Abstract
Objective:
To develop a grading scale to predict the risk of intracerebral hemorrhage (ICH) and prognosis after treatment with IV tissue-plasminogen activator (t-PA) in patients with ischemic stroke.
Methods:
We constructed a five-point scale based on NIH Stroke Scale score, extent of hypodensity on CT scan, serum glucose at baseline, and history of diabetes to predict the risk of hemorrhage after thrombolysis (HAT score). We evaluated the predictive ability of this scale, using c-statistics, in two independent cohorts: the t-PA treated group in the National Institute of Neurological Disorders and Stroke study, and consecutive patients treated with IV t-PA at our institution.
Results:
The percentage of patients who developed any ICH after t-PA increased with higher scores in both cohorts. Collectively, the rate of any symptomatic ICH was 2% (0 point), 5% (1 point), 10% (2 points), 15% (3 points), and 44% (>3 points). The c-statistic was 0.72 (95% CI 0.65–0.79; p < 0.001) for all hemorrhages; 0.74 (0.63–0.84; p < 0.001) for symptomatic hemorrhages; and 0.79 (0.70–0.88; p < 0.001) for hemorrhages with final fatal outcome. Similar results were obtained when each cohort was analyzed separately. The score also reasonably predicted good (mRS ≤ 2) (c-statistic 0.75; 0.69–0.80; p < 0.001) and catastrophic (mRS ≥ 5) (0.78; 0.72–0.84; p < 0.001) functional outcomes on day 90 in the National Institute of Neurological Disorders and Stroke t-PA-treated patients.
Conclusions:
The hemorrhage after thrombolysis (HAT) score is a practical, quick, and easy-to-perform scale that allows reasonable risk stratification of intracerebral hemorrhage after IV tissue-plasminogen activator (t-PA). However, the prognostic value of this scale and its use to predict the net benefit from t-PA needs to be refined and prospectively confirmed in a larger cohort of patients before it can be used in clinical decision-making.
GLOSSARY
- AIS
= acute ischemic stroke;
- DWI
= diffusion-weighted imaging;
- HAT score
= hemorrhage after thrombolysis score;
- HFFO
= hemorrhage with final fatal outcome;
- IA
= intra-arterial;
- ICH
= intracerebral hemorrhage;
- MCA
= middle cerebral artery;
- mRS
= modified Rankin Score;
- NIHSS
= National Institutes of Health Stroke Scale;
- NINDS
= National Institute of Neurological Disorders and Stroke;
- ROC
= receiver-operator curves;
- SICH
= symptomatic ICH;
- t-PA
= tissue-plasminogen activator;
- TPI
= Thrombolytic Predictive Instrument.
Intracerebral hemorrhage (ICH) is the most feared complication of tissue-plasminogen activator (t-PA) therapy for acute ischemic stroke (AIS). In a pooled analysis of 6 IV t-PA stroke trials, 5.9% of t-PA-treated patients compared to 1.1% of placebo cases had ICH associated with neurologic deterioration.1 Only 2–4% of patients with AIS receive IV t-PA in the United States.2 Concern that ICH after t-PA might worsen an individual patient’s prognosis partly limits more widespread use of t-PA. Multiple studies of patients with AIS receiving t-PA have identified several risk factors for ICH after thrombolysis. These include stroke severity as assessed by baseline National Institutes of Health Stroke Scale (NIHSS) score,3 older age,4 history of diabetes,5 presence and extent of ischemic changes on CT6 or diffusion-weighted imaging (DWI),7 high baseline serum glucose,8 admission fibrinolytic profile,9 and current smoking.10 However, none has explored the ability to predict the risk of ICH and prognosis for each thrombolytic candidate based on the cumulative grouping of these individual variables before initiating t-PA.
Although a number of baseline factors and individual variables that modify the effect of t-PA play a predominant role in influencing the net benefit from thrombolysis,11 stratification of the risk of ICH after t-PA might facilitate patient selection for thrombolytic therapy. Therefore, we aimed to develop and validate a simple grading scale that can be performed at the bedside during the hyperacute phase to predict the risk of ICH and prognosis after treatment with IV t-PA.
