Abstract
Objective:
To investigate the relationship between white matter tract integrity and language and memory performances in patients with temporal lobe epilepsy (TLE).
Methods:
Diffusion tensor imaging (DTI) was performed in 17 patients with TLE and 17 healthy controls. Fractional anisotropy (FA) and mean diffusivity (MD) were calculated for six fiber tracts (uncinate fasciculus [UF], arcuate fasciculus [AF], fornix [FORX], parahippocampal cingulum [PHC], inferior fronto-occipital fasciculus [IFOF], and corticospinal tract [CST]). Neuropsychological measures of memory and language were obtained and correlations were performed to evaluate the relationship between DTI and neuropsychological measures. Hierarchical regression was performed to determine unique contributions of each fiber tract to cognitive performances after controlling for age and hippocampal volume (HV).
Results:
Increases in MD of the left UF, PHC, and IFOF were associated with poorer verbal memory in TLE, as were bilateral increases in MD of the AF, and decreases in FA of the right AF. Increased MD of the AF and UF, and decreased FA of the AF, UF, and left IFOF were related to naming performances. No correlations were found between DTI measures and nonverbal memory or fluency in TLE. Regression analyses revealed that several fibers, including the AF, UF, and IFOF, independently predicted cognitive performances after controlling for HV.
Conclusions:
The results suggest that structural compromise to multiple fiber tracts is associated with memory and language impairments in patients with temporal lobe epilepsy. Furthermore, we provide initial evidence that diffusion tensor imaging tractography may provide clinically unique information for predicting neuropsychological status in patients with epilepsy.
GLOSSARY
- AF
= arcuate fasciculus;
- BNT
= Boston Naming Test;
- CST
= corticospinal tract;
- DTI
= diffusion tensor imaging;
- FA
= fractional anisotropy;
- FORX
= fornix;
- HV
= hippocampal volume;
- ICHV
= intracranial-adjusted HV;
- IFOF
= inferior fronto-occipital fasciculus;
- LM
= Logical Memory;
- MD
= mean diffusivity;
- MTS
= mesial temporal sclerosis;
- PHC
= parahippocampal cingulum;
- TLE
= temporal lobe epilepsy;
- UF
= uncinate fasciculus;
- WMS-III
= Wechsler Memory Scale–Third Edition.
Global and lobar white matter atrophy has been reported in patients with temporal lobe epilepsy (TLE),1,2 and an association between global white matter volume loss and generalized cognitive dysfunction has been described.2 However, the relationships among local white matter changes and cognitive impairments in TLE have not been established. Diffusion-tensor imaging (DTI) is a relatively new MRI technique for investigating white matter microstructure by measuring the relative motility of water within a voxel (mean diffusivity [MD]) and its directionality (fractional anisotropy [FA]).3,4 Higher MD and lower FA values are thought to reflect factors such as demyelination and axonal injury that are important for understanding neurologic disease.5 DTI tractography is an extension of DTI that provides an in vivo method of quantifying and visualizing the integrity of white matter tracts, offering a unique tool for investigating the relationship between compromise of specific white matter tracts and cognitive impairment. [Note: The term tractography in this study refers to the quantitative assessment of the microstructural organization of fiber tracts in vivo from MRI.21 It should be noted that the more specific use of the term is reserved for streamline fiber tracking based on connectivity between a seeded source and an identified projection area].
Few studies have investigated the link between DTI tractography and cognition in epilepsy. One study demonstrated that increased diffusivity in the left uncinate fasciculus (UF) was related to poorer auditory memory, whereas increased diffusivity and reduced FA in the right UF were related to poorer visual memory in left TLE.6 In another study, FA of the left arcuate fasciculus (AF) was associated with fMRI language lateralization in TLE.7 In a subsequent study, the same researchers showed that preoperative FA of the AF was useful in predicting postoperative naming decline.8 These studies provide initial evidence that pathology within two frontotemporal fiber tracts is associated with memory and language functioning in TLE.
Due to increasing evidence of diffuse white matter pathology in TLE, it is likely that numerous fiber tracts are affected, and that pathology within these fiber tracts contributes to memory and language impairments. These fibers include the UF, AF, fornix (FORX), parahippocampal fibers of the cingulum (PHC), and the inferior fronto-occipital fasciculus (IFOF). Whereas previous studies have implicated the PHC,9,10 UF,6,11,12 and FORX13 in learning and memory, the AF7,14,15 and IFOF15,16 have been implicated in language functioning. The corticospinal tract (CST) was also included in this study to serve as a “control” fiber that was not expected to contribute to cognition. The purpose of the study was to evaluate the relationships among memory and language impairments and white matter pathology in TLE. We also evaluated these fibers as predictors of memory and language in TLE after controlling for hippocampal volume (HV).
