Abstract
Background:
Older adults get lost, in many cases because of recognized or incipient Alzheimer disease (AD). In either case, getting lost can be a threat to individual and public safety, as well as to personal autonomy and quality of life. Here we compare our previously described real-world navigation test with a virtual reality (VR) version simulating the same navigational environment.
Methods:
Quantifying real-world navigational performance is difficult and time-consuming. VR testing is a promising alternative, but it has not been compared with closely corresponding real-world testing in aging and AD. We have studied navigation using both real-world and virtual environments in the same subjects: young normal controls (YNCs, n = 35), older normal controls (ONCs, n = 26), patients with mild cognitive impairment (MCI, n = 12), and patients with early AD (EAD, n = 14).
Results:
We found close correlations between real-world and virtual navigational deficits that increased across groups from YNC to ONC, to MCI, and to EAD. Analyses of subtest performance showed similar profiles of impairment in real-world and virtual testing in all four subject groups. The ONC, MCI, and EAD subjects all showed greatest difficulty in self-orientation and scene localization tests. MCI and EAD patients also showed impaired verbal recall about both test environments.
Conclusions:
Virtual environment testing provides a valid assessment of navigational skills. Aging and Alzheimer disease (AD) share the same patterns of difficulty in associating visual scenes and locations, which is complicated in AD by the accompanying loss of verbally mediated navigational capacities. We conclude that virtual navigation testing reveals deficits in aging and AD that are associated with potentially grave risks to our patients and the community.
GLOSSARY
- AD
= Alzheimer disease;
- EAD
= early Alzheimer disease;
- MCI
= mild cognitive impairment;
- MMSE
= Mini-Mental State Examination;
- ONC
= older normal control;
- std. wt.
= standardized weight;
- THSD
= Tukey honestly significant difference;
- VR
= virtual reality;
- YNC
= young normal control.
Functional disability early in the course of Alzheimer disease (AD) often involves navigational deficits having life-threatening complications that emerge from wandering and getting lost while driving.1 Navigational impairment is a harbinger of AD-related cognitive decline, unrelated to the degree of verbal memory impairment, as a symptomatic variant of mild cognitive impairment (MCI).2 Navigational impairment is also a key feature of an AD variant that presents with disorders of spatial cognition, memory, and orientation.3,4 This syndrome is associated with posterior cortical atrophy5,6 linked to the accumulation of AD neuropathology in peristriate cortices7 and imaging evidence of decreased tissue volume in the right posterior hippocampal and parietal areas.8
In practice, AD is substantially defined by the presence of verbal memory deficits. The lack of available behavioral measures of navigational capacity undermines our ability to detect early AD (EAD) and assess the risk of driving and independent living. We have previously shown that selective deficits of visual motion processing in AD and MCI are associated with disorders of ambulatory and vehicular navigation.2,9,10 These impairments lead to getting lost and accidental collisions while driving,11,12 as well as wandering and the loss of independent living.13,14 We have previously characterized navigational disorders in AD using a standardized real-world test environment, identifying task and subject variables that influence navigational strategies and navigational success. Those studies identified both visuospatial and verbal capacities that contribute to navigational success, and the ability to link a recognized scene with a place in the environment as a critical predictor of a subject's getting lost in the test environment.15,16 We hypothesize that a computer-based virtual reality (VR) test environment might yield comparable measures of broad applicability to the early detection of navigational impairment in MCI and AD.
METHODS
Subject groups.
