Abstract
Objective:
To model the long-term risks and benefits of natalizumab in individuals with relapsing multiple sclerosis (MS).
Methods:
We created a Markov model to evaluate treatment effects on reducing relapses and slowing disease progression using published natural history data and clinical trial results. Health changes, measured in quality-adjusted life-years (QALYs), were based on patient health preferences. Patient cohorts treated with no disease-modifying treatment, natalizumab, subcutaneous interferon β-1a, and a theoretical “perfect” MS treatment were modeled. Sensitivity analysis was used to explore model uncertainty, including varying risks of developing progressive multifocal leukoencephalopathy (PML).
Results:
Treatment with natalizumab resulted in 9.50 QALYs over a 20-year time horizon, a gain of 0.80 QALYs over the untreated cohort and 0.38 QALYs over interferon β-1a. The health loss due to PML was small (−0.06 QALYs). To offset natalizumab’s incremental health gain over interferon β-1a, the risk had to increase from 1 to 7.6 PML per 1,000 patients treated over 17.9 months. The “perfect” MS treatment accumulated 10.59 QALYs over the 20-year time horizon, 1.89 QALYs above the untreated cohort. Interferon β-1a resulted in greater QALY gains compared with natalizumab if natalizumab’s relative relapse reduction was reduced from 68% to 35% or if interferon β-1a’s relative reduction was increased from 32% to 65%.
Conclusions:
A more than sevenfold increase in actual risk of progressive multifocal leukoencephalopathy was required to decrease natalizumab’s health gain below that of interferon β-1a, and there remains considerable room for additional gains in health (>50%) beyond those already achieved with current therapies.
GLOSSARY
- AFFIRM
= Natalizumab Safety and Efficacy in Relapsing Remitting Multiple Sclerosis;
- EDSS
= Expanded Disability Status Scale;
- FDA
= Food and Drug Administration;
- IFN
= interferon;
- MS
= multiple sclerosis;
- PML
= progressive multifocal leukoencephalopathy;
- PRISMS
= Prevention of Relapses and Disability by Interferon Beta-1a Subcutaneously in Multiple Sclerosis;
- QALY
= quality-adjusted life-year;
- TOUCH
= Tysabri Outreach: Unified Commitment to Health;
- TYGRIS
= Tysabri Global Observation Program in Safety.
Natalizumab (Tysabri), an α4 integrin antagonist, is the most recent multiple sclerosis (MS) disease-modifying drug shown to be effective for relapsing forms of MS, but is associated with a small risk of progressive multifocal leukoencephalopathy (PML), a usually fatal disease.1,2 When choosing between treatment options, the majority of MS patients prefer active roles in medical decision making.3 However, while patients must have “sufficient and appropriate” information to express treatment preferences,4 a great deal of uncertainty surrounds the risk of PML associated with natalizumab.
Two studies are currently being conducted to evaluate the long-term safety of natalizumab. The Tysabri Outreach: Unified Commitment to Health (TOUCH) system is a US Food and Drug Administration (FDA)–mandated restricted distribution and aggressive PML-monitoring program in the United States, and the Tysabri Global Observation Program in Safety (TYGRIS) is a worldwide observational study.5 As of February 2007, more than 5,700 MS patients receiving natalizumab were currently enrolled in these studies, and no additional cases of PML were identified, although the treatment duration was short (mean of 3.4 infusions for those in the TOUCH system). Although in the future both studies will be able to provide more accurate measures of short- and long-term PML risk, patients and clinicians presently considering natalizumab therapy have limited information regarding the health impacts of natalizumab’s risks and benefits.
To decrease uncertainty regarding the long-term safety of natalizumab (defined by the FDA as the benefits outweighing the risks of treatment)6 and to investigate natalizumab’s treatment profile and its relationship to patient health, we created a risk-benefit model using the quality-adjusted life-year (QALY) as an outcome metric. We then compared long-term health changes for the natalizumab cohort with the health profiles modeled for a natural history cohort, a cohort treated with interferon (IFN) β-1a, and a cohort treated with a “perfect” MS treatment.
METHODS
Model description.