METHODS
Scale development.
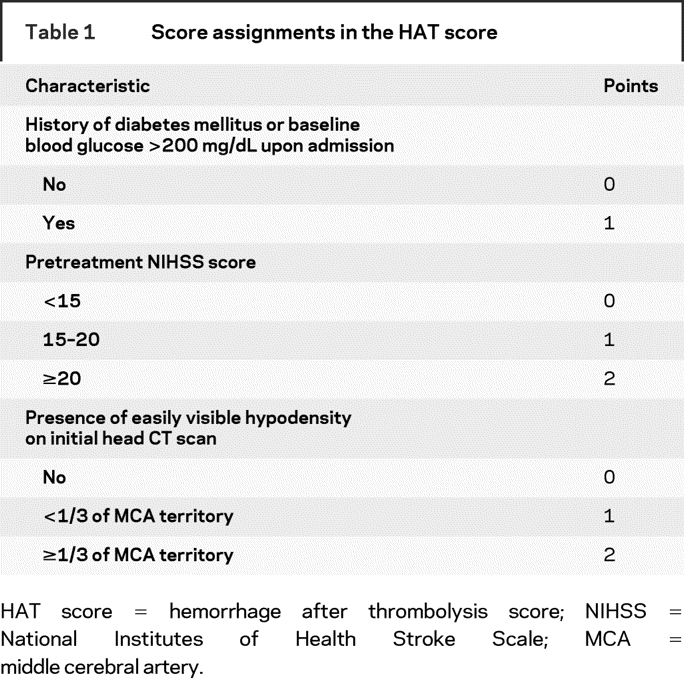
We performed a PubMED literature search and reviewed the publications studying the predictors of hemorrhagic transformation after t-PA in patients with AIS.1,3,4–6,8,10,12,13 Four of these studies were limited to patients treated with IV t-PA within 3 hours; three to patients treated with IV t-PA within 6 hours; and two included patients receiving either IV or intra-arterial (IA) t-PA. We identified the pretreatment factors associated with greater risk of ICH in at least two publications and ranked them according to their cumulative OR as derived from the reviewed studies. The following pretreatment factors emerged as predictors of ICH: age; baseline NIHSS; history of diabetes; baseline serum glucose; visible hypodensity in middle cerebral artery (MCA) territory on CT; current smoking; and use of antithrombotics prior to t-PA. We examined the prognostic ability of various combinations of these factors, using different thresholds and cutoff points based on reported values in the literature.1,3,4–6,8,10,12,13 We ultimately constructed a five-point scale to predict the risk of hemorrhage after t-PA, HAT score, incorporating the top four predictors that had the highest ORs. These were the baseline NIHSS score, presence and extent of hypodensity on initial CT scan, history of diabetes, and high baseline serum glucose. We assigned points for each of the four predictors, based on the weight of its prognostic value, as shown in table 1. This combination had the highest predictive capability for ICH.
Table 1 Score assignments in the HAT score
Scale validation.
We examined the predictive ability of the HAT score in two independent cohorts of IV t-PA-treated patients with AIS. First, we tested the HAT score in the t-PA treated cohorts of the National Institute of Neurological Disorders and Stroke (NINDS) Stroke Trials 1–2. The details of these trials have been published.12–15 Clinical data were collected at baseline, 24 hours, 7–10 days, 3 months, and 1 year after randomization. CT scans were obtained prior to the initiation of t-PA, at 24 hours, and 7–10 days after the onset of stroke, and whenever any clinical worsening was noted to detect ICH. Hypodensity on the baseline CT scans was identified as focal or diffuse area, which on visual inspection is less than the white matter density but greater than CSF density, excluding areas of chronic white matter ischemic changes or areas considered chronic or old and closer to CSF density and artifacts.16 Symptomatic ICH (SICH) within 36 hours were defined as any CT-documented ICH that was temporally related to any deterioration in the patient’s clinical condition in the judgment of clinical investigator. We used the public-access data files from National Technical Information Services to abstract all variables used for the HAT score from the data of the two NINDS trials cohorts. We also retrieved the modified Rankin Score (mRS) at 3 months to identify subjects with excellent (mRS ≤1), good (mRS ≤2), and catastrophic (mRS ≥5) outcomes. We calculated the HAT score for each patient by summing the total number of weighted risk predictors present (table 1). We excluded patients with missing data on any of the components of the HAT score from our analysis.