We studied young normal controls (YNCs), older normal controls (ONCs), patients with MCI, and patients with EAD (figure 1). All subjects were free of other neurologic, ophthalmologic, or psychiatric illnesses. Corrected binocular visual acuity of 20/40 and a Mini-Mental State Examination (MMSE) score >17 were required; approximately half of the subjects (52%) were women. Patients were recruited from outpatient programs affiliated with the University of Rochester Medical Center and were diagnosed by a geriatric neurologist or psychiatrist specializing in dementia as meeting the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association criteria for AD17 or meeting the American Academy of Neurology criteria for MCI.18
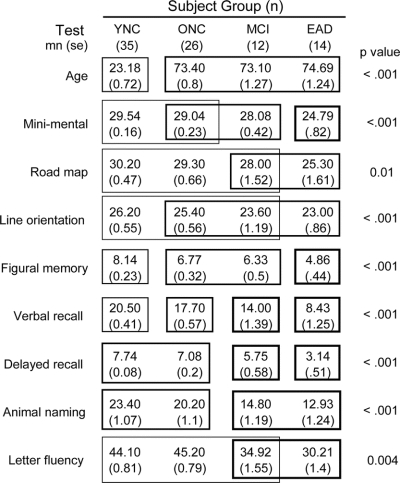
Figure 1 Summary statistics for each subject group showing sample size, age, and the results of neuropsychological tests
Two-way analysis of variance revealed that all tests yielded significant between-subject group differences (p values shown at right). Post hoc tests of group differences for each test were performed using the Tukey honestly significant difference (THSD; p < 0.05). The THSDs for each test showed which groups are not significantly different from one another (included in the same box frame) and which are significantly different from one another (not in the same frame). YNC = young normal control; ONC = older normal control; MCI = mild cognitive impairment; EAD = early Alzheimer disease.
Specifically, AD patients were required to show impairments of verbal or spatial memory on clinical or neuropsychological testing along with similarly characterized deficits of language, praxis, recognition, or executive function that impacted on daily living and were not attributable to other causes of cognitive dysfunction. MCI patients were required to have substantiated memory impairment without other deficits or losses of customary roles or capacities. The final ascertainment of distinctions between AD and MCI were often made after repeated clinical assessments and formal testing. We cannot presume that MCI patients will develop AD. ONCs were mainly the spouses of patients who did not meet criteria for AD or MCI. YNCs were mainly graduate students. Each subject gave informed consent at recruitment. The institutional Human Subjects Review Board approved all procedures and protocols.
Navigation testing.
The real-world and virtual environment navigation tests each consisted of eight corresponding subtests. We randomly assigned subjects to undergo real-world or virtual testing first; both were completed over 3 days. In an earlier study, we examined the effect of our subjects' previous exposure to the hospital lobby without finding a significant effect, so that questionnaire was not repeated.15
Real-world environment.
The real-world navigational battery has been described extensively.15,16 Briefly, we use the lobby of Strong Memorial Hospital as a test environment in a 90-minute test that begins with an experimenter-directed tour of the lobby on a fixed path with subjects seated in a wheelchair. We used a wheelchair to avoid interference by discomfort or disability in older subjects when walking a substantial distance, anticipating the distraction and the nonuniformity of route exposure that it might entail. Subjects were instructed to attend to the route because they would later undergo testing related to it. Their wheelchair was pushed along the 1,000-ft path for approximately 4 minutes. On completion of the route, eight subtests were administered to assess navigational capacity; each subtest consisted of 10 questions.
Virtual reality environment.
The virtual environment was created with a gaming engine (Quake3, ID Software) with detailed floor plans and photographs of the hospital lobby. A laptop PC was used to present a three-dimensional view of the lobby from wheelchair height.
Navigational subtests.
The eight subtests were administered in the order of presentation below.
Route learning.
After completing the route demonstration, the route was begun again. The subjects were asked whether they had gone left, right, or straight at each of 10 choice points. Mistakes were recorded and corrected to maintain the integrity of the path. In the virtual environment, subjects were shown the route on the PC screen. On the second presentation, the video was stopped at the 10 choice points.
Free recall.
After the test trip around the route, subjects had 1 minute to name as many objects or landmarks as they could recall. In both real-world and virtual testing, this was scored as the total number of items up to a maximum of 10.
Self-orientation.
Subjects were shown pictures of 10 different objects or locations from the test route, chosen to be distributed as two sequentially presented sets of five sites distributed at ∼45° intervals to the front and sides of the subject's position, the subject's back being toward an outside wall. They were asked to point in the direction of the location depicted as if there were no walls between themselves and the target. Responses were scored as correct if their responses were within 22.5° of the correct direction, respecting the 45° placement intervals. In the virtual environment, subjects used the arrow keys to rotate the direction of view in a manner corresponding to pointing in the desired direction.
Route drawing.
Subjects drew the route, one choice segment at a time, on a scale outline of the lobby. Each subsequent outline included a depiction of the previous segments. In the virtual environment, subjects used a mouse to indicate the location of the next choice point while viewing a scale map of the lobby on the video screen.
Landmark recall.
