Abstract
Objective:
Patients with cortical dysplasia (CD) are difficult to treat because the MRI abnormality may be undetectable. This study determined whether fluorodeoxyglucose (FDG)-PET/MRI coregistration enhanced the recognition of CD in epilepsy surgery patients.
Methods:
Patients from 2004–2007 in whom FDG-PET/MRI coregistration was a component of the presurgical evaluation were compared with patients from 2000–2003 without this technique. For the 2004–2007 cohort, neuroimaging and clinical variables were compared between patients with mild Palmini type I and severe Palmini type II CD.
Results:
Compared with the 2000–2003 cohort, from 2004–2007 more CD patients were detected, most had type I CD, and fewer cases required intracranial electrodes. From 2004–2007, 85% of type I CD cases had normal non–University of California, Los Angeles (UCLA) MRI scans. UCLA MRI identified CD in 78% of patients, and 37% of type I CD cases had normal UCLA scans. EEG and neuroimaging findings were concordant in 52% of type I CD patients, compared with 89% of type II CD patients. FDG-PET scans were positive in 71% of CD cases, and type I CD patients had less hypometabolism compared with type II CD patients. Postoperative seizure freedom occurred in 82% of patients, without differences between type I and type II CD cases.
Conclusions:
Incorporating fluorodeoxyglucose-PET/MRI coregistration into the multimodality presurgical evaluation enhanced the noninvasive identification and successful surgical treatment of patients with cortical dysplasia (CD), especially for the 33% of patients with nonconcordant findings and those with normal MRI scans from mild type I CD.
GLOSSARY
- AED
= antiepileptic drug;
- CD
= cortical dysplasia;
- FDG
= fluorodeoxyglucose;
- mMCD
= mild malformations of cortical development;
- TLE
= temporal lobe epilepsy;
- UCLA
= University of California, Los Angeles.
Cortical dysplasia (CD), first described in 1971,1 is the most common malformation of cortical development identified in surgically treated patients with therapy-resistant epilepsy.2,3 The histopathology of CD is classified into mild Palmini type I and severe Palmini type II.4,5 Type I CD is characterized by cortical dyslamination and columnar disorganization often associated with excessive subcortical white matter neurons. Type II CD shows cortical disorganization plus abnormal dysmorphic or cytomegalic neurons with or without balloon cells.6 In the past decade, improvements in MRI have increased awareness that CD is a frequent substrate causing epilepsy. This is especially true for patients with type II CD in whom structural MRI and functional neuroimaging often identify the lesion.7–10 However, patients with type I CD are a challenge in that they often have negative MRI scans, making surgical treatment difficult without knowing the exact location and borders necessary for complete lesion removal.11–16
Our center has recently focused on exploring newer neuroimaging methods to detect cortical lesions in the multimodality presurgery evaluation of patients with refractory epilepsy.17,18 One promising tool has been fluorodeoxyglucose (FDG)-PET/MRI coregistration, where FDG-PET images are fused onto the structural MRI and the degree of hypometabolism is color coded. This technique seemed to distinguish subtle lesions not appreciated by MRI or PET alone that turned out to be CD at histopathology. This study was designed to evaluate our initial experience with FDG-PET/MRI coregistration as a technique to noninvasively identify CD in the evaluation of patients with therapy-resistant epilepsy.
METHODS
See e-Methods on the Neurology® Web site at www.neurology.org.
RESULTS
Patient cohorts.
The 2004–2007 cohort in which FDG-PET/MRI coregistration studies were a routine part of the presurgical evaluation consisted of 45 patients with CD. Most CD patients (35; 78%) were younger than 18 years at surgery, similar to previous reports from this center.6 Eighty-seven percent of patients were from the Pediatric Epilepsy Surgery program at the University of California, Los Angeles (UCLA). Of the 45 CD patients, 27 (60%) were classified by histopathology as mild Palmini type I (all type IA), and 18 (40%) were classified as severe Palmini type II (8 as type IIA and 10 as type IIB). Two of the type I CD patients also had mild malformations of cortical development (mMCD).5 No patient had type IB CD. Intracranial electrodes were used in 1 patient (2%) to identify the ictal onset zone.
