Abstract
Objective:
To test the hypotheses that older community dwelling men taking non–enzyme-inducing antiepileptic drugs (NEIAEDs) and those taking enzyme-inducing antiepileptic drugs (EIAEDs) have increased rates of hip bone loss.
Methods:
We ascertained antiepileptic drug (AED) use (interviewer-administered questionnaire with verification of use by containers) and measured hip bone mineral density (BMD) (using dual energy x-ray absorptiometry) at baseline and an average of 4.6 years later in a cohort of 4,222 older community-dwelling men enrolled in the Osteoporotic Fractures in Men study. Men were categorized as nonusers (no AED use at either examination, n = 4060), NEIAED user (use of NEIAED only at either examination, n = 100), or EIAED user (use of EIAED only at either examination, n = 62).
Results:
After adjustment for multiple potential confounders (age, race, clinic site, health status, pain interfering with work or activity, physical activity, smoking status, alcohol use, total calcium intake, diabetes, chronic kidney disease, vitamin D supplement use, bisphosphonate use, selective serotonin reuptake inhibitor use, inability to rise from a chair, body mass index, and baseline BMD), the average rate of decline in total hip BMD was −0.35%/year among nonusers compared with −0.53%/year among NEIAED users (p = 0.04) and −0.46%/year among EIAED users (p = 0.31). Multivariable adjusted rate of loss was −0.60%/year among men taking NEIAED at both examinations, −0.51%/year among men taking NEIAED at one examination only, and −0.35%/year among nonusers (p for trend = 0.03). Findings were similar at hip subregions.
Conclusion:
Use of non–enzyme-inducing antiepileptic drugs was independently associated with increased rates of hip bone loss in this cohort of older community-dwelling men.
GLOSSARY
- AED
= antiepileptic drug;
- BMD
= bone mineral density;
- CKD
= chronic kidney disease;
- CV
= coefficient of variation;
- DXA
= dual energy x-ray absorptiometry;
- EIAED
= enzyme-inducing antiepileptic drugs;
- IDIS
= Iowa Drug Information Service;
- MrOS
= Osteoporotic Fractures in Men;
- NEIAED
= non–enzyme-inducing antiepileptic drugs;
- PASE
= Physical Activity Scale for the Elderly;
- SOF
= Study of Osteoporotic Fractures;
- SSRI
= selective serotonin reuptake inhibitors.
Antiepileptic drug (AED) use may be associated with higher rates of bone loss because AED use may have adverse effects on bone metabolism.1 On the other hand, AED use may be a marker of factors such as poor health, medical conditions, lifestyle behaviors, and neuromuscular impairments that are associated with greater rates of bone loss. Thus, an apparent association between AED use and bone loss might be due to these confounding factors rather than an effect of AED use.
The prevailing “induction” model explaining AED-related bone disease postulates that use of enzyme-inducing AEDs (EIAEDs) that increase activity of hepatic mixed function oxidase enzymes accelerate the metabolism of vitamin D3, resulting in inactive metabolites, leading to decreased fractional calcium absorption, secondary hyperparathyroidism with greater bone resorption, and higher rates of bone loss.2 Evidence linking phenytoin (EIAED)3–7 and phenobarbital (EIAED)4,5 to lower bone mineral density (BMD) is generally consistent with this theory. However, carbamazepine (EIAED)6–10 has not been associated with lower BMD, while valproic acid (non–enzyme-inducing AED [NEIAED])3,7–9 has been associated with lower BMD. Thus, multiple mechanisms underlying AED-related bone loss appear to exist, and all types of AED are potentially implicated.11
Despite increasing utilization of newer NEIAEDs,12 there is little data on the effect of newer NEIAEDs on BMD.13,14 A cross-sectional study7 reported that BMD did not differ between premenopausal women with epilepsy taking carbamazepine (EIAED), phenytoin (EIAED), valproic acid (older NEIAED), or lamotrigine (newer NEIAED). However, cross-sectional studies examining associations yield weaker evidence for causality and are more subject to potential biases than prospective cohort studies.
To test the hypotheses that older men taking NEIAEDs and older men taking EIAEDs have increased rates of hip bone loss, we ascertained AED use and measured hip BMD at baseline and an average of 4.6 years later in a cohort of 4,222 older community-dwelling men enrolled in the Osteoporotic Fractures in Men (MrOS) study.
METHODS
Participants.
