Abstract
Background:
Deficits in working memory are commonly observed after traumatic brain injury (TBI), with executive control processes preferentially impacted relative to storage and rehearsal. Previous activation functional neuroimaging investigations of working memory in patients with TBI have reported altered functional recruitment, but methodologic issues including sample heterogeneity (e.g., variability in injury mechanism, severity, neuropathology or chronicity), underspecified definitions of “working memory,” and behavioral differences between TBI and control groups have hindered interpretation of these changes.
Methods:
Executive control processing in working memory was explicitly engaged during fMRI in a sample of carefully selected chronic-stage, moderate-to-severe TBI patients with diffuse axonal injury (DAI) but without focal lesions.
Results:
Despite equivalent task performance, we observed a pattern of greater recruitment of interhemispheric and intrahemispheric regions of prefrontal cortex (PFC) and posterior cortices in our DAI sample. Enhanced activations were recorded in the left dorsolateral PFC (middle frontal gyrus), right ventrolateral PFC (inferior frontal gyrus), bilateral posterior parietal cortices, and left temporo-occipital junction. Region-of-interest analyses confirmed that these effects were robust across individual patients and could not be attributed to load factors or slowed speed of processing.
Conclusions:
Augmented functional recruitment in the context of normal behavioral performance may be a neural marker of capacity or efficiency limits that can affect functional outcome after traumatic brain injury with diffuse injury.
GLOSSARY
- BA
= Brodmann area;
- BOLD
= blood oxygen level–dependent;
- DAI
= diffuse axonal injury;
- DLPFC
= dorsolateral prefrontal function;
- GCS
= Glasgow Coma Scale;
- IFG
= inferior frontal gyrus;
- ITI
= intertrial interval;
- MFG
= middle frontal gyrus;
- NA
= not applicable;
- NS
= not significant;
- PFC
= prefrontal cortex;
- ROI
= region of interest;
- TBI
= traumatic brain injury.
Deficits in working memory are commonly observed in patients with traumatic brain injury (TBI).1 Working memory, however, is not a unitary process; it can be separated into storage and executive control components.2 There is substantial evidence that these components can be dissociated at the neural as well as the behavioral level,3,4 with the executive control component strongly associated with dorsolateral prefrontal function (DLPFC).5
This functional localization creates a puzzle when considering patients with TBI, where ventral, not dorsal, frontal regions are vulnerable to focal lesions,6 and where focal lesions may be absent even in the presence of marked cognitive deficits.7 This raises the possible influence of diffuse axonal injury (DAI). DAI is a ubiquitous consequence of rapid deceleration of the head, followed by disrupted ionic homeostasis, cytoskeleton compromise, and ultimately disconnection of the distal axonal segment from the soma.8 DLPFC functions may be preferentially impacted by DAI as a result of its extensive reciprocal connections with almost all cortical and subcortical structures in the brain.9
Although DAI is likely to substantially contribute to the executive control deficits in TBI, extant evidence to support this hypothesis is weak. Investigations of the functional neuroanatomy of working memory after significant TBI have suggested increased recruitment of frontal regions,10–12 but these are complicated by methodologic factors such as task complexity (in particular, confounding working memory load effects with the critical executive control processes), performance impairments in patients, and the combination of patients with focal and diffuse injuries (for a review, see reference 13). More evidence is found in mild TBI,12,14 but these studies were conducted at the acute phase, with little expected long-term functional consequences relative to patients with more significant TBI.
In this study, we assessed patients with moderate and severe TBI and documented DAI but without large focal lesions. We have adopted a working memory paradigm with well-established functional neuroanatomy that is capable of separating executive control and load factors, with good convergent validity with other measures of executive control in working memory.15 Finally, we have used a standardized prescan training regimen to equate task performance between TBI and control subjects during fMRI scanning.
METHODS
Subjects.
Eight patients (6 men) with moderate to severe TBI were recruited along with 12 healthy control subjects (8 men). TBI patients and control subjects were matched on age [t(18) = 0.785 (p > 0.05, not significant [NS])] and education [t(18) = −1.99 (p > 0.05, NS)]. All subjects were right-handed, native English speakers and were screened for previous neurologic injury, major medical conditions affecting cognition, history of psychiatric illness, and the use of medications affecting cognition.16 Two other sets of control subjects were used for the purposes of characterizing TBI patients’ neuropsychological status and structural neuroimaging data (table 1).