We used TreeAge 4.0 software (TreeAge software, Inc., Boston, MA) to create a Markov probability model to assess the long-term treatment effects of natalizumab on QALYs in patients with clinically definite relapsing MS (figure 1). A Markov model is a type of decision model that is used to model transitions from one health state to another over time.7 Health states were defined using the Expanded Disability Status Scale (EDSS).8 Markov models have been used in MS disease modeling since 19859 and are increasingly being used in chronic conditions such as MS to incorporate the progressive and fluctuating nature of the disease process.10 We adhered to principles of good practice for decision analytic modeling in health care evaluations.11 A full technical report is available from the author upon request.
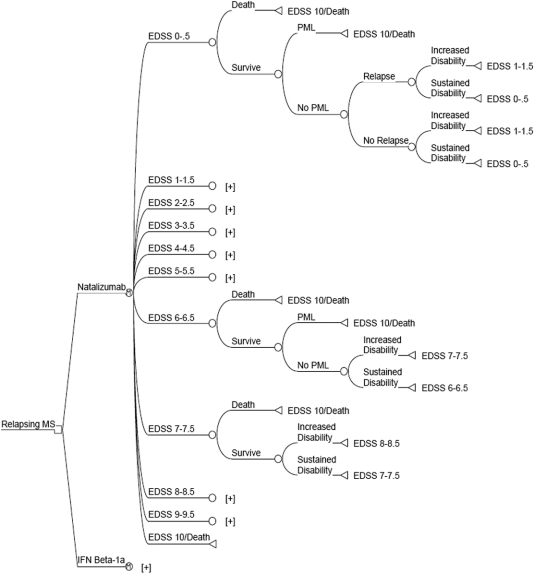
Figure 1 Semi-Markov probability model
After the treatment decision node (square node), the cohort is split into groups based on Expanded Disability Status Scale (EDSS) states. During each 6-month cycle, subjects may die, develop progressive multifocal leukoencephalopathy (PML) (if taking natalizumab), have a relapse and progress in disability, or have a relapse without disability progression. All subtrees ending with [+] are identical to the subtree above; the interferon (IFN) β-1a arm is identical to the natalizumab arm, except that the probability of developing PML is zero. MS = multiple sclerosis.
The model time horizon was divided into 6-month cycles, during which patients may have a relapse, progress to a more disabled health state, or both. After each cycle, the cohort was redistributed among EDSS scores based on natural history progression probabilities.12,13 Data inputs for treatment effects and utility values were obtained from the literature (table 1). Three major assumptions were adopted for the base case model:
Table 1 Base case annual probabilities and utilities
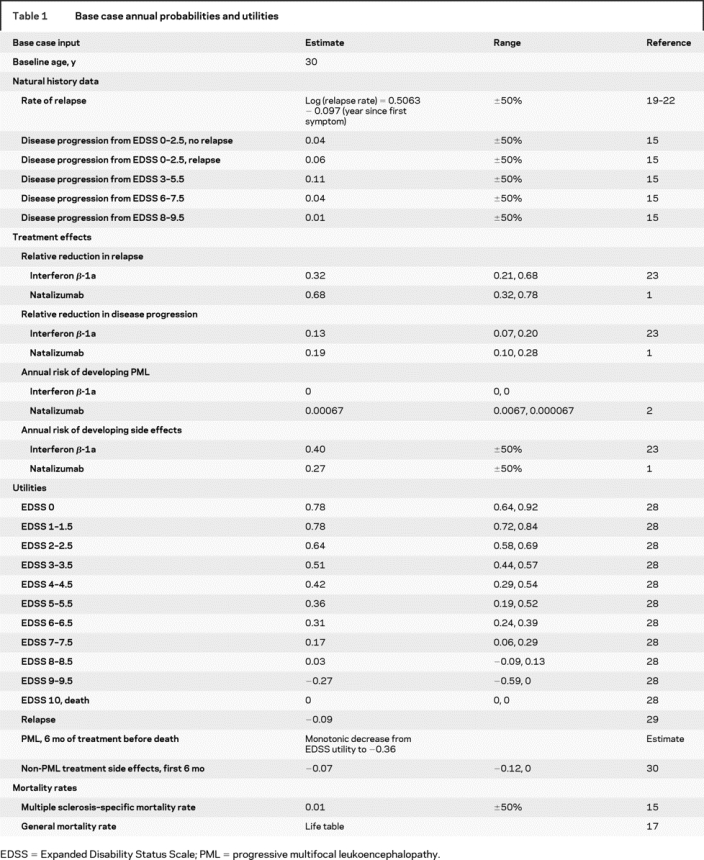
Relapses occurred only in the lower disability states (EDSS 0–5.5). The transformation of relapsing-remitting MS to secondary progressive MS occurs over time and was not directly specified in the natural history data. Thus, as previous models have done, we assumed that this transformation occurred between EDSS 3.0 and EDSS 7.5, and that relapses did not occur after EDSS 6.0.14,15
Patients could not transition to a less-disabled EDSS health state, an assumption that is consistent with long-term natural history data.13
Treatment discontinuation was not directly modeled, because these effects were implicit in published effectiveness data (intent-to-treat analysis).
