Abstract
Objective:
To investigate longitudinal change in the medical decision-making capacity (MDC) of patients with amnestic mild cognitive impairment (MCI) under different consent standards.
Methods:
Eighty-eight healthy older controls and 116 patients with MCI were administered the Capacity to Consent to Treatment Instrument at baseline and at 1 to 3 (mean = 1.7) annual follow-up visits thereafter. Covariate-adjusted random coefficient regressions were used to examine differences in MDC trajectories across MCI and control participants, as well as to investigate the impact of conversion to Alzheimer disease on MCI patients’ MDC trajectories.
Results:
At baseline, MCI patients performed significantly below controls only on the three clinically relevant standards of appreciation, reasoning, and understanding. Compared with controls, MCI patients experienced significant declines over time on understanding but not on any other consent standard. Conversion affected both the elevation (a decrease in performance) and slope (acceleration in subsequent rate of decline) of MCI patients’ MDC trajectories on understanding. A trend emerged for conversion to be associated with a performance decrease on reasoning in the MCI group.
Conclusions:
Medical decision-making capacity (MDC) decline in mild cognitive impairment (MCI) is a relatively slow but detectable process. Over a 3-year period, patients with amnestic MCI show progressive decline in the ability to understand consent information. This decline accelerates after conversion to Alzheimer disease (AD), reflecting increasing vulnerability to decisional impairment. Clinicians and researchers working with MCI patients should give particular attention to the informed consent process when conversion to AD is suspected or confirmed.
GLOSSARY
- AD
= Alzheimer disease;
- ADRC
= Alzheimer’s Disease Research Center;
- CCTI
= Capacity to Consent to Treatment Instrument;
- CDR
= Clinical Dementia Rating scale;
- DRS-2
= Dementia Rating Scale, second edition;
- GDS
= Geriatric Depression Scale;
- MCI
= mild cognitive impairment;
- MDC
= medical decision-making capacity;
- MMSE
= Mini-Mental State Examination;
- UAB
= University of Alabama at Birmingham.
Functional change is critical to understanding both progression in mild cognitive impairment (MCI) and conversion to Alzheimer disease (AD) and other dementias.1–3 Although MCI criteria continue to be refined,4,5 the diagnostic tenet that MCI patients evidence largely intact functional abilities continues to be central. This reflects the clinical view that frank restriction in functional activities is a hallmark of AD and other dementias.6,7 However, an increasing number of cross-sectional studies have shown that persons with MCI experience limitations in functional abilities relative to cognitively healthy older adults.1–3,8 The longitudinal course of such functional restrictions has received less attention.9–12
Medical decision-making capacity (MDC) (also referred to as treatment consent capacity or consent capacity) is a higher-order functional capacity. It refers to an individual’s cognitive and emotional capacity to accept a medical treatment, refuse treatment, or select among treatment alternatives.13 Declining consent capacity in MCI patients poses important clinical, legal, and ethical challenges,14 because these patients increasingly are treated pharmacologically and are involved in clinical research trials.15,16 Thus, longitudinal investigation of MDC among patients with MCI has scientific, clinical, and public policy value.
Recent studies suggest that measurable decline in MDC occurs over time in MCI patients. In a cross-sectional study,8 we found that patients with MCI exhibited significant impairments on complex and clinically relevant consent standards of appreciating the consequences of a treatment choice, providing reasoning for a treatment choice, and understanding the treatment situation and choices. In addition, in a 2-year longitudinal study,17 we found that mild AD patients demonstrated significant decline on these three complex standards.
In the present study, we investigated MDC over a 3-year period in a sample of older controls and patients with MCI. We hypothesized that (1) relative to controls, MCI patients would demonstrate declines on the three complex consent standards but not on simpler consent standards; and (2) that conversion from MCI to AD would be associated with a decrease in scores, and an acceleration in subsequent rate of decline, on the three complex standards.
METHODS
Participants.
The present analyses involved the 204 participants—88 controls and 116 MCI patients—who had been diagnostically characterized through the University of Alabama at Birmingham (UAB) Alzheimer’s Disease Research Center (ADRC) consensus conference as of March 2008 as part of an ongoing longitudinal study of functional change in MCI. The ADRC diagnostic team consisted of neurologists, neuropsychologists, and nursing staff.
Control participants were cognitively healthy older adults who underwent neurologic, neuropsychological, and neuroradiologic evaluations to ensure absence of medical and psychiatric conditions that could compromise cognition. Their Mini-Mental State Examination (MMSE)18 scores ranged from 25 to 30, and their Dementia Rating Scale, second edition (DRS-2),19 total scores ranged from 124 to 144.
MCI participants were either patients who presented for clinical evaluation through the UAB Memory Disorders Clinic or community volunteers recruited into the ADRC. Diagnosis of MCI was made using original Petersen/Mayo criteria20 (appendix e-1 on the Neurology® Web site at www.neurology.org). MCI participants in the present study were all of the amnestic subtype. Their MMSE scores ranged from 22 to 30, and their DRS-2 total scores ranged from 113 to 142.