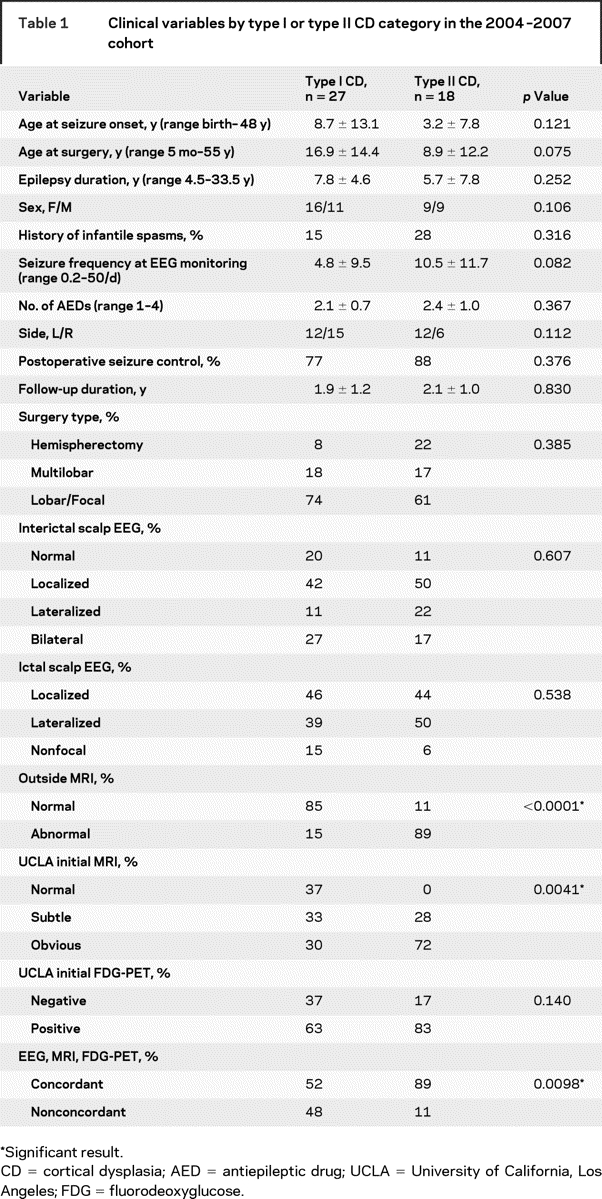
From 2000 to 2003, before the routine use of FDG-PET/MRI coregistration, the same inclusion and exclusion criteria identified 38 patients with CD. Nine (24%) were classified as type I (all type IA), and 29 (76%) were classified as type II (15 as type IIA and 14 as type IIB). Intracranial electrodes were used in 8 patients (21%). Comparing the 2000–2003 cohort with the 2004–2007 cohort showed no differences in age at seizure onset (p = 0.04), age at surgery (p = 0.08), epilepsy duration (p = 0.67), seizure frequency at EEG monitoring (p = 0.61), or type of surgery (p = 0.05). That is, compared with the 2000–2003 cohort before the regular use of FDG-PET/MRI coregistration techniques, from 2004 to 2007 more CD patients had surgery (+18%), a higher percentage of patients had type I CD on histopathology (24% vs 60%; χ2, p = 0.0009), and fewer patients with CD had intracranial electrode studies (21% vs 2%; p = 0.0060).
2004–2007 cohort.
For the 2004–2007 cohort, most clinical variables did not substantially differ between patients with type I and type II CD (table 1). Compared with patients with type II CD, those with type I CD tended to be older at seizure onset and surgery, and had lower seizure frequencies during EEG monitoring. Epilepsy duration, sex, history of infantile spasms, number of AEDs, and side of resection were not different between those with type I and those with type II CD. The type of surgery was hemispherectomy (n = 6), multilobar resection (portions of two or three lobes; n = 8), or lobar–focal (n = 31), and there were no differences between patients with type I or type II CD (table 1). There were no differences in the type of surgery for CD patients with normal, subtle, or obvious MRI lesions on UCLA scans (p = 0.41); normal or abnormal outside MRI reports (p = 0.31); negative or positive initial FDG-PET reports (p = 0.50); and concordant or nonconcordant EEG and neuroimaging findings (p = 0.34). Localized interictal and ictal scalp EEG abnormalities were found in 44% of CD patients. There were no differences in interictal and ictal EEG findings between those with type I and type II CD (table 1); normal, subtle, or obvious MRI lesions (p > 0.31); normal or abnormal outside MRI reports (p > 0.65); positive or negative initial FDG-PET reports (p > 0.28); and concordant or nonconcordant findings (p = 0.07).
