Abstract
Objective:
Evidence of a relation between use of lipid lowering drugs and cognitive outcomes is mixed. This study aimed to test the association between use of statins and incidence of dementia and cognitive impairment without dementia (CIND) over 5 years of follow-up.
Methods:
Data were from a population-based cohort study comprising 1,789 older Mexican Americans. All participants had cognitive and clinical evaluations performed every 12 to 15 months. Participants who fell below specified cutpoints on cognitive tests were then evaluated clinically. Dementia diagnoses were finalized by an adjudication team. A total of 1,674 participants free of dementia/CIND at baseline were included in these analyses. Statin use was verified at each participant's home by medicine cabinet inspection. Cox proportional hazards models were used to evaluate the association between statin use and incidence of dementia/CIND.
Results:
Overall, 452 of 1,674 participants (27%) took statins at any time during the study. Over the 5-year follow-up period, 130 participants developed dementia/CIND. In Cox proportional hazards models adjusted for education, smoking status, presence of at least one APOE ɛ4 allele, and history of stroke or diabetes at baseline, persons who had used statins were about half as likely as those who did not use statins to develop dementia/CIND (HR = 0.52; 95% CI 0.34, 0.80).
Conclusion:
Statin users were less likely to have incident dementia/cognitive impairment without dementia during a 5-year follow-up. These results add to the emerging evidence suggesting a protective effect of statin use on cognitive outcomes.
GLOSSARY
- 3MSE
= Modified Mini-Mental State Examination;
- AD
= Alzheimer disease;
- ATP
= Adult Treatment Panel;
- CDC
= Centers for Disease Control;
- CIND
= cognitive impairment without dementia;
- DSM-IV
= Diagnostic and Statistical Manual of Mental Disorders–IV;
- FPG
= fasting plasma glucose;
- IQCODE
= Informant Questionnaire on Cognitive Decline in the Elderly;
- LDL-C
= low density-lipoprotein cholesterol;
- LLT
= lipid lowering therapy;
- MCI
= mild cognitive impairment;
- NINCDS-ADRDA
= National Institute of Neurologic and Communicative Disorders and Stroke–Alzheimer Disease and Related Disorders Association;
- PROSPER
= Prospective Study of Pravastatin in the Elderly;
- SALSA
= Sacramento Area Latino Study on Aging;
- SENAS
= Spanish English Neuropsychological Assessment Scales;
- SEVLT
= Spanish and English Verbal Learning Test.
The primary treatment benefit of statins is considered to be the reduction of low density-lipoprotein cholesterol (LDL-C) levels.1–8 In most trials of cardiovascular morbidity/mortality, statin treatment was shown to reduce cardiovascular events 20% to 30%.1–6 Other studies have indicated that statins have multiple actions beyond cholesterol lowering7,9,10; these actions offer potential biologic mechanisms for the effect of statins on dementia.11 However, the evidence has been inconsistent about the relation of statin use and cognitive impairment.12 The pattern of risk reductions seen in epidemiologic studies13–20 continue to raise questions regarding the impact of statins on dementia. Earlier case-control studies13–15 showed a protective effect of lipid lowering agents on the incidence of dementia. A later study19 provided evidence that indication bias may have been present in the earlier reports. The second wave of observational studies showed the same pattern of reductions, although results of the main analyses were not generally significant.16,18–20
In this article, we report the results from the Sacramento Area Latino Study on Aging (SALSA), a prospective cohort study of older (≥60 years of age) Mexican Americans from the Sacramento, CA, area. SALSA is an ongoing study, started in 1998–1999, designed to examine whether vascular and lifestyle risk factors increase the risk of dementia and decline in cognitive and physical functioning. Our objective in this study was to assess the relation between use of statins and incidence of combined dementia and cognitive impairment without dementia (CIND).
METHODS
Participants.