Informed consent was obtained from all control and MCI participants as part of this institutional review board–approved research.
Study design and longitudinal assessment of participants.
The 204 participants described above were recruited in four consecutive annual cohorts (cohort 1: July 2004 to June 2005; cohort 2: July 2005 to June 2006; cohort 3: July 2006 to June 2007; and cohort 4 [still being enrolled]: July 2007 to June 2008). Therefore, members of cohort 1 have completed baseline assessment and 3 subsequent annual follow-up assessments, members of cohort 2 have completed baseline assessment and two subsequent annual follow-up assessments, members of cohort 3 have completed baseline assessment and one subsequent annual follow-up assessment, and members of cohort 4 have only completed baseline assessment. In addition, members of any one cohort were not enrolled simultaneously but rather over the course of the cohort year. Thus, depending on when “data freeze” occurred before analyses, persons belonging to the same annual cohort may not have the same number of annual follow-up assessments. Although such a recruitment approach results in unbalanced data, modern statistical approaches have been developed for analyzing such data and reducing, to the extent possible, any potential bias due to the imbalance (see Data analyses section).
Of the 204 participants who completed a baseline evaluation, 133 (71 controls, 62 MCI) subsequently had a first annual follow-up evaluation, 79 (37 controls, 42 MCI) subsequently had a second annual follow-up evaluation, and 23 (12 controls, 11 MCI) subsequently had a third annual follow-up evaluation. The variability in sample size across time is not due to attrition but rather reflects the fact that participants were recruited in annual cohorts and on a rolling basis within each cohort, as described above.
Participants’ diagnostic status across time.
Of the 116 patients with MCI, 22 (19%) progressed to AD during the period they were under observation. Of the 88 cognitively healthy control participants, 9 (10.2%) received a follow-up diagnosis of MCI during the period they were under observation.
Consent capacity measure.
Consent capacity was assessed with the Capacity to Consent to Treatment Instrument (CCTI),14 a reliable and valid instrument for assessing MDC in older adults.14,21 The CCTI consists of two clinical vignettes that each present a hypothetical medical problem (A: neoplasm, B: cardiovascular disease) and symptoms, and two treatment alternatives with associated risks and benefits. The vignettes were presented to patients in both oral and written formats, and the patients then answered questions designed to test consent capacity under each of four core consent standards derived from legal and medical literature22,23:
S1: expressing a treatment choice (expressing choice);
S3: appreciating the consequences of a treatment choice (appreciation);
S4: providing rational reasons for a treatment choice (reasoning); and
S5: understanding the treatment situation, treatment choices, and respective risks/benefits (understanding).
In addition, we tested a fifth standard described as making the “reasonable” treatment choice [S2]. [S2] is not a clinically accepted consent standard because of concerns about the arbitrariness of the operative term reasonable.22 However, it is a useful standard in research because it contributes to our understanding of the treatment preferences of patients with neurocognitive disorders. Therefore, we treat [S2] (reasonable choice) as experimental and use brackets to distinguish it from the four core consent standards.
Data analyses.
Group differences on baseline demographic, clinical, and CCTI variables were examined using one-way analysis of variance or χ2/Fisher exact tests.
To examine differences in trajectories of change in MDC across MCI and control participants on the four interval-level standards (S1, S3, S4, and S5), we fitted separate random coefficient regressions that modeled changes in performance on these standards across the four time points under consideration.24,25 Random coefficient regression, also called individual growth curve modeling, provides a robust and very flexible approach to analyzing longitudinal data. It easily accommodates variability in the number and spacing of assessment points across study participants.25–27 In addition, because random coefficient models assume that each individual’s observed scores are a random sample of data from their underlying true growth curve,25,27 they facilitate analyses that include all participants, even those with only one wave of data.25 The random regression models we fitted included terms for group, time, and the group × time interaction (appendix e-2). Group differences in trajectories of change on [S2] were tested using generalized estimating equations. For all trajectories of change analyses, the interaction was the primary term of interest because it would indicate whether rates of change in MDC across time differ between MCI and control participants.
To test the effect of conversion to dementia on MDC trajectory, we fitted another set of random coefficient regressions in which group membership was treated as a time-varying variable, to capture the fact that conversion entails a change in a person’s diagnostic status across time. These set of analyses included terms for conversion (a time-varying variable indicating diagnostic status at each assessment point), time, and the conversion × time interaction. Baseline diagnosis was entered as a covariate. The terms of interest in these analyses were conversion and the conversion × time interaction. A significant conversion term would indicate that conversion affects the elevation of the trajectory (e.g., it results in a loss of points on the standard under investigation), whereas a significant conversion × time term would indicate that conversion affects the slope of the trajectory (e.g., it accelerates subsequent rate of decline on the standard being tested). Because it is a contradiction in terms for conversion to occur at the baseline assessment, the time variable was recentered at the first annual follow-up, the earliest time point at which conversion may occur, to preserve the meaningfulness of the models’ coefficients.25
All analyses were performed using SAS 9.1 (SAS Institute Inc., Cary, NC). Only findings with a two-tailed p value ≤ 0.05 were considered significant.