Disease modeling.
The baseline cohort included 30-year-old patients with clinically definite, relapsing MS, and a 1:2 male-to-female ratio based on the approximate US prevalence of MS.16 Patients entered the model with minimal or mild disability (57% with EDSS scores of 1–1.5 and 43% with EDSS scores of 2–2.5), the relative distribution based on natural history data from patients at disease onset.13 Age-specific general mortality rates were used for all patients,17 except those at EDSS 9–9.5, who were assigned an additional MS-specific mortality rate based on natural history data.12,13
Disease progression was based on the probability of increasing in disability by 1 EDSS point. Estimates were reported by a previous MS cost-effectiveness model,15 which used data from two natural history studies (excluding data for primary progressive MS subjects).12,13 Both published natural history studies reported progression based on EDSS groupings of 0–2.5, 3–5.5, 6–7.5, and 8–9.5. We assumed that all patients within each EDSS grouping progressed at the same rate. We estimated a higher probability of disease progression for mildly disabled patients (EDSS 0–2.5) that experienced a relapse compared with those who did not experience a relapse (table 1).18
Because relapse rates have been shown to be time dependent,19,20 we developed a predictive regression model using natural history relapse rates from four prospective studies (total of 618 patients).19–22 Using the available data (13 of 25 years), we regressed the relapse rate by years after MS onset using a log transformation to maximize the model’s predictive ability (formula in table 1). The predicted annual relapse rate was 1.5 for the year after diagnosis, declined to 1.1 by the fifth year (the approximate mean disease duration for subjects in the Natalizumab Safety and Efficacy in Relapsing Remitting Multiple Sclerosis [AFFIRM] and Prevention of Relapses and Disability by Interferon Beta-1a Subcutaneously in Multiple Sclerosis [PRISMS] trials),1,23 and further declined logarithmically to 0.24 over the next 15 years.
Treatment modeling.
The two treatment options were 300 mg natalizumab (Tysabri, Biogen Idec and Elan Pharmaceuticals) administered by IV infusion every 4 weeks or 44 μg IFNβ-1a (Rebif, Ares-Serono) administered subcutaneously three times weekly.1 Subcutaneous IFNβ-1a was chosen as a reference treatment because it was evaluated in studies with similar design, enrollment criteria, and endpoints to natalizumab.23 We applied relative treatment effects from the pivotal clinical trials to the untreated natural history cohort, because prior research has shown that relative treatment effects are usually constant across spectrums of underlying risk.24 Because no treatment termination guidelines exist in the United States, we followed UK prescribing guidelines and assumed treatments were continued until EDSS 7 was reached.25 We also modeled a “perfect” MS treatment in which patients experienced no relapses, disease progression, or side effects. The “perfect” treatment had no restorative attributes, and patients maintained their baseline level of disability and utility throughout the time horizon.
We assumed that monotherapy with natalizumab was sufficient for developing PML, and calculated an annual risk based on the published risk estimate (1 per 1,000 patients treated for an average of 17.9 months).2 The probability of developing non-PML side effects (e.g., injection-site reactions, flulike symptoms, and fatigue) was estimated using the most common significant side effect reported in the clinical trials.1,23 All non-PML side effects were assumed to abate after 6 months.15
Health impact modeling.
Net health changes over time were measured in QALYs, which is a time-weighted measure of utility states; 1 QALY is equal to 1 year in perfect health.26,27 Utility values were obtained from North American patient preferences for EDSS disability states and relapses (table 1).28,29 The utility for a patient developing PML was estimated by monotonically decreasing the patient’s utility over 6 months from their present EDSS health state to the worst possible health state measured by the Health Utilities Index III (−0.36), a health state considered worse than death. All PML cases were then assumed to be fatal after one 6-month cycle (death has a utility of 0). The disutility of non-PML side effects for IFNβ-1a was obtained from a patient survey.30 The disutility of natalizumab’s side effects was assumed to be the same as that for IFNβ-1a. Future utilities were discounted by 3% annually.26
Analyses.