A detailed description of sampling and recruitment in the SALSA study has been published.21 Briefly, eligible study participants were community-dwelling, non-institutionalized Latinos, primarily Mexican Americans, aged 60 years and older in 1998 who lived in the Sacramento area. About 49% of the participants were born in Mexico or another Latin American country. A total of 1,789 participants were enrolled in the study. Each participant answered questions about lifestyle, depressive symptoms, acculturation, and medical diagnoses in the language of choice at the participants' homes. At baseline, 115 participants had dementia or CIND so were excluded from analysis of incidence rates. Of the 1,674 remaining eligible participants, 130 developed dementia or CIND over 5 years of follow-up.
Exposure measurement.
Statin use, including dose, duration, frequency, and source, was ascertained at each participant's home at baseline and updated on a semiannual phone call and reviewed and updated at each annual visit by direct inspection of all prescription medication. Medication codes were assigned by trained study staff using the Centers for Disease Control (CDC) Ambulatory Care Drug Database System (http://www2.cdc.gov/drugs/). Statin use was defined as use of any drug in the class of HMG-CoA reductase inhibitors including atorvastatin, cerivastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin.
Other covariates.
Other covariates included baseline measurements of diabetes status, previous stroke, education, nativity, smoking status, insurance, and APOE genotyping. Participants were considered to have diabetes if they met any of the following three criteria: fasting plasma glucose (FPG) level ≥126 mg/dL (7.0 mmol/L) (fasting was defined as no caloric intake for at least 8 hours) or use of an antidiabetic medication or self-report of a doctor's diagnosis of diabetes. Previous stroke was obtained by medical history. Smoking status (current, former, never), nativity, insurance status, and years of education were obtained during interviews in the participants' homes. APOE genotyping was done using buccal cell DNA. The method for genotyping followed a modification of PCR amplification/HhaI restriction isotyping method.
Outcome measurement.
Cognitive testing and evaluations were performed yearly at each annual follow-up visit. Dementia was diagnosed using Diagnostic and Statistical Manual of Mental Disorders–IV (DSM-IV)22 and National Institute of Neurologic and Communicative Disorders and Stroke–Alzheimer Disease and Related Disorders Association (NINCDS-ADRDA) criteria.23 The Modified Mini-Mental State Examination (3MSE), the Spanish and English Verbal Learning Test (SEVLT), the Spanish English Neuropsychological Assessment Scales (SENAS), and the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) were used in a multistage process to evaluate participants for cognitive impairment and dementia. Participants were referred for further evaluation if test scores from the SEVLT test were below a prespecified threshold (≤77 3MSE or ≤ 5 Word List Delayed Recall) or had decreased from baseline by ≥ 8 points (3MSE) or 3 points (Word List). Accuracy of the screening test for identifying dementia for those participants meeting criteria and a randomly selected subsample of the entire population was examined by sensitivity, specificity, and positive predictive values examined by status. There were no differences in the sensitivity or specificity of the screening criteria for detecting dementia whether the sample was selected randomly or met screening criteria.21 A single case review team including a geriatrician, neurologist, and a neuropsychologist adjudicated all potential dementia cases. All participants were classified as normal, CIND, or having dementia by the adjudication team. Diagnoses were established on the basis of neuropsychological test scores and IQCODE scores, but also included the history, mental status examination, and findings from the neurologic examination when available. DSM-IV criteria22 were used to establish a diagnosis of dementia. Cases with dementia were referred for MRI and appropriate laboratory tests. Prior to any analyses used in this article, dementia and CIND were combined into one variable: dementia/CIND. Those who were initially CIND but who progressed to dementia were classified as CIND and their follow-up time was calculated based on the date and age of CIND diagnosis. The use of the combined endpoint (incident CIND with incident dementia) improved statistical power. It also allowed the analyses of the clinically important cognitive changes which may occur over the considerable heterogeneity of the expression or onset of dementia. Longitudinal studies have shown that individuals diagnosed with mild cognitive impairment (MCI) have a much higher risk than cognitively normal people of progressing to dementia or Alzheimer disease (AD).24 In addition, the use of incident CIND (thereby excluding any participants with baseline evidence of CIND) may reduce indication bias since earlier studies showed that statins may not be prescribed as often to individuals with signs and symptoms of dementia.19
Statistical analyses.
