Abstract
Objective:
Mutations in the gene encoding phospholipase A2 group VI (PLA2G6) are associated with two childhood neurologic disorders: infantile neuroaxonal dystrophy (INAD) and idiopathic neurodegeneration with brain iron accumulation (NBIA). INAD is a severe progressive psychomotor disorder in which axonal spheroids are found in brain, spinal cord, and peripheral nerves. High globus pallidus iron is an inconsistent feature of INAD; however, it is a diagnostic criterion of NBIA, which describes a clinically and genetically heterogeneous group of disorders that share this hallmark feature. We sought to delineate the clinical, radiographic, pathologic, and genetic features of disease resulting from defective phospholipase A2.
Methods:
We identified 56 patients clinically diagnosed with INAD and 23 with idiopathic NBIA and screened their DNA for PLA2G6 mutations.
Results:
Eighty percent of patients with INAD had mutations in PLA2G6, whereas mutations were found in only 20% of those with idiopathic NBIA. All patients with two null mutations had a more severe phenotype. On MRI, nearly all mutation-positive patients had cerebellar atrophy, and half showed brain iron accumulation. We observed Lewy bodies and neurofibrillary tangles in association with PLA2G6 mutations.
Conclusion:
Defects in phospholipase A2 lead to a range of phenotypes. PLA2G6 mutations are associated with nearly all cases of classic infantile neuroaxonal dystrophy but a minority of cases of idiopathic neurodegeneration with brain iron accumulation, and genotype correlates with phenotype. Cerebellar atrophy predicts which patients are likely to be mutation-positive. The neuropathologic changes that are caused by defective phospholipase A2 suggest a shared pathogenesis with both Parkinson and Alzheimer diseases.
GLOSSARY
- INAD
= infantile neuroaxonal dystrophy;
- NBIA
= idiopathic neurodegeneration with brain iron accumulation;
- PKAN
= pantothenate kinase-associated neurodegeneration.
The neuroaxonal dystrophies are degenerative disorders that share the pathologic feature of axonal spheroids in brain. Spheroids are poorly understood axonal swellings that occur in infantile neuroaxonal dystrophy (INAD), pantothenate kinase-associated neurodegeneration (PKAN, formerly Hallervorden-Spatz syndrome), idiopathic neurodegeneration with brain iron accumulation (NBIA), and Schindler disease. INAD is a severe psychomotor disorder with early onset and rapid progression of hypotonia, hyperreflexia, and tetraparesis.1 Spheroids are found in both the central and peripheral nervous systems in INAD, and iron accumulates in brain in a subset of these patients.2,3 The term “neurodegeneration with brain iron accumulation” is used both as a descriptor for the set of neurologic disorders with high basal ganglia iron and as a specific diagnostic category for patients whose molecular basis of disease is unknown.4,5 As causative genes are identified, the stratification of patients with idiopathic NBIA will shift to reflect new understanding of etiology.
Recently, we discovered that some cases of idiopathic NBIA are caused by mutations in the same gene that underlies INAD.6 This gene, PLA2G6, encodes a phospholipase A2 group VI protein that is suspected to play a key role in cell membrane homeostasis.7 Since INAD and some cases of NBIA share a causative gene, we sought to characterize the range of clinical features associated with PLA2G6 mutations in these two populations. In the current study, we genotyped patients with INAD or idiopathic NBIA and reviewed their medical records to assess whether there was a correlation between clinical, radiographic, or pathologic findings and the presence of a PLA2G6 mutation.
METHODS
Subjects.