Table 1 Clinical variables by type I or type II CD category in the 2004–2007 cohort
The frontal lobe was involved in 56%, the temporal lobe was involved in 49%, the parietal lobe was involved in 31%, and the occipital lobe was involved in 22% of patients with CD. Numerically, the temporal lobe was a more frequent location for type I CD (63%) compared with type II (28%), and the frontal lobe was a more frequent location for type II (89%) compared with type I CD (33%) (p = 0.02). By epilepsy syndrome, 12 patients had temporal lobe epilepsy (TLE) without hippocampal sclerosis, and 32 had extratemporal lobe epilepsy. Of those with TLE associated with CD, 11 (92%) had type I CD, compared with 15 (47%) of those with extratemporal lobe epilepsy (p = 0.0071).
Neuroimaging and presurgery evaluation.
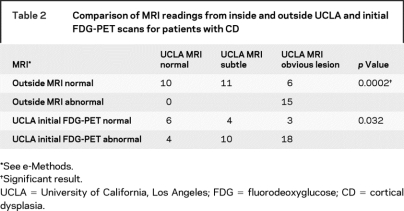
From 2004 to 2007, structural MRI and FDG-PET scans by themselves had variable specificity in identifying patients with CD. Outside MRI reports were available for all 45 patients and correctly identified a lesion in 18 (40%). By comparison, 35 initial MRI scans (78%) blindly reviewed within UCLA showed subtle or obvious lesions. Of note, 11 subtle and 6 obvious lesions identified on UCLA MRI scans were reported as normal on outside MRI reports (table 2). Initial blinded review of the grayscale FDG-PET scans correctly identified the lesion in 32 patients (71%) with CD (table 2). Combining scalp EEG findings, structural MRI, and initial FDG-PET results found that 30 (67%) of the CD patients had concordant findings that would typically allow patients to proceed to surgery without additional tests to localize the lesion or ictal onset zone. However, 15 (33%) of the CD patients had nonconcordant findings that would usually require secondary tests (e.g., intracranial electrodes, magnetic source imaging) to confirm the focus for surgery.
Table 2 Comparison of MRI readings from inside and outside UCLA and initial FDG-PET scans for patients with CD
Results of the standardized multimodal presurgery evaluation without considering the FDG-PET/MRI coregistration results were less sensitive in identifying patients with type I compared with type II CD. MRI reports from outside of UCLA correctly identified a lesion in 15% of type I CD cases, compared with 89% of type II CD cases (table 1). Even within UCLA, initial blinded review of the structural MRI correctly identified 63% of patients with type I CD, compared with 100% of patients with type II CD (subtle and obvious; table 1). Initial blinded review of the grayscale FDG-PET scans was slightly better than that of the structural MRI scans in that 63% of patients with type I CD had positive scans, compared with 83% of patients with type II CD (table 1). Finally, 48% of patients with type I CD were found to have concordant EEG and neuroimaging findings, compared with 89% of those with type II CD (table 1).
FDG-PET/MRI coregistration.
The addition of FDG-PET/MRI coregistration to the presurgical protocol enhanced our ability to detect CD in epilepsy surgery patients. The technique was especially helpful for the 48% of patients with type I CD (figures 1–3), and the 11% of type II cases (both had focal type IIA lesions) with nonconcordant EEG and MRI findings. All but one patient (98%) in the 2004–2007 cohort showed true-positive FDG-PET/MRI coregistration results (all with CD at histopathology). There was one potential false-positive FDG-PET/MRI scan involving a patient where CD was highly suspected clinically (focal EEG with subtle MRI lesion), but the surgical specimen was too small and distorted to make an adequate assessment of type I CD. This case was not included in this series, although this patient was seizure-free after surgery. Thus, FDG-PET/MRI coregistration studies added value for the 33% of patients in the nonconcordant clinical group, and was especially helpful for those with type I CD where the structural MRI was considered normal (n = 10).