RESULTS
Baseline demographic, clinical, and CCTI variables.
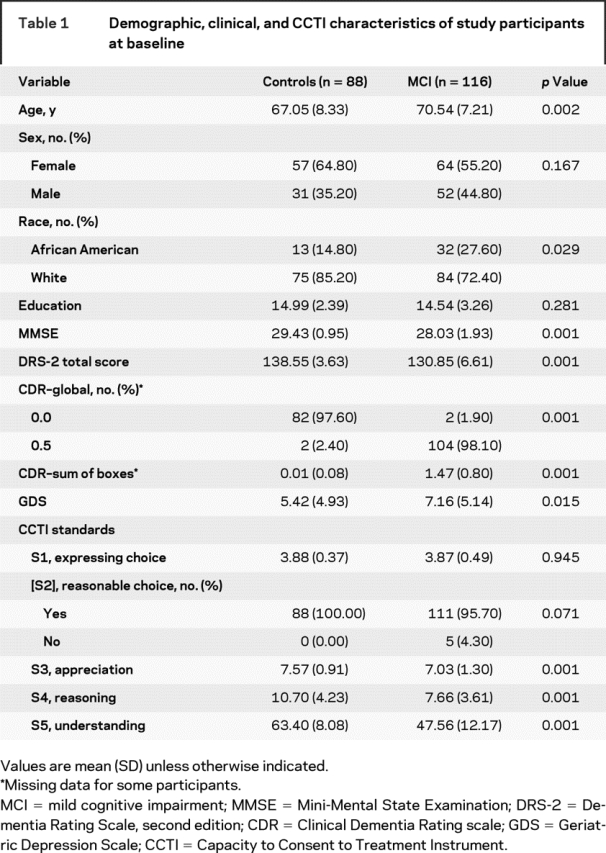
Table 1 shows the result of group comparisons on baseline demographic, clinical, and CCTI variables. As expected, MCI patients differed significantly from controls on all measures of global mental status and dementia staging. MCI patients also were older, reported significantly more depressive symptoms, and were composed of proportionately more African Americans than controls. On the CCTI, MCI patients performed worse than control participants only on appreciation (S3), reasoning (S4), and understanding (S5), which are the more complex and clinically relevant consent standards.21
Table 1 Demographic, clinical, and CCTI characteristics of study participants at baseline
Group differences in trajectories of change in MDC.
Consistent with these unadjusted group differences in CCTI performance at baseline, the random coefficient regressions revealed that MCI patients evidenced significantly worse consent abilities at baseline relative to control participants on appreciation (S3), reasoning (S4), and understanding (S5) after controlling for group differences in age, depressive symptoms, and race. This is shown by the effect for control in table 2.
Table 2 Effect of baseline diagnostic status on trajectories of change in MDC
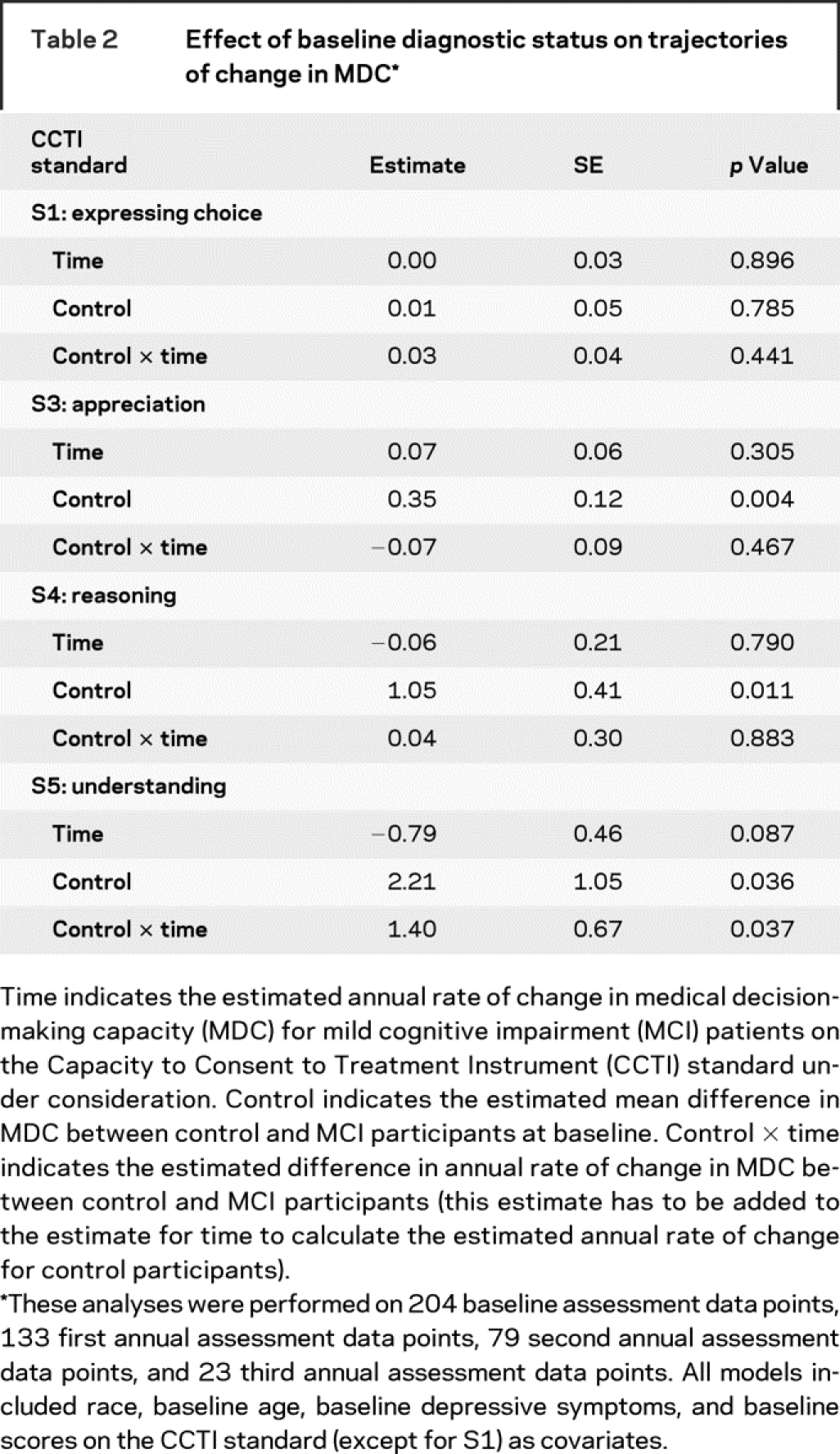
After adjusting for group differences in race, baseline age, depressive symptoms, and CCTI scores, MCI patients had a significantly greater rate of decline in MDC only on understanding (S5). On average, there was a 0.79-point annual decline in MCI patients’ understanding abilities, as shown by the estimate for time in table 2. In contrast, control