We recruited subjects through a research listing on GeneTests8 and on the Oregon Health & Science University Web site. Using data collected from physicians by questionnaire and medical records, we identified 56 patients from 53 families with a clinical diagnosis of INAD and 23 patients from 20 families with a clinical diagnosis of idiopathic NBIA. Most patients underwent neuroimaging, but the specific clinical studies varied widely, and images or interpretation information were sometimes limited or unavailable. Clinical criteria1 were used to help classify patients with INAD within the limits of the clinical information available. All the patients classified as INAD had onset before 3 years of age with psychomotor regression and progression of disease. Each patient with INAD had one or more of the following: axonal spheroids on peripheral nerve biopsy, truncal hypotonia, and tetraparesis. Truncal hypotonia was reported in 48 of the patients with INAD (unknown for 8), and 39 of these developed the typical pattern of subsequent tetraparesis. Axonal spheroids were present in most cases (39 of 47, 9 additional patients had not been biopsied). Individuals with iron accumulation in the basal ganglia (most often globus pallidus and substantia nigra), without an eye-of-the-tiger sign on T2-weighted brain MRI, and progressive dystonia and dysarthria were classified as idiopathic NBIA. Patients with idiopathic NBIA were negative for mutations in the PANK2 gene, confirming that none had PKAN. Although some patients classified with INAD had iron accumulation in the globus pallidus, none had dystonia. Referring physicians provided DNA samples and medical information after participants had given written informed consent according to a protocol approved by the institutional review board at Oregon Health & Science University. Only partial clinical data were available on some patients.
Analysis of mutations.
We used primer sets to amplify all PLA2G6 exons and adjacent intronic sequences including splice signals and sequenced DNA in at least one affected individual from each family. Mutations were not routinely confirmed in parental samples of mutation-positive individuals.
Statistical analysis.
We performed Fisher exact tests using an online tool (http://home.clara.net/sisa/fisher.htm) with significance set at p ≤ 0.01.
Neuropathologic studies.
We reviewed the pathology of patients clinically diagnosed with INAD, and we performed a postmortem examination of brain in a case classified in life as idiopathic NBIA (previously reported as case 2 in Ostergaard et al.9) but subsequently found to be due to mutations in PLA2G6. On this specimen, we performed immunohistochemistry studies as previously described using mouse monoclonal antibodies syn303 for detection of α-synuclein, and PHF-1 for detection of hyperphosphorylated tau protein.10
RESULTS
Genetic findings.
Of the 79 patients tested, we found PLA2G6 mutations in 45 individuals from 42 families with INAD (79% of unrelated cases) and in 6 members of 4 families with idiopathic NBIA (20% of unrelated cases). We were unable to identify a PLA2G6 mutation in 11 patients with clinical evidence of INAD, including 5 in whom spheroids were reported on peripheral nerve biopsy. Analysis for gene dosage alterations is currently underway for these cases. All 28 patients with two null mutations or homozygous for any mutation had early onset, rapidly progressive disease. No mutation accounted for >10% of cases in any population. In two patients, we found only a single mutant allele; one patient had INAD and the other idiopathic NBIA. Individual patients and their corresponding mutations are summarized in table 1.
Table 1PLA2G6mutations identified in patients
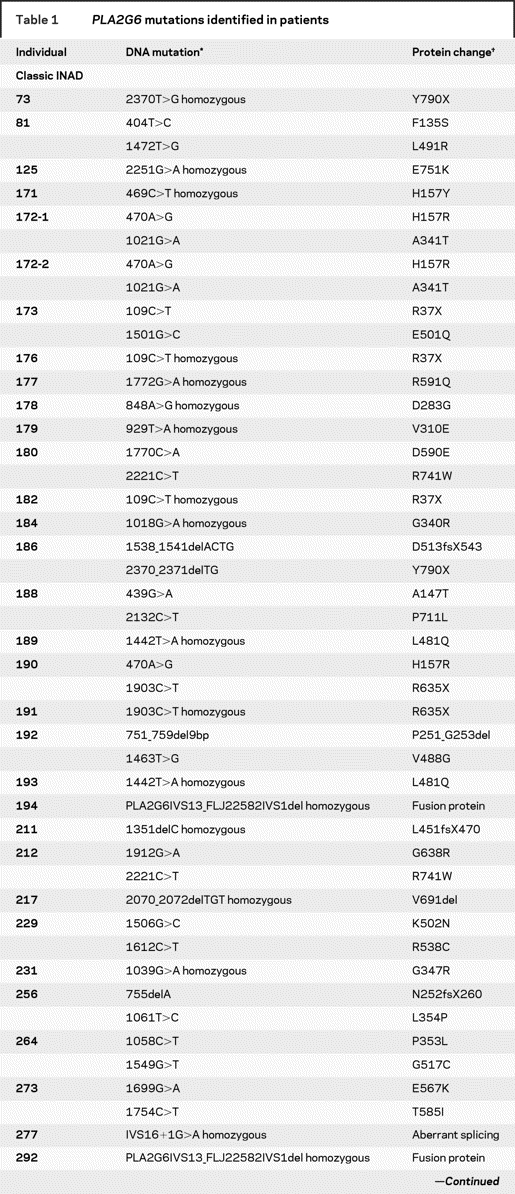
Table 1 Continued
Clinical findings.