Subjects were asked to name only those objects or fixtures that were helpful in finding their way on the self-directed, second trip around the lobby. The number of objects listed, up to a maximum of 10 objects, was used as a dependent measure in the analysis. This test was identical in the VR version.
Photograph recognition.
Ten photographs (figure 2B) were presented singly, five from the test route and five from other locations in the Medical Center. Subjects identified whether each photo was from the test route or not. Responses were scored as correct, false positive, or false negative. In the virtual environment, the photographs were presented on screen.
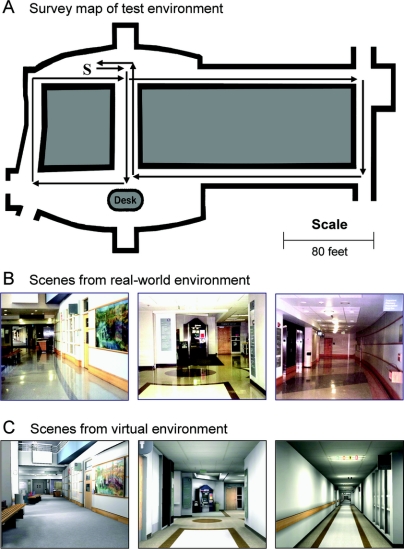
Figure 2 Schematic representation of the spatial orientation test route in the Strong Hospital Lobby
(A) Survey map of the test environment showing the outline of the lobby (bold lines) and the route traveled in testing. Subjects started and stopped at the location of the S on the map. Each arrow represents a segment of the route, beginning and ending at a decision point. (B and C) Examples of three scenes from the test route as seen in the real-world (B) and virtual (C) versions of the test environment. The scenes correspond to the main lobby (left), a typical intersection (middle), and an extended hallway (right).
Photograph location.
Another set of 10 photographs from the test route was presented while subjects used a scale outline of the lobby with 10 locations marked by letters to indicate the location corresponding to the scene, scored as the number correct. In the virtual environment, the photographs and drawing of the lobby were presented on the screen, and subjects used the mouse to indicate the correspondence.
Video location.
Ten short video clips, taken from the subject's view of the test route, were presented with three repetitions. After each display was completed, subjects drew an X on a blank map where the clip began and an arrow coming from the X showing the direction and extent of the depicted movement. Responses were considered correct if the X was placed in the correct location and the arrow indicated the correct direction. In the virtual environment, a numbered map was presented after each video clip, and subjects responded using the mouse.
Neuropsychological tests.
The neuropsychological battery assessed aspects of general cognition, visual–perceptual skills, and memory. Categorical Name Retrieval assessed verbal fluency. The Money Road Map test19 assessed topographic orientation along a drawn route by reporting whether turns were to the traveler's left or right. Judgment of Line Orientation20 tested aspects of spatial perception. Two tests were from the Wechsler Memory Scale–Revised21: Figural Memory uses designs for immediate visual recognition, and Verbal Paired-Associates Test I and II assess immediate and delayed recall for a list of word pairs. The MMSE22 was part of the diagnostic assessment.
Data analysis.
Results from the navigational and neuropsychological tests were analyzed using multivariate analyses of variance in mixed designs with subject group as between-subject factor and various subtests as within-subject factors. The resulting F scores are reported with subscripted test and error degrees of freedom and the resulting p values. All significant effects were followed up with post hoc analyses using the Tukey honestly significant difference (THSD) to maintain α levels at p = 0.05.
Discriminant function analysis was used for the identification of the subtest that most effectively distinguished between subject groups. This approach was applied separately to both the real-world and VR tests. All statistical analyses were run using SPSS 15.0 statistical software.23
RESULTS
ONC, MCI, and EAD subjects were of similar ages, with declining MMSE scores across groups (figure 1). Aging effects were evident as the better performance of YNCs over ONCs on figural memory and verbal recall. ONCs performed better than MCI patients did on immediate and delayed verbal recall and on verbal fluency (animal naming), whereas MCI patients performed better than EAD patients did on figural memory and on immediate and delayed verbal recall. These neuropsychological findings are consistent with the group assignments established by neurologic criteria.