METHODS
Participants.
Participants included 17 patients with TLE and 17 age-, education-, and gender-matched controls. All patients were undergoing presurgical evaluation at the University of California, San Diego, Epilepsy Center. Patients with TLE were prospectively enrolled in the study if they demonstrated strong evidence of unilateral left (n = 9) or right (n = 8) temporal lobe seizure onset during ictal video-EEG recordings, and if video-EEG was supported by seizure semiology and neuroimaging. In 11 patients, ictal onset was confirmed with intracranial electrodes. Diagnoses were supported in 14 patients by the presence of mesial temporal sclerosis (MTS). Seven of the 17 patients have undergone temporal lobe resections—all with Class I seizure outcomes. Controls were recruited through open advertisement and screened neurologic or psychiatric illness.
Procedure.
Neuropsychological evaluation.
The Logical Memory (LM) I and II subtests from the Wechsler Memory Scale–Third Edition (WMS-III)17 were used to evaluate immediate and delayed verbal memory. The Faces I and II subtests from the WMS-III were used to evaluate immediate and delayed nonverbal memory. The Verbal Fluency subtest from the Delis-Kaplan Executive Functioning System18 was used to determine letter fluency, and the Boston Naming Test (BNT)19 was used as a measure of confrontational naming.
Image acquisition.
MRI was performed on a General Electric 1.5T EXCITE HD scanner with an eight-channel phased-array head coil. Image acquisitions included a conventional three-plane localizer, GE calibration scan, two T1-weighted three-dimensional structural scans (echo time = 4.8 msec, repetition time = 10.7 msec, flip angle = 8 deg, bandwidth = 31.25 Hz/pixel, field of view = 25.6 cm, matrix = 256 × 256, slice thickness = 1.0 mm), and five diffusion-weighted sequences. Hippocampal and total white matter volumes were obtained according to procedures described previously.20 Diffusion data were acquired using single-shot echoplanar imaging with isotropic 2.5 mm voxels (matrix size = 96 × 96, field of view = 24 cm, 47 axial slices, slice thickness = 2.5 mm), covering the entire cerebrum and brainstem without gaps. Three volume series were acquired with 51 diffusion gradient directions using b-values of 600, 800, and 1,000 mm2/s, each with an additional b = 0 volume. For use in nonlinear B0 distortion correction, two additional volume series were acquired with one b = 0 volume and a single diffusion direction (b = 800 mm2/s), with either forward or reverse phase-encode polarity. All patients were seizure-free for a minimum of 24 hours prior to the MRI scan in order to avoid the possible effects of acute postictal changes on diffusion parameters.21
Image processing.
Image files in DICOM format were transferred to a Linux workstation for processing. The two T1-weighted images were rigid body registered to each other and reoriented into a common space, roughly similar to alignment based on the AC-PC line. Images were corrected for nonlinear warping caused by nonuniform fields created by the gradient coils.22 Image intensities were corrected for spatial sensitivity inhomogeneities by normalizing with the ratio of a body coil scan to a head coil scan. Preprocessing of the diffusion-weighted images was performed according to previously described procedures.23 Images were resampled using linear interpolation to 1.875 mm3 isotropic voxels.
Fiber tracking and calculations.
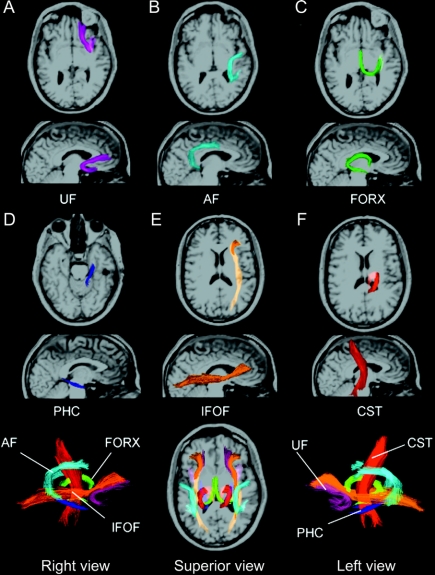
Fiber FA and MD values for the six fiber tracts were derived using a probabilistic diffusion tensor atlas that was based on manual tracings as described previously23 (figure 1). Results obtained from the atlas-generated fibers correlate highly with those obtained from manual tracings (average R2= 0.98). However, the atlas has the advantage of eliminating the time demands of manual tracings.