In mutation-positive patients classified as having INAD, the average age at onset was 1.1 years (range 5 months to 2.5 years, n = 44) presenting with psychomotor regression characterized by early truncal hypotonia progressing to tetraparesis (95%, n = 39). These data are consistent with previous clinical descriptions of the disorder.1,11 The weakness was usually spastic (70%) but sometimes areflexic (30%). Nearly half of the patients (46%) presented with ataxia or gait instability. Neuro-ophthalmologic abnormalities were common: 79% (n = 25) of patients developed optic atrophy at an average age of 3.7 years, with nystagmus and strabismus developing in 73% and 67% of patients, respectively. Most patients had evidence of denervation on electromyelogram (78%, n = 36) and fast rhythms on EEG (77%, n = 30). Only one-third had decreased nerve conduction velocity (35%, n = 37) with the same fraction manifesting generalized seizures (29%, n = 38; average onset at 7 years, range 3 to 18 years). The average age at death of the 11 patients who had died was 9.4 years (range 5 to 16 years). Clinical features in the 11 mutation-negative INAD patients did not differ significantly from those of patients with mutations (table 2).
Table 2 Frequency of major features in proposed diagnostic categories
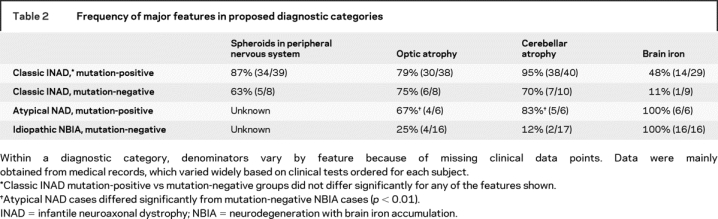
In contrast to those with INAD, the cohort of patients classified as having idiopathic NBIA had widely varying phenotypes, and mutation-positive patients differed significantly from those lacking mutations (table 2). Patients with mutations had an average age at onset of 4.4 years (range 1.5 to 6.5, n = 6), vs 6.8 years (range 1 to 31, n = 15) for mutation-negative patients. Four presented with gait instability or ataxia, three had speech delay, and two were also noted to have diminished social interaction. One maintained a diagnosis of autism until 8 years of age, when his parents noted toe-walking and gait instability. Two-thirds of mutation-positive patients had optic atrophy, but only 25% of those without mutations showed this feature. The frequencies of tetraparesis (spastic or areflexic), nystagmus, and seizures in mutation-positive patients with NBIA did not differ significantly from those in patients with INAD. However, three other common features of INAD were not observed in any mutation-positive patient with NBIA, including truncal hypotonia (p = 0.0001), strabismus (p = 0.008), and fast rhythms on EEG (p = 0.002). Two patients (40%, n = 5) had evidence of denervation on EMG.
The natural history of disease for patients with mutation-positive NBIA included progressive dystonia and dysarthria and neurobehavioral disturbances with impulsivity, distractibility and poor attention span, hyperactivity, and emotional lability.
Radiographic findings.
Radiographic data were collected through use of a questionnaire completed by the referring physicians. Whenever possible, the corresponding author also reviewed images. Imaging approaches varied and some studies were lost, leading to incomplete data and varying denominators for the features described.