Our subject groups showed significant differences in navigational performance [F(3,76) = 47.85, p < 0.001]. In both the real-world and virtual environments, YNC subjects performed best, with successively lower scores in first the ONC and MCI groups and then the EAD group (THSDs: YNC > ONC = MCI > EAD). There was a significant difference in performance between the two environments, with the virtual test yielding somewhat lower scores across all groups [F(1,76) = 19.65, p < 0.001]. However, this environment difference affected all groups equally such that there were no significant group-by-environment interactions [F(3,76) = 1.47, p < 0.23] (figure 3A).
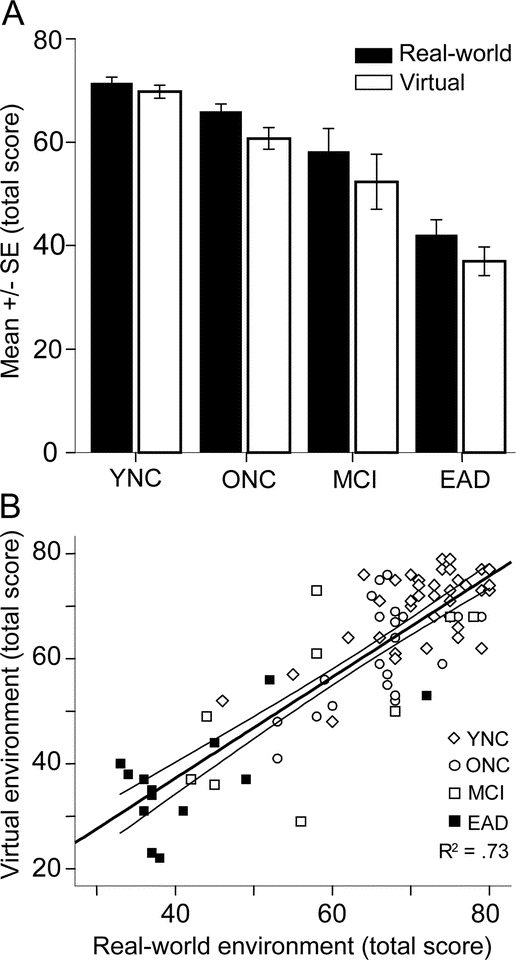
Figure 3 Total score on the navigation task by subject and navigational environment
(A) Mean scores (± SEM) from the navigation test conducted in the real-world (solid bars) and virtual (open bars) test environments are shown for young normal controls (YNC), older normal controls (ONC), patients with mild cognitive impairment (MCI), and patients with Alzheimer disease (AD). Mean total scores were significantly different between subject groups, with mean scores decreasing from the YNC to ONC, to MCI, and to AD groups. In each subject group, the mean total score was not significantly different between the real-world and virtual environments. (B) The correlation between navigation test scores from the real-world (abscissa) and virtual (ordinate) test environments for each of the four subject groups. A high overall correlation was obtained between individual subjects' scores in the two environments (R2 = 0.73), mainly driven by group differences.
Differences in navigational performance across subject groups provided a substantial range of test scores in both the real-world and virtual environments (figure 3B). The absence of significant group-by-environment interaction effects is consistent with the strong correlation between real-world and virtual tests scores across all subjects (r = 0.73). This leads us to conclude that virtual navigation testing is a good indicator of navigational performance as seen in a corresponding real-world environment and that this conclusion applies to all subject groups. The order of testing, real-world or virtual environment first, did not have a substantial effect on test scores, but there was a slightly better correlation between real-world and virtual environment scores (incremental R2 = 0.05) when real-world testing occurred first.
The overall profiles of navigation subtest performance from the real-world and virtual environments were similar in all four subject groups. There were significant effects of subtest in all but the MCI group [F(7,2) = 1.39, p = 0.48] [YNC: F(7,30) = 19.07, p < 0.001; ONC: F(7,14) = 9.79, p < 0.001; EAD: F(7,5) = 26.46, p = 0.001], reflecting the relatively greater variance seen in almost all subtests in the MCI group. This suggests that the MCI group is less homogenous than the other groups, possibly because MCI is a multifarious transition state between healthy aging and AD.