From March 2000 to April 2002, 5,995 men at least 65 years old were recruited for participation in the baseline examination of the prospective MrOS study. Men were recruited primarily from population-based listings in six regions of the United States.15,16 We excluded men who were unable to walk without assistance and men with a history of bilateral hip replacement.
From March 2005 through April 2006, 4,530 men (85% of active survivors) attended a second clinic examination. Of these, 4,230 men completed a medication inventory and technically adequate hip BMD measurements at baseline and second examinations. We excluded 8 men from our analysis who were taking an EIAED in combination with a NEIAED at one or both examinations. The remaining 4,222 men were included in the analytical cohort.
The institutional review boards for all participating institutions approved the study protocol and the consent forms. All men provided written informed consent.
AED use.
Participants attending the baseline and second examinations were asked to bring all current (any use within last 4 weeks) prescription and nonprescription medications. Interviewers completed a medication history for each participant, including name of medication and frequency of use. All medications recorded were stored in an electronic medications inventory database. Each medication was matched to its ingredients based on the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City). A computerized dictionary was used to categorize type of medication from product brand and generic names obtained from containers.17 Subsequently, two physicians blinded to outcome status reviewed the computerized drug data for AED use and verified classification of each medication as a NEIAED (gabapentin, valproic acid, lamotrigine, levetiracetam, pregabalin, and tiagabine) or EIAED (phenytoin, phenobarbital, primidone, carbamazepine, oxcarbazepine, topiramate). No participant reported taking ethosuximide, felbamate, methsuximide, or ethotoin at either examination.
After excluding eight men who were taking an EIAED in combination with a NEIAED at one or both examinations (there were no men taking EIAED at one examination and NEIAED at the other), men were classified as NEIAED users (reported NEIAED use at either examination, n = 100), EIAED users (reported EIAED use at either examination, n = 62), or nonusers (reported no AED use at both examinations, n = 4,060). In addition, men who reported NEIAED or EIAED use at only one examination were categorized as intermittent users of that class of AED, while men who reported use at both examinations were classified as continuous users of that class of AED.
Measurement of BMD.
BMD at the total hip and two subregions (femoral neck, trochanter) was measured at both examinations (mean ± SD, 4.6 ± 0.4 years between examinations) using dual energy x-ray absorptiometry (DXA) with QDR-4500 W scanners (Hologic, Inc., Bedford, MA). Repeat measurements were performed on the same instruments as were used for the initial measurements. A central quality control laboratory, certification of DXA technicians, and standardized procedures for scanning were implemented to insure reproducibility of DXA measurements. At baseline, a hip phantom was circulated and scanned at the six clinical centers. Cross-calibration studies indicated no linear differences across scanners and the inter-scanner coefficient of variation (CV) was 0.9%. Each clinic scanned a hip phantom throughout the study to monitor longitudinal changes, and correction factors were applied to participant data as appropriate. In addition, multivariable models included an indicator variable for the individual center to adjust for interclinic differences. The rate of change in hip BMD was expressed as an annualized percentage of the initial value as percentage change in BMD per year.
Other measurements.
Participants completed a questionnaire and were interviewed at the baseline examination by trained staff, who asked about health status, a physician diagnosis of selected medical conditions including diabetes, pain interfering with normal work in the past month, smoking status, and alcohol use. Physical activity was assessed using the Physical Activity Scale for the Elderly (PASE).18 Total calcium intake from foods and supplements was estimated by using a modified Block semiquantitative food-frequency questionnaire (Block Dietary Systems, Berkeley, CA).19 Current use of vitamin D supplements, hypoglycemic agents, selective serotonin reuptake inhibitors (SSRI), and bisphosphonates was determined using the method described for ascertainment of AED use. Tests of neuromuscular function included the ability to rise up from a chair (without using the arms) five times. Weight was measured using a calibrated scale and height was measured using a standard held-expiration technique with a wall-mounted Harpenden stadiometer (Holtain, UK). Height and weight were used to calculate a standard body mass index. At the second examination, participants were asked about a physician diagnosis of chronic kidney disease (CKD).
Statistical analysis.
Characteristics of participants at the baseline examination by category of AED use were compared (nonusers of AEDs vs NEIAED users, nonusers vs EIAED users) using χ2 tests for dichotomous variables, t tests for continuous variables, and Wilcoxon rank sum test for categorical variables with skewed distributions.