Most mutation-positive patients had cerebellar atrophy (95% of INAD cases, n = 40; 83% of NBIA cases, n = 6). It is noteworthy that the two patients with INAD without cerebellar atrophy were studied at less than 2 years of age, perhaps before the development of this radiographic feature. Among mutation-negative patients, we saw cerebellar atrophy in 70% (n = 10) of those diagnosed with INAD but in only 12% (n = 17) of those with idiopathic NBIA. Eleven of 18 patients with mutation-positive INAD had cerebellar hyperintensity on proton density, FLAIR, or T2-weighted MRI, which was not found in any patients with NBIA. The entire cohort of patients with mutations had a multitude of additional MRI abnormalities including thin corpus callosum, mixed white and gray matter atrophy, mega cisterna magna, thin optic chiasma, enlarged ventricles, vermal atrophy, and on T2-weighted imaging, hyperintense putamen and cerebral cortex.
Forty-eight percent of mutation-positive patients with INAD had abnormal iron accumulation in the globus pallidus (14 of 29 patients), and 4 had increased iron in the substantia nigra as well. Among mutation-negative patients, globus pallidus iron was observed in only 1 of 9 patients (11%) with INAD. By definition, all of the cases classified as idiopathic NBIA regardless of mutation status had high iron in the globus pallidus. The pattern of iron accumulation was similar to that seen in other forms of NBIA but did not include globus pallidus central hyperintensity on T2-weighted imaging.
Neuropathologic findings.
In PLA2G6 mutation-positive INAD, peripheral nerve biopsies (skin, conjunctiva, sural nerve, muscle, areola, and rectal mucosa) demonstrated axonal spheroids in 87% of patients (n = 39). While one child who was negative for spheroids was tested only once at 2 years of age, a second individual had a negative biopsy at 12 years despite the finding of a positive skin biopsy in his affected sibling at 11 years of age.1 Five of 8 mutation-negative patients (63%) also had spheroids reported on biopsy. Two patients with NBIA and mutations in PLA2G6 underwent a biopsy for spheroids, only one of which was positive.
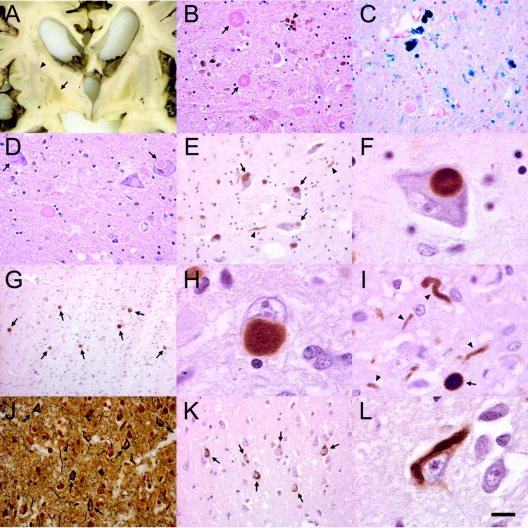
A postmortem examination was performed on one mutation-positive case of NBIA. The patient presented at 3 years of age with toe walking and lower extremity spasticity. She developed optic atrophy and became nonambulatory by 5 years of age with dystonia and dysarthria, progressing to profound sensorimotor impairment by age 9 years. She died at age 23 years. She had two PLA2G6 mutations (238G>A, 2370_2371delTG). On postmortem gross examination the cerebral cortex, basal ganglia, white matter, and cerebellum were severely atrophic, and the globus pallidus and pars reticulata of substantia nigra stained brown (figure 1A). Microscopy revealed widespread loss of neurons and accompanying gliosis in most cerebral cortical areas and in striatum. The cerebellar cortex showed a pronounced cell loss involving both the Purkinje and granular cell layers, with degenerating Purkinje cells (torpedoes).