Cox proportional hazards models were used to assess the association between statin use and the risk of dementia/CIND. The time variable was participant age,25 and participants were considered at risk for dementia/CIND in the analysis beginning with their age at study entry.26 Ties were broken using the discrete option in PHREG; the discrete model assumes that when two or more events appear to happen at the same time, there is no underlying ordering. Participants without a diagnosis of dementia/CIND during the 5-year study period were censored in the analysis at the age of their last available contact up to and including visit 5. Any statin use was modeled as a time-dependent variable with values of 0 at times prior to exposure and values of 1 after exposure began. Once statin use was assigned as 1, values did not return to 0, even if statin use was stopped. Other lipid lowering therapies (i.e., fibrates, niacin, dietary supplements, or bile acid sequestrants) were not categorized as statin use and were therefore assigned a value of 0. If the participant had a diagnosis of dementia/CIND, the statin exposure must have occurred at baseline or at visits prior to the diagnosis; therefore, statin use for all participants was counted up to and including visit 4 to ensure consistency between those with a diagnosis of dementia/CIND (since exposure had to have occurred prior to diagnosis) and those without. Potential confounders were tested as fixed covariates and included gender, education, medical insurance, nativity (born in the United States vs Mexico or Latin America), smoking status, presence of at least one APOE ɛ4 allele (a major genetic risk factor for AD), and baseline blood pressure, history of stroke, diabetes, use of antihypertensive medication, and cognitive test results (3MSE and Word list Delayed Recall Trial). Baseline comparisons between groups were made by use of Fisher exact tests for discrete variables and a t test for continuous variables. Models were checked for the proportional hazards assumption graphically and with statistical tests. All statistical analyses were performed using SAS version 9.1.
RESULTS
Of the 1,674 participants free of dementia or CIND at baseline, 452 (27%) took statins at some time during the study. Forty-three participants (43/1674 [<3%]) were on lipid lowering therapies other than statins at any time during the study, including fibrates, niacin, dietary supplements, or bile acid sequestrants; these participants were counted as no statin use. Of the participants who used statins, 58% (n = 263) used them for two or more years of the study.
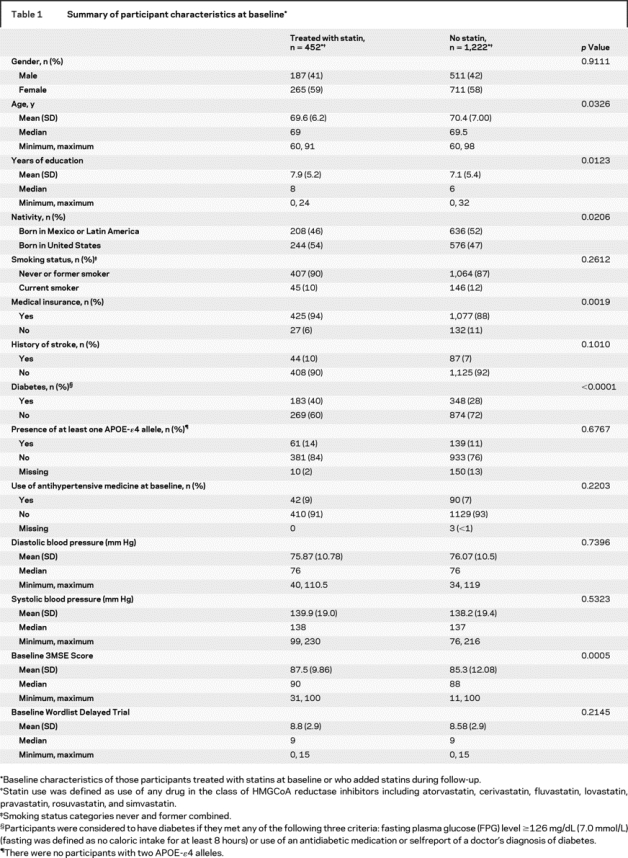
The demographic and baseline characteristics for those participants who used statins during the study and those who did not use statins are shown in table 1. The two groups were comparable with respect to gender, smoking status, history of stroke, presence of any APOE-ɛ4 allele, baseline blood pressure and use of antihypertensive medication, and the Word List Delayed Recall Trial results. Participants in the statin group were slightly younger, had more education, a greater percentage were born in the United States, and more were covered by medical insurance. A greater percentage of statin-treated participants had a history of diabetes at baseline, and higher mean baseline 3MSE scores. Participants using statins experienced a significantly greater decline in LDL-C than those not using statins (−5.34 μg/dL per year, p < 0.0001).