Second, we tested the HAT score in a consecutive cohort of IV t-PA-treated patients from our institution. We reviewed our prospectively collected stroke database from 2001 to 2007. We included consecutive patients with AIS, treated with IV t-PA within 3 hours from symptom onset according to the NINDS trials protocol and the American Stroke/Heart Association guidelines,17 who had CT scan before treatment and MRI with T2* sequence (n = 58) or CT scan (n = 40) 24–48 hours later to assess for ICH. We derived a cumulative HAT score for each patient. Patients who were treated after 3 hours from stroke onset, those who had MRI but not CT prior to t-PA treatment, those who received antiplatelet agents or anticoagulants before their second CT or MRI, and those treated with combined IV/IA t-PA or IA t-PA alone were excluded. Results of the official neuroradiology report were used to determine the presence and extent of visible hypodensity on initial head CT scan and the presence vs absence of ICH on follow-up scans. Hemorrhages on follow-up MRI were identified as areas of susceptibility effect (signal loss; darkening) on T2* images.7 SICH in our patients was defined as ICH associated with an increase of ≥4 points on the NIHSS score from baseline assessment. Hemorrhage with final fatal outcome (HFFO) refers to death during hospitalization after the development of ICH.
Statistical analysis.
We developed receiver-operator curves (ROC) models for each of the two cohorts for any ICH, SICH, and HFFO, and for excellent, good, and catastrophic outcomes at day 90 in NINDS patients. Areas under ROC (c-statistics) and 95% CI were calculated as a measure of predictive ability. The c-statistic integrates sensitivity and specificity of the range of a variable, and estimates how well a prediction rule can correctly rank-order patients by risk. Ideal prediction produces a c-statistic of 1.00; prediction no better than chance is associated with c-statistic of ≤0.50.
We used Student t and Fisher exact tests for continuous and categorical variables, respectively, to compare the demographic characteristics of patient population in various cohorts. All analyses were done with SAS; p < 0.05 was considered significant.
RESULTS
Complete data were missing in 10 of 312 patients who received t-PA in the NINDS trials, leaving 302 with complete dataset. Baseline characteristics between excluded and included patients were similar, except for higher prevalence of diabetes among excluded patients (62% vs 21%; p = 0.01). A total of 307 of 312 patients in the placebo cohorts had complete data. Meanwhile, 98 consecutive patients from our prospectively collected database met all of our predetermined inclusion/exclusion criteria. Table 2 shows the baseline characteristics of our patients and NINDS t-PA-treated patients. Our patients were older and had higher frequency of visible hypodensity (<1/3 of the MCA) on initial CT scan. Baseline blood glucose >200 mg/dL was present in a higher percentage of NINDS patients. Table 3 shows that follow-up CT revealed ICH in 15% of NINDS patients (SICH in 6% and HFFO in 3%); 20% of our patients had ICH on follow-up scans (7% were considered SICH and 6% HFFO).