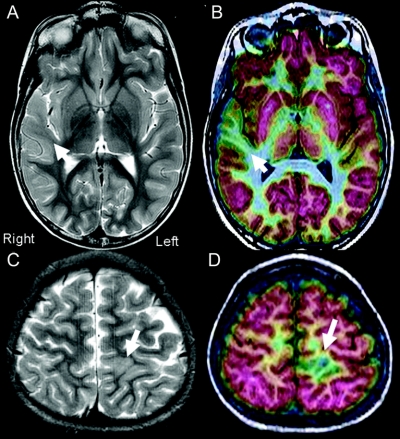
Figure 1 Examples of difficult-to-identify type I cortical dysplasia from two patients with epilepsy
(A and B) This 15-year-old patient began with seizures at age 4 years. Interictal scalp EEG disclosed synchronous spikes from F4, C4, and T4 and rarer independent spikes from F3 and C3. Ictal events were associated with broad EEG changes over the right frontal–temporal electrodes. Two previous outside MRI scans had been read as normal, as had his initial fluorodeoxyglucose (FDG)-PET scan (grayscale). FDG-PET/MRI coregistration indicated a well-defined area of focal hypometabolism in the right superior temporal gyrus (B, arrow). Closer inspection of the structural MRI indicated less white matter signal on T2 imaging in the same area (A, arrow). (C and D) This 8-year-old patient presented with seizure onset at age 3 years. The seizures were characterized by right leg and arm tonic events with eye fluttering lasting from 15 to 45 seconds. Scalp EEG showed fairly regular synchronous interictal discharges coming from the Cz, C3, and Pz electrodes, with rarer spikes from C4. Scalp EEG ictal onsets were difficult to localize secondary to movement artifacts. A previous outside MRI scan had been read as normal. University of California, Los Angeles MRI was read as showing a subtle lesion consistent with CD (C, arrow). FDG-PET/MRI coregistration indicated a focal area of hypometabolism in the left superior parietal region just behind the sensory cortex (D, arrow).
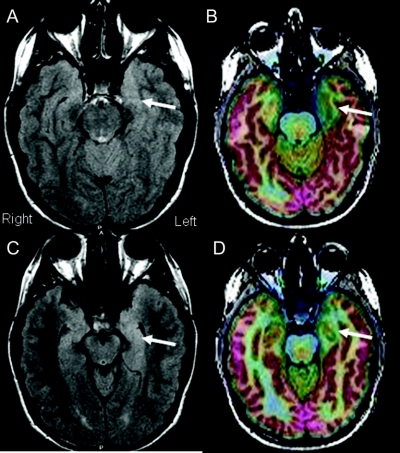
Figure 2 Example of mesial temporal lobe epilepsy without hippocampal sclerosis from type I cortical dysplasia
This 17-year-old patient presented with a history of seizure onset at age 7 years. Scalp EEG found rare interictal T1 spikes, and ictal events were associated with 4- to 5-Hz high amplitude slowing over the T1 and T3 electrodes. Two previous MRI scans performed at outside hospitals had been read as normal. Fluorodeoxyglucose-PET/MRI coregistration indicated an area of well-defined hypometabolism involving the left anterior mesial temporal region (B and D, arrows). Structural MRI indicated slightly increased fluid-attenuated inversion recovery changes in the same region without increased T2 signal in the hippocampus (A and C, arrows).
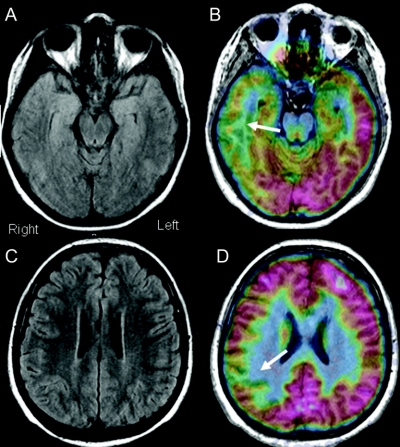
Figure 3 Example of multilobar type I cortical dysplasia with normal structural MRI
This 11-year-old patient began with seizures at age 5 years. Scalp EEG disclosed intermittent background slowing over the temporal–parietal–central region, with interictal spikes localized to C4, T4, T6, and O2 electrodes. Initial ictal onsets were difficult to localize, but after a few seconds, there were phase reversals at T6 through O2. MRI was read as normal within the University of California, Los Angeles (B and C). However, fluorodeoxyglucose-PET/MRI coregistration demonstrated multiple areas of hypometabolism involving the right temporal and parietal regions (B and D).
Postoperative outcomes.
Our approach incorporating FDG-PET/MRI coregistration into the presurgical evaluation resulted in similar rates of postoperative seizure control and complications in patients with type I and type II CD. In the 2004–2007 cohort, 82% of patients were seizure-free after surgery, with an average (±SD) of 2.0 ± 1.1 years of follow-up (range 0.5–4 years). There were no differences in postoperative seizure freedom between patients with type I (77% seizure-free) and type II (88%) CD (table 1). Similarly, there were no differences in seizure outcome if the UCLA MRI scan was classified as normal, subtle, or obvious (p = 0.80); normal or abnormal based on outside MRI reports (p = 0.43); positive or negative initial FDG-PET scans (p = 0.45); and concordant or nonconcordant (p = 0.34). The 9 patients with persistent seizures after surgery were cases where, based on the FDG-PET/MRI coregistration scans, the lesion could not be totally removed because it involved regions of the motor, sensory, language, or visual cortex or their white matter pathways where resection would have resulted in neurologic deficits that patients or their parents were unwilling to accept (n = 7), or the surgical resection was incomplete based on postoperative MRI images (n = 2; one recently undergoing a second surgery).