Figure 1 Horizontal (A–F) and sagittal views of the six fiber tracts in a healthy, 38-year-old, right-handed man
All fibers were obtained from a probabilistic DTI atlas based on manual tracings that were performed in DTI Studio. Individual fiber tracts are shown projected on their corresponding T1-weighted images using Tractoview software. Color-coding is included to assist with identification of the fibers in the superimposed images (bottom row). Colors do not provide information with respect to fiber orientation.
Statistical analysis.
First, univariate analyses of variance were performed to examine group differences in FA, MD, and each neuropsychological measure. Second, the relationships among age- and education-adjusted test scores and FA/MD of each fiber tract were evaluated using Spearman rho correlations. Third, hierarchical regression analyses were then performed to determine the contribution of each fiber tract to cognitive performances in patients with TLE, after regressing out age and HV. In order to control for Type I error rates, only correlations with a p value <0.01 were considered significant.
RESULTS
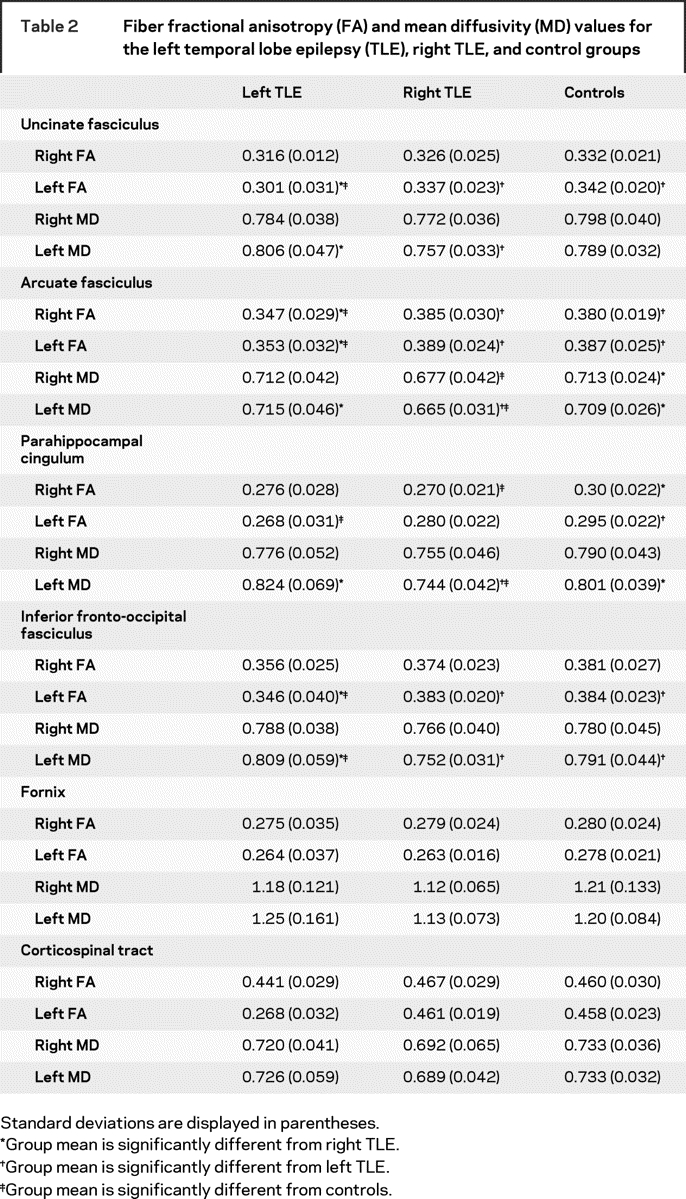
There were no group differences between the patients and controls in age (TLE mean = 34.1 years; control mean = 38.7 years), years of education (TLE mean = 13.2; control mean = 14.3), or gender distribution (7 men per group). Mann–Whitney U test revealed no group differences between the right and left TLEs in duration of illness, number of anticonvulsant medications, seizure frequency, time since last seizure, or age at seizure onset. There were no group differences in the number of anticonvulsant medications with known cognitive side effects (e.g., phenobarbital) vs those without cognitive side effects (e.g., lamotrigine). Neuropsychological testing revealed that patients with left TLE were impaired on LM I, LM II, and the BNT (table 1). Left and right TLEs were both impaired on Verbal Fluency. Patients did not differ from each other on any measure. However, table 1 reveals a trend toward greater impairment on all verbal measures in left TLE. Group differences in fiber FA and MD are shown in table 2.