Table 2 Characteristics of patients included in two cohorts used to derive the HAT score
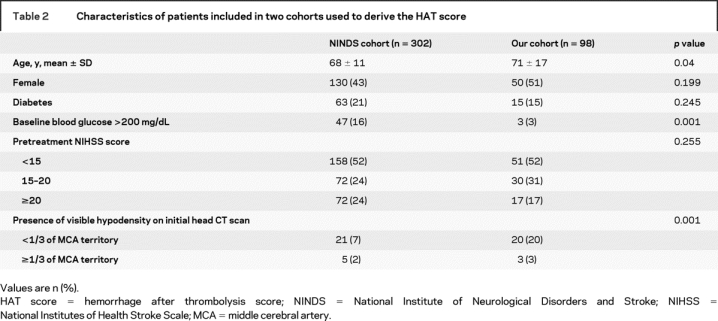
Table 3 Risk of ICH after t-PA therapy by HAT score
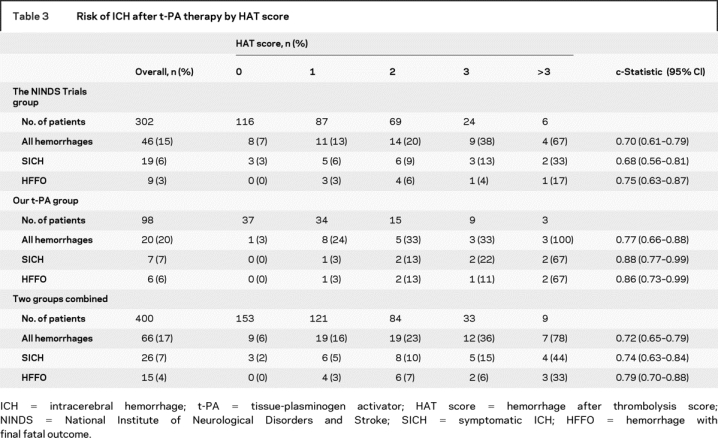
The mean HAT score was 1.07 ± 1.06 for the NINDS t-PA cohorts, and 1.07 ± 1.15 in our patients. As table 3 shows, the percentage of NINDS patients who developed any ICH after t-PA increased with higher HAT scores. The corresponding c-statistic was 0.70 (CI 0.61–0.79; p < 0.001). The same trend was seen in patients with SICH (c-statistic 0.68; 0.56–0.81; p < 0.001). Similarly, the risk of any ICH after t-PA tended to be greater with higher than lower HAT scores in our cohort. The c-statistic for predicting any ICH was 0.77 (0.66–0.88; p < 0.001), and 0.88 (0.77–0.99; p < 0.001) for SICH.
Given the similar patterns for the HAT score validity in the two cohorts, we evaluated its prognostic capability in the two cohorts combined (400 patients). The risk of any ICH after t-PA also increased with increasing HAT scores (figure). Seventy-eight percent of patients with HAT score >3 developed ICH (SICH in 44% and HFFO in 33%). The c-statistic in the combined cohorts was 0.72 (0.65–0.79; p < 0.001) for any hemorrhage; 0.74 (0.63–0.84; p < 0.001) for SICH; and 0.79 (0.70–0.88; p < 0.001) for HFFO, indicating that this score can reasonably predict the risk of any ICH, including SICH and HFFO, after IV t-PA.
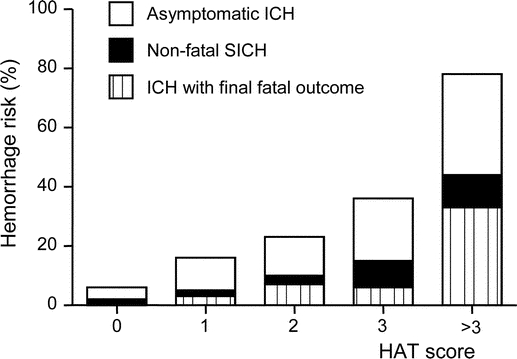
Figure Risk of intracerebral hemorrhage after tissue-plasminogen activator therapy in two cohorts combined (n = 400)
ICH = intracerebral hemorrhage; SICH = symptomatic ICH; HAT score = hemorrhage after thrombolysis score.
We also assessed the predictive value of alternative models with different combinations and cutoff points for each of the HAT score variables, and after incorporating other additional variables. None of these resulted in significant changes in the scale’s predictive ability. For example, using NIHSS score cutoff point of 15 alone to predict the risk of ICH was associated with a c-statistic of 0.65 (0.55–0.75; p = 0.004) for any ICH. Using NIHSS cutoff points of 5 or 10 were associated with c-statistics of 0.63–0.64 (0.53–0.75; p = 0.01); and serum glucose threshold of >180 mg/dL with c-statistic of 0.69 (0.60–0.78; p < 0.001) for any ICH. Similarly, c-statistics were 0.65–0.66 when age >75, current smoking, or use of antithrombotics prior to t-PA were added as predictive variables.