Table 1 TBI patient demographics, acute injury characteristics, structural neuroimaging data, and neuropsychological test data
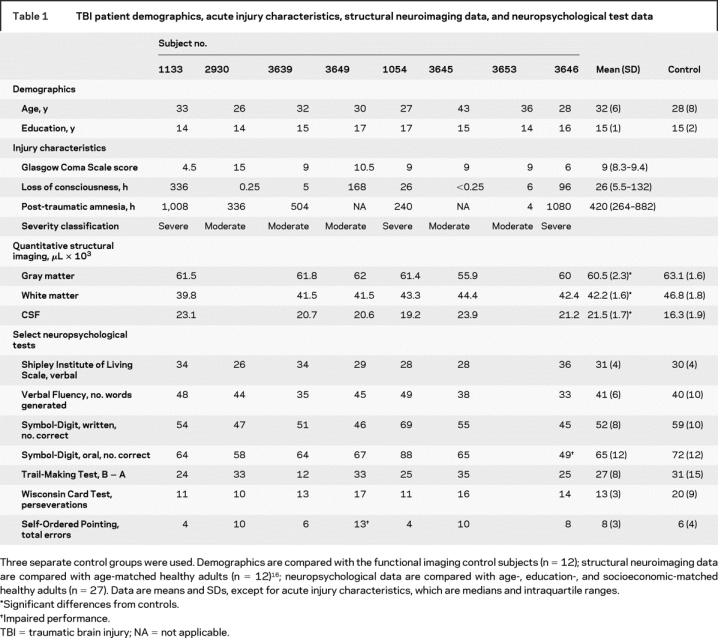
TBI patients were recruited from consecutive admissions as part of the Toronto TBI study.16,17 All patients had sustained a TBI as a result of a motor vehicle accident and were in the chronic stage of recovery at the time of study participation (table 1). Despite their significant TBIs, all patients demonstrated good functional recovery as evidenced by a return to preinjury employment or academic status. Injury severity was determined by Glasgow Coma Scale (GCS) as documented by the trauma team leader’s score at the time of discharge from the trauma unit, corresponding to the recommended 6-hour GCS score.18 Severity in two cases (1054, 2930) was upgraded from that indicated by the GCS because of extended loss of consciousness, extended post-traumatic amnesia, or both. Seven of 8 patients underwent a separate structural MRI TBI protocol (for details, see reference 16). Radiologic interpretation indicated evidence of DAI-related neuropathology (hemosiderin deposits) localized to the frontal lobes in all 7 patients, with additional pathology visualized in the parietal lobe (2 patients), the temporal lobe (1 patient), the corpus callosum (1 patient), the basal ganglia (2 patients), and the thalamus (1 patient). No patient had lesions greater than 3 mm in diameter. Whole-brain volumetric measures of gray matter, white matter, and CSF following our published image analysis methods16 were available for 6 patients, all of whom showed evidence of atrophy relative to age- and education-matched control subjects. Taken together, the radiologic interpretation and significant volume loss in the TBI patients are consistent with DAI.
Neuropsychological test data were compared with a local normative sample of age-, education-, and socioeconomic-matched control subjects. TBI patients demonstrated average to high-average performance on the verbal subtask of the Shipley Institute of Living Scale.19 This premorbid estimate, combined with matched education levels between our TBI and fMRI control subjects, minimizes group differences in native verbal capacity limitations, an important criterion for a study of verbal working memory. TBI patients also performed normally on other neuropsychological tests of attention and executive functioning, including (with one exception) a task explicitly measuring executive control within working memory.20
Behavioral task.