Table 1 Summary of participant characteristics at baseline
A total of 130 participants developed dementia or CIND over the 5-year follow-up period (58 CIND and 82 dementia [10 of the CIND progressed to dementia]). No participants were reclassified from CIND to unimpaired, or from dementia to CIND. CIND cases were reevaluated by the adjudication team until they converted to dementia. Dementia cases were also followed after diagnosis but not re-evaluated by the team so any reversions would not be observed. No CIND cases converted to normal during follow-up. Etiology was recorded for participants with dementia: 48% had possible or probable AD, 23% had undetermined etiology, 13% had ischemic vascular dementia, 13% had mixed AD or vascular dementia, and 3% had other. Table 2 provides a summary of the overall number and percent of incident dementia/CIND cases by visit, as well as statin use, deaths, and number of participants lost to follow-up over the 5-year follow-up period. The figure shows a flow diagram of participants at baseline and at follow-up by statin use.
Table 2 Summary of statin use and incidence of dementia/CIND, deaths, and lost to follow-up by study visit
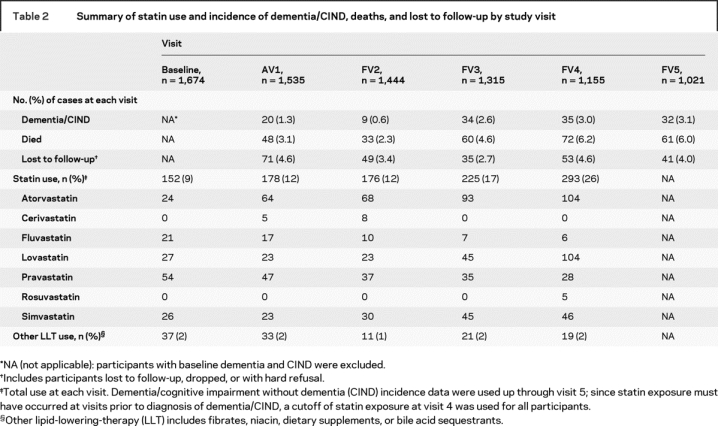
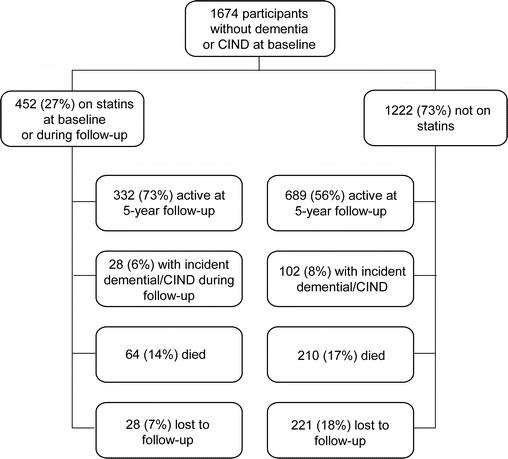
Figure Flow diagram of participants at baseline and at follow-up by statin use
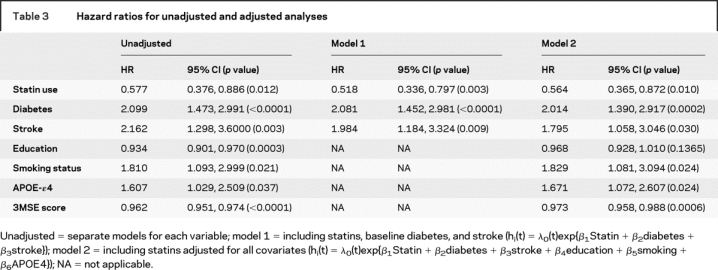
Table 3 shows the results of a series of Cox proportional hazards models (unadjusted analyses and two adjusted models) used to examine the relationship between dementia/CIND, use of statins, and each of the significant baseline covariates listed in table 1. Baseline diabetes, stroke, years of education, smoking status, presence of any APOE-ɛ4 allele, and 3MSE score were all significantly associated with dementia/CIND in unadjusted regression models. Of the covariates significantly associated with dementia/CIND, baseline diabetes and stroke were also associated with statin use and were therefore included in model 1 as potential confounders. All significant covariates were included in model 2. There were no significant interactions between statin use and any of the covariates. In the unadjusted analyses, statin use was associated with a 43% lower rate of dementia/CIND. In model 1, statin use remained associated with a 48% lower rate of dementia/CIND. In model 2, with all important covariates included in the model, rates of dementia/CIND were 44% lower in statin users. Although baseline 3MSE score did not predict statin use, and there was no interaction between the two variables, we stratified model 1 on median 3MSE score (<88 and ≥88) to determine whether the protective effect of statins on dementia persisted in both the low and high functioning groups. We found that the relationship persisted in both groups (<88 median score: HR = 0.546, 95% CI = 0.327, 0.912; ≥88 median score: HR = 0.444, 95% CI = 0.200, 0.988).
Table 3 Hazard ratios for unadjusted and adjusted analyses
DISCUSSION