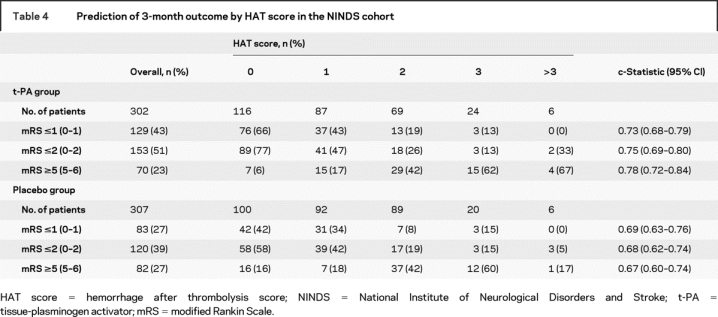
Table 4 summarizes the functional outcome in both t-PA and placebo-treated cohorts of the NINDS trials by HAT score grouping. As it illustrates, the percentage of patients with excellent (mRS ≤1) and good (mRS ≤2) outcomes decreased, while those with catastrophic outcomes (mRS ≥5) increased, with rising HAT scores in both cohorts. However, c-statistics were 0.73 for excellent, 0.75 for good, and 0.78 for poor outcome in t-PA-treated patients compared to 0.69, 0.68, and 0.67 in the placebo cohorts, suggesting that HAT score can also be used to predict the functional outcome, particularly in t-PA treated patients.
Table 4 Prediction of 3-month outcome by HAT score in the NINDS cohort
DISCUSSION
The HAT score provides a quick and easy-to-perform scale to predict the risk of ICH and prognosis after treatment with IV t-PA. The scale can reasonably predict the rate of any ICH after t-PA, including SICH and HFFO, and functional outcome at day 90; and demonstrates different risk for different categories of the scale. The consistency of the predictive capability of the scale in two independent cohorts of t-PA-treated patients, and the fact that it was derived from various cohorts of t-PA-treated patients and not limited to the NINDS cohorts, indicate that this score is likely to have good external validity.
A risk assessment scale must be simple to use and generally applicable, without extensive time commitment, especially when it is used during the hyperacute phase of AIS. The HAT score comprises four factors related to baseline neurologic examination (NIHSS score), patient characteristics (history of diabetes and admission glucose level), and neuroimaging (presence vs absence and extent of easily visible hypodensity on head CT scan). All of these elements are part of routine clinical assessments for pretreatment screening of t-PA candidates, which can be easily and rapidly determined at the bedside. Studies suggest that the use of advanced MRI techniques, such as hyperintense acute reperfusion marker (HARM),18 T2* permeability imaging,19 or voxel-by-voxel volumetric analysis of the acute diffusion coefficient maps,7 and determining the level of endogenous fibrinolysis inhibitors9 at admission may have prognostic value for predicting ICH after treatment with t-PA. These were not included in our scale because they are either not readily assessable or available within the time window of thrombolytic therapy or might require more complex judgment and interpretation.
Elements of the HAT score are reliable in patient risk stratification. Each component in the scale had been reported to be an independent predictor of SICH after t-PA for AIS by various investigators and in different cohorts.1,3,4–6,8,10,12,13 The NIHSS score is widely used for assessment of clinical stroke severity. Severity of initial clinical deficit was associated with increased risk of ICH after t-PA in the European Cooperative Acute Stroke Study.6 Higher NIHSS score is thought to correlate with the volume of ischemic tissue at risk for hemorrhagic transformation.3,5,13 The presence and extent of visible hypodensity on baseline CT, which is thought to represent cytotoxic edema and possibly the development of irreversible injury, have also been associated with ICH after t-PA.20 In one study, early CT hypodensity <1/3 (OR 3.17) and >1/3 of MCA territory (OR 9.38) emerged as independent predictors of SICH after t-PA.3 Because both baseline NIHSS score and presence and extent of hypodensity on CT scan are overwhelmingly associated with the risk for SICH, it is justified to weigh them more than others and to divide them into three subcategories to adjust for variations in severity across a continuous scale. Glucose may accelerate blood–brain barrier disruption by increasing matrix metalloproteinase-9 expression.21 Admission hyperglycemia, especially >200 mg/dL, or diabetes mellitus have been found to be significant risk factors for SICH in two registries.3,5 Therefore, they were dichotomized to reflect the strength of association with hemorrhage risk and weighted accordingly.