We used a modified version of the Alphaspan task,21 previously used in neuroimaging studies to separate executive control processes from storage and rehearsal in verbal working memory.22,23 On each trial, subjects studied a letter set consisting of 3 or 5 consonant letter strings (set size or “load” manipulation) and were asked to “maintain” the letter set over a brief delay or “alphabetize” the letters into the correct alphabetical position during the delay (executive demand manipulation). At the end of the delay, a probe was presented consisting of a letter and an ordinal position (e.g., L-4: “Was L the fourth letter in the set?”). On maintain trials, the probe referred to the ordinal position in the original letter set, whereas on alphabetize trials, the probe referred to the letter position following alphabetization of the list. Probability of a correct probe was set at 0.5 for all trials in all conditions. Executive control was operationalized as the difference between alphabetize and maintain conditions.23
Before scanning, each participant completed 20 practice trials (5 trials in each of the 4 conditions). During scanning, subjects completed 28 trials of each of the four task conditions (alphabetize 3- and 5-letter sets, maintain 3- and 5-letter sets) during a single scan session. Within each session, four 12-minute scans were acquired. Trials were grouped by executive demand with two blocks of seven trials at each level of executive demand presented during a single scan acquisition. Set size was randomized within each block. A schematic of a single trial is presented in figure 1. All trials at each load level were matched on timing and stimulus display characteristics and varied only on task demand (i.e., maintain or alphabetize).
Figure 1 Schematic of fMRI behavioral paradigm
Warning cue (alphabetize or maintain) was presented at the beginning of each trial block. ITI = intertrial interval.
fMRI scanning and analyses.
Scanning was performed on a 3.0-tesla MRI system (Signa 3T94 hardware, VH3M3 software; General Electric Healthcare, Waukesha, WI). A volumetric anatomic MRI was performed before functional scanning, using standard high-resolution three-dimensional T1-weighted fast spoiled gradient echo images. Functional scans were obtained using a single-shot T2*-weighted pulse with spiral in–out, achieving 26 slices, each 5 mm thick. Data processing and analyses were performed using Analysis of Functional NeuroImages software,24 and a standard voxel-wise, mixed effects analysis was implemented to identify brain regions exhibiting main effects of group and group × executive control demand interactions. Complete details of the scan acquisition sequences and statistical analysis can be found in the e-Methods on the Neurology® Web site at www.neurology.org.
RESULTS
Behavior.
A 2-group (control vs TBI) × 2 (executive demand) × 2 (set size) repeated-measures analysis of variance for accuracy (percent correct) revealed a significant main effect of executive demand and set size [F(1) = 18.51 (executive demand), F(1) = 11.42 (set), p < 0.01 for both comparisons] with poorer performance observed during the alphabetize and set size 5 conditions. The interaction between executive demand and set was not significant [F(1) = 0.02, NS]. Importantly, there was no main effect of group [F(1) = 2.54, NS] and no group × executive demand [F(1) = 0.094, NS] or group × set [F(1) = 0.182, NS] interactions, indicating that the main effects of condition were equivalent across both the control and TBI groups (figure 2, top panel). Post hoc analyses revealed that there were no group differences on any of the tasks (p > 0.05, all comparisons). As seen in figure e-1, there was considerable overlap in task performance across the groups, with at least half of the TBI participants performing within or above the 95% CI of the mean performance of our control group across all tasks. Therefore, the lack of group differences in behavior reflects the effectiveness of the pretraining regimen in matching performance accuracy, rather than low statistical power to detect group differences.
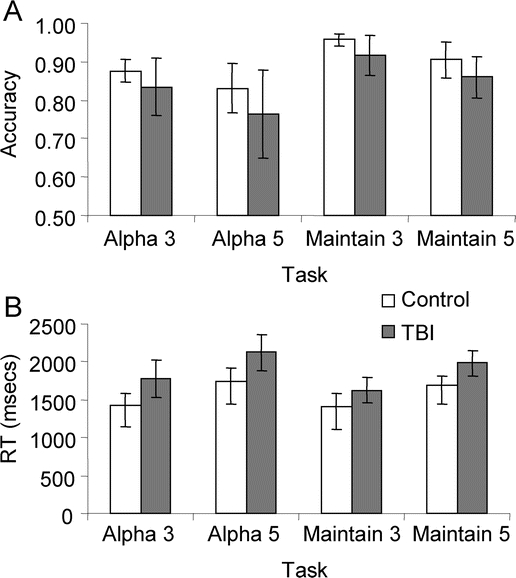
Figure 2 Behavioral data for Alphaspan task during fMRI scanning
(A) Task accuracy (% correct) for traumatic brain injury (TBI) and control subjects. (B) Reaction time (RT, msec). Error bars represent 95% CIs.