We found statin use to be associated with a significant reduction in the incidence of dementia/CIND among a representative sample of community-dwelling Mexican Americans which was unaffected by confounding for key covariates. Compared to our study, the results of other epidemiologic studies evaluating the effect of statins on dementia have been variable,13–20 with frequent debate regarding methodologic issues in these studies, including the presence of indication bias in early case-control studies.12 It is striking to note, however, that risk reductions were seen in all epidemiologic studies, with the exception of one study.19 However, the reductions were not significant in the main analyses of most of the cohort studies.16,18–20
Two major clinical trials are often cited as providing evidence that statins do not have an effect on the incidence of dementia: the Prospective Study of Pravastatin in the Elderly (PROSPER)5 and the Medical Research Council/British Heart Foundation Heart Protection Study27; however, because of methodologic limitations in relation to dementia outcomes in these two trials, the results of these trials are difficult to evaluate. Dementia incidence or cognitive outcomes were not preplanned endpoints in either of them, neither study included a clinical cognitive evaluation, and numbers of patients with follow-up information for cognitive evaluations were not reported in either study manuscript. In PROSPER a post hoc analysis compared changes in cognitive scores over a 3-year period between statin-treated and placebo patients and found no significant differences. In the MRC/BHF HPS trial,27 similar percentages of participants (0.3% in each—statin vs placebo—group) developed dementia during the 5-year follow-up period. The report did not state how the outcome of dementia was determined (e.g., reported as an adverse event or by follow-up phone interview).
Four cohort studies also examined the relationship between lipid lowering therapy (LLT) use and risk of dementia.16,18–20 All of these studies had differing study designs, and only one study evaluated statin use as a time-dependent covariate.18 In the three studies using a single timepoint to ascertain statin use, the percentages of participants on statins were low (ranging from 4% to 12%).16,19–20 In the study with design similar to the SALSA study,18 similarities include the length of follow-up time (5 years) and the method of analysis (Cox proportional hazards models with statin use as a time-dependent covariate in the model). Some of the differences in this study include the outcome variable (dementia vs CIND and dementia), a lower percentage of statin use (17%), less frequent ascertainment of statin use (every 2 years), use of a pharmacy database compared to visual inspection, a population that was mostly white (91%), and an average age at study entry that was older (75 years of age compared to 70 years of age in SALSA). Of note, this study found that statin use may provide some benefit for younger individuals (evaluation of effect of statin use on dementia after stratification by age [<80 and ≥ 80 years] and with one APOE ɛ4 allele: HR = 0.33, 95% CI = 0.10, 1.04). 16,18 Comparison of clinical trials to observational studies are made difficult by the kinds of participants that can be included in each.