We calculated the net health gain over 20 years for the natural history cohort and the treatment cohorts (natalizumab, IFNβ-1a, and the “perfect” MS treatment). For each treatment, we calculated the QALY gains associated with reduction in relapses and delayed progression, and the QALY losses associated with PML- and non–PML-related side effects. Shorter time horizons of 2 and 10 years were conducted during sensitivity analysis. We also performed one-way sensitivity analyses for each model input over the range of values displayed in table 1.
We also assessed the impact on the treatment decision by varying the risk of PML over time and also by doubling the risk of PML every 18 months (for 9 years) due to the potential for cumulative exposure to natalizumab. Last, we modeled a cohort with increased disability progression, with progression rates approximately equal to the rate observed in the AFFIRM clinical trial.1
RESULTS
Base case analysis.
The natural history cohort accumulated 8.70 QALYs over the 20-year time horizon. Natalizumab resulted in an additional 0.80 QALYs gained for a total of 9.50 QALYs, whereas IFNβ-1a resulted in an additional 0.42 QALYs for a total of 9.12 QALYs gained (table 2 and figure 2). The majority of health gains for both treatments were derived from reducing relapse rates, and the health loss due to developing PML while taking natalizumab was small (−0.06 QALYs). The “perfect” MS treatment accumulated 10.59 QALYs over the 20-year time horizon, 1.89 QALYs above the untreated cohort. Therefore, treatment with natalizumab resulted in 43% of the theoretical health gain for an MS disease-modifying drug, and treatment with IFNβ-1a resulted in 22% of the theoretical health gain.
Table 2 Base case results
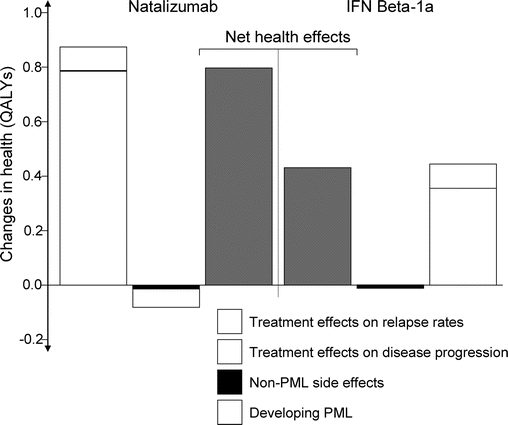
Figure 2 Treatment effects over 20 years compared with a natural history cohort
IFN = interferon; PML = progressive multifocal leukoencephalopathy; QALY = quality-adjusted life-year.
Sensitivity analyses.
Varying the risk of developing PML while taking natalizumab had considerable influence on the results, but a more than sevenfold increase in the risk was required to decrease natalizumab’s health gain below that of IFNβ-1a’s. The increase in PML risk was from 1 patient developing PML to 7.6 patients developing PML per 1,000 patients treated over 17.9 months. The results were also sensitive to changes in each treatment’s relative relapse rate reduction. For example, natalizumab resulted in fewer QALYs gained compared with IFNβ-1a if the relative risk reduction of relapses associated with natalizumab was reduced from 68% to 35% or if the relative risk reduction of relapses associated with IFNβ-1a was increased from 32% to 65% (table 3). In addition, increasing the disutility associated with relapses (which is analogous to more severe or longer relapses) favored natalizumab. Finally, the results were not sensitive to changes in the probability or disutility of non-PML side effects for either treatment.
Table 3 Model inputs and scenarios that influenced results
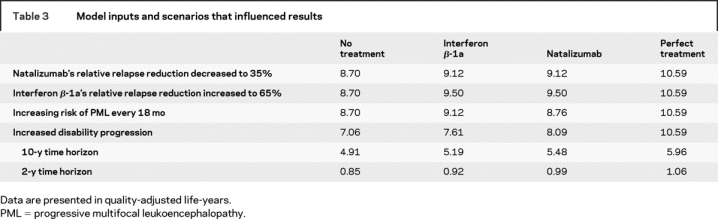
Both natalizumab and IFNβ-1a resulted in health gains over shorter time horizons of 2 and 10 years (table 3). Varying baseline disability status or the utilities associated with EDSS disability states caused the absolute size of health gains to change, but the relative difference between natalizumab and IFNβ-1a remained approximately the same. Larger treatment benefits were also accrued for the high disability progression cohort, but the relative health gains compared with a theoretical perfect treatment decreased to 29% for natalizumab and 16% for IFNβ-1a.