participants experienced a 0.61-point annual increase in understanding, as shown by the sum of the estimates for time and control × time in table 2. Figure 1 illustrates the relationship between diagnostic group and change in understanding over the four assessment points.
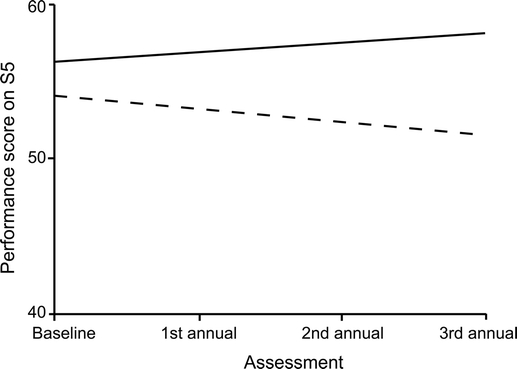
Figure 1 Covariate-adjusted performance scores on S5, by diagnostic group, across assessment occasion
This analysis was performed on 204 baseline assessment data points, 133 first annual assessment data points, 79 second annual assessment data points, and 23 third annual assessment data points. Control (solid line) and MCI (dashed line).
The generalized estimating equations performed to assess rates of change on [S2] did not converge to an admissible solution because, at all four assessment points, all control participants successfully performed the task assessed under this standard, resulting in cells with zero counts. Consequently, we reanalyzed the data by using the Fisher exact test to assess group differences in the proportion of individuals who could not perform the [S2] task (appendix e-3).
Effect of conversion to dementia on MDC trajectory.
After adjusting for baseline diagnosis, we found that conversion at the first annual follow-up significantly affected the elevation of an individual’s growth curve on understanding (S5) but not on any other CCTI standard, as shown by the conversion term in table 3. For example, an MCI patient who converted to AD at the first annual follow-up experienced, at the same time, a loss of 3.07 points on understanding. There was a nonsignificant trend for conversion at the first annual follow-up to be associated with a loss of 1.06 points on the reasoning standard.