To examine the association between NEIAED use and rate of change in BMD at the total hip and subregions (femoral neck, trochanter), the annualized mean change in BMD and its 95% CI was calculated for NEIAED users and nonusers using the least-square-means procedure. Similar analyses were performed to examine the association between EIAED use and rate of change in BMD. Initial models adjusted for age alone. Known risk factors for bone loss in the MrOS cohort and characteristics related to AED use were considered to be potential confounders and examined for inclusion in multivariable models. We included in our multivariable models age, clinic, baseline total hip BMD, and those variables (race, health status, pain interfering with work, physical activity, smoking status, alcohol use, total calcium intake, diabetes, CKD, vitamin D supplement use, bisphosphonate use, SSRI use, inability to rise from a chair, and BMI) related to use of NEIAED or use of EIAED at p ≤ 0.10 or rate of change in total hip BMD at p ≤ 0.10 independent of age and AED (NEIAED or EIAED) use.
In a secondary analysis performed to explore whether there was evidence of an effect of duration of therapy, the annualized mean change in BMD was calculated by category of NEIAED use (nonusers of AED, intermittent NEIAED users, continuous NEIAED users). A p value for linear trend in annualized mean percent change in hip BMD between use groups was calculated. Similar analyses were performed to examine the association between category of EIAED use and change in hip BMD. Since prior studies have reported an association between valproic acid use and lower BMD,3,7–9 we performed a secondary analysis examining the association between NEIAED use and rate of change in hip BMD excluding men taking valproic acid at either examination (n = 8). Since most of the NEIAED users in the cohort reported taking gabapentin, we also performed an analysis limited to gabapentin users (use of gabapentin alone at either examination, n = 84) and nonusers. Similarly, we examined the association between phenytoin use and rate of change in hip BMD limiting the analysis to phenytoin users (n = 29) and nonusers. Finally, analyses were performed in which propensity scores were calculated indicating the likelihood of AED use (e.g., NEIAED use, EIAED use, gabapentin use, phenytoin use) based on logistic regression with the use variable as the outcome and the covariates in the multivariate model.20 This propensity score was then used in place of the covariates in the models. Because use of propensity scores did not substantially alter the findings, models adjusted for multiple covariates are presented in this article.
RESULTS
Characteristics of the study population.
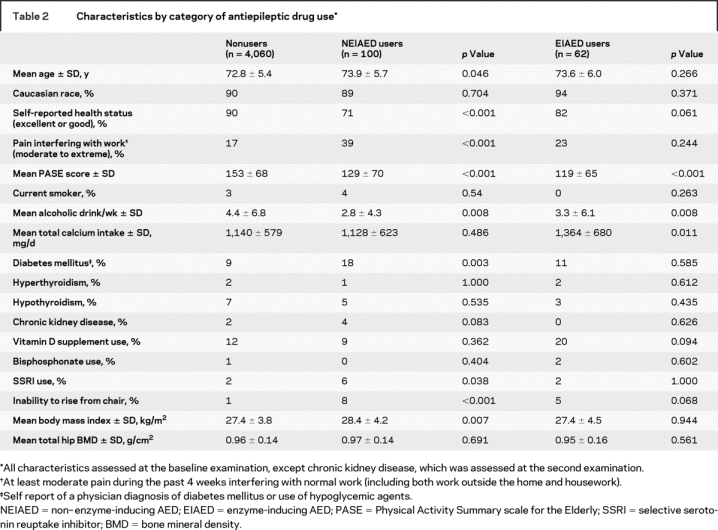
The cohort included 4,222 men, of whom 100 (2.4%) were NEIAED users and 62 (1.5%) were EIAED users. The remaining 4,060 men (96.2%) reported no AED use. Of the 100 NEIAED users, 78 reported NEIAED use at either examination (intermittent user) and 22 reported NEIAED use at both examinations (continuous user). A total of 85 of the 100 NEIAED users reported gabapentin use at either examination; of these, one man took valproic acid at baseline and gabapentin at the second examination. Of the 62 EIAED users, 29 were intermittent and 33 were continuous users. A total of 29 of the 62 EIAED users were taking phenytoin either alone (n = 26) or in combination with phenobarbital (n = 3). Specific drug use among men taking NEIAEDs and men taking EIAEDs is listed in table 1. Characteristics of the 4,222 participants by category of AED use (nonuser of AED, NEIAED user, EIAED user) are shown in table 2.