The effects of statin use on the incidence of dementia/CIND seen in this analysis may be due in part to some of the methodologic strengths in this study. Important strengths include the prospective collection of data, including medication data ascertained by semiannual phone updates and annual visual inspection of medications (allowing for use of a time-dependent statin covariate in the model with evaluations at five timepoints including baseline), and clinical evaluation with adjudication of dementia cases used for diagnosis of dementia and CIND. There was a large proportion of participants with diabetes in this population with high risk of cardiovascular disease, and therefore these participants had greater overall statin use (27%) compared to most of the previously reported cohort studies. In SALSA, the annual follow-up visits were conducted from 2000 to 2006, during which time there was a significant increase in statin use in the general population as well as an increase in statin use among the elderly, especially following the issuance of 2001 Adult Treatment Panel (ATP) III guidelines.28 During this period, adherence to statin therapy was also reported to be improved for patients with high cardiovascular risk or with previous stroke, albeit still somewhat poor (<60% 6 months after initiation).28 The data from our study reflect these trends including an increase in statin use (9% at baseline increasing to 26% at visit 4; see table 2) and adherence to therapy (58% used statins two or more follow-up periods of the study).
Although we combined dementia and CIND to improve statistical power, longitudinal studies have shown that individuals diagnosed with MCI have a much higher risk than cognitively normal people of progressing to dementia or AD.24 One of the potential limitations of this study is the lack of power to evaluate the effects of statins on specific dementia etiologies. In addition, while the non mortality loss to follow-up was generally low in this study (<17% overall), there was less loss to follow-up in statin users (7%) compared to nonusers (18%). In the nonusers group, participants lost to follow-up and those remaining in the study were similar with respect to gender, age, and history of heart failure, diabetes, stroke, myocardial infarction, and high blood pressure at baseline. Statin users had higher baseline prevalence of type 2 diabetes, myocardial infarction, and high blood pressure. There was no difference by use group for baseline heart failure or stroke. Although there are some differences in health status, we adjusted for these factors in analysis. The difference in mortality attrition by user group is slight. Statin users were only slightly less likely to die (14%) compared to the nonusers group (17%). Statin use was not associated with survival in a Cox regression model even when adjusted for covariates (HR: 0.92; 95% CI: 0.69–1.20) among participants not lost to follow-up. Nevertheless, indication bias, differential loss to follow-up, and competing risk could bias the estimate of effect for statin use away from the null.
To date, there are no primary prevention trials of statins for any kind of dementia or cognitive decline in normal people. An ongoing trial of statins as potential treatment for delaying progression of AD29 has not so far provided clear evidence of benefit. Additional questions and future research suggested in particular by this analysis involve the investigation of differences of statin use and association of dementia and CIND in individuals with stroke and diabetes, and the impact of statins on CIND and subtypes of dementia.
Supplementary Material
Address correspondence and reprint requests to Dr. Mary N. Haan, University of Michigan, School of Public Health, Department of Epidemiology, Ann Arbor, MI 48104 mnhaan@umich.edu.
Supported by NIA AG12975, DK 60753.
Disclosure: Caryn Cramer was employed by Pfizer Corporation during completion of her doctoral degree during which time this study was conducted. Pfizer did not provide any material support for this study, and did not participate in the design, conduct, management, analysis, interpretation, review, or approval of the study or the manuscript. The other authors have reported no conflicts of interest.
Received October 23, 2007. Accepted in final form April 22, 2008.