Figure 1 Pathologic changes from a case of atypical neuroaxonal dystrophy due to a PLA2G6 mutation
(A) Coronal section through basal ganglia. Note brownish discoloration of inner segment of globus pallidus (arrow) contrasting to the more gray putamen (arrowhead). (B) Microscopic examination demonstrated neuronal loss, iron pigment accumulation (star), and large eosinophilic spheroids (arrows) on hematoxylin-eosin (H-E) stain. (C) Perl’s Prussian blue staining revealed extensive iron accumulation (dark blue grains), mostly in large extracellular deposits and in perivascular distribution. (D) Lewy bodies (arrows) were observed in substantia nigra with H-E staining and by immunostaining with syn303 (E, F). α-Synuclein-positive Lewy bodies (G, H), as well as spheroids and dystrophic neurites (I, arrowheads) were abundant in cingulate cortex. (J) Neurofibrillary tangles (arrows) were observed by Bielschowsky silver stain in insular cortex and (K, L) by immunostaining with PHF-1 for hyperphosphorylated tau in temporal cortex. Scale bar: 50 μm.
We found axonal swellings or spheroids throughout the cerebral cortex, striatum, cerebellum, brainstem, and spinal cord (figure 1B), but none in rectal mucosa and skin biopsy. Spheroids appeared as rounded eosinophilic swellings with a diameter of 30–100 μm that were also stained with Bielschowsky silver stain. We saw brown granular iron deposits in a perivascular morula-like pattern within globus pallidus and substantia nigra (figure 1C). Large spheroids were also present in globus pallidus and stained with neurofilament antibodies.
We also observed pathologic features in this case that define Parkinson disease and dementia with Lewy bodies. Most pigmented neurons in the substantia nigra pars compacta contained classic Lewy bodies (figure 1D) that were strongly labeled with α-synuclein antibodies (figure 1, E and F). Many cortical regions, including cingulate, prefrontal, midfrontal, and temporal cortex, contained Lewy bodies that were accompanied by synuclein-positive dystrophic neurites and spheroids (figure 1, G–I). Lewy bodies were present in the dorsal raphe nucleus, thalamus, and locus coeruleus, while synuclein-positive spheroids and dystrophic neurites were abundant in locus coeruleus, caudate, putamen, globus pallidus, dorsal raphe nucleus, and hypothalamic nuclei. Many of the spheroids identified on hematoxylin and eosin staining were not stained with synuclein antibodies.
We also observed neurofibrillary tangles in cortical neurons, a pathologic hallmark of Alzheimer disease. Neurofibrillary tangles were visualized by Bielschowsky silver stain (figure 1J) and by immunohistochemistry with PHF-1 antibody to hyperphosphorylated tau (figure 1, K and L). They were most abundant in entorhinal and temporal cortex but were also present in cingulate, motor, and midfrontal cortex. Tau-positive dystrophic neurites were infrequently observed with PHF-1 staining.
DISCUSSION
In this study, we identified mutations in the phospholipase A2 group VI gene (PLA2G6) in the majority of patients clinically diagnosed with INAD and in a smaller fraction of patients with idiopathic NBIA. This latter condition is thought to encompass several different disorders, accounting for the lower frequency of mutations observed in this group. We found that genotype correlates with phenotype: mutations predicted to lead to absent protein were associated with the INAD profile of early onset and rapid progression, whereas compound heterozygous missense mutations correlated with the less severe phenotype of NBIA, consistent with residual function in the mutant protein (table 1).
The term “infantile neuroaxonal dystrophy” is well established in the literature, as with other disorders historically described based on their clinical and pathologic features. Rather than abandon this terminology in the face of the PLA2G6 gene discovery, we instead propose a functional classification of mutation-positive patients to reflect the shared molecular etiology of their disease. Children presenting with psychomotor regression in the first year of life and loss of ambulation within 5 years should be designated as having “classic INAD.” When disease onset is later and progression slower, the term “atypical neuroaxonal dystrophy” should be used. For patients with brain iron on imaging but without a defined genetic basis for their disease, we propose continuing to use the term “idiopathic NBIA.” As more people are discovered to have mutations in PLA2G6, we anticipate that the phenotypic spectrum of atypical neuroaxonal dystrophy will probably expand but that this classification will remain useful.