On measures of reaction time, there was a main effect of set size [F(3) = 43.81, p < 0.001] and group [F(1) = 6.41, p < 0.05], but no group × set size interaction [F(3) = 1.54, NS]. Post hoc testing revealed that control subjects exhibited faster responding at probe for all tasks with the exception of maintain 3 (p < 0.05, all comparisons). Task main effects were driven by longer reaction times at probe on 5-letter vs 3-letter set size trials, irrespective of executive demand (p < 0.001 for all comparisons; figure 2).
Neuroimaging analyses.
Executive demand was associated with increased activity in left lateral frontal, posterior parietal, and insular cortices, replicating prior work with this paradigm.23 The same contrast in TBI patients revealed a more extensive pattern of suprathreshold activation (figure 3B and table 2). Brain voxels demonstrating greater alphabetization vs maintain activity (t > 4.96, p < 10−4) in this contrast represented 0.004% of total brain volume in the TBI sample as compared with 0.0008% in control subjects. Specific areas of activation included the lateral prefrontal cortex (PFC) bilaterally, the insular and superior parietal cortices bilaterally, and the right temporo-occipital junction.
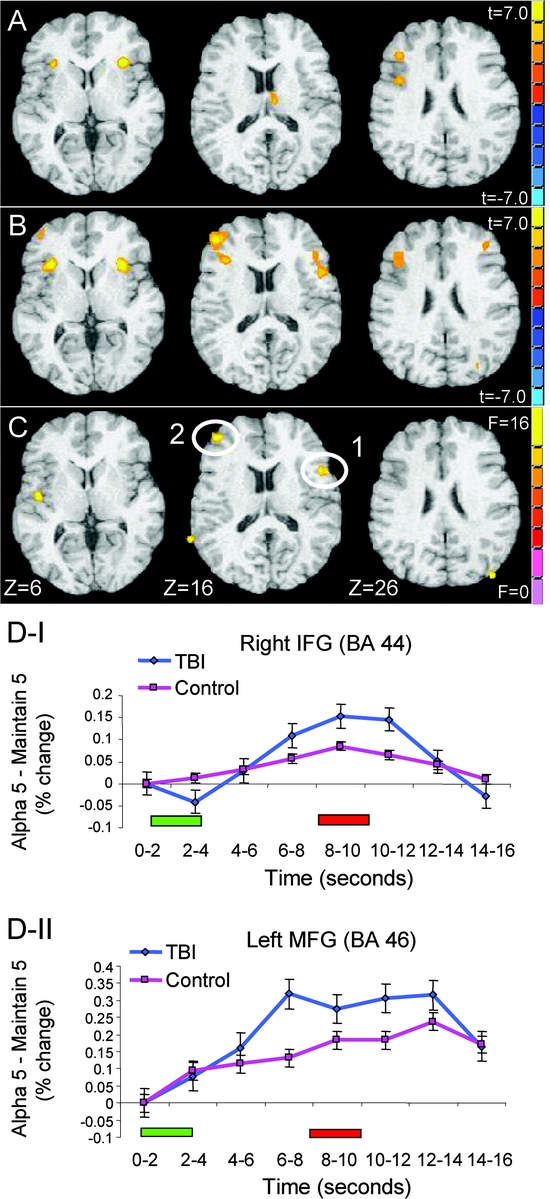
Figure 3 Areas of maximal BOLD signal change in the executive demand contrast (i.e., alphabetize > maintain conditions), collapsed across set size
(A) Control subjects. (B) Traumatic brain injury (TBI) patients. (C) Areas demonstrating a significant group × executive demand interaction. All suprathreshold cluster maxima for each group and the interaction are reported in table 2. The right side of the brain is displayed on the right side of the image. (D.1 and D.2) Temporal differences (% change in blood oxygen level–dependent [BOLD] signal) between alphabetize and maintain set size 5 conditions for control subjects and TBI patients, cross-referenced to numbers as specified in panel C. The green bar below the x-axis represents the onset of stimulus presentation; the red bar represents probe onset. IFG = inferior frontal gyrus; MFG = middle frontal gyrus.