Table 1 Neuropsychological performance on language and memory tests in patients with temporal lobe epilepsy (TLE) and controls
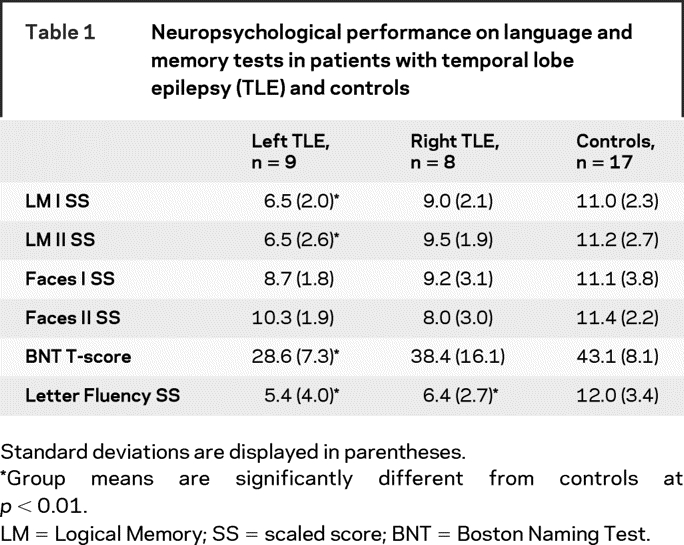
Table 2 Fiber fractional anisotropy (FA) and mean diffusivity (MD) values for the left temporal lobe epilepsy (TLE), right TLE, and control groups
Correlational analyses.
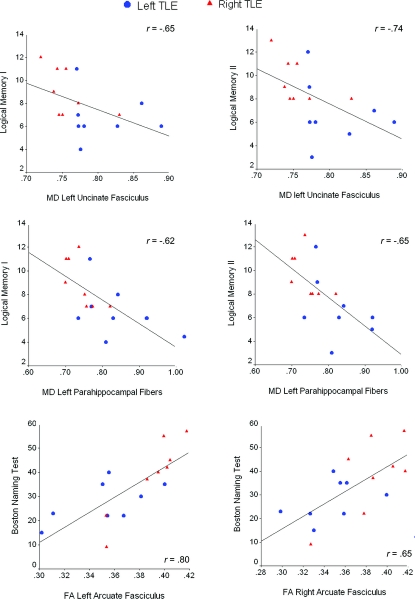
No significant correlations emerged among fiber FA or MD values and age- and education-adjusted language or memory performances in the controls. Therefore, all remaining results are reported for patients only. Due to the lack of group differences between patients with right and left TLE in cognitive performances and many of the FA/MD scores, patients were combined in the correlation and regression analyses. Qualitative differences between left and right TLEs can be seen in selected scatterplots (figure 2).
Figure 2 Scatterplots displaying the relationship between neuropsychological test performances and fractional anisotropy (FA)/mean diffusivity (MD) of selected left and right fiber tracts
All FA and MD values are plotted against age- and education-adjusted scaled scores.
Memory.
There were no significant correlations between left, right, or total white matter volume and memory performances in patients with TLE. For verbal memory, lower LM I scores were associated with higher MD of the left UF, PHC, and IFOF (table 3). Lower LM II scores were associated with lower FA of the right AF, and with higher MD of the left UF, PHC, and IFOF, and higher MD of the left and right AF. There were no significant correlations between any of the FA or MD fiber measures and Faces I or II.