Prior to the discovery of the causal gene, a diagnosis of INAD was confirmed by demonstrating axonal spheroids in a peripheral nerve biopsy. In our study, we found that some patients with mutations lacked spheroids and some without mutations had spheroids (table 2), indicating incomplete detection using either pathologic or molecular methods. We were unable to identify a mutation in nearly one-fifth of patients with a strong clinical profile of INAD, including five patients with peripheral spheroids. We presume these patients to have undetected PLA2G6 mutations, indicating an 87% detection rate using current molecular and pathologic methods in combination. With the development of methods to identify large deletions, duplications, and insertions, we predict that the PLA2G6 mutation detection rate will exceed 95% by molecular methods alone, as has been achieved for other rare autosomal recessive disorders. Such assays are being developed for clinical use, and preliminary studies in patients with strong clinical and radiographic features for INAD suggest that up to 25% of alleles negative by sequencing contain large deletions. When INAD is suspected, we recommend that genetic testing precede tissue biopsy, which would be unnecessary when PLA2G6 mutations are identified by DNA analysis. In rare cases, biopsy of peripheral tissue (conjunctiva, rectal mucosa, skin, or sural nerve) may still be useful in the diagnostic evaluation of mutation-negative patients.
The spectrum of clinical features associated with mutations in PLA2G6 is broader than previously described, and genotype correlates with phenotype. We found the clinical profile of classic INAD to be a fairly uniform one of early hypotonia and tetraparesis that progresses rapidly to complete psychomotor retardation. We observed optic atrophy, nystagmus, and strabismus in most patients. We found abnormalities on various electrophysiologic measures, including a decrease in nerve conduction velocity, denervation on EMG, and fast rhythms on EEG. In contrast, we found atypical neuroaxonal dystrophy to be clinically heterogeneous, with patients showing later onset disease and slower progression, with variable ataxia, spasticity, and neurobehavioral abnormalities.
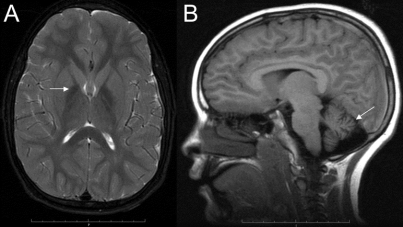
The imaging findings in classic infantile and atypical neuroaxonal dystrophy will guide diagnostic molecular testing. We observed cerebellar atrophy in nearly all PLA2G6 mutation-positive patients, and globus pallidus iron accumulation in half, evident as low signal intensity on T2-weighted MRI (figure 2).2 Since cerebellar atrophy is not typically found in other disorders associated with high brain iron, this combination of features is strongly predictive of a PLA2G6 mutation.12 Thus, if neuroimaging shows cerebellar atrophy with or without evidence of iron accumulation in a patient with psychomotor regression, molecular testing for a PLA2G6 mutation is indicated.
Figure 2 Pattern on brain MRI
(A) High globus pallidus iron (arrow) on T2-weighted imaging in a 9-year-old patient with atypical neuroaxonal dystrophy and PLA2G6 mutations. (B) Cerebellar atrophy (arrow) is evident in a case of classic infantile neuroaxonal dystrophy.
The pathology associated with mutations in PLA2G6 suggests a shared pathogenesis with both Parkinson disease and Alzheimer disease. In this study, we saw not only the expected peripheral and central spheroids, but also α-synuclein-positive Lewy bodies, dystrophic neurites, and neurofibrillary tangles in the brain tissue of a 23-year-old patient with atypical neuroaxonal dystrophy. Interestingly, although synuclein-positive spheroids have been observed in classic early onset cases with death before age 10,13 Lewy bodies and neurofibrillary tangles have not been reported in association with this phenotype. This suggests that in order to form these structures either exposure to the neuropathologic process must be prolonged, or the brain must reach a certain stage of maturation. Our patient is among the youngest with any disease in whom Lewy bodies and neurofibrillary tangles have been described.