Table 1 Individual drug use within category of antiepileptic drug (AED) use
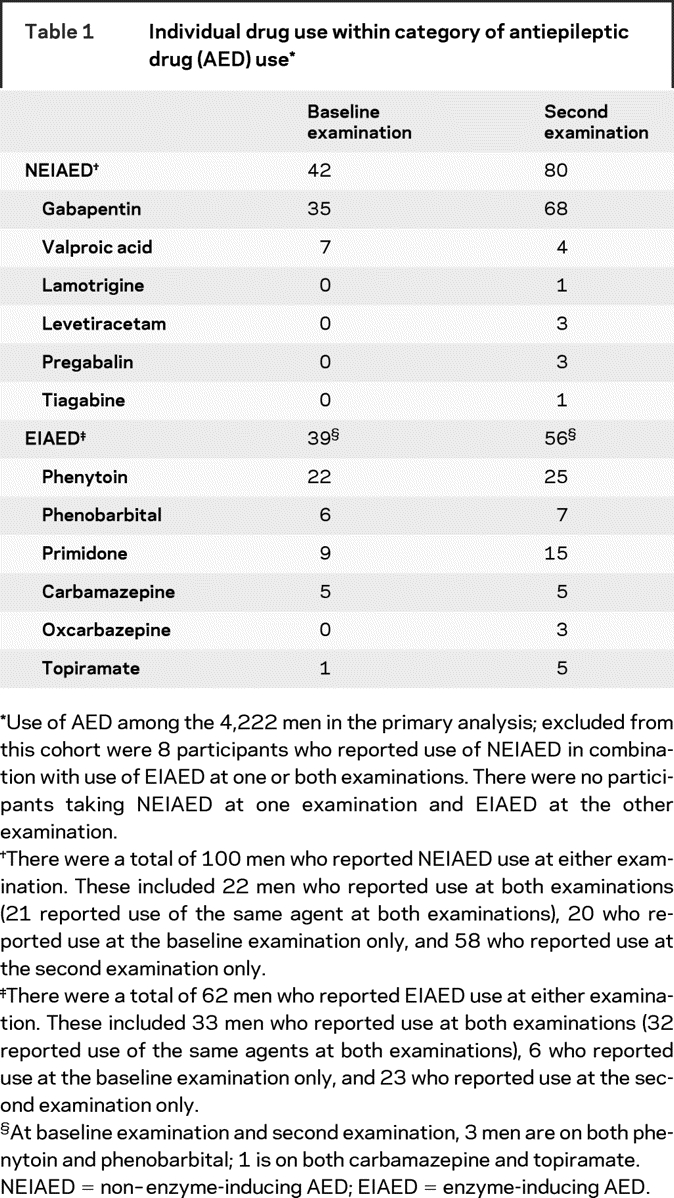
Table 2 Characteristics by category of antiepileptic drug use
NEIAED use and rate of hip bone loss.
On average, men taking NEIAEDs had a higher age-adjusted rate of bone loss at the total hip than nonusers of AEDs (−0.63%/year vs −0.35%/year; p = 0.001) (table 3). After adjusting for multiple potential confounders (including age, race, clinic, health status, pain interfering with work, physical activity, smoking status, alcohol use, total calcium intake, diabetes, CKD, vitamin D supplement use, bisphosphonate use, SSRI use, inability to rise from a chair, BMI, and baseline total hip BMD), the difference was somewhat attenuated (−0.53%/year vs −0.35%/year, p = 0.04). Findings were similar at the femoral neck and trochanter.
Table 3 Mean annualized rate of change in hip BMD by category of antiepileptic drug use
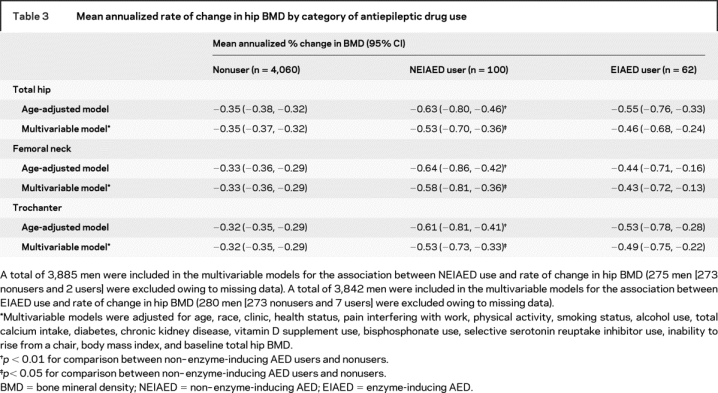
In secondary analyses exploring whether there was evidence of an effect of treatment duration, the mean multivariable-adjusted annualized percent rate of bone loss at the total hip was −0.35 (95% CI, −0.37 to −0.32) among nonusers, −0.51 (95% CI, −0.70 to −0.32) among intermittent NEIAED users, and −0.60 (95% CI, −0.96 to −0.25) among continuous NEIAED users (p for trend = 0.03). Findings were similar at hip subregions (p for trend < 0.05).