There were no operative deaths in this series. Surgical complications occurred in 10 patients (22%), and the incidence was not different between patients with type I (18%) and type II (28%) CD (p = 0.49); normal, subtle, or obvious MRI lesions (p = 0.08); normal and abnormal outside MRI scans (p = 0.40); positive and negative initial FDG-PET scans (p = 0.48); and concordant or nonconcordant presurgery findings (p = 0.45). Surgical complications included 5 patients who had incomplete motor, sensory, visual, or language deficits that resolved within 3 months after surgery, 3 patients with infections (1 meningitis, 1 urinary tract infection, and 1 mastoiditis ipsilateral to the side of the craniotomy) all successfully treated with antibiotics, and 2 patients who required CSF shunts after cerebral hemispherectomy.
FDG-PET hypometabolism.
FDG-PET hypometabolism was less severe for patients with type I compared with type II CD (figure e-1). For individual CD patients, FDG-PET hypometabolism ranged from 10% to 75% less than measurements from homotopic contralateral control cerebral cortex. Repeating the analysis comparing patients with normal, subtle, or obvious lesions on MRI showed no differences between patient groups in FDG-PET hypometabolism (p = 0.02; data not shown). As might be expected given that many normal outside MRI scans were patients with type I CD, those with reportedly normal outside MRI reports had less FDG-PET hypometabolism than did patients with abnormal scans (p < 0.0001; data not shown).
Correlations between neuroimaging and clinical variables.
FDG-PET rates of hypometabolism (Bq/mL) were compared with clinical variables (table 1) to determine whether these factors showed correlations. None of these univariate comparisons reached our statistical threshold (p > 0.02; data not shown).
DISCUSSION
For epilepsy surgery patients, this study found that incorporating FDG-PET/MRI coregistration into the multimodality presurgical evaluation enhanced the noninvasive detection and successful surgical treatment of patients with CD, especially those with mild Palmini type I dysplasia. Compared with the 2000–2003 cohort before the regular use of FDG-PET/MRI coregistration, from 2004 to 2007 more patients with CD had surgery (+18%), a higher percentage had type I CD (15% vs 60%), and fewer patients had intracranial electrode studies (phase II; 21% vs 2%). In the 2004–2007 cohort, 60% had normal outside MRI reports, and 85% of patients with type I had normal MRI reports, compared with 11% for type II CD cases. UCLA MRI protocols identified more cases of CD (78%) compared with outside MRI reports (40%). However, 70% of the type I CD cases were classified as normal or subtle, compared with 28% for type II CD patients. Interpretation of the grayscale FDG-PET scans correctly identified patients with CD in 71% of cases, without a difference in those with type I or type II CD. Concordant EEG and neuroimaging findings were found in 67% of CD patients, and fewer patients with type I CD had concordant findings. Using FDG-PET/MRI coregistration and electrocorticography to guide surgical resection resulted in postoperative seizure control in 82% of CD patients, without a difference between type I and type II CD cases. Coregistration tools were more helpful for mild CD cases because the color-coded technique seemed to identify subtle decreases in FDG-PET hypometabolism for type I compared with type II CD.