Table 3 Spearman rho correlations between atlas-generated fiber fractional anisotropy (FA)/mean diffusivity (MD) values and age- and education-corrected neuropsychological test scores for patients with temporal lobe epilepsy
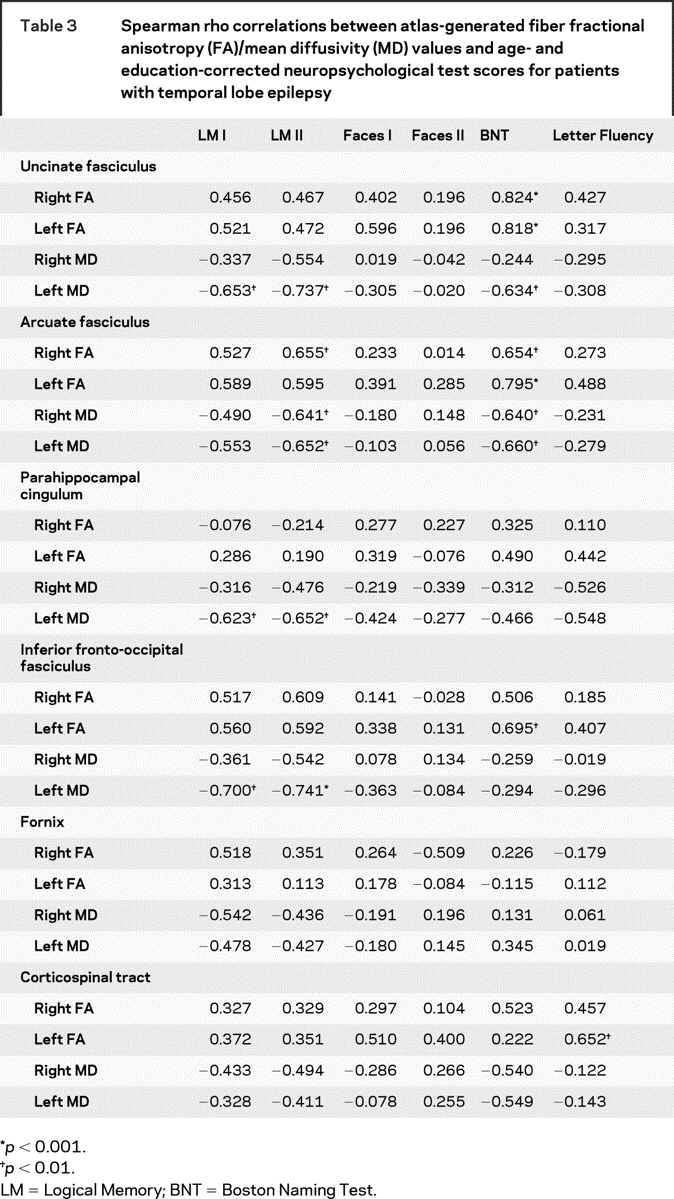
Language.
There were no significant correlations between left, right, or total white matter volume and language performances in patients with TLE. Lower BNT T scores were associated with lower FA of the left IFOF, and of the left and right AF and UF. Lower BNT scores were also associated with higher MD of the left UF, left AF, and right AF. Lower Verbal Fluency scores were associated with lower FA of the left CST.
Regression analyses.
Memory.
Smaller left intracranial-adjusted HV (ICHV) predicted lower LM I (b = 0.647; p < 0.01) and LM II (b = 0.625; p < 0.01) scores. After regressing out age and ICHV in a hierarchical model, MD of the left IFOF (b = 0.403; p < 0.05) added to the prediction of LM II, accounting for an additional 12% of the variance. Together, ICHV and MD of the left IFOF explained 66% of the variance in LM II scores. No other FA/MD variables remained significant contributors to the prediction of memory scores.
Language.
Left ICHV was not correlated with age- and education-adjusted BNT scores. Therefore, after accounting for the contributions of age and left ICHV, FA of the left UF (b = 0.910; p < 0.01), right UF (b = 0.904; p < 0.001), left AF (b = 0.866; p < 0.01), and left IFOF (b = 0.718; p < 0.01) all remained significant predictors of BNT scores.
DISCUSSION
The goal of this study was to use DTI tractography to investigate the associations among microstructural compromise to white matter tracts and memory and language performances in TLE. We found that increased MD of numerous fiber tracts was associated with poorer verbal, but not nonverbal, memory performances in TLE. These associations were strongly left-lateralized for the UF, PHC, and IFOF. In addition, decreased FA and increased MD of the left IFOF, and bilateral AF and UF were associated with poorer confrontational naming, but not fluency, in TLE. These findings are supported by previous research demonstrating that increased diffusivity and decreased FA within frontotemporal fiber tracts is linked to neuropsychological impairments in TLE.6–8 Our findings extend the literature by demonstrating that 1) damage to fiber tracts other than the AF and UF are associated with language and memory impairments in TLE, and 2) many of these fibers appear to contribute to cognition independent of HV, thereby providing clinically unique information in the prediction of cognitive deficits. Finally, our results revealed that language and memory performances are not associated with the integrity of all left hemisphere white matter in that most did not correlate with a fiber tract implicated in motor functioning, nor did they correlate with total left hemisphere white matter volume. Together, these findings provide evidence that a network of fiber tracts within the left and right hemisphere contribute to language and memory in TLE, and that these associations can be revealed by DTI.