DISCUSSION
Understanding the long-term risks and benefits of treatment has never been more important given the serious limits to the old paradigm of short-term clinical trials, FDA approval, and weak postmarketing oversight. The expanded legislative authority given to the FDA to improve its ability to track long-term safety of approved therapeutics speaks to the importance of this issue.31 Decision modeling is one approach to guide evidence-based decision making and to highlight areas in need of future research.
Our results show that the benefit of long-term treatment with natalizumab far outweighed the risk of developing PML. A more than sevenfold increase in the risk of PML was required (from 1 to 7.6 patients per 1,000 treated over 17.9 months) to reduce natalizumab’s health gain below that of IFNβ-1a’s. This increase in risk is outside the 95% CI of the current PML risk estimate (0.2–2.8 per 1000 over 17.9 months of treatment).2 It may not be, however, outside the tolerance of many MS patients. Approximately 55% of MS patients indicated in a recent survey that they definitely or probably would use a “treatment for MS that was significantly more effective than currently available drugs,” even with a 1 in 1,000 chance of a fatal side effect.32 Approximately 18% of patients surveyed would tolerate a risk of 1 in 100, and 14% would tolerate a risk of 1 in 10. Thus, even if there were an increased risk over time due to cumulative exposure to natalizumab, it would likely be tolerated by some MS patients.
The health gains associated with natalizumab and IFNβ-1a were less than 1 QALY gained over 20 years. As shown in a previous model, the majority of the health gains came from reducing relapse rates.33 In fact, only 8% of the potential QALY gains associated with the treatments was due to delaying disease progression. In addition, natalizumab and IFNβ-1a accounted for less than 50% of potential QALY gains compared with a “perfect” treatment, emphasizing that short-term treatment trials showing a considerable effect on intermediate endpoints may have a much more modest effect on quality of life over the entire course of treating the disease.
The model results were sensitive to changes in the relative risk reductions of natalizumab and IFNβ-1a. In this model, we applied the relative risk reduction found in the pivotal clinical trials to a natural history cohort that experienced more disease activity than did the AFFIRM placebo group and less activity compared with the PRISMS placebo group, after taking into account disease duration. The differences in health gains between the two treatments, therefore, would be reduced if natalizumab’s relative risk reduction is less in patients with greater disease activity or if IFNβ-1a’s relative risk reduction is greater in patients with less disease activity. A near convergence of the relative treatment effect on relapses would be required for the health gains associated with each treatment to be equal.
Prior research has shown that short-term QALY gains associated with natalizumab and IFNβ-1a were similar when analyzing the absolute treatment differences within the pivotal trial populations.33 Similar QALY gains occurred despite the twofold greater relative risk reductions associated with natalizumab because the natalizumab trial population experienced more than 50% less disease activity than did the IFNβ-1a trial population. Although we do not fully know how relative and absolute risk reductions behave outside the clinical trial setting, relative risk reductions have been shown to usually be constant across risk spectrums.24 A randomized trial is the only way to definitely understand treatment effects in the same population.
Decision models are inherently limited by the data and the measures used. For example, the EDSS does not capture all aspects of disability, and QALYs may be insensitive to small changes in physical and psychological function. Therefore, the model results must be interpreted with caution. The purpose of a model, however, is not to predict future events, but to provide a structured, transparent, and quantitative method for framing a complex decision that is open to debate and modification. Our study has additional limitations. First, we assumed that monotherapy with natalizumab was sufficient for developing PML, although no cases were reported in the AFFIRM clinical trial. In all PML cases detected, natalizumab was used in combination with, or after, immunomodulating therapy.34–36 Future results from the TOUCH and TYGRIS studies will decrease this uncertainty. Second, we did not model the exact scenario of patients currently enrolled within the TOUCH program, because 68% switched from another disease-modifying drug.5 Increasing the cohort’s baseline disability or the probability of disease progression, however, did not influence the base case results. Third, we assumed that treatment effects remained constant over time, because no diminished treatment effect over time for IFNβ-1a has been reported.