The medical literature and our results indicate that there are differences in clinical presentation and the ability of structural MRI to identity mild type I from severe type II CD in epilepsy surgery patients.19 Patients with type I CD tend to be older at seizure onset and undergo surgery at a later age, seizure frequency is less, and the lesion is often in the temporal lobe associated with TLE, compared with those with type II CD, where the lesion is often extratemporal.11,20–22 Studies report that 30% to 70% of patients with type I CD have positive structural MRI scans, compared with 80% to 100% of those with type II CD.9,10,12,16,23,24 Put another way, 20% to 60% of epilepsy surgery patients with normal MRI scans are reported to have type I CD at histopathology.13,14,25,26 Thus, structural MRI underreports CD in patients with refractory epilepsy, especially in those with type I CD.27 By comparison, FDG-PET alone is reported to correctly identify CD in 70% to 90% of cases, which is slightly better than MRI.28 Like MRI, however, interpretation of grayscale FDG-PET scans is less sensitive for type I compared with type II CD.15,29–32 Furthermore, intracranial EEG ictal onset zones are often diffuse and ill-defined in patients with CD.33,34 Consequently, determining the location and borders of the surgical resection is challenging in CD patients to completely remove the lesion, especially for patients with type I CD, where the area generating seizures often overlaps with functional cortex and the structural brain abnormalities are relatively subtle.35 These clinical challenges help to explain the wide variability in outcomes after epilepsy surgery that range from 40% to 90% of CD patients reported as seizure-free.8,10,12,36–38 Seizure freedom is reported in 30% to 66% for patients with type I CD, compared with 67% to 79% for those with type II CD, where the lesion is often MRI positive.21,22,39
The results of our study support the concept that adding advanced neuroimaging techniques, such as FDG-PET/MRI coregistration, into the multimodality presurgical evaluation can enhance the noninvasive detection of patients with CD, especially those with type I CD and normal MRI scans.15 Our protocol using FDG-PET/MRI coregistration added value for the 33% of patients with nonconcordant EEG and neuroimaging findings who otherwise would have required other tests, such as intracranial electrodes, to identify the focus. An additional advantage of the FDG-PET/MRI coregistration technique was that it allowed for more precise surgical planning because the borders of the cortical lesions could be more clearly identified, thus reducing the chance of an incomplete resection.40 The improvement in detecting CD using FDG-PET/MRI coregistration was likely the result of better sensitivity in recognizing subtle areas of hypometabolism using the color scale, and the more precise localization over anatomic regions with the structural MRI.
The reader should be aware of possible limitations in study design and methods in interpreting our report. For example, although FDG-PET/MRI coregistration seemed to show higher specificity in detecting CD, our study design cannot determine the number of false-negative scans. That is, we do not know how many patients with CD, especially those with mMCD and type I CD, were missed using our presurgical protocol. We also do not know what contribution experience had in detecting CD over the time course of this study, or precisely which factors in the evaluation process might have contributed most to our ability to identify patients with CD. In addition, our study was designed to address the question of whether our imaging technique was useful for the clinical treatment of patients with CD. We do not know whether FDG-PET/MRI coregistration might be helpful for assessing other etiologies in patients being considered for epilepsy surgery. Finally, although our FDG-PET/MRI coregistration results are promising, these findings are based on a relatively small cohort studied retrospectively. Our presurgical protocol needs to be confirmed in a larger clinical series where these tools are applied prospectively to predict which patients with refractory epilepsy harbor CD. Furthermore, it will be important for future studies to discern possible histologic mechanisms to explain the differences in FDG-PET hypometabolism between patients with type I and type II CD.
For the practicing neurologist, our results indicate that a negative structural MRI report in patients with refractory epilepsy does not mean that a subtle CD does not exist. The challenge for epilepsy specialists will be to design presurgical protocols that do not depend exclusively on structural MRI to find these subtle lesions with high specificity and low patient risk. Thus, future advances in the surgical treatment of patients with refractory epilepsy will likely involve development and validation of newer imaging and other techniques and incorporating these tools into the multimodality presurgical evaluation process so that more patients have the opportunity to be considered for surgical therapy and the chance to become seizure-free.
Supplementary Material
Received April 28, 2008. Accepted in final form August 8, 2008.
Address correspondence and reprint requests to Dr. Gary W. Mathern, Reed Neurological Research Center, 710 Westwood Plaza, Room 2123, Los Angeles, CA 90095-1769 gmathern@ucla.edu
Supplemental data at www.neurology.org
This study was supported in part by NIH grants R01 NS38992 and P05 NS02808 to G.W.M. and J.E. H.M. was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (17689040) and the Japan Epilepsy Research Foundation (H16-009).
Disclosure: The authors report no disclosures.