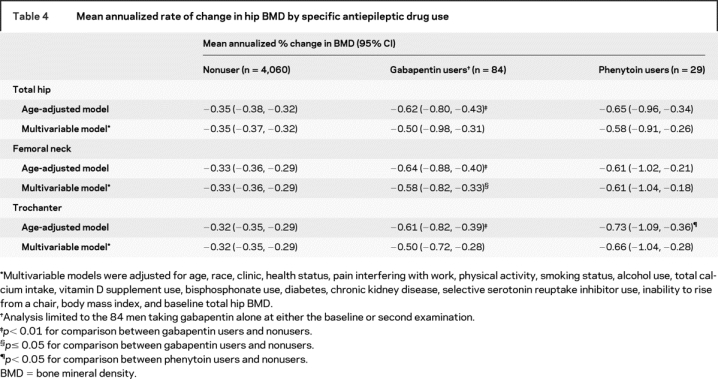
Excluding users of valproic acid did not alter the findings. In analyses limited to gabapentin users and nonusers of AEDs (table 4), the magnitude of the difference in age-adjusted rates of bone loss at the hip was similar to that between men taking any NEIAEDs and nonusers (−0.62%/year vs −0.35%/year at total hip, p = 0.005). After adjustment for multiple potential confounders, the p value for the comparison between gabapentin users and nonusers was 0.05 at the femoral neck and 0.11 at the total hip and trochanter.
Table 4 Mean annualized rate of change in hip BMD by specific antiepileptic drug use
EIAED use and rate of hip bone loss.
Compared with nonusers of AEDs, men taking EIAEDs appeared to have a higher age-adjusted rate of bone loss at the total hip (−0.55%/year vs −0.35%/year, p = 0.07) (table 3). The difference was smaller after adjustment for multiple potential confounders and not different from zero (−0.46%/year vs −0.35%/year, p = 0.31). Findings were similar at hip subregions.
In secondary analyses examining for evidence of an effect of treatment duration, the mean adjusted annualized percent rate of decline at the total hip was −0.34 (95% CI, −0.37 to −0.32) among nonusers, −0.53 (95% CI, −0.84 to −0.21) among intermittent EIAED users, and −0.40 (95% CI, −0.71 to −0.09) among continuous EIAED users (p for trend = 0.43). Findings were similar at hip subregions.
In analyses limited to phenytoin users and nonusers of AEDs (table 4), men taking phenytoin appeared to have a higher age-adjusted rate of hip bone loss (−0.73%/year vs −0.32%/year at the trochanter, p = 0.03; −0.65%/year vs −0.35%/year at the total hip, p = 0.06; and 0.61%/year vs −0.32%/year at the femoral neck, p = 0.17). While these differences were only slightly attenuated in magnitude after adjustment for multiple potential confounders, the p value was 0.08 at trochanter, 0.15 at total hip, and 0.19 at femoral neck.
DISCUSSION
Nearly all previous investigations of AED use and BMD are cross-sectional studies of select populations with inadequate control of confounders. Three prior prospective studies5,10,21 examined AED use and rates of bone loss, but were limited by small sample size, selection bias, absence of a comparison group of subjects not taking AEDs, and lack of adjustment for potential confounders. A prior analysis22 from the Study of Osteoporotic Fractures (SOF), a prospective study of a large cohort of older community-dwelling women similar in design to MrOS, reported that phenytoin users had higher adjusted rates of hip bone loss compared with nonusers of AEDs. The 1.8-fold higher rate of loss among women taking phenytoin in SOF was similar in magnitude to the 1.7-fold higher rate of loss among men taking phenytoin in this study, though the latter comparison did not reach the level of significance in multivariable models. Reasons for the apparent discrepancy include more complete control of confounding in MrOS, gender differences in effects of AEDs on bone loss, or inadequate power in MrOS to detect an effect of phenytoin on bone loss. The MrOS analysis had an 80% power to detect a difference of 0.35 or more in the annualized percent change in total hip BMD between EIAED users and nonusers of AEDs after adjustment for multiple confounders. Thus, our results do not exclude an effect of EIAED use on rates of change in hip BMD. Finally, although the adjusted average rate of loss at each hip site was higher among men taking NEIAEDs than among men taking EIAEDs, 95% CIs around the point estimates overlapped due to the relatively small numbers of men taking AEDs. Our study is not adequately powered to directly compare these two AED user groups.