Table 2 Activation cluster maxima corresponding to maximal BOLD signal changes during the alphabetize vs maintain conditions (i.e., main effect of executive demand) for each group and for the group × executive demand (ED) interaction
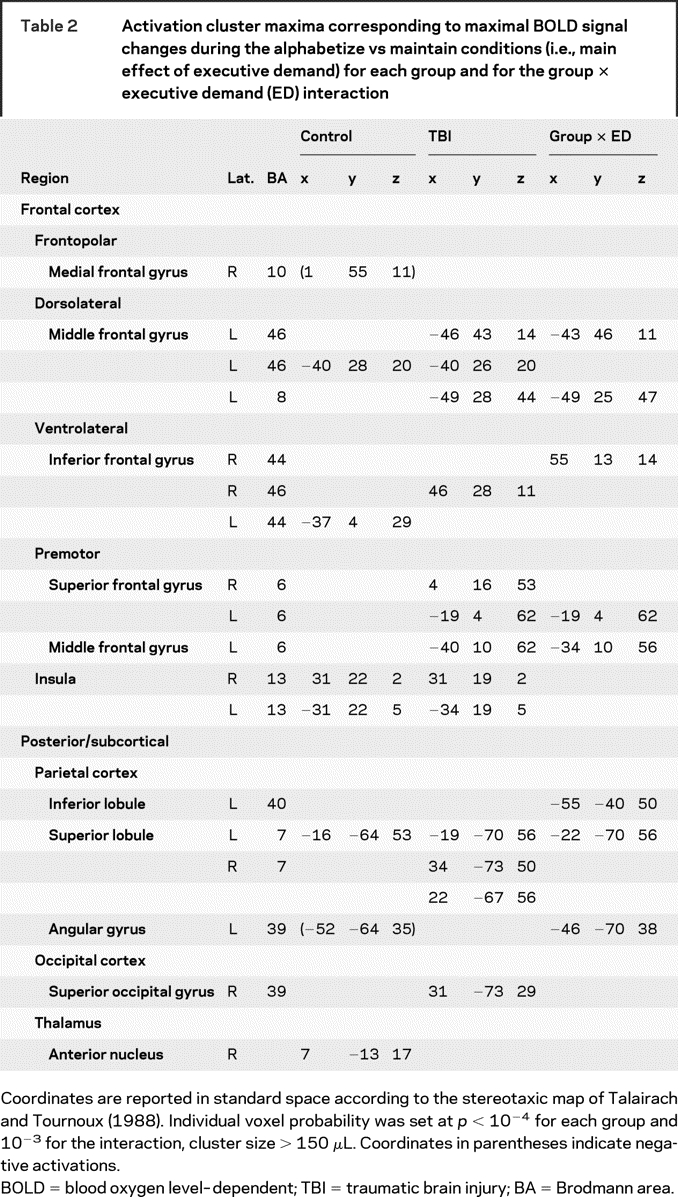
Group (TBI vs control) × executive demand (alphabetize vs maintain) interactions were observed in the left middle frontal gyrus, right inferior frontal gyrus, and left posterior regions, including inferior and superior parietal cortices. Activity in these areas was significantly greater during alphabetize trials than during maintain trials, and this difference was significantly greater in TBI patients relative to control subjects (figure 3C and table 2). An area of the left angular gyrus (Brodmann area [BA] 39) exhibited a significant group × executive demand interaction associated with greater activation during maintain in the control subjects. We used region-of-interest (ROI) analyses to confirm that these interactions were reliable (see Methods). Across ROIs, an average of 77% of the TBI sample showed an increase in blood oxygen level–dependent (BOLD) signal during alphabetize trials that was greater than the 95th percentile for controls (table 2 and figure e-2). A small cluster of activation in the right medial frontal polar cortex (BA 10) demonstrated a significant group × executive demand × set size interaction. Post hoc analyses revealed that this interaction was driven by a disproportionate BOLD signal increase during alphabetize 5 relative to maintain 5 trials in TBI patients relative to controls. A similar, albeit attenuated, pattern was observed in the 3-letter set conditions. No similar interactions were observed within lateral PFC, suggesting that group differences in executive control processes mediated by this region were not impacted by load as defined in this study.