REFERENCES
- 1.Freeman D, Norrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation 2001;103:357–362. [DOI] [PubMed] [Google Scholar]
- 2.Keech A, Colquhoun D, Best J, et al. Secondary prevention of cardiovascular events with long-term pravastatin in patients with diabetes or impaired fasting glucose. Results from the LIPID trial. Diabetes Care 2003;26:2713–2721. [DOI] [PubMed] [Google Scholar]
- 3.Sever P, Dahlof B, Poulter N, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower than average cholesterol concentrations in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003;361:1149–1158. [DOI] [PubMed] [Google Scholar]
- 4.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003;361:2005–2016. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd J, Blauw G, Murphy MB. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002;360:1623–1630. [DOI] [PubMed] [Google Scholar]
- 6.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–696. [DOI] [PubMed] [Google Scholar]
- 7.Miida T, Hirayama S, Nakamura Y. Cholesterol-independent effects of statins and new therapeutic targets: ischemic stroke and dementia. J Atheroscler Thromb 2004;11:253–264. [DOI] [PubMed] [Google Scholar]
- 8.Schacter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fund Clin Pharmacol 2004;19:117–125. [DOI] [PubMed] [Google Scholar]
- 9.Massy ZA, Deane WF, Kasiske BL. Inhibition of the mevalonate pathway: benefits beyond cholesterol reduction? Lancet 1996;347:102–103. [DOI] [PubMed] [Google Scholar]
- 10.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation 2004;109:39–43. [DOI] [PubMed] [Google Scholar]
- 11.Li G, Larson EB, Sonnen JA, et al. Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology 2007;69:878–885. [DOI] [PubMed] [Google Scholar]
- 12.Rockwood K. Epidemiological and clinical trials evidence about a preventive role for statins in Alzheimer's disease. Acta Neurol Scand 2006;114(suppl 185):71–77. [DOI] [PubMed] [Google Scholar]
- 13.Jick H, Zornberg GL, Jick SS, et al. Statins and the risk of dementia. Lancet 2000;356:1627–1631. [DOI] [PubMed] [Google Scholar]
- 14.Hajjar I, Schumpert J, Hirth V, et al. The impact of the use of statins on the prevalence of dementia and the progression of cognitive impairment. J Gerontol 2002;(57A):M414–M418. [DOI] [PubMed]
- 15.Wolozin B, Kellman W, Russeau P. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3methyglutaryl coenzyme A reductase inhibitors. Arch Neurol 2000;57:1439–1443. [DOI] [PubMed] [Google Scholar]
- 16.Rockwood K, Kirkland S, Hogan D, et al. Use of lipid-lowering agents, indication bias, and the risk of dementia in community-dwelling elderly people. Arch Neurol 2002;59:223–227. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez E, Hiroko D, Birzescu M, et al. Use of lipid-lowering drugs in older adults with and without dementia: a community-based epidemiological study. JAGS 2002;50:1852–1856. [DOI] [PubMed] [Google Scholar]
- 18.Li G, Higdon R, Kukuss WA, et al. Statin therapy and risk of dementia in the elderly. Neurology 2004;63:1624–1628. [DOI] [PubMed] [Google Scholar]
- 19.Zandi P, Sparks L, Khachaturian A, et al. Do statins reduce risk of incident dementia and Alzheimer disease? The Cache County Study. Arch Gen Psychiatry 2005;62:217–224. [DOI] [PubMed] [Google Scholar]
- 20.Reitz C, Tang MX, Luchsinger J, Mayeux R. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch Neurol 2004;61:705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haan M, Mungas D, Gonzalez H, et al. Prevalence of dementia in older Latinos. JAGS 2003;51:169–177. [DOI] [PubMed] [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV). Washington, DC: American Psychiatric Association, 1994:143–147. [Google Scholar]
- 23.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 24.Petersen RC, Stevens JC, Ganguli M, et al. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Neurology 2001;56:1133–1142. [DOI] [PubMed] [Google Scholar]
- 25.Commenges D, Letenneur L, Joly P, Alioum A, Dartigues J-F. Modelling age-specific risk: application to dementia. Stat Med 1998;17:1973–1988. [DOI] [PubMed] [Google Scholar]
- 26.Allison Paul. Survival Analysis Using SAS: A Practical Guide. Cary, NC: SAS Press, 1995. [Google Scholar]
- 27.Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;9326:7–22. [Google Scholar]
- 28.Ovbiagele B, Saver JL, Bang H, et al. Statin treatment and adherence to national cholesterol guidelines after ischemic stroke. Neurology 2006;66:1164–1170. [DOI] [PubMed] [Google Scholar]
- 29.Sparks L, Sabbagh M, Connor D, et al. Atorvastatin for the treatment of mild to moderate Alzheimer disease. Arch Neurol 2005;62:753–757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