There is a paucity of longitudinal data examining the rate of change in BMD among NEIAED users. Our results suggest that NEIAED use is associated in a graded manner with rates of hip bone loss in older men with lower rates of loss among nonusers of AEDs, intermediate rates of loss among intermittent users, and higher rates of loss among continuous users. These findings are supported by prior studies reporting higher fracture rates among NEIAED users compared with nonusers of AEDs23 or a similar fracture risk between patients taking NEIAEDs vs those taking EIAEDs.24 In particular, our results suggest that gabapentin users compared with nonusers of AEDs have a 1.4- to 1.8-fold higher adjusted rate of loss depending on the specific hip site.
Rates of bone loss may increase with AED use for several reasons. Existing data both support and refute the prevailing “induction” model,1,11,14 suggesting that this model alone cannot account for higher rates of bone loss among AED users. Some evidence supports deleterious effects of EIAEDs25 including phenytoin and carbamazepine and NEIAEDs26–29 including valproic acid and levetiracetam on cultured bone cells. We are not aware of studies examining the effect of gabapentin on bone cells. However, gabapentin increases cerebral and spinal cord norepinephrine release30 and activation of osteoblastic adrenergic receptors by norepinephrine decreases osteoblast numbers and reduces bone formation.31 Gabapentin mediated sympathetic nervous system activation might at least partially explain a detrimental effect of gabapentin on BMD. Several other potential biologic mechanisms of AED-related bone loss have been proposed.11,32–36 Detrimental effects of AED use on bone may in part explain the higher incidence of fractures in people with epilepsy.37,38
On the other hand, observational studies examining the association between AED use and rates of bone loss are subject to confounding and AED use may be a marker of other conditions or medications that increase the risk of bone loss. To address potential confounding, our analyses were adjusted for several factors associated with AED use or rates of change in BMD. In addition, findings were consistent in analyses adjusted for propensity scores indicating likelihood of using AEDs.
Strengths of this study include its prospective design; study population comprised of a large cohort of older men not selected on the basis of AED treatment; verification of AED use; and adjustment for several potential confounders. However, this study has limitations. Participants were older men, and our results may not apply to other populations. With the exception of gabapentin, we had limited or inadequate power to examine the association between a specific AED and bone loss. Dose, indications for use, and compliance data were not available. Unmeasured changes in AED use may have occurred between examinations which would bias our estimates of the association toward the null hypothesis of no association. Finally, analyses were adjusted for several factors associated with use of NEIAEDs, use of EIAEDs, or rates of change in hip BMD, but the possibility of confounding cannot be eliminated without the use of a randomized, controlled trial design.
We found that use of NEIAEDs is independently associated with increased rates of hip bone loss in this cohort of older men. Given the increasing utilization of NEIAEDs, additional prospective studies of the association between AED use (including NEIAED and EIAED) and rates of change in BMD are warranted. In addition, future investigations should examine alternative biologic mechanisms underlying an association.
Address correspondence and reprint requests to Dr. Kristine E. Ensrud, VA Medical Center, One Veterans Drive (111-0), Minneapolis, MN 55417 ensru001@umn.edu
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01 AG027810, and UL1 RR024140.
Disclosure: The authors report no disclosures.
Received February 18, 2008. Accepted in final form May 23, 2008.
