Abstract
Objective:
Small uncontrolled series suggest that treatment of obstructive sleep apnea (OSA) in patients with epilepsy may improve seizure control. Prior to conducting a definitive randomized controlled trial, we addressed critical design issues in a pilot study.
Methods:
We identified a cohort of adult patients with medically refractory epilepsy and coexisting OSA, documented by polysomnography (PSG). After an 8-week baseline period, subjects with OSA were randomized to therapeutic or sham continuous positive airway pressure (CPAP) for 10 weeks. Subjects maintained seizure calendars and antiepileptic drug dosages were held constant.
Results:
Sixty-eight subjects with suspected OSA were enrolled and 35 subjects randomized to therapeutic CPAP (22 subjects) or sham (13 subjects) CPAP. Male gender and an elevated sleep apnea questionnaire score were predictive of OSA on PSG. Nineteen subjects in the therapeutic group and all 13 subjects in the sham group completed the trial. Baseline apnea-hypopnea index (AHI) and CPAP adherence were comparable between groups. A significant reduction in AHI was observed in the therapeutic CPAP group as compared to the sham group. Subjects, study coordinators, and principal investigators were unable to predict treatment allocation.
Conclusions:
This pilot study provided critical information related to study design and feasibility for planning a comprehensive trial to test the hypothesis that treating obstructive sleep apnea in patients with epilepsy improves seizure control.
GLOSSARY
- AEDs
= antiepileptic drugs;
- AHI
= apnea-hypopnea index;
- BMI
= body mass index;
- CPAP
= continuous positive airway pressure;
- OSA
= obstructive sleep apnea;
- PSG
= polysomnography.
Epilepsy has a prevalence of 0.5–1.01 and 30% of affected individuals continue to have seizures despite antiepileptic drugs (AEDs).2 Reducing factors which may promote seizures, such as sleep deprivation,3–5 could lead to new and more effective treatment strategies.
Obstructive sleep apnea (OSA), a common disorder resulting in sleep disruption and deprivation,6 is present in as many as 33% of patients with refractory partial epilepsy. 7 In older adults with epilepsy, the presence of sleep apnea is associated with worsening seizure control or late onset seizures.8 While several clinical series have reported improvements in seizure control after identification and treatment of comorbid OSA,9–12 none of these studies involved randomization, double-blind design, or the use of sham continuous positive airway pressure (CPAP) as a control condition. A definitive clinical trial with these features could establish the importance of screening for and treating OSA within an epilepsy population. Several unanswered questions have complicated planning such a trial, however. These include efficient identification of OSA, adherence to and blinding of CPAP, and the magnitude of seizure reduction that might be anticipated after treatment.
To address these questions and effectively plan for such a definitive randomized multicenter study, we performed a prospective, randomized, double-blind, preliminary trial of CPAP for seizure reduction in patients with refractory epilepsy with coexisting OSA.
METHODS
Screening and enrollment.
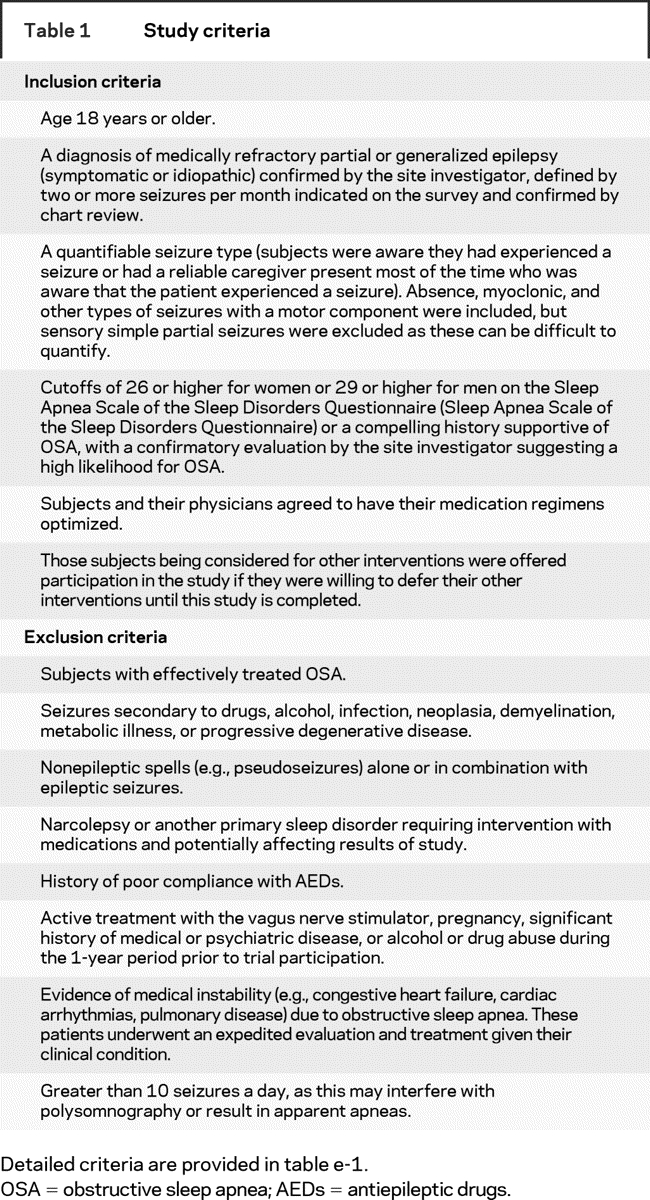
This pilot clinical trial was conducted between September 2002 and July 2005 at University of Michigan, Cleveland Clinic, University of North Carolina at Chapel Hill, and Vanderbilt University, with a Data Coordinating Center at the University of Michigan. The study was approved by each site’s Institutional Review Board and a National Institute of Neurological Disorders and Stroke Data Safety and Monitoring Board also oversaw conduct of the trial. The Food and Drug Administration granted us an Investigational Device Exemption to study sham CPAP in our subjects. Epilepsy clinic patients initially meeting trial eligibility on the basis of chart review completed the Sleep Apnea Scale of the Sleep Disorders Questionnaire,13 and underwent a sleep and epilepsy evaluation by the site investigator. Subjects continuing to meet eligibility criteria (table 1, table e-1 on the Neurology® Web site at www.neurology.org), including two or more quantifiable partial or generalized seizures per month (excluding simple partial seizures), were invited to provide informed consent to participate in the trial. During the initial enrollment visit, subjects were given basic sleep hygiene information, including avoiding sleep deprivation and alcohol, and minimizing sleeping in the supine position. Subjects were given standardized seizure calendars and instructed how to tabulate each specific seizure type.