Table 3 Effect of conversion on trajectories of change in MDC
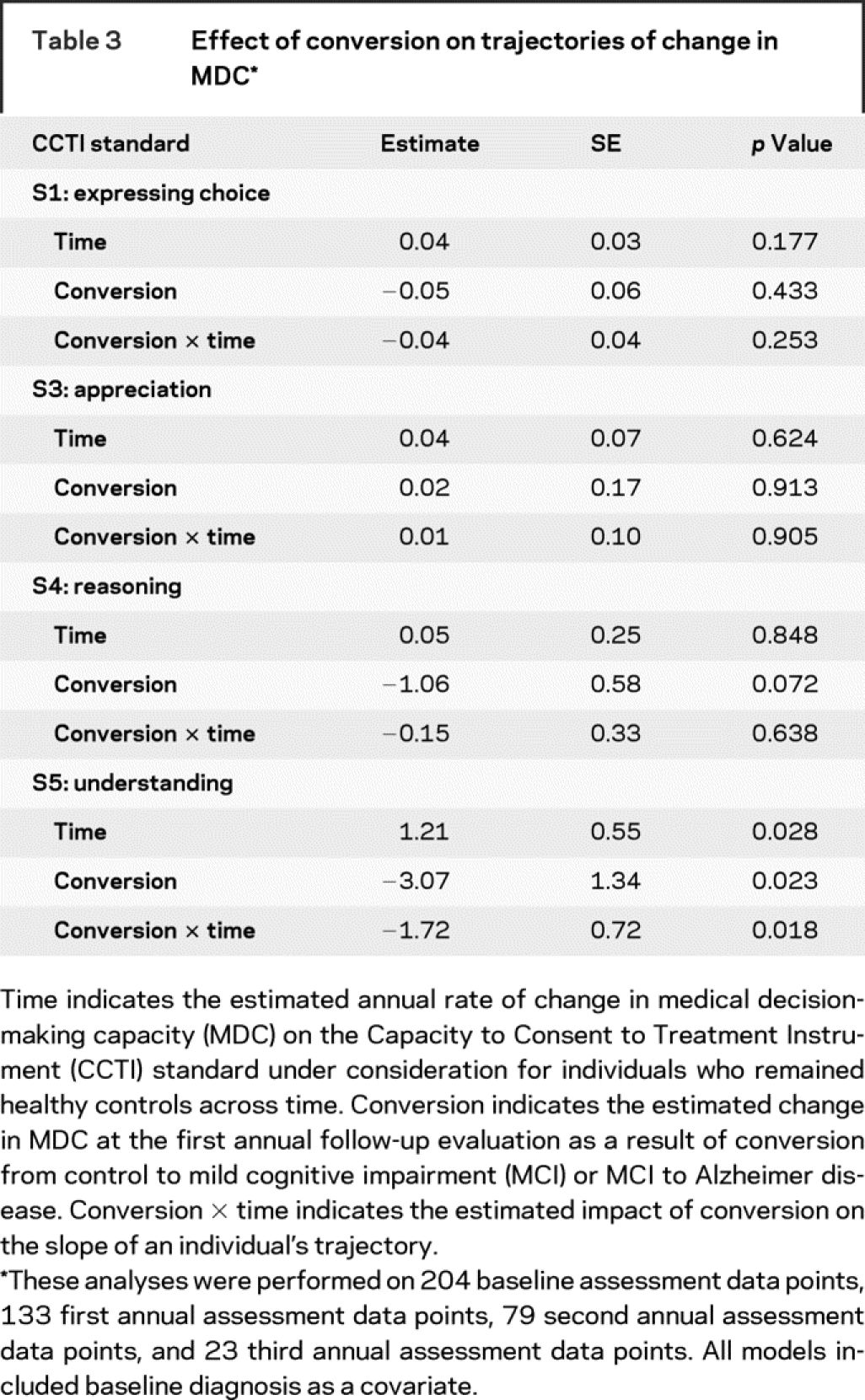
Furthermore, after conversion to dementia, there was an acceleration in an individual’s subsequent rate of decline in understanding, as shown by the conversion × time term in table 3. To illustrate, nonconverting MCI patients’ performance on understanding decreased an average of 0.51 points (1.21–1.72) per year. In contrast, for the MCI patient who converted to AD at the first annual follow-up, her subsequent (i.e., postconversion) rate of decline in understanding was 2.23 points [1.21 − (2 × 1.72)] per year. This impact of conversion to AD, at the first annual follow-up, on the elevation and slope of a converting MCI patient’s understanding growth curve is depicted in figure 2.