Studies examining the effects of age on the risk of ICH after t-PA have inconsistent results.4,22 Older age was reported to be an independent risk factor for ICH in a pooled analysis of major IV t-PA trials.1 However, post hoc analysis in the NINDS trials showed that age was not a significant predictor of SICH.13 In our study, the addition of age did not improve the scale’s predictive ability. We therefore did not include age as a component of the HAT score.
Hemorrhagic transformation after thrombolysis must be put in the context of the overall clinical outcome. Therefore, although it is helpful to identify patients at increased risk for ICH, the ability to predict functional outcome after t-PA is important. The Stroke-Thrombolytic Predictive Instrument (TPI) has been previously reported to predict good vs catastrophic functional outcomes in patients with AIS, with or without thrombolysis, using seven variables including diabetes and stroke severity.23 Although direct comparisons and validations of the stroke-TPI and HAT score are yet to be performed, the HAT score is simpler and appears to have reasonable capability in predicting both the risk of ICH and 3-month functional outcome after treatment with t-PA. Our risk prediction score can be potentially useful at two stages in the management of t-PA eligible patients with stroke. The HAT score might be useful in identifying patients at high vs low risk of SICH, and for individualized counseling of patients and families to make an informed decision regarding the potential risks of thrombolysis, by providing valuable prognostic information. Based on our results, patients with HAT score >3 tend to have higher rates of SICH and catastrophic outcomes at 3 months. On the other hand, HAT score ≤2 may assure reluctant physicians that the risk of ICH is low and could facilitate treatment with t-PA.
It is important to note that the HAT score alone should not be used to withhold treatment, if deemed appropriate, since most patients who have severe infarcts are destined for poor outcomes. It is also important to consider that several other baseline variables, such as symptom-to-treatment time, which can modify the degree of benefit from t-PA and its impact upon final outcome, are not incorporated in the HAT score.11 These factors, together with the risk of ICH, should be carefully considered for treatment decision-making.
The reported rates of early ICH after thrombolysis vary from 5% to 50%.4,13,24–26 In this study, the rate of any ICH in our cohort was higher than that in the NINDS trials. This could be attributed to differences in the imaging technique used to detect ICH and patient population. Using T2* MRI in some of our patients may have allowed us to identify a different group of asymptomatic hemorrhages by detecting subtle petechial hemorrhages that would have been missed on conventional CT scans that were exclusively used in the NINDS trials.27,28 The HAT score more accurately predicted the risk of SICH in our cohort than in the NINDS trials (c-statistic 0.88 vs 0.68). This could be attributed to the differences in defining neurologic deterioration between the two cohorts. The NINDS trials used a definition of any neurologic deterioration as assessed by NIHSS, while we used a definition of worsening by ≥4 points on NIHSS.
Our study has limitations attributed to its retrospective nature and small number of patients experiencing ICH, especially SICH. The definitions of SICH in the two cohorts were different, and information on the occurrence of parenchymal hematoma type-2, which is often associated with worse outcome,4 was not reported in the public-access NINDS trials database. Furthermore, we could not fully ascertain a causal relationship between ICH and symptomatic worsening or death given the retrospective nature of our analysis. We only included patients for whom all elements required to calculate HAT score were available and excluded those with missing elements. This restriction could potentially limit general application of the HAT score to all patients with stroke. These limitations can only be addressed in a prospective study. Besides, the c-statistics in some of the models may be too low to be used for individual prediction. It is important to point out that the HAT score applies only to patients who match the NINDS trials t-PA treatment protocol. Our study only included patients receiving IV t-PA within 3 hours after stroke onset. Using HAT score to predict ICH risk and prognosis in patients receiving IV t-PA after the 3 hours time window and in patients receiving IA t-PA needs further exploration. We were only able to examine the HAT risk prediction score in the NINDS cohorts because the data are publicly available.