Our study has strengths. First, we chose natural history data for disease modeling rather than clinical trial placebo data to increase the generalizability to all newly diagnosed relapsing patients. Generalizability is also improved by using health state preferences from a large North American cohort of MS patients. Finally, the model can easily allow for periodic updates as new data emerge or for the addition of cost information to conduct cost-effectiveness analyses from various perspectives.
As newer and higher risk therapies emerge for many chronic neurologic conditions, developing alternative approaches to technology assessment should be of high priority for researchers, clinicians, patients, policy makers, and the public.37 Natalizumab, in addition to its importance to the MS community, is an example for neurology and the medical field as a whole.
Address correspondence and reprint requests to Dr. Robert G. Holloway, University of Rochester Medical Center, 601 Elmwood Ave., Box 673, Rochester, NY 14642 robert_holloway@urmc.rochester.edu
Disclosure: K.N., R.G.H., and S.R.S. were supported in part by contract HC0071 from the National Multiple Sclerosis Society. K.N. was supported in part by research grant K01 AG 20980 from the National Institute on Aging. E.R.D. was supported in part by an American Academy of Neurology Clinical Research Training Fellowship. R.G.H. was supported in part by grant K24 NS4 2098 from the National Institute of Neurological Disorders and Stroke. S.R.S. has received research funding from Biogen, Serono, and Teva and honoraria for educational and consulting activities from Berlex, Biogen, Serono, and Teva. The project described was partially supported by grant number 1 UL1 RR024160-01 from the National Center for Research Resources (NCRR), a component of the NIH and the NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Received August 23, 2007. Accepted in final form April 1, 2008.
REFERENCES
- 1.Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354:899–910. [DOI] [PubMed] [Google Scholar]
- 2.Yousry TA, Major EO, Ryschkewitsch C, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med 2006;354:924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heesen C, Kasper J, Segal J, Kopke S, Muhlhauser I. Decisional role preferences, risk knowledge and information interests in patients with multiple sclerosis. Mult Scler 2004;10:643–650. [DOI] [PubMed] [Google Scholar]
- 4.Coulter A, Entwistle V, Gilbert D. Sharing decisions with patients: is the information good enough? BMJ 1999;318:318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozic CBG, Kooijmans M, Kim R, Lynn F, Panzara MA. The safety of natalizumab in patients with relapsing multiple sclerosis: an update from TOUCH and TYGRIS. 59th Annual Meeting of the American Academy of Neurology; April 28–May 5, 2007; Boston, MA.
- 6.Meadows M. The FDA’s drug review process: ensuring drugs are safe and effective. FDA Consum 2002;36:19–24. [PubMed] [Google Scholar]
- 7.Naimark D, Krahn MD, Naglie G, Redelmeier DA, Detsky AS. Primer on medical decision analysis, part 5: working with Markov processes. Med Decis Making 1997;17:152–159. [DOI] [PubMed] [Google Scholar]
- 8.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 9.Wolfson C, Confavreux C. A Markov model of the natural history of multiple sclerosis. Neuroepidemiology 1985;4:227–239. [DOI] [PubMed] [Google Scholar]
- 10.Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology 2007;68:2059–2065. [DOI] [PubMed] [Google Scholar]
- 11.Weinstein MC, O’Brien B, Hornberger J, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices–Modeling Studies. Value Health 2003;6:9–17. [DOI] [PubMed] [Google Scholar]
- 12.Runmarker B, Andersen O. Prognostic factors in a multiple sclerosis incidence cohort with twenty-five years of follow-up. Brain 1993;116:117–134. [DOI] [PubMed] [Google Scholar]
- 13.Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study, I: clinical course and disability. Brain 1989;112:133–146. [DOI] [PubMed] [Google Scholar]