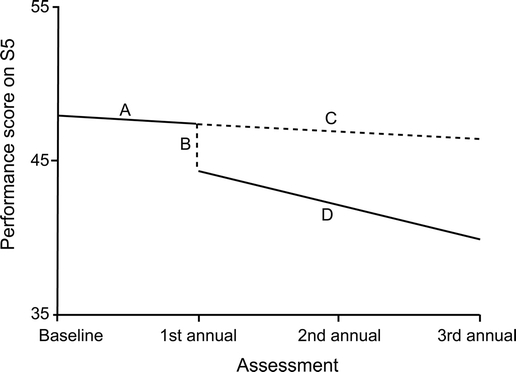
Figure 2 Effect of conversion, from MCI to AD at the first annual follow-up evaluation, on S5 performance in a prototypical MCI patient
A represents a prototypical mild cognitive impairment (MCI) patient’s slope before conversion. B represents the decrease in understanding that occurs for this patient after conversion to Alzheimer disease (AD) at the first annual follow-up evaluation. C represents what this patient’s slope would have been had she not converted to AD. Note that this slope has the same inclination as her preconversion slope. D represents this patient’s slope after she converted to AD. Note that this slope is considerably steeper than her preconversion slope. This analysis was performed on 204 baseline assessment data points, 133 first annual assessment data points, 79 second annual assessment data points, and 23 third annual assessment data points.
DISCUSSION
A high proportion of MCI patients eventually progress to some form of dementia.4,28 Because functional impairment is a defining feature of dementia,6 individuals with MCI are accordingly at increased risk for progressive functional decline. In this study, we longitudinally investigated functional change in MCI by focusing on MDC, a critically important functional domain in the dementia spectrum.14 We found that, compared with cognitively healthy older adults, patients with MCI had significantly poorer MDC at baseline on the complex and clinically relevant consent standards of appreciation (S3), reasoning (S4), and understanding (S5). These findings persisted after adjusting for group differences in age, depressive symptoms, and race. Patients with MCI did not differ from controls on the simple consent standards of expressing choice (S1) and reasonable choice [S2]. These findings replicate an earlier cross-sectional examination of MDC among patients with MCI by our research group.8 The data analyzed in that investigation8 are a subset of those analyzed in this article.
We hypothesized that, compared with controls, patients with MCI would exhibit longitudinal declines on the complex standards of appreciation (S3), reasoning (S4), and understanding (S5). However, over the 3-year study period, decline emerged only on understanding. It is not surprising that understanding was the standard showing decline. As assessed using the CCTI vignette method, this standard requires comprehensive factual knowledge and understanding of the treatment situation and choices, and, accordingly, relies heavily on intact short-term verbal memory.8,21,29 The reliance on short-term memory makes it a uniquely challenging standard for persons with AD and related dementias.8,14,17,21 The selective decline in understanding is, therefore, consistent with the cardinal amnestic deficits of patients with MCI.4,29,30 This interpretation is supported by longitudinal studies of cognitive change in MCI that have demonstrated greater rates of decline in episodic and semantic memory relative to other cognitive abilities.27,31
These findings provide insights into how MDC declines in MCI. Essentially, MDC decline seems to be a slow but detectable process, with cumulative change building over time. At baseline, the control and MCI groups already differed on the three clinically relevant standards (S3, S4, and S5), indicating that group separation (i.e., functional change) was under way before study onset. However, significant decline over the 3-year period emerged on only the most complex and memory-intensive consent standard: understanding. It is likely that a longer observational period is needed to detect declines in reasoning, appreciation, and the simpler consent abilities.
The study also examined the impact of conversion from MCI to dementia on the longitudinal trajectory of MDC. We found that when a prototypical MCI patient converted to AD at her first annual follow-up assessment, she experienced an associated loss of 3.07 points on the understanding standard. In addition to this associated decrease in performance, she also experienced acceleration in the rate of postconversion decline in understanding. In contrast to her preconversion 0.51-point annual decrement in understanding, she now experienced an average 2.23-point annual decrement in understanding after conversion. This suggests that MCI patients become increasingly vulnerable to decisional impairment after conversion to AD. From clinical and ethical standpoints, therefore, clinicians and researchers working with the MCI population should pay particular attention to the consent process when conversion to AD is suspected or established.32,33 Reduction of information load, repetition of materials, involvement of family members, and other procedures for optimizing the consent provided by persons with cognitive impairment have been detailed elsewhere.8,34–36
The present study contributes to understanding the natural history of functional change in MCI. Prior studies9–12 have examined longitudinal changes in functional abilities in MCI using generic self-report measures of functional ability such as the Lawton and Brody Instrumental Activities of Daily Living Scale. The convergent evidence from these studies indicates that persons with MCI experience increasing levels of difficulty in the performance of daily activities over time, that restriction in complex activities of daily living precedes limitations in more basic functional abilities, and that MCI patients who demonstrate functional difficulties at baseline have a higher risk of progressing to dementia over time.9–12 We built on these studies by using a performance-based instrument that decomposes a specific functional domain—MDC—into component abilities. We found that the consent ability of understanding declined over 3 years, whereas other consent abilities remained more stable. This approach reflects current attempts to delineate specific functional abilities that are susceptible to decline in MCI5,37 using structured assessment protocols.30,38
The present study also demonstrated that conversion impacts the elevation and slope of functional trajectories in MCI, a phenomenon not previously identified. Reassessing an MCI patient’s higher-order functional abilities at the time of AD diagnosis may be prudent to ensure that the patient’s autonomy is adequately safeguarded.39 In a similar vein, the ability to prevent, decelerate, or reverse (higher-order) functional restrictions among persons with MCI might be considered a target outcome for pharmacologic trials and other intervention studies.