REFERENCES
- 1.Taylor DC, Falconer MA, Bruton CJ, Corsellis JA. Focal dysplasia of the cerebral cortex in epilepsy. J Neurol Neurosurg Psychiatry 1971;34:369–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker AJ, Blumcke I, Urbach H, Hans V, Majores M. Molecular neuropathology of epilepsy-associated glioneuronal malformations. J Neuropathol Exp Neurol 2006;65:99–108. [DOI] [PubMed] [Google Scholar]
- 3.Harvey AS, Cross JH, Shinnar S, Mathern BW. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia 2008;49:146–155. [DOI] [PubMed] [Google Scholar]
- 4.Mischel PS, Nguyen LP, Vinters HV. Cerebral cortical dysplasia associated with pediatric epilepsy: review of neuropathologic features and proposal for a grading system. J Neuropathol Exp Neurol 1995;54:137–153. [DOI] [PubMed] [Google Scholar]
- 5.Palmini A, Najm I, Avanzini G, et al. Terminology and classification of the cortical dysplasias. Neurology 2004;62 (suppl 3):S2–S8. [DOI] [PubMed] [Google Scholar]
- 6.Cepeda C, Andre VM, Levine MS, et al. Epileptogenesis in pediatric cortical dysplasia: the dysmature cerebral developmental hypothesis. Epilepsy Behav 2006;9:219–235. [DOI] [PubMed] [Google Scholar]
- 7.Chugani HT, Shields WD, Shewmon DA, et al. Infantile spasms, I: PET identifies focal cortical dysgenesis in cryptogenic cases for surgical treatment. Ann Neurol 1990;27:406–413. [DOI] [PubMed] [Google Scholar]
- 8.Mathern GW, Giza CC, Yudovin S, et al. Postoperative seizure control and antiepileptic drug use in pediatric epilepsy surgery patients: the UCLA experience, 1986–1997. Epilepsia 1999;40:1740–1749. [DOI] [PubMed] [Google Scholar]
- 9.Lawson JA, Birchansky S, Pacheco E, et al. Distinct clinicopathologic subtypes of cortical dysplasia of Taylor. Neurology 2005;64:55–61. [DOI] [PubMed] [Google Scholar]
- 10.Kral T, von Lehe M, Podlogar M, et al. Focal cortical dysplasia: long term seizure outcome after surgical treatment. J Neurol Neurosurg Psychiatry 2007;78:853–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fauser S, Schulze-Bonhage A, Honegger J, et al. Focal cortical dysplasias: surgical outcome in 67 patients in relation to histological subtypes and dual pathology. Brain 2004;127:2406–2418. [DOI] [PubMed] [Google Scholar]
- 12.Chung CK, Lee SK, Kim KJ. Surgical outcome of epilepsy caused by cortical dysplasia. Epilepsia 2005;46(suppl 1):25–29. [DOI] [PubMed] [Google Scholar]
- 13.Lee SK, Lee SY, Kim KK, et al. Surgical outcome and prognostic factors of cryptogenic neocortical epilepsy. Ann Neurol 2005;58:525–532. [DOI] [PubMed] [Google Scholar]
- 14.McGonigal A, Bartolomei F, Regis J, et al. Stereoelectroencephalography in presurgical assessment of MRI-negative epilepsy. Brain 2007;130:3169–3183. [DOI] [PubMed] [Google Scholar]
- 15.Kurian M, Spinelli L, Delavelle J, et al. Multimodality imaging for focus localization in pediatric pharmacoresistant epilepsy. Epileptic Disord 2007;9:20–31. [DOI] [PubMed] [Google Scholar]
- 16.Widdess-Walsh P, Jeha L, Nair D, et al. Subdural electrode analysis in focal cortical dysplasia: predictors of surgical outcome. Neurology 2007;69:660–667. [DOI] [PubMed] [Google Scholar]
- 17.Chandra PS, Salamon N, Huang J, et al. FDG-PET/MRI coregistration and diffusion-tensor imaging distinguish epileptogenic tubers and cortex in patients with tuberous sclerosis complex: a preliminary report. Epilepsia 2006;47:1543–1549. [DOI] [PubMed] [Google Scholar]
- 18.Wu JY, Sutherling WW, Koh S, et al. Magnetic source imaging localizes epileptogenic zone in children with tuberous sclerosis complex. Neurology 2006;66:1270–1272. [DOI] [PubMed] [Google Scholar]
- 19.Kasper BS. Mild forms of focal cortical dysplasia: how certain are we? Brain 2005;128:E22; author reply E23. [DOI] [PubMed]
- 20.Sisodiya SM. Surgery for malformations of cortical development causing epilepsy. Brain 2000;123:1075–1091. [DOI] [PubMed] [Google Scholar]
- 21.Tassi L, Colombo N, Garbelli R, et al. Focal cortical dysplasia: neuropathological subtypes, EEG, neuroimaging and surgical outcome. Brain 2002;125:1719–1732. [DOI] [PubMed] [Google Scholar]