It is important to emphasize that the use of the HAT score to predict the net benefit of IV t-PA cannot be determined from our study. The score should be further examined in other large IV t-PA trials cohorts and in prospective studies to increase its precision and applicability before it can be recommended to make clinical decisions regarding the use of thrombolysis.
Supplementary Material
Address correspondence and reprint requests to Dr. Magdy Selim, Department of Neurology, Stroke Division, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Palmer 127, Boston, MA 02215 mselim@bidmc.harvard.edu
Dr. Lou is supported by Zhejiang University Academic Stars Research Fellowship and grants from the National Natural Science Foundation of China (30500175); Dr. Selim is supported in part by grants from NINDS (1R01-NS 057127-01A1 and 1R01-NS 045754-01A2) and NIH (5R01-HL46690-14).
Disclosure: The authors report no disclosures.
Received October 1, 2007. Accepted in final form June 6, 2008.
REFERENCES
- 1.Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004;363:768–774. [DOI] [PubMed] [Google Scholar]
- 2.Douglas VC, Tong DC, Gillum LA, et al. Do the Brain Attack Coalition’s criteria for stroke centers improve care for ischemic stroke? Neurology 2005;64:422–427. [DOI] [PubMed] [Google Scholar]
- 3.Tanne D, Kasner SE, Demchuk AM, et al. Markers of increased risk of intracerebral hemorrhage after intravenous recombinant tissue plasminogen activator therapy for acute ischemic stroke in clinical practice: the Multicenter rt-PA Stroke Survey. Circulation 2002;105:1679–1685. [DOI] [PubMed] [Google Scholar]
- 4.Larrue V, von Kummer RR, Müller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke 2001;32:438–441. [DOI] [PubMed] [Google Scholar]
- 5.Demchuk AM, Morgenstern LB, Krieger DW, et al. Serum glucose level and diabetes predict tissue plasminogen activator-related intracerebral hemorrhage in acute ischemic stroke. Stroke 1999;30:34–39. [DOI] [PubMed] [Google Scholar]
- 6.Larrue V, von Kummer R, del Zoppo G, Bluhmki E. Hemorrhagic transformation in acute ischemic stroke: potential contributing factors in the European Cooperative Acute Stroke Study. Stroke 1997;28:957–960. [DOI] [PubMed] [Google Scholar]
- 7.Selim M, Fink JN, Kumar S, et al. Predictors of hemorrhagic transformation after intravenous recombinant tissue plasminogen activator: prognostic value of the initial apparent diffusion coefficient and diffusion-weighted lesion volume. Stroke 2002;33:2047–2052. [DOI] [PubMed] [Google Scholar]
- 8.Kidwell CS, Saver JL, Carneado J, et al. Predictors of hemorrhagic transformation in patients receiving intra-arterial thrombolysis. Stroke 2002;33:717–724. [DOI] [PubMed] [Google Scholar]
- 9.Ribo M, Montaner J, Molina CA, et al. Admission fibrinolytic profile is associated with symptomatic hemorrhagic transformation in stroke patients treated with tissue plasminogen activator. Stroke 2004;35:2123–2127. [DOI] [PubMed] [Google Scholar]
- 10.Bang OY, Saver JL, Liebeskind DS, et al. Cholesterol level and symptomatic hemorrhagic transformation after ischemic stroke thrombolysis. Neurology 2007;68:737–742. [DOI] [PubMed] [Google Scholar]
- 11.Kent DM, Selker HP, Rithazer R, et al. Can multivariable risk-benefit profiling be used to select treatment-favorable patients for thrombolysis in stroke in the 3-to-6 hour time window? Stroke 2006;37:2963–2969. [DOI] [PubMed] [Google Scholar]
- 12.Thomalla G, Sobesky J, Köhrmann M, et al. Two tales: hemorrhagic transformation but not parenchymal hemorrhage after thrombolysis is related to severity and duration of ischemia: MRI study of acute stroke patients treated with intravenous tissue plasminogen activator within 6 hours. Stroke 2007;38:313–318. [DOI] [PubMed] [Google Scholar]