A limitation of the present study is the relatively lower proportion of participants who had undergone second and third annual follow-up assessments. Although random coefficient regression is quite robust to imbalance,25–27 balance facilitates the precision with which model parameters are estimated. Thus, it will be useful to revisit the analyses reported here at a time when more data are available across more time points. Secondly, because our study only included amnestic MCI patients, we do not know whether the findings generalize to other MCI subtypes. There is some evidence to suggest that trajectories of functional change may vary by MCI subtype.10 Finally, we acknowledge that the treatment consent abilities exhibited by individuals facing real, personal medical problems might differ somewhat from those used in relation to research involving hypothetical vignettes. Nonetheless, vignette-based MDC instruments are widely used in dementia research13,14,33 and have demonstrable face, content, and construct validities.40
AUTHOR CONTRIBUTIONS
Statistical analyses were conducted by O.C.O.
ACKNOWLEDGMENT
The authors thank the staff of the Neuropsychology Laboratory in the Department of Neurology for their assistance with data collection.
Supplementary Material
Address correspondence and reprint requests to Dr. Daniel C. Marson, Department of Neurology, SC 650K, University of Alabama at Birmingham, Birmingham, AL 35294-0017 dmarson@uab.edu
Supplemental data at www.neurology.org
Supported by grants 1R01 AG021927 (to D.C.M., Principal Investigator) and 1P50 AG16582 (Alzheimer’s Disease Research Center) (to D.C.M., Principal Investigator) from the National Institute on Aging.
Disclosure: The capacity outcome measure used in the study is owned by the University of Alabama at Birmingham Research Foundation (UABRF). D.C.M. and L.E.H. receive royalty income through UABRF. The other authors report no disclosures.
Received May 12, 2008. Accepted in final form July 22, 2008.
REFERENCES
- 1.Griffith HR, Belue K, Sicola A, et al. Impaired financial abilities in mild cognitive impairment. Neurology 2003;60:449–457. [DOI] [PubMed] [Google Scholar]
- 2.Tuokko H, Morris C, Ebert P. Mild cognitive impairment and everyday functioning in older adults. Neurocase 2005;11:40–47. [DOI] [PubMed] [Google Scholar]
- 3.Missotten P, Squelard G, Ylieff M, et al. Quality of life in older Belgian people: comparison between people with dementia, mild cognitive impairment, and controls. Int J Geriatr Psychiatry Epub 2008 Jan 22. [DOI] [PubMed]
- 4.Petersen RC, Negash S. Mild cognitive impairment: an overview. CNS Spectrums 2008;13:45–53. [DOI] [PubMed] [Google Scholar]
- 5.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004;256:240–246. [DOI] [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., text revision. Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
- 7.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–194. [DOI] [PubMed] [Google Scholar]
- 8.Okonkwo OC, Griffith HR, Belue K, et al. Medical decision-making capacity in patients with mild cognitive impairment. Neurology 2007;69:1528–1535. [DOI] [PubMed] [Google Scholar]
- 9.Artero S, Touchon J, Ritchie K. Disability and mild cognitive impairment: a longitudinal population-based study. Int J Geriatr Psychiatry 2001;16:1092–1097. [DOI] [PubMed] [Google Scholar]
- 10.Wadley VG, Crowe M, Marsiske M, et al. Changes in everyday function in individuals with psychometrically defined mild cognitive impairment in the Advanced Cognitive Training for Independent and Vital Elderly Study. J Am Geriatr Soc 2007;55:1192–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peres K, Chrysostome V, Fabrigoule C, Orgogozo JM, Dartigues JF, Barberger-Gateau P. Restriction in complex activities of daily living in MCI: impact on outcome. Neurology 2006;67:461–466. [DOI] [PubMed] [Google Scholar]
- 12.Purser JL, Fillenbaum GG, Pieper CF, Wallace RB. Mild cognitive impairment and 10-year trajectories of disability in the Iowa Established Populations for Epidemiologic Studies of the Elderly cohort. J Am Geriatr Soc 2005;53:1966–1972. [DOI] [PubMed] [Google Scholar]
- 13.Grisso T. Evaluating Competencies: Forensic Assessments and Instruments. New York: Kluwer/Academic/Plenum Publishers, 2003. [Google Scholar]
- 14.Marson DC, Ingram K, Cody H, Harrell LE. Assessing the competency of patients with Alzheimer’s disease under different legal standards. Arch Neurol 1995;52:949–954. [DOI] [PubMed] [Google Scholar]