We tested the suggestion that virtual navigational testing might predict the results of real-world navigational testing, adding in the results of our battery of eight standard neuropsychological tests to determine their combined predictive capacity. Stepwise multiple linear regression selected the virtual navigation (βstd = 0.4, p < 0.001) and delayed verbal memory (βstd = 0.4, p < 0.001) scores as the two best predictors of real-world navigation testing to results with an adjusted r2 of 0.82. The inclusion of MMSE (βstd = 0.14, p = 0.045) and judgment of line orientation (βstd = 0.13, p = 0.012) scores enhanced the regression model to yield an adjusted r2 of 0.84. Thus, it seems that real-world testing might reflect the influence of factors well described by virtual testing along with factors well described by delayed verbal memory testing.
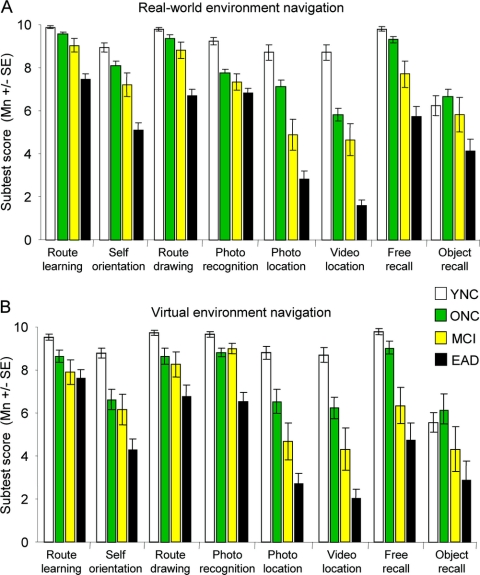
We found a consistent trend of relative performance across all subtests in the four subject groups (THSDs: YNC > ONC > MCI > EAD). Nevertheless, there was a wide range of subject group differentiation achieved by the eight subtests (figure 4A). The same general trend across subject groups and the same variation in the range of performance across subtests was seen with the eight virtual environment navigation subtests (figure 4B). The similarity of all four subject groups' subtest performances in the real-world and the virtual environments is reflected in the lack of a significant interaction between group, environment, and subtest [F(21,99) = 1.47, p = 0.092].
Figure 4 Mean scores on each subtest from the real-world (A) and virtual (B) navigation test environments
Across subject groups, the same pattern of relative performance was apparent on subtests as was seen on total test scores; poorer performance from young normal controls (YNC) to older normal controls (ONC), to patients with mild cognitive impairment (MCI), and to patients with early Alzheimer disease (EAD). The real-world and virtual environments yielded similar patterns and magnitudes of differences across subject groups. The photo and video location subtests yielded the lowest mean scores from both the MCI and AD groups.
We did not find that the total or subtest scores provided a reliable approach to separating subject groups. Instead, we created composite scores to identify the combination of measures that best differentiated between subject groups. To do so, we used stepwise discriminant analysis for group classification applied separately to data from real-world and virtual environment testing. The YNC and ONC groups were used to identify subtest changes associated with aging, and the ONC and combined MCI and EAD groups were used to identify subtest changes associated with disease; the latter grouping avoided the limitations inherent in the smaller sample size of the MCI and EAD groups. All of these group comparisons, based on the results of both real-world and virtual navigation testing, yielded single discriminant functions.
Real-world navigational testing distinguished between the YNC and ONC groups using photo recognition (standardized weight [std. wt.] = 0.59, p < 0.001) and video location (std. wt. = 0.60, p < 0.001) subtests to correctly classify 80% of the subjects, whereas virtual navigation testing used photo recognition (std. wt. = 0.64, p < 0.001) and self-orientation (std. wt. = 0.77, p < 0.001) to correctly classify 79% of the subjects. Real-world navigational testing distinguished between the ONC and MCI/EAD groups using photo location (std. wt. = 0.64, p < 0.001) and free recall (std. wt. = 0.65, p < 0.001) subtests to correctly classify 83% of the subjects, whereas virtual navigation testing used the same subtests (photo location std. wt. = 0.49, p < 0.001; free recall std. wt. = 0.78, p < 0.001) to correctly classify 85% of the subjects.