The pathologic and imaging features shared by disorders of distinct molecular etiology may lend further insights into pathogenesis. The similar appearance of spheroids and iron in classic and atypical neuroaxonal dystrophy and in PKAN, another inherited neurodegenerative disorder with brain iron accumulation, suggests that defects in the respective mutant proteins lead to a common mechanism of iron dyshomeostasis in basal ganglia. The PLA2G6 protein is critical for cellular membrane phospholipid homeostasis and remodeling,7 and defects in this protein may cause accumulation of membranes, organelles, and protein in distal axons that represent spheroids. The protein that is defective in PKAN normally regulates the biosynthesis of coenzyme A, which is critical to fatty acid metabolism. Both proteins associate with mitochondria and play a role in lipid metabolism, which when perturbed may alter the regulation of iron transport and utilization. By identifying genes that underlie neurodegenerative disorders with high brain iron, we have improved diagnostic testing and gained insights into pathogenesis. This new knowledge may guide the development of therapeutic strategies not only for these rare disorders but also for pathogenically related more common illnesses such as Parkinson disease and Alzheimer disease.
NOTE ADDED IN PROOF
Mutations have now been found in adult-onset parkinsonism-dystonia syndrome (Paisan-Ruiz C, Bhatia KP, Li A, et al. Characterization of PLA2G6 as a locus for dystonia-parkinsonism. Ann Neurol Epub ahead of print 2008 Jun 20).
AUTHORS’ AFFILIATIONS
From the Departments of Molecular and Medical Genetics, Pediatrics and Neurology (A.G., S.K.W., P.H., S.S., T.M.N., S.J.H.), School of Medicine, Oregon Health and Science University, Portland; the Department of Pathology (I.E.H.), Aalborg Hospital, Aarhus University Hospital, Aalborg, Denmark; the Department of Neurology and Hope Center for Neurological Disorders (P.T.K., I.M.), Washington University School of Medicine, St. Louis, MO; the Department of Pediatrics (J.C.C.), School of Medicine, University of Washington, Seattle; the Department of Child Neurology (N.N., G.Z.), Istituto Nazionale Neurologico “Carlo Besta,” Milan, Italy; Neuropediatric Service (D.R.), Assistance Publique-Hôpitaux de Paris, Hôpital Armand Trousseau and Université Pierre et Marie Curie, Paris, France; the Department of Pediatric Neurology (I.D.), Hôpital Necker Enfants Malades, Paris, France; the Unit of Molecular Medicine (E.B.), Bambino Gesù Children’s Research Hospital, Rome, Italy; the Department of Neurological and Visual Sciences (A.S.), University of Verona School of Medicine, Italy; the Departments of Medicine and Pediatrics (B.L., J.G.), University of California, San Francisco; and Instituto de Genetica Medica (C.D.) and Neuropediatria (C.B., I.C., M.S.), Hospital Maria Pia, Porto, Portugal.
ACKNOWLEDGMENT
The authors thank the patients and their families, and clinical collaborators (Drs. C. Schanen, M. Korson, J. Terryde, A. Hoyland, S. Torp, A. Mansoor, S. Devine, B. Melaiki, V. Puri, R. Torkelson, J. Hahn, W. Dobyns, M. Filocamo, H. Mandel, M. Vanasse, J. Michaud, J. Fan, I. Baric, I. Karacic, M. Tennison, M. Wilson, K. Nørgaard Hansen, S. Norberg, M. Wiznitzer, A. Feigenbaum, M. de Jong, M. Hausler, F. Collins, A. Malandrini, M. Salih, J.A. Urtizberea, C. Schrander-Stumpel, J.W.D. de Jong, C. Haenggli, A. Bottani, B. Maria, M. Reyes, F. Hisama, B. Russman, A. Macaya, S. Bohlega, W. Ondo, C. Dean, E. Spence, C. Wilson and genetic counselors J. Hendrick, P. Devers, A. Salvino, B. Todd, I. Gosselin, S. Schelley, T. Berry, and R. Anderson).
Address correspondence and reprint requests to Dr. Susan J. Hayflick, Department of Molecular and Medical Genetics, Oregon Health & Science University, L103a, 3181 SW Sam Jackson Park Rd., Portland, OR 97239-3098 hayflick@ohsu.edu
e-Pub ahead of print on September 17, 2008, at www.neurology.org.