Based on previous research and temporal lobe connectivity, we hypothesized that the UF, PHC, and FORX would be associated with memory performances in TLE. The UF is considered to be the major fiber tract connecting the inferior frontal and anterior temporal lobe,24,25 and has been implicated in binding stimuli into specific episodes for subsequent retrieval and conscious recollection.26 Our findings are in line with previous DTI research demonstrating the importance of the UF in episodic memory,6 and our data reveal a strong, material-specific association between increased MD of the left UF and poorer verbal memory in TLE.
We also found a strong association between MD of left PHC fibers and verbal memory in TLE. The PHC runs along the ventral aspect of the parahippocampal gyrus and connects medial temporal lobe regions to the posterior cingulate cortex.3 These fibers are a crucial part of Papez Circuit27—a group of interconnected gray and white matter limbic structures that has long been implicated in memory.9 The contribution of PHC fibers to learning and memory is further supported by studies demonstrating that damage to this pathway disrupts learning and retention of information in rats.28
Although the rather strong association between MD of the left IFOF and verbal memory was not anticipated, it has been suggested that the IFOF is a subcortical pathway underlying the semantic system with a putative role in providing a link between phonology and sentence comprehension.15 Given the nature of our verbal memory task (i.e., recall of paragraph information), it is possible that the high demands placed on semantic processing account for the strong association. Alternatively, our verbal memory task has high attentional demands and likely recruits frontal lobe regions involved in attention.29 Thus, increased diffusivity of the left IFOF may have led to impaired attention, resulting in poorer performances on both immediate and delayed recall.
Contrary to our hypotheses, we did not find a correlation between FA or MD of the FORX and memory performances. However, FORX values in patients with TLE did not differ from controls. Therefore, damage to the FORX may have been too subtle to reveal a significant relationship with memory scores in our patient group.
With respect to language performances, the association between integrity of the left AF and confrontational naming is well-established and has been documented in DTI studies.7,8,14 However, we also found a strong correlation between integrity of the right AF and confrontational naming in our patients. These data are commensurate with studies demonstrating right hemisphere contributions to language processing,30 and with studies suggesting partial reorganization of language to the right hemisphere in some patients with left TLE.7 In addition to the AF, there is emerging evidence that the left IFOF plays a key role in language. Electrostimulation of the IFOF induces semantic paraphasias during picture naming, and this phenomenon occurs regardless of which portion of the IFOF is stimulated.16 It has been suggested that the IFOF may be a subcortical pathway underlying the semantic system, i.e., part of a ventral semantic stream involved in language and semantic processing that complements the dorsal language system that includes the AF. Finally, bilateral integrity of the UF was associated with higher naming scores. Although primarily associated with episodic retrieval, the UF has also been implicated in lexical-semantic retrieval processes that are fundamental to confrontational naming.31
Taken together, our results provide additional insight into underlying structure-function relationships in TLE, and demonstrate how DTI can be used to delineate the neurocognitive correlates of localized white matter damage. We also propose that DTI can be used in conjunction with HC volumes to predict cognitive impairments in TLE. Despite the potential clinical appeal of these findings, several other points and limitations should be addressed. First, given the correlational nature of this study, causation is only assumed and cannot be completely determined from the analyses. Second, we observed a general trend for patients with left TLE to have lower FA and higher MD values bilaterally in many tracts relative to patients with right TLE. This is consistent with some evidence of greater bilateral pathology in left TLE.32,33 However, these results should be replicated in a larger group of patients to rule out the possibility that this finding is limited to our sample. Third, given the lack of significant differences between right and left TLEs in cognitive performances, we