- 22.Widdess-Walsh P, Kellinghaus C, Jeha L, et al. Electro-clinical and imaging characteristics of focal cortical dysplasia: correlation with pathological subtypes. Epilepsy Res 2005;67:25–33. [DOI] [PubMed] [Google Scholar]
- 23.Chapman K, Wyllie E, Najm I, et al. Seizure outcome after epilepsy surgery in patients with normal preoperative MRI. J Neurol Neurosurg Psychiatry 2005;76:710–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siegel AM, Cascino GD, Meyer FB, et al. Surgical outcome and predictive factors in adult patients with intractable epilepsy and focal cortical dysplasia. Acta Neurol Scand 2006;113:65–71. [DOI] [PubMed] [Google Scholar]
- 25.Siegel AM, Jobst BC, Thadani VM, et al. Medically intractable, localization-related epilepsy with normal MRI: presurgical evaluation and surgical outcome in 43 patients. Epilepsia 2001;42:883–888. [DOI] [PubMed] [Google Scholar]
- 26.Jayakar P, Dunoyer C, Dean P, et al. Epilepsy surgery in patients with normal or nonfocal MRI scans: integrative strategies offer long-term seizure relief. Epilepsia 2008;49:758–764. [DOI] [PubMed] [Google Scholar]
- 27.Von Oertzen J, Urbach H, Jungbluth S, et al. Standard magnetic resonance imaging is inadequate for patients with refractory focal epilepsy. J Neurol Neurosurg Psychiatry 2002;73:643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olson DM, Chugani HT, Shewmon DA, Phelps ME, Peacock WJ. Electrocorticographic confirmation of focal positron emission tomographic abnormalities in children with intractable epilepsy. Epilepsia 1990;31:731–739. [DOI] [PubMed] [Google Scholar]
- 29.Kim SK, Na DG, Byun HS, et al. Focal cortical dysplasia: comparison of MRI and FDG-PET. J Comput Assist Tomogr 2000;24:296–302. [DOI] [PubMed] [Google Scholar]
- 30.Casse R, Rowe CC, Newton M, Berlangieri SU, Scott AM. Positron emission tomography and epilepsy. Mol Imaging Biol 2002;4:338–351. [DOI] [PubMed] [Google Scholar]
- 31.Ollenberger GP, Byrne AJ, Berlangieri SU, et al. Assessment of the role of FDG PET in the diagnosis and management of children with refractory epilepsy. Eur J Nucl Med Mol Imaging 2005;32:1311–1316. [DOI] [PubMed] [Google Scholar]
- 32.Yun CH, Lee SK, Lee SY, et al. Prognostic factors in neocortical epilepsy surgery: multivariate analysis. Epilepsia 2006;47:574–579. [DOI] [PubMed] [Google Scholar]
- 33.Boonyapisit K, Najm I, Klem G, et al. Epileptogenicity of focal malformations due to abnormal cortical development: direct electrocorticographic-histopathologic correlations. Epilepsia 2003;44:69–76. [DOI] [PubMed] [Google Scholar]
- 34.Turkdogan D, Duchowny M, Resnick T, Jayakar P. Subdural EEG patterns in children with Taylor-type cortical dysplasia: comparison with nondysplastic lesions. J Clin Neurophysiol 2005;22:37–42. [DOI] [PubMed] [Google Scholar]
- 35.Najm IM, Tilelli CQ, Oghlakian R. Pathophysiological mechanisms of focal cortical dysplasia: a critical review of human tissue studies and animal models. Epilepsia 2007;48(suppl 2):21–32. [DOI] [PubMed] [Google Scholar]
- 36.Kloss S, Pieper T, Pannek H, Holthausen H, Tuxhorn I. Epilepsy surgery in children with focal cortical dysplasia (FCD): results of long-term seizure outcome. Neuropediatrics 2002;33:21–26. [DOI] [PubMed] [Google Scholar]
- 37.Hader WJ, Mackay M, Otsubo H, et al. Cortical dysplastic lesions in children with intractable epilepsy: role of complete resection. J Neurosurg 2004;100:110–117. [DOI] [PubMed] [Google Scholar]
- 38.Alexandre V Jr, Walz R, Bianchin MM, et al. Seizure outcome after surgery for epilepsy due to focal cortical dysplastic lesions. Seizure 2006;15:420–427. [DOI] [PubMed] [Google Scholar]
- 39.Fauser S, Bast T, Altenmuller DM, et al. Factors influencing surgical outcome in patients with focal cortical dysplasia. J Neurol Neurosurg Psychiatry 2008;79: 103–105105. [DOI] [PubMed] [Google Scholar]
- 40.Juhasz C, Chugani DC, Muzik O, et al. Is epileptogenic cortex truly hypometabolic on interictal positron emission tomography? Ann Neurol 2000;48:88–96. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.