The subtest scores from both the real-world and virtual environment were used in a factor analysis to better assess the underlying components of subject performance. In a combined analysis, a two-factor solution (eigenvalues >1.0 after varimax rotation) emerged with robustly uniform results. The first factor, which explained approximately 56% of the total variance, contained four variables. These corresponded to the same two subtests, Photo Location and Video Location, from both environments: (factor loadings of Photo Location = 0.815 and Video Location = 0.867 from the real-world environment, and Photo Location = 0.876 and Video Location = 0.882 from the virtual environment). A second factor, which explained only 9% percent of the residual variance, was the Free Recall test of objects from each environment: (factor loadings of 0.797 from the real-world environment and 0.820 from the virtual environment). Adding the neuropsychological test results to the factor analysis grouped immediate and delayed verbal memory with the second component and added three additional components at still lower eigenvalues. These findings are consistent with a strong visuospatial component and a less strong verbal component.
DISCUSSION
VR technology has expanded the range of studies exploring human spatial behavior, showing that people create cognitive maps as they do when exploring real-world environments.24 Few studies have analyzed the effects of aging and AD on real-world and virtual navigation. We find that strong correlations between navigational capacities in real-world and virtual environments25 are not fundamentally altered by aging or AD. This supports the use of virtual environment testing to detect impaired navigational capacities, with the caveat that virtual environment testing yields somewhat lower scores in all groups.26
The learning of navigational landmarks is equivalent in real-world and virtual environments,26 suggesting that cognitive mechanisms are similarly engaged under both conditions. This is consistent with the high correlation between spatial knowledge measured in a VR paradigm and that measured in the real-world. Such correlations reflect individual differences in spatial ability, sex, and prior computer experience.25 The specific environment (city street, building) designed is not thought to be a critical factor.
When VR technology is used for training, allowing the advantage of exact control over environmental variation for experimental purposes, its effectiveness depends on the ability of subjects to make active navigational decisions; passive VR experience does not offer training advantages over studying maps.27 Exploration of virtual space has been shown to reflect both subject memory for object location, as well as for route direction, and the subject's ability to find a novel path to a goal, reverse a route back to the origin, or find a shortcut.28
Navigational testing revealed declining performance from YNC to ONC, to MCI, and to EAD groups (figure 3), reflecting a significant, deleterious impact of both aging and AD. Subtest scores revealed differences between the four subject groups (figure 4) characterized by discriminant analyses in which we focused on comparing aging and AD. Cognitive aging effects were concentrated in photo recognition scores from real-world and virtual testing, with additional contributions from video location in the real-world environment and self-orientation in the virtual environment.
Photo recognition consistently distinguished young and older adults, suggesting that aging impairs visual memory for scenes, consistent with aging effects on figural memory (figure 1). Previous descriptions of age-related declines in visual memory have shown deficits with faces but not other figures,29 or environmental scenes.30 Our finding of figural and scene memory impairments in normal aging may reflect the more naturalistic demands of a navigational environment.
The additional effects of aging on video location and self-orientation subtests suggest specific decline in linking scene memory to mental representations of locations. This is consistent with studies showing the impact of aging on visual associative capacities,31 including associations between two faces and between faces and locations,32 that is not explained by other cognitive declines.33 Nevertheless, it remains unclear whether normal aging selectively impacts nonverbal visual associative functions34 or equally burdens all associative domains.35
No single measure from our navigational testing reliably predicted a subject's membership in any of the test groups. However, real-world and virtual testing both identified photo location and free recall as distinguishing ONCs from the combined MCI/EAD group. Photo location reflects associative deficits for linking scenes and locations, much like that dominating aging. Distinctions between aging and AD in visual associative capacity may relate to the difficulty of the photo location, self-orientation, and video location subtests: the ONC, MCI, and EAD groups all had trouble with self-orientation and video location, but only the MCI/EAD group had trouble with photo location. The ONC and MCI/EAD groups are more clearly distinguished by the inclusion of free recall scores in discriminant functions. Thus, the decline in verbal memory for environmental features reveals a discontinuity between our older adult subjects and our AD spectrum patients.
Navigation relies on the complementarity of visual self-movement perception for path integration36 and the identification of named places for landmark registration.37 The path integration and landmark registration strategies are combined in cognitive mapping to create a mental representation of the relative position of landmarks by their sequential observation along a path. The combination of visual and verbal losses in AD undermines both strategies, impairing route repetition and drawing, the subtests closely related to real-world way-finding.15 The core cognitive deficit that we observed in EAD, therefore, was actually a dual one consisting of visuospatial deficits and verbal memory deficits.