- 13.Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. The NINDS t-PA Stroke Study Group. Stroke 1997;28:2109–2118. [DOI] [PubMed] [Google Scholar]
- 14.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 1995;333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 15.Kwiatkowski TG, Libman RB, Frankel M, et al. Effects of tissue plasminogen activator for acute ischemic stroke at one year. National Institute of Neurological Disorders and Stroke Recombinant Tissue Plasminogen Activator Stroke Study Group. N Engl J Med 1999;340:1781–1787. [DOI] [PubMed] [Google Scholar]
- 16.Patel SC, Levine SR, Tilley BC, et al. Lack of clinical significance of early ischemic changes on computed tomography in acute stroke. JAMA 2001;286:2830–2838. [DOI] [PubMed] [Google Scholar]
- 17.Adams HP Jr, Brott TG, Furlan AJ, et al. Guidelines for thrombolytic therapy for acute stroke: a supplement to the guidelines for the management of patients with acute ischemic stroke: a statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Heart Association. Circulation 1996;5:1167–1174. [DOI] [PubMed] [Google Scholar]
- 18.Kim EY, Na DG, Kim SS, Lee KH, Ryoo JW, Kim HK. Prediction of hemorrhagic transformation in acute ischemic stroke: role of diffusion weighted imaging and early parenchymal enhancement. AJNR Am J Neuroradiol 2005;26:1050–1055. [PMC free article] [PubMed] [Google Scholar]
- 19.Bang OY, Buck BH, Saver JL, et al. Prediction of hemorrhagic transformation after recanalization therapy using T2*-permeability magnetic resonance imaging. Ann Neurol Epub 2007 Aug 7. [DOI] [PubMed]
- 20.Grond M, von Kummer R, Sobesky J, et al. Early x-ray hypoattenuation of brain parenchyma indicates extended critical hypoperfusion in acute stroke. Stroke 2000;31:133–139. [DOI] [PubMed] [Google Scholar]
- 21.Garg R, Chaudhuri A, Munschauer F, Dandona P. Hyperglycemia, insulin, and acute ischemic stroke: a mechanistic justification for a trial of insulin infusion therapy. Stroke 2006;37:267–273. [DOI] [PubMed] [Google Scholar]
- 22.Berrouschot J, Rother J, Glahn J, Kucinski T, Fiehler J, Thomalla G. Outcome and severe hemorrhagic complications of intravenous thrombolysis with tissue plasminogen activator in very old (≥80 years) stroke patients. Stroke 2005;36:2421–2425. [DOI] [PubMed] [Google Scholar]
- 23.Kent DM, Selker HP, Ruthazer R, Bluhmki E, Hacke W. The stroke-thrombolytic predictive instrument: a predictive instrument for intravenous thrombolysis in acute ischemic stroke. Stroke 2006;37:2957–2962. [DOI] [PubMed] [Google Scholar]
- 24.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke: the European Cooperative Acute Stroke Study (ECASS). JAMA 1995;274:1017–1025. [PubMed] [Google Scholar]
- 25.Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (alteplase) for ischemic stroke 3 to 5 hours after symptom onset: the ATLANTIS Study: a randomized controlled trial: Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA 1999;21:2019–2026. [DOI] [PubMed] [Google Scholar]
- 26.Molina CA, Montaner J, Abilleira S, et al. Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke 2001;32:1079–1084. [DOI] [PubMed] [Google Scholar]
- 27.Linfante I, LIinas RH, Caplan LR, Warach S. MRI features of intracerebral hemorrhage within 2 hours from symptom onset. Stroke 1999;30:2263–2267. [DOI] [PubMed] [Google Scholar]
- 28.Schellinger PD, Jansen O, Fiebach JB, Hacke W, Sartor K. A standardized MRI stroke protocol: comparison with CT in hyperacute intracerebral hemorrhage. Stroke 1999;30:765–768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


