schabus M, Doppelmayr M, Hanslmayr S, Gruber WR, Birbaumer N. A shift in visual spatial attention is selectively associated with human EEG alpha activity. Eur J Neurosci. 2005;22:2917–2926. doi: 10.1111/j.1460-9568.2005.04482.x. [DOI] [PubMed] [Google Scholar]
- Sharbrough F, Chatrian GE, Lesser RP, Luders H, Nuwer M, Picton TW. Guidelines for standard electrode position nomenclature. J Clinical Neurophysiol. 1991;8:200–202. [PubMed] [Google Scholar]
- Sikes RW, Vogt BA. Nociceptive neurons in area 24 of rabbit cingulate cortex. J Neurophysiol. 1992;68:1720–1732. doi: 10.1152/jn.1992.68.5.1720. [DOI] [PubMed] [Google Scholar]
- Stancak A, Svoboda J, Rachmanova R, Vrana J, Kralik J, Tintera J. Desynchronization of cortical rhythms following cutaneous stimulation: effects of stimulus repetition and intensity, and of the size of the corpus callosum. Clinical Neurophysiol. 2003;114:1936–1947. doi: 10.1016/s1388-2457(03)00201-3. [DOI] [PubMed] [Google Scholar]
- Steriade M, Gloor P, Llinas RR, Lopes da Silva FH, Mesulam MM. Basic mechanisms of cerebral rhythmic activities. Electroencephalogr clinical Neurophysiol. 1990;76:481–508. doi: 10.1016/0013-4694(90)90001-z. [DOI] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. α-Band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Sikes RW. The medial pain system, cingulate cortex and parallel processing of nociceptive information. Prog Brain Res. 2000;122:223–235. doi: 10.1016/s0079-6123(08)62141-x. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Sikes RW, Vogt LJ. Anterior cingulate cortex and the medial pain system. In: Vogt BA, Gabriel M, editors. Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook. Boston: Birkhauser; 1993. pp. 313–344. [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific α-band electroencephalography increases over occipital cortex. J Neurosci. 2000;20(RC63):1–6. doi: 10.1523/JNEUROSCI.20-06-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi N, Callan DE, Goda N, Anderson SJ, Yoshida Y, Kawato M. Attention modulation of oscillatory activity in human visual cortex. NeuroImage. 2003;20:98–113. doi: 10.1016/s1053-8119(03)00341-0. [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MW, Cohen JD. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psych Rev. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]