Thus, the fundamental distinction between navigation in aging and AD may be related to the definitional involvement of verbal memory impairment in MCI and AD. Still, it is not clear that verbal memory dysfunction is fundamentally different in AD spectrum disorders and normal aging, with verbal memory deficits and reactions to them (i.e., complaints) being similar in MCI and older adults.38 Thus, the use of verbal memory as a line of demarcation between aging and AD might be more a matter of practical limits on testing rather than of distinct cognitive pathophysiologies.
ACKNOWLEDGMENT
The authors thank Teresa Steffenella, MA, for her expertise in patient assessment and William Vaughn for his expertise in computer systems development and management. The authors thank Drs. Voyko Kavcic, Mark Mapstone, and William K. Page, as well as Michael Jacob and Anthony Monacelli, for comments on the manuscript.
Address correspondence and reprint requests to Dr. Charles J. Duffy, University of Rochester Medical Center, 601 Elmwood Ave., Rochester, NY 14642 charles_duffy@urmc.rochester.edu
Disclosure: Supported by the National Eye Institute, National Institute on Aging, and Alzheimer's Association grants to C.J.D. The authors have not received other support related to these studies.
Received February 22, 2008. Accepted in final form June 12, 2008.
REFERENCES
- 1.Silverstein NM, Flaherty G, Tobin TS. Dementia and Wandering Behavior: Concern for the Lost Elder. New York: Springer Publishing, 2002. [Google Scholar]
- 2.Mapstone M, Steffenella TM, Duffy CJ. A visuospatial variant of mild cognitive impairment: getting lost between aging and AD. Neurology 2003;60:802–808. [DOI] [PubMed] [Google Scholar]
- 3.Henderson VW, Mack W, Williams BW. Spatial disorientation in Alzheimer's disease. Arch Neurol 1989;46:391–394. [DOI] [PubMed] [Google Scholar]
- 4.Renner JA, Burns JM, Hou CE, McKeel DW, Storandt M, Morris JC. Progressive posterior cortical dysfunction: a clinicopathologic series. Neurology 2004;63:1175–1180. [DOI] [PubMed] [Google Scholar]
- 5.Cogan DG. Visual disturbances with focal progressive dementing disease. Am J Ophthalmol 1985;100:68–72. [DOI] [PubMed] [Google Scholar]
- 6.Benson DF, Davis RJ, Snyder BD. Posterior cortical atrophy. Arch Neurol 1988;45:789–793. [DOI] [PubMed] [Google Scholar]
- 7.Tang-Wai DF, Graff-Radford NR, Boeve BF, et al. Clinical, genetic, and neuropathological characteristics of posterior cortical atrophy. Neurology 2004;63:1168–1174. [DOI] [PubMed] [Google Scholar]
- 8.deIpolyi AR, Rankin KP, Mucke L, Miller BL, Gorno-Tempini ML. Spatial cognition and the human navigation network in AD and MCI. Neurology 2007;69:986–997. [DOI] [PubMed] [Google Scholar]
- 9.Tetewsky S, Duffy CJ. Visual loss and getting lost in Alzheimer's disease. Neurology 1999;52:958–965. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien HL, Tetewsky S, Avery LM, Cushman LA, Makous W, Duffy CJ. Visual mechanisms of spatial disorientation in Alzheimer's disease. Cereb Cortex 2001;11:1083–1092. [DOI] [PubMed] [Google Scholar]
- 11.Dubinsky RM, Stein AC, Lyons K. Practice parameter: risk of driving and Alzheimer's disease (an evidence-based review): report of the quality standards subcommittee of the American Academy of Neurology. Neurology 2000;54:2205–2211. [DOI] [PubMed] [Google Scholar]
- 12.Uc EY, Rizzo M, Anderson SW, Shi Q, Dawson JD. Driver route-following and safety errors in early Alzheimer disease. Neurology 2004;63:832–837. [DOI] [PubMed] [Google Scholar]
- 13.Aud MA. Dangerous wandering: elopements of older adults with dementia from long-term care facilities. Am J Alzheimers Dis Other Demen 2004;19:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duchek JM, Carr DB, Hunt L, et al. Longitudinal driving performance in early-stage dementia of the Alzheimer type. J Am Geriatr Soc 2003;51:1342–1347. [DOI] [PubMed] [Google Scholar]