chose to combine the patient groups. With a larger sample, perhaps group differences would have emerged and revealed different DTI-cognitive relationships within each group. Fourth, we did not find any correlations among DTI variables and performances on measures of nonverbal memory. It is of note that neither patient group was impaired in memory for faces. Therefore, the lack of a correlation may have been due to a restricted range of scores. However, the association between nonverbal memory and right temporal lobe function is known to be more tenuous than the association between verbal memory and left temporal lobe function.34 Fifth, we adopted a statistical threshold of p < 0.01 to control for Type I errors. This value may have resulted in some error inflation, accounting for one or more atypical findings (i.e., the correlations between left CST and verbal fluency or right AF and LM II). Similarly, although FA and MD values are inversely related, they bore somewhat different relationships to cognition. In general, fiber MD correlated with memory performances, whereas fiber FA and MD correlated with naming. Inspection of table 3 reveals that all FA and MD correlations were in the expected direction. However, the magnitude of the relationships differed in that some values exceeded our statistical threshold of p < 0.01, whereas others fell just below (i.e., possible Type II errors). Other findings may have reflected Type I errors that would not emerge with a more stringent p value. A final possibility is that controlling for age in our relationships attenuated the correlations with FA and MD. It is well known that white matter integrity correlates with age.35 However, we choose to co-vary out age to isolate the effects of TLE on structure–function relationship. Finally, the validity of DTI as a measure of white matter integrity remains difficult to determine due to the lack of a gold standard. DTI measurements are subject to inaccuracies due to crossing fibers, partial voluming, noise, and limitations of the particular tracking algorithm applied.36 Although one can assume that these measurement errors are uniform across participants and would have minimally affected our analyses, the degree to which measurement or instrumentation-related factors influenced our results is unknown. Additional research is needed establishing the validity of DTI tractography in patients with epilepsy and other neurologic diseases before its clinical value can be fully appreciated.
ACKNOWLEDGMENT
The authors thank J. Kuperman for assistance with Tractoview software.
Address correspondence and reprint requests to Dr. C.R. McDonald, Multimodal Imaging Laboratory, Suite C101, 8950 Villa La Jolla Drive, La Jolla, CA 92037 camcdonald@ucsd.edu
Editorial, page 1854
e-Pub ahead of print on October 22, 2008, at www.neurology.org.
Supported by grant number K23NS056091 from the National Institute of Neurological Disorders and Stroke (NINDS) and GE Healthcare.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIH.
Disclosure: The authors report no disclosures.
Received March 19, 2008. Accepted in final form June 23, 2008.
REFERENCES
- 1.Yu A, Li K, Li L, Shan B, Wang Y, Xue S. Whole-brain voxel-based morphometry of white matter in medial temporal lobe epilepsy. Eur J Radiol 2008;65:86–90. [DOI] [PubMed] [Google Scholar]
- 2.Hermann B, Seidenberg M, Bell B, et al. Extratemporal quantitative MR volumetrics and neuropsychological status in temporal lobe epilepsy. J Int Neuropsychol Soc 2003;9:353–362. [DOI] [PubMed] [Google Scholar]
- 3.Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 2007;36:630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Concha L, Beaulieu C, Gross DW. Bilateral limbic diffusion abnormalities in unilateral temporal lobe epilepsy. Ann Neurol 2005;57:188–196. [DOI] [PubMed] [Google Scholar]
- 5.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed 2002;15:435–455. [DOI] [PubMed] [Google Scholar]
- 6.Diehl B, Busch RM, Duncan JS, Piao Z, Tkach J, Luders HO. Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia 2008 (in press). [DOI] [PubMed]
- 7.Powell HW, Parker GJ, Alexander DC, et al. Abnormalities of language networks in temporal lobe epilepsy. Neuroimage 2007;36:209–221. [DOI] [PubMed] [Google Scholar]
- 8.Powell HW, Parker GJ, Alexander DC, et al. Imaging language pathways predicts postoperative naming deficits. J Neurol Neurosurg Psychiatry 2008;79:327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia. Brain 1987;110:1631–1646. [DOI] [PubMed] [Google Scholar]
- 10.Morris R, Pandya DN, Petrides M. Fiber system linking the mid-dorsolateral frontal cortex with the retrosplenial/presubicular region in the rhesus monkey. J Comp Neurol 1999;407:183–192. [DOI] [PubMed] [Google Scholar]