Although TBI subjects did not show significant evidence of slowing on neuropsychological tasks (table 1), the reaction time differences between our groups on three of the four experimental tasks suggested that slowed processing speed during alphabetization may have contributed to a cumulative enhancement of the hemodynamic response. To test this hypothesis, we extracted ROI time series data for the 5-letter set sizes for the alphabetize and maintain conditions (where we expected speed of processing differences to be most prominent) in two anterior lateral PFC ROIs demonstrating reliable group × executive demand interactions. In the right inferior frontal gyrus, differences in BOLD signal between the two conditions peaked between 8 and 10 seconds after stimulus onset in both groups (figure 3D.1), whereas time to peak occurred earlier in our TBI sample in the left middle frontal gyrus ROI (6–8 seconds after stimulus onset; figure 3D.2). Neither of these anterior lateral PFC regions demonstrated a significant interaction of group (TBI, control) × trial time (TR1–TR8) [right middle frontal gyrus: F(1,7) = 1.83, p < 0.05; left middle frontal gyrus: F(1,7) = 1.67, p < 0.05]. Thus, we could find no evidence that “time on task” significantly altered the hemodynamic response in our TBI patients relative to the control group. In sum, our group-level and single-subject ROI analyses confirm that the neural signature of executive control in working memory is altered after TBI and that these brain changes cannot be accounted for by differences in behavioral performance, variability within our patient sample, or time-on-task differences.
DISCUSSION
Our results demonstrate that DAI after TBI is associated with altered functional brain response during executive control processing in working memory. Previous studies investigating the functional neuroanatomy of working memory in TBI have been confounded by heterogeneity in patient selection or task characteristics (e.g., references 11 and 12). Focal lesions in our sample were limited to scattered, small hemosiderin deposits, which reflect DAI. It is possible that larger lesions may have been detected using more sensitive sequences, such as susceptibility weighted imaging.25 However, the consistency of the functional brain changes across the TBI sample (figure e-2) suggests that DAI, common to all of our TBI subjects, and not focal lesions, which were inconsistently localized across TBI subjects, accounts for the augmented functional recruitment. We were thus able to specifically examine the impact of DAI on executive control processes to a greater degree than heretofore possible.
Prescan training ensured comparable performance across groups on the working memory task, circumventing potential confounds associated with group differences evident in earlier reports. Even TBI subjects who performed at or above the mean of the control sample on the most challenging condition (alphabetize 5) demonstrated augmented functional recruitment in the right PFC in the alphabetize 5–maintain 5 contrast (figures e-1 and e-2). Thus, executive control processes may be facilitated by supplemental recruitment within this region after DAI. Although group differences in performance cannot explain the group differences in functional activation reported here, this is not to say that performance differences are unrelated to functional activation patterns. We are currently investigating how such brain–behavior correlations are affected by TBI. Moreover, although the patients had sustained moderate to severe brain injury, all had returned to preinjury employment or academic status and were several years after injury, thus avoiding confounds related to recovery phase.
Finally, the functional neuroanatomy of our tasks is well characterized, has good convergent validity with other executive measures, and allowed us to independently manipulate load and executive control demands. By eliminating these sources of patient- and task-related variance, we showed that the functional neuroanatomy of executive control processing is specifically altered by DAI after moderate to severe brain injury. Follow-up analyses indicated that our results were reliable across the TBI sample and could not be attributed to slowing.
These data are consistent with the characterization of working memory as an emergent property of coordinated activity within a distributed network of brain regions.26,27 We have demonstrated that diffuse injury, in the absence of focal brain pathology, is sufficient to alter brain networks subserving working memory processing. Our findings replicate earlier work demonstrating the involvement of PFC and left perisylvian regions during performance of healthy subjects on this task.23 In TBI, however, this network is augmented by additional recruitment of lateral prefrontal regions bilaterally as well as left posterior parietal regions. We observed a significant group × executive demand interaction within lateral PFC regions bilaterally as well as in left posterior parietal cortices. These data demonstrate that DAI is associated with augmented functional recruitment within this network of brain regions, particularly during tasks requiring high executive control. The localization of this augmented functional recruitment implicates interhemispheric frontal and intrahemispheric frontoparietal pathways in the altered functional neuroanatomy of executive control in working memory after TBI. We are currently examining this hypothesis using diffusion tensor imaging data in combination with fMRI.