Authors’ affiliations are listed at the end of the article.
Supported by grants from the National Institute of Child Health and Human Development, the National Eye Institute, L’Association Internationale De Dystrophie Neuro Axonale Infantile, and the NBIA Disorders Association. Additional support was provided by the Paolo Zorzi Foundation and the Italian National Ministry of Health. Samples were obtained from the “Cell line and DNA bank from patients affected with genetic diseases” collection at the Giannina Gaslini Institute (http://www.gaslini.org/labdppm.htm) supported by Italian Telethon grants (project #GTF04002) and the Coriell Cell Repositories. This publication was made possible with support from the Oregon Clinical and Translational Research Institute (OCTRI), grant number UL1 RR024140 01 from the National Center for Research Resources (NCRR), a component of the NIH, and NIH Roadmap for Medical Research.
Disclosure: The authors report no disclosures.
Received November 27, 2007. Accepted in final form May 29, 2008.
REFERENCES
- 1.Nardocci N, Zorzi G, Farina L, et al. Infantile neuroaxonal dystrophy: clinical spectrum and diagnostic criteria. Neurology 1999;52:1472–1478. [DOI] [PubMed] [Google Scholar]
- 2.Farina L, Nardocci N, Bruzzone MG, et al. Infantile neuroaxonal dystrophy: neuroradiological studies in 11 patients. Neuroradiol 1999;41:376–380. [DOI] [PubMed] [Google Scholar]
- 3.Simonati A, Trevisan C, Salviati A, Rizzuto N. Neuroaxonal dystrophy with dystonia and pallidal involvement. Neuropediatrics 1999;30:151–154. [DOI] [PubMed] [Google Scholar]
- 4.Hayflick SJ. Pantothenate kinase-associated neurodegeneration (formerly Hallervorden-Spatz syndrome). J Neurol Sci 2003;207:106–107. [DOI] [PubMed] [Google Scholar]
- 5.Hayflick SJ, Westaway SK, Levinson B, et al. Genetic, clinical, and radiographic delineation of Hallervorden-Spatz syndrome. N Engl J Med 2003;348:33–40. [DOI] [PubMed] [Google Scholar]
- 6.Morgan NV, Westaway SK, Morton JE, et al. PLA2G6, encoding a phospholipase A(2), is mutated in neurodegenerative disorders with high brain iron. Nat Genet 2006;38:752–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baburina I, Jackowski S. Cellular responses to excess phospholipid. J Biol Chem 1999;274:9400–9408. [DOI] [PubMed] [Google Scholar]
- 8.GeneTests. Medical Genetics Information Resource (database online); Seattle: University of Washington; 1993–2007.
- 9.Ostergaard JR, Christensen T, Hansen KN. In vivo diagnosis of Hallervorden-Spatz disease. Dev Med Child Neurol 1995;37:827–833. [DOI] [PubMed] [Google Scholar]
- 10.Kotzbauer PT, Giasson BI, Kravitz AV, et al. Fibrillization of alpha-synuclein and tau in familial Parkinson’s disease caused by the A53T alpha-synuclein mutation. Exp Neurol 2004;187:279–288. [DOI] [PubMed] [Google Scholar]
- 11.Aicardi J, Castelein P. Infantile neuroaxonal dystrophy. Brain 1979;102:727–748. [DOI] [PubMed] [Google Scholar]
- 12.Hayflick SJ, Hartman M, Coryell J, et al. Brain MRI in neurodegeneration with brain iron accumulation with and without PANK2 mutations. AJNR Am J Neuroradiol 2006;27:1230–1233. [PMC free article] [PubMed] [Google Scholar]
- 13.Newell KL, Boyer P, Gomez-Tortosa E, et al. Alpha-synuclein immunoreactivity is present in axonal swellings in neuroaxonal dystrophy and acute traumatic brain injury. J Neuropathol Exp Neurol 1999;58:1263–1268. [DOI] [PubMed] [Google Scholar]