- 15.Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol 2005;62:1160–1163. [DOI] [PubMed] [Google Scholar]
- 16.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 2005;352:2379–2388. [DOI] [PubMed] [Google Scholar]
- 17.Huthwaite JS, Martin RC, Griffith HR, Anderson B, Harrell LE, Marson DC. Declining medical decision-making capacity in mild AD: a two-year longitudinal study. Behav Sci Law 2006;24:453–463. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 19.Jurica PJ, Leitten CL, Mattis S. Dementia Rating Scale, 2nd ed. Lutz, FL: Psychological Assessment Resources, 2001. [Google Scholar]
- 20.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001;58:1985–1992. [DOI] [PubMed] [Google Scholar]
- 21.Marson DC, Chatterjee A, Ingram K, Harrell LE. Towards a neurologic model of competency: cognitive predictors of capacity to consent in Alzheimer’s disease using three different legal standards. Neurology 1996;46:666–672. [DOI] [PubMed] [Google Scholar]
- 22.Roth LH, Meisel A, Lidz CW. Tests of competency to consent to treatment. Am J Psychiatry 1977;134:279–284. [DOI] [PubMed] [Google Scholar]
- 23.Appelbaum PS, Grisso T. Assessing patients’ capacities to consent to treatment. N Engl J Med 1988;319:1635–1638. [DOI] [PubMed] [Google Scholar]
- 24.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS® for Mixed Models, 2nd ed. Cary, NC: SAS Institute, 2007. [Google Scholar]
- 25.Singer JD, Willett JB. Applied Longitudinal Data Analysis. New York: Oxford University Press, 2003. [Google Scholar]
- 26.Wilson RS, Gilley DW, Bennett DA, Beckett LA, Evans DA. Person-specific paths of cognitive decline in Alzheimer’s disease and their relation to age. Psychol Aging 2000;15:18–28. [DOI] [PubMed] [Google Scholar]
- 27.Albert M, Blacker D, Moss MB, Tanzi R, McArdle JJ. Longitudinal change in cognitive performance among individuals with mild cognitive impairment. Neuropsychology 2007;21:158–169. [DOI] [PubMed] [Google Scholar]
- 28.Grundman M, Petersen RC, Bennett DA, et al. Alzheimer’s Association research roundtable meeting on mild cognitive impairment: what have we learned? Alzheimers Dementia 2006;2:220–233. [DOI] [PubMed] [Google Scholar]
- 29.Okonkwo OC, Griffith HR, Belue K, et al. Cognitive models of medical decision-making capacity in patients with mild cognitive impairment. J Int Neuropsychol Soc 2008;14:297–308. [DOI] [PubMed] [Google Scholar]
- 30.Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet 2006;367:1262–1270. [DOI] [PubMed] [Google Scholar]
- 31.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology 2002;59:198–205. [DOI] [PubMed] [Google Scholar]
- 32.Kim SYH, Karlawish JHT. Ethics and politics of research involving subjects with impaired decision-making abilities. Neurology 2003;62:1645–1646. [DOI] [PubMed] [Google Scholar]
- 33.Kim SY, Caine ED, Currier GW, Leibovici A, Ryan JM. Assessing the competence of persons with Alzheimer’s disease in providing informed consent for participation in research. Am J Psychiatry 2001;158:712–717. [DOI] [PubMed] [Google Scholar]
- 34.Taub H, Kline G, Baker M. The elderly and informed consent: effects of vocabulary level and corrected feedback. Exp Aging Res 1991;17:137–146. [DOI] [PubMed] [Google Scholar]
- 35.Mittal D, Palmer B, Dunn L, et al. Comparison of two enhanced consent procedures for patients with mild Alzheimer disease or mild cognitive impairment. Am J Geriatr Psychiatry 2007;15:163–167. [DOI] [PubMed] [Google Scholar]
- 36.Karlawish JHT, Casarett D. Addressing the ethical challenges of clinical trials that involve patients with dementia. J Geriatr Psychiatry Neurol 2001;14:222–228. [DOI] [PubMed] [Google Scholar]
- 37.Fisk JD, Merry HR, Rockwood K. Variations in case definition affect prevalence but not outcomes of mild cognitive impairment. Neurology 2003;61:1179–1184. [DOI] [PubMed] [Google Scholar]
- 38.Luis CA, Loewenstein DA, Acevedo A, Barker WW, Duara R. Mild cognitive impairment: directions for future research. Neurology 2003;61:438–444. [DOI] [PubMed] [Google Scholar]
- 39.Marson DC. Competency assessment and research in an aging society. Generations 2002;26:99–102. [Google Scholar]
- 40.Fitten LJ, Lusky R, Hamann C. Assessing treatment decision-making capacity in elderly nursing home residents. J Am Geriatr Soc 1990;38:1097–1104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