- 14.Bell C, Graham J, Earnshaw S, Oleen-Burkey M, Castelli-Haley J, Johnson K. Cost-effectiveness of four immunomodulatory therapies for relapsing-remitting multiple sclerosis: a Markov model based on long-term clinical data. J Manag Care Pharm 2007;13:245–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prosser LA, Kuntz KM, Bar-Or A, Weinstein MC. Cost-effectiveness of interferon beta-1a, interferon beta-1b, and glatiramer acetate in newly diagnosed non-primary progressive multiple sclerosis. Value Health 2004;7:554–568. [DOI] [PubMed] [Google Scholar]
- 16.Mayr WT, Pittock SJ, McClelland RL, Jorgensen NW, Noseworthy JH, Rodriguez M. Incidence and prevalence of multiple sclerosis in Olmsted County, Minnesota, 1985–2000. Neurology 2003;61:1373–1377. [DOI] [PubMed] [Google Scholar]
- 17.Arias E. United States life tables, 2002. Natl Vital Stat Rep 2004;53:1–38. [PubMed] [Google Scholar]
- 18.Lublin FD, Baier M, Cutter G. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology 2003;61:1528–1532. [DOI] [PubMed] [Google Scholar]
- 19.Broman T, Andersen O, Bergmann L. Clinical studies on multiple sclerosis, I: presentation of an incidence material from Gothenburg. Acta Neurol Scand 1981;63:6–33. [PubMed] [Google Scholar]
- 20.Patzold U, Pocklington PR. Course of multiple sclerosis: first results of a prospective study carried out of 102 MS patients from 1976-1980. Acta Neurol Scand 1982;65:248–266. [DOI] [PubMed] [Google Scholar]
- 21.Fog T, Linnemann F. The course of multiple sclerosis in 73 cases with computer-designed curves. Acta Neurol Scand Suppl 1970;47:3–175. [PubMed] [Google Scholar]
- 22.Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study, 2: predictive value of the early clinical course. Brain 1989;112:1419–1428. [DOI] [PubMed] [Google Scholar]
- 23.PRISMS Study Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet 1998;352:1498–1504. [PubMed] [Google Scholar]
- 24.McAlister FA. Commentary: relative treatment effects are consistent across the spectrum of underlying risks … usually. Int J Epidemiol 2002;31:76–77. [DOI] [PubMed] [Google Scholar]
- 25.Chilcott J, McCabe C, Tappenden P, et al. Modelling the cost effectiveness of interferon beta and glatiramer acetate in the management of multiple sclerosis. Commentary: evaluating disease modifying treatments in multiple sclerosis. BMJ 2003;326:522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gold MR SJ, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press, 1996. [Google Scholar]
- 27.Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. JAMA 1996;276:1172–1177. [PubMed] [Google Scholar]
- 28.Brown MG, Kirby S, Fisk JD, et al. Clinical effectiveness of disease modifying therapies that delay disability progression in relapsing remitting-onset multiple sclerosis. International Society for Pharmacoeconomics and Outcomes Research; May 22, 2006; Philadelphia, PA. Abstract.
- 29.Kobelt G, Berg J, Atherly D, Hadjimichael O. Costs and quality of life in multiple sclerosis: a cross-sectional study in the United States. Neurology 2006;66:1696–1702. [DOI] [PubMed] [Google Scholar]
- 30.Prosser LA, Kuntz KM, Bar-Or A, Weinstein MC. Patient and community preferences for treatments and health states in multiple sclerosis. Mult Scler 2003;9:311–319. [DOI] [PubMed] [Google Scholar]
- 31.Schultz WB. Bolstering the FDA’s drug-safety authority. N Engl J Med 2007;357:2217–2219. [DOI] [PubMed] [Google Scholar]
- 32.Calfee J. A Representative Survey of M.S. Patients on Attitudes toward the Benefits and Risks of Drug Therapy. Washington, DC: AEI-Brookings Joint Center for Regulatory Studies, 2006. [Google Scholar]
- 33.Dorsey ER, Thompson JP, Noyes K, Dick AW, Holloway RG, Schwid SR. Quantifying the risks and benefits of natalizumab in relapsing multiple sclerosis. Neurology 2007;68:1524–1528. [DOI] [PubMed] [Google Scholar]
- 34.Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med 2005;353:369–374. [DOI] [PubMed] [Google Scholar]
- 35.Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med 2005;353:375–381. [DOI] [PubMed] [Google Scholar]
- 36.Van Assche G, Van Ranst M, Sciot R, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med 2005;353:362–368. [DOI] [PubMed] [Google Scholar]
- 37.Garrison LP Jr, Towse A, Bresnahan BW. Assessing a structured, quantitative health outcomes approach to drug risk-benefit analysis. Health Aff (Millwood) 2007;26:684–695. [DOI] [PubMed] [Google Scholar]