REFERENCES
- 1. Petty SJ O’Brien TJ Wark JD Anti-epileptic medication and bone health. Osteoporos Int. 2007;18:129–142. doi: 10.1007/s00198-006-0185-z. [DOI] [PubMed] [Google Scholar]
- 2. Tannirandorn P Epstein S Drug-induced bone loss. Osteoporos Int. 2000;11:637–659. doi: 10.1007/s001980070062. [DOI] [PubMed] [Google Scholar]
- 3. Sato Y Kondo I Ishida S et al. Decreased bone mass and increased bone turnover with valproate therapy in adults with epilepsy. Neurology. 2001;57:445–449. doi: 10.1212/wnl.57.3.445. [DOI] [PubMed] [Google Scholar]
- 4. Chung S Ahn C Effects of anti-epileptic drug therapy on bone mineral density in ambulatory epileptic children. Brain Dev. 1994;16:382–385. doi: 10.1016/0387-7604(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 5. Kubota F Kifune A Shibata N et al. Bone mineral density of epileptic patients on long-term antiepileptic drug therapy: a quantitative digital radiography study. Epilepsy Res. 1999;33:93–97. doi: 10.1016/s0920-1211(98)00077-1. [DOI] [PubMed] [Google Scholar]
- 6. Valimaki MJ Tiihonen M Laitinen K et al. Bone mineral density measured by dual-energy x-ray absorptiometry and novel markers of bone formation and resorption in patients on antiepileptic drugs. J Bone Miner Res. 1994;9:631–637. doi: 10.1002/jbmr.5650090507. [DOI] [PubMed] [Google Scholar]
- 7. Pack AM Morrell MJ Marcus R et al. Bone mass and turnover in women with epilepsy on antiepileptic drug monotherapy. Ann Neurol. 2005;57:252–257. doi: 10.1002/ana.20378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sheth RD Wesolowski CA Jacob JC et al. Effect of carbamazepine and valproate on bone mineral density. J Pediatr. 1995;127:256–262. doi: 10.1016/s0022-3476(95)70304-7. [DOI] [PubMed] [Google Scholar]
- 9. Kafali G Erselcan T Tanzer F Effect of antiepileptic drugs on bone mineral density in children between ages 6 and 12 years. Clin Pediatr (Phila) 1999;38:93–98. doi: 10.1177/000992289903800205. [DOI] [PubMed] [Google Scholar]
- 10. Kim SH Lee JW Choi KG Chung HW Lee HW A 6-month longitudinal study of bone mineral density with antiepileptic drug monotherapy. Epilepsy Behav. 2007;10:291–295. doi: 10.1016/j.yebeh.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 11. Fitzpatrick LA Pathophysiology of bone loss in patients receiving anticonvulsant therapy. Epilepsy Behav. 2004;5:S3–15. doi: 10.1016/j.yebeh.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 12. LaRoche SM Helmers SL The new antiepileptic drugs: clinical applications. JAMA. 2004;291:615–620. doi: 10.1001/jama.291.5.615. [DOI] [PubMed] [Google Scholar]
- 13. McCorry D Chadwick D Marson A Current drug treatment of epilepsy in adults. Lancet Neurol. 2004;3:729–735. doi: 10.1016/S1474-4422(04)00935-4. [DOI] [PubMed] [Google Scholar]
- 14. Pack AM Morrell MJ Epilepsy and bone health in adults. Epilepsy Behav. 2004;5:S24–S29. doi: 10.1016/j.yebeh.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 15. Orwoll E Blank JB Barrett-Connor E et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 16. Blank JB Cawthon PM Carrion-Petersen ML et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 17. Pahor M Chrischilles EA Guralnik JM Brown SL Wallace RB Carbonin P Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 18. Washburn RA Ficker JL Physical Activity Scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. J Sports Med Phys Fitness. 1999;39:336–340. [PubMed] [Google Scholar]
- 19. Block G Subar AF Estimates of nutrient intake from a food frequency questionnaire: the 1987 National Health Interview Survey. J Am Diet Assoc. 1992;92:969–977. [PubMed] [Google Scholar]
- 20. Rubin DB Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997. 1278 Pt 2757 763 [DOI] [PubMed] [Google Scholar]
- 21. Andress DL Ozuna J Tirschwell D et al. Antiepileptic drug-induced bone loss in young male patients who have seizures. Arch Neurol. 2002;59:781–786. doi: 10.1001/archneur.59.5.781. [DOI] [PubMed] [Google Scholar]