Altered functional recruitment after brain injury has been characterized as a decline in the efficiency of neural processing operations.28,29 TBI patients often report subjective changes associated with cognitively demanding tasks in spite of normal task performance, a dissociation that presents significant challenges with respect to diagnostics and treatment planning in the absence of measurable signs of cognitive dysfunction in patients with TBI, as well as in patients with other neuropathologies causing diffuse or multifocal damage (e.g., dementia, multiple sclerosis). Reduced cognitive efficiency, as indexed by altered functional recruitment during neuropsychologically normal task performance, may serve as a novel metric, reflecting the costs of normal behavioral performance at the neural level. Indeed, these measures may correlate well with subjective complaints of patients with diffuse damage, but without demonstrable neuropsychological deficits.
Two further clinical implications emerge from these findings. First, although augmented recruitment was observed here in a sample of well-recovered patients without focal injury, we predict that patients with more severe behavioral or functional deficits would similarly demonstrate this pattern of enhanced neural recruitment during cognitively demanding tasks, albeit with diminishing behavioral gains. Such a pattern has recently been reported30 and suggests that these data may have clinical relevance across the spectrum of TBI severity. Second, TBI is commonly associated with deficits in arousal, chronic pain, anxiety and depressive symptomology, each of which has been associated with similar patterns of augmented functional neural recruitment to those reported here (for a review, see reference 31). Although the cumulative impact of such comorbidity on higher cognitive functions after TBI has yet to be fully investigated, it is plausible that any reduction in neural processing efficiency resulting from brain injury may be exacerbated by secondary impacts of comorbid physical or psychological impairments. The impact of such interactions may impact the pace or extent of recovery in these patients and presents a challenge to both clinicians and researchers in assessing the implications of such comorbidity.
AUTHOR CONTRIBUTIONS
All statistical analyses were conducted by the lead author.
ACKNOWLEDGMENT
The authors thank Adriana Restagno and Marina Mandic for their invaluable assistance with the participant recruitment and image analysis.
Supplementary Material
Address correspondence and reprint requests to Dr. Brian Levine, Rotman Research Institute, Baycrest Center for Geriatric Care, 3560 Bathurst St., Toronto, ON, M6A 2E1, Canada blevine@rotman-baycrest.on.ca
Supplemental data at www.neurology.org
Supported by a grant from the NIH–National Institute of Child Health and Human Development (no. HD42385-01) to B.L.
Disclosure: The authors report no disclosures.
Received January 8, 2008. Accepted in final form May 30, 2008.
REFERENCES
- 1.McAllister TW, Flashman LA, Sparling MB, Saykin AJ. Working memory deficits after traumatic brain injury: catecholaminergic mechanisms and prospects for treatment—a review. Brain Inj 2004;18:331–350. [DOI] [PubMed] [Google Scholar]
- 2.Baddeley A. Working Memory. New York: Oxford University Press, 1986. [Google Scholar]
- 3.Goldman-Rakic PS. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Plum F, Mountcastle V, eds. Handbook of Physiology: The Nervous System. Bethesda, MD: American Physiological Society, 1987:373–417. [Google Scholar]
- 4.D’Esposito M, Cooney JW, Gazzaley A, Gibbs SE, Postle BR. Is the prefrontal cortex necessary for delay task performance? Evidence from lesion and FMRI data. J Int Neuropsychol Soc 2006;12:248–260. [DOI] [PubMed] [Google Scholar]
- 5.Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci 2003;7:415–423. [DOI] [PubMed] [Google Scholar]
- 6.Courville CB. Pathology of the Central Nervous System, Part 4. Mountain View, CA: Pacific Press Publishing, 1937. [Google Scholar]
- 7.Scheid R, Walther KR, Guthke T, Preul C, von Cramon DY. Cognitive sequelae of diffuse axonal injury. Arch Neurol 2006;63:418–424. [DOI] [PubMed] [Google Scholar]
- 8.Povlishock JT, Katz DI. Update of neuropathology and neurological recovery after traumatic brain injury. J Head Trauma Rehabil 2005;20:76–94. [DOI] [PubMed] [Google Scholar]