- 11.Nestor PG, Kubicki M, Gurrera RJ, et al. Neuropsychological correlates of diffusion tensor imaging in schizophrenia. Neuropsychology 2004;18:629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szeszko PR, Robinson DG, Ashtari M, et al. Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology 2008;33:976–984. [DOI] [PubMed] [Google Scholar]
- 13.Nestor PG, Kubicki M, Kuroki N, et al. Episodic memory and neuroimaging of hippocampus and fornix in chronic schizophrenia. Psychiatry Res 2007;155:21–28. [DOI] [PubMed] [Google Scholar]
- 14.Powell HW, Parker GJ, Alexander DC, et al. Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. Neuroimage 2006;32:388–399. [DOI] [PubMed] [Google Scholar]
- 15.Duffau H. The anatomo-functional connectivity of language revisited: new insights provided by electrostimulation and tractography. Neuropsychologia 2008;46:927–934. [DOI] [PubMed] [Google Scholar]
- 16.Duffau H, Gatignol P, Mandonnet E, Peruzzi P, Tzourio-Mazoyer N, Capelle L. New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain 2005;128:797–810. [DOI] [PubMed] [Google Scholar]
- 17.Wechsler D. WMS-III Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation, 1997. [Google Scholar]
- 18.Delis DC, Kaplan E, Kramer JH. Manual for the Delis-Kaplan Executive Function System (D-KEFS). San Antonio, TX: The Psychological Corporation, 2001. [Google Scholar]
- 19.Kaplan EF, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lea & Febiger, 1983. [Google Scholar]
- 20.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–355. [DOI] [PubMed] [Google Scholar]
- 21.Yogarajah M, Duncan JS. Diffusion-based magnetic resonance imaging and tractography in epilepsy. Epilepsia 2008;49:189–200. [DOI] [PubMed] [Google Scholar]
- 22.Jovicich J, Czanner S, Greve D, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage 2006;30:436–443. [DOI] [PubMed] [Google Scholar]
- 23.Hagler DJ Jr, Ahmadi ME, Kuperman J, et al. Automated white matter tractography using a probabilistic diffusion tensor atlas: application to temporal lobe epilepsy. Hum Brain Mapp 2008 Jul 31 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 24.Petrides M, Pandya DN. Association fiber pathways to the frontal cortex from the superior temporal region in the rhesus monkey. J Comp Neurol 1988;273:52–66. [DOI] [PubMed] [Google Scholar]
- 25.Makris N, Papadimitriou GM, van der Kouwe A, et al. Frontal connections and cognitive changes in normal aging rhesus monkeys: a DTI study. Neurobiol Aging 2007;28:1556–1567. [DOI] [PubMed] [Google Scholar]
- 26.Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science 1991;253:1380–1386. [DOI] [PubMed] [Google Scholar]
- 27.Papez JW. A proposed mechanism of emotion. 1937. J Neuropsychiatry Clin Neurosci 1995;7:103–112. [DOI] [PubMed] [Google Scholar]
- 28.Warburton EC, Aggleton JP, Muir JL. Comparing the effects of selective cingulate cortex lesions and cingulum bundle lesions on water maze performance by rats. Eur J Neurosci 1998;10:622–634. [DOI] [PubMed] [Google Scholar]
- 29.Knight RT, Grabowecky MF, Scabini D. Role of human prefrontal cortex in attention control. Adv Neurol 1995;66:21–34; discussion 34–26. [PubMed]
- 30.Raboyeau G, De Boissezon X, Marie N, et al. Right hemisphere activation in recovery from aphasia: lesion effect or function recruitment? Neurology 2008;70:290–298. [DOI] [PubMed] [Google Scholar]
- 31.Lu LH, Crosson B, Nadeau SE, et al. Category-specific naming deficits for objects and actions: semantic attribute and grammatical role hypotheses. Neuropsychologia 2002;40:1608–1621. [DOI] [PubMed] [Google Scholar]
- 32.Keller SS, Mackay CE, Barrick TR, Wieshmann UC, Howard MA, Roberts N. Voxel-based morphometric comparison of hippocampal and extrahippocampal abnormalities in patients with left and right hippocampal atrophy. Neuroimage 2002;16:23–31. [DOI] [PubMed] [Google Scholar]
- 33.Bonilha L, Alessio A, Rorden C, et al. Extrahippocampal gray matter atrophy and memory impairment in patients with medial temporal lobe epilepsy. Hum Brain Mapp 2007;28:1376–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaz SA. Nonverbal memory functioning following right anterior temporal lobectomy: a meta-analytic review. Seizure 2004;13:446–452. [DOI] [PubMed] [Google Scholar]
- 35.Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage 2004;21:1174–1181. [DOI] [PubMed] [Google Scholar]
- 36.Nucifora PG, Verma R, Lee SK, Melhem ER. Diffusion-tensor MR imaging and tractography: exploring brain microstructure and connectivity. Radiology 2007;245:367–384. [DOI] [PubMed] [Google Scholar]