- 22. Ensrud KE Walczak TS Blackwell T Ensrud ER Bowman PJ Stone KL Antiepileptic drug use increases rates of bone loss in older women: a prospective study. Neurology. 2004;62:2051–2057. doi: 10.1212/01.wnl.0000125185.74276.d2. [DOI] [PubMed] [Google Scholar]
- 23. Vestergaard P Rejnmark L Mosekilde L Fracture risk associated with use of antiepileptic drugs. Epilepsia. 2004;45:1330–1337. doi: 10.1111/j.0013-9580.2004.18804.x. [DOI] [PubMed] [Google Scholar]
- 24. Souverein PC Webb DJ Weil JG Van Staa TP Egberts AC Use of antiepileptic drugs and risk of fractures: case-control study among patients with epilepsy. Neurology. 2006;66:1318–1324. doi: 10.1212/01.wnl.0000210503.89488.88. [DOI] [PubMed] [Google Scholar]
- 25. Feldkamp J Becker A Witte OW Scharff D Scherbaum WA Long-term anticonvulsant therapy leads to low bone mineral density: evidence for direct drug effects of phenytoin and carbamazepine on human osteoblast-like cells. Exp Clin Endocrinol Diabetes. 2000;108:37–43. doi: 10.1055/s-0032-1329213. [DOI] [PubMed] [Google Scholar]
- 26. Cho HH Park HT Kim YJ Bae YC Suh KT Jung JS Induction of osteogenic differentiation of human mesenchymal stem cells by histone deacetylase inhibitors. J Cell Biochem. 2005;96:533–542. doi: 10.1002/jcb.20544. [DOI] [PubMed] [Google Scholar]
- 27. Gracious BL Bossert RP Puza J Valproic acid inhibits osteoblast and osteoclast activity in therapeutic dose ranges. J Bone Miner Res. 2004;19:S440Abs. [Google Scholar]
- 28. Schroeder TM Westendorf JJ Histone deacetylase inhibitors promote osteoblast maturation. J Bone Miner Res. 2005;20:2254–2263. doi: 10.1359/JBMR.050813. [DOI] [PubMed] [Google Scholar]
- 29. Nissen-Meyer LS Svalheim S Tauboll E et al. Levetiracetam, phenytoin, and valproate act differently on rat bone mass, structure, and metabolism. Epilepsia. 2007;48:1850–1860. doi: 10.1111/j.1528-1167.2007.01176.x. [DOI] [PubMed] [Google Scholar]
- 30. Hayashida K DeGoes S Curry R Eisenach JC Gabapentin activates spinal noradrenergic activity in rats and humans and reduces hypersensitivity after surgery. Anesthesiology. 2007;106:557–562. doi: 10.1097/00000542-200703000-00021. [DOI] [PubMed] [Google Scholar]
- 31. Takeda S Elefteriou F Levasseur R et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 32. Koch HU Kraft D von HD Schaefer K Influence of diphenylhydantoin and phenobarbital on intestinal calcium transport in the rat. Epilepsia. 1972;13:829–834. doi: 10.1111/j.1528-1157.1972.tb05167.x. [DOI] [PubMed] [Google Scholar]
- 33. Elliott JO Jacobson MP Haneef Z Homocysteine and bone loss in epilepsy. Seizure. 2007;16:22–34. doi: 10.1016/j.seizure.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 34. Pack AM Morrell MJ Flynn KL Done S Possible mechanisms of bone disease in women receiving antiepileptic drug (AED) monotherapy. Epilepsia. 2001;42:292. [Google Scholar]
- 35. Isojarvi JI Pakarinen AJ Ylipalosaari PJ Myllyla VV Serum hormones in male epileptic patients receiving anticonvulsant medication. Arch Neurol. 1990;47:670–676. doi: 10.1001/archneur.1990.00530060082023. [DOI] [PubMed] [Google Scholar]
- 36. Kruse K Kracht U Inhibitory effect of calcium on serum prolactin. Acta Endocrinol (Copenh) 1981;98:339–344. doi: 10.1530/acta.0.0980339. [DOI] [PubMed] [Google Scholar]
- 37. Souverein PC Webb DJ Petri H Weil J Van Staa TP Egberts T Incidence of fractures among epilepsy patients: a population-based retrospective cohort study in the General Practice Research Database. Epilepsia. 2005;46:304–310. doi: 10.1111/j.0013-9580.2005.23804.x. [DOI] [PubMed] [Google Scholar]
- 38. Vestergaard P Epilepsy, osteoporosis and fracture risk: a meta-analysis. Acta Neurol Scand. 2005;112:277–286. doi: 10.1111/j.1600-0404.2005.00474.x. [DOI] [PubMed] [Google Scholar]