- 9.Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci 1999;11:1011–1036. [DOI] [PubMed] [Google Scholar]
- 10.Perlstein WM, Cole MA, Demery JA, et al. Parametric manipulation of working memory load in traumatic brain injury: behavioral and neural correlates. J Int Neuropsychol Soc 2004;10:724–741. [DOI] [PubMed] [Google Scholar]
- 11.Christodoulou C, DeLuca J, Ricker JH, et al. Functional magnetic resonance imaging of working memory impairment after traumatic brain injury. J Neurol Neurosurg Psychiatry 2001;71:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAllister TW, Saykin AJ, Flashman LA, et al. Brain activation during working memory 1 month after mild traumatic brain injury: a functional MRI study. Neurology 1999;53:1300–1308. [DOI] [PubMed] [Google Scholar]
- 13.Levine B, Fujiwara E, O’Connor C, et al. In vivo characterization of traumatic brain injury neuropathology with structural and functional neuroimaging. J Neurotrauma 2006;23:1396–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen JK, Johnston KM, Frey S, Petrides M, Worsley K, Ptito A. Functional abnormalities in symptomatic concussed athletes: an fMRI study. Neuroimage 2004;22:68–82. [DOI] [PubMed] [Google Scholar]
- 15.Kane MJ, Hambrick DZ, Tuholski SW, Wilhelm O, Payne TW, Engle RW. The generality of working memory capacity: a latent-variable approach to verbal and visuospatial memory span and reasoning. J Exp Psychol Gen 2004;133:189–217. [DOI] [PubMed] [Google Scholar]
- 16.Levine B, Kovacevic N, Nica I, Cheung G, Schwartz ML, Black SE. Toronto traumatic brain injury study: injury severity and quantified MRI. Neurology 2008;70:771–778. [DOI] [PubMed] [Google Scholar]
- 17.Fujiwara E, Schwartz ML, Gao F, Black SE, Levine B. Ventral frontal cortex functions and quantified MRI in traumatic brain injury. Neuropsychologia (in press). [DOI] [PMC free article] [PubMed]
- 18.Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet 1974;2:81–84. [DOI] [PubMed] [Google Scholar]
- 19.Zachary RA. Shipley Institute of Living Scale, Revised Manual. Los Angeles: Western Psychological Services, 1986. [Google Scholar]
- 20.Petrides M, Milner B. Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia 1982;20:249–262. [DOI] [PubMed] [Google Scholar]
- 21.Craik FIM. A functional account of differences in memory. In: Klix F, Hagendorf H, eds. Human Memory and Cognitive Capacities. North Holland: Elsevier Science Publishers, 1986:409–421. [Google Scholar]
- 22.D’Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn 1999;41:66–86. [DOI] [PubMed] [Google Scholar]
- 23.Postle BR, Berger JS, D’Esposito M. Functional neuroanatomical double dissociation of mnemonic and executive control processes contributing to working memory performance. Proc Natl Acad Sci USA 1999;96:12959–12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox RW, Hyde JS. Software tools for analysis and visualization of fMRI data. NMR Biomed 1997;10:171–178. [DOI] [PubMed] [Google Scholar]
- 25.Tong KA, Ashwal S, Holshouser BA, et al. Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: improved detection and initial results. Radiology 2003;22:332–339. [DOI] [PubMed] [Google Scholar]
- 26.Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience 2006;139:23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci 2003;3:255–274. [DOI] [PubMed] [Google Scholar]
- 28.Chen AJW, Abrams GM, D’Esposito M. Functional reintegration of prefrontal neural networks for enhancing recovery after brain injury. J Head Trauma Rehabil 2006;21:107–118. [DOI] [PubMed] [Google Scholar]
- 29.Hillary FG, Genova HM, Chiaravalloti ND, Rypma B, DeLuca J. Prefrontal modulation of working memory performance in brain injury and disease. Hum Brain Mapp 2006;27:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newsome MR, Scheibel RS, Steinberg JL, et al. Working memory brain activation following severe traumatic brain injury. Cortex 2007;43:95–111. [DOI] [PubMed] [Google Scholar]
- 31.Levine B, Katz D, Black SE, Dade L. New approaches to brain-behavior assessment in traumatic brain injury. In: Stuss DT, Knight R, eds. Principles of Frontal Lobe Function. New York: Oxford University Press, 2002:448–465. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