- 15.Monacelli AM, Cushman LA, Kavcic V, Duffy CJ. Spatial disorientation in Alzheimer's disease: the remembrance of things passed. Neurology 2003;61:1491–1497. [DOI] [PubMed] [Google Scholar]
- 16.Cushman LA, Duffy CJ. The sex specificity of navigational strategies in Alzheimer disease. Alzheimer Dis Assoc Disord 2007;21:122–129. [DOI] [PubMed] [Google Scholar]
- 17.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 18.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–194. [DOI] [PubMed] [Google Scholar]
- 19.Money J. A Standardized Road Map Test of Direction Sense. San Rafael, CA: Academic Therapy Publications, 1976. [Google Scholar]
- 20.Benton A, Hamsher K, Varney NR, Spreen O. Contributions to Neuropsychological Assessment: A Clinical Manual. New York: Oxford University Press, 1983. [Google Scholar]
- 21.Wechsler D. Wechsler Memory Scale, Revised Manual. San Antonio, TX: Psychological Corp., 1987. [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 23.SPSS. SPSS v15. Upper Saddle River, NJ: Prentice Hall, 2007. [Google Scholar]
- 24.Gillner S, Mallot HA. Navigation and acquisition of spatial knowledge in a virtual maze. J Cogn Neurosci 1998;10:445–463. [DOI] [PubMed] [Google Scholar]
- 25.Waller D, Knapp D, Hunt E. Spatial representations of virtual mazes: the role of visual fidelity and individual differences. Hum Factors 2001;43:147–158. [DOI] [PubMed] [Google Scholar]
- 26.Richardson AE, Montello DR, Hegarty M. Spatial knowledge acquisition from maps and from navigation in real and virtual environments. Mem Cognit 1999;27:741–750. [DOI] [PubMed] [Google Scholar]
- 27.Farrell MJ, Arnold P, Pettifer S, Adams J, Graham T, MacManamon M. Transfer of route learning from virtual to real environments. J Exp Psychol Appl 2003;9:219–227. [DOI] [PubMed] [Google Scholar]
- 28.Janzen G. Memory for object location and route direction in virtual large-scale space. Q J Exp Psychol (Colchester) 2006;59:493–508. [DOI] [PubMed] [Google Scholar]
- 29.Boutet I, Faubert J. Recognition of faces and complex objects in younger and older adults. Mem Cognit 2006;34:854–864. [DOI] [PubMed] [Google Scholar]
- 30.Gutchess AH, Welsh RC, Hedden T, et al. Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. J Cogn Neurosci 2005;17:84–96. [DOI] [PubMed] [Google Scholar]
- 31.Naveh-Benjamin M. Adult age differences in memory performance: tests of an associative deficit hypothesis. J Exp Psychol Learn Mem Cogn 2000;26:1170–1187. [DOI] [PubMed] [Google Scholar]
- 32.Bastin C, Van der Linden M. The effects of aging on the recognition of different types of associations. Exp Aging Res 2006;32:1–77. [DOI] [PubMed] [Google Scholar]
- 33.Naveh-Benjamin M, Guez J, Kilb A, Reedy S. The associative memory deficit of older adults: further support using face-name associations. Psychol Aging 2004;19:541–546. [DOI] [PubMed] [Google Scholar]
- 34.Tubi N, Calev A. Verbal and visuospatial recall by younger and older subjects: use of matched tasks. Psychol Aging 1989;4:493–495. [DOI] [PubMed] [Google Scholar]
- 35.Kemps E, Newson R. Comparison of adult age differences in verbal and visuo-spatial memory: the importance of “pure”, parallel and validated measures. J Clin Exp Neuropsychol 2006;28:341–356. [DOI] [PubMed] [Google Scholar]
- 36.Kavcic V, Fernandez R, Logan DJ, Duffy CJ. Neurophysiological and perceptual correlates of navigational impairment in Alzheimer's disease. Brain 2006;129:736–746. [DOI] [PubMed] [Google Scholar]
- 37.Golledge RG. Wayfinding Behavior: Cognitive Mapping and Other Spatial Processes. Baltimore: The Johns Hopkins University Press, 1999. [Google Scholar]
- 38.Cargin JW, Collie A, Masters C, Maruff P. The nature of cognitive complaints in healthy older adults with and without objective memory decline. J Clin Exp Neuropsychol 2007;21:1–13. [DOI] [PubMed] [Google Scholar]