Table 1 Study criteria
Baseline and treatment phases.
After enrollment, subjects participated in an 8-week baseline phase which included two nights of diagnostic PSG. Those meeting study criteria for OSA by PSG were randomized in a 2:1 ratio to either therapeutic or sham treatment with CPAP using a computerized random number generator at the Data Coordinating Center, with envelopes containing the specified assignment (therapeutic vs sham CPAP) mailed to each site. The sleep technologist at each site opened the randomization envelope prior to the titration nights. Subjects then underwent two nights of either therapeutic or sham CPAP titration, during which subjects randomized to therapeutic CPAP were titrated to the appropriate pressure that resolved their apneas and hypopneas. Those randomized to sham CPAP slept with the sham CPAP both nights. Our rationale for including two nights was to document an AHI on night 2 of the therapeutic or sham titration that could be compared with the subjects’ AHI from their baseline PSGs; as night 1 of a titration involves attempting a variety of pressure settings in the therapeutic group as well as adjustment of masks in both therapeutic and sham groups, night 2 provided a clearer indicator of AHI on an effective CPAP setting. After the two nights of titration, subjects were given a home CPAP unit (either therapeutic or sham). Sham CPAP methodology is described in appendix e-1 and figure e-1.
To preserve blinding, subjects were loaned CPAP machines (Respironics REMstar Pro CPAP System, Respironics, Inc, Murrysville, PA) and observed during a 10-week treatment phase (2 weeks of CPAP acclimation followed by 8 additional weeks of treatment). At the end of the study, all subjects were placed on therapeutic CPAP (those on sham were titrated to the appropriate pressure that resolved their apneas and hypopneas). Subjects received a weekly phone call from the site coordinator inquiring about adherence to CPAP and the presence of adverse events. The study coordinators at each site were blinded to the intervention, as were the principal site investigators and sleep technologists who analyzed the studies for staging and respiratory events. The only individuals aware of the intervention were the sleep technologists who performed the studies and a physician at each site, separate from the principal investigator, who reviewed the results of the therapeutic CPAP titrations with the sleep technologists prior to initiating CPAP.
Polysomnography.
The PSG recordings included EEG, electrooculogram, submental EMG, nasal-oral airflow, respiratory effort, pulse oximetry, and anterior tibialis EMG. At all sites, studies were staged according to standard criteria14 by a registered PSG technologist who was blinded to the treatment status. Respiratory events were scored using the following criteria: an obstructive apnea was defined by an decrease in the thermistor channel by 90% or greater for 10 or more seconds; a hypopnea was defined by a decrease in the nasal pressure transducer channel by 50% or greater for 10 or more seconds, associated with either a 3% oxygen desaturation or an EEG arousal lasting 3 seconds or longer.15 All studies were reviewed by the site investigator. To further enhance the quality of the measurements and ensure reliability among sites, one of the registered sleep technologists visited each of the other sites to review records and confirm consistency in the scoring of sleep stages and respiratory events. To meet eligibility for the study, an AHI of 5 or greater on at least one night of study was required. To expedite treatment in subjects with severe sleep apnea, we planned to exclude from randomization subjects whose AHIs were greater than 50 on at least one night of study, although no enrolled subjects met this criterion. In prior work, we have shown that the AHI on nights 1 and 2 of study were comparable.16 Therefore, we averaged the AHI from nights 1 and 2 to determine the baseline AHI for each subject.
CPAP adherence.
CPAP adherence was measured using cards built into the CPAP systems. These cards were downloaded into a commercially available software package (Respironics, Inc.) and provided the time used in a 4-week period.
Data collection and statistical analysis.
Our analyses focused on design and feasibility issues rather than efficacy of CPAP in reducing seizure frequency. Therefore, we did not power our trial to have sufficient sample size to test efficacy. Instead, we screened outpatient clinic rosters and contacted eligible subjects arriving in our epilepsy clinics to determine the ratio of subjects screened to subjects enrolled and reasons for exclusion. This information was collected to evaluate whether specific study criteria (e.g., seizure frequency) needed to be modified to maximize enrollment in a larger scale trial.
Data were collected on case report forms completed by site personnel and entered electronically onto a Web-based data entry system with second data entry and verification by the Data Coordinating Center. Adverse events were tabulated (table e-2).
To answer the study-related design questions stated in the Introduction, the Mann-Whitney test (for continuous variables) and the Mantel-Haenszel test (for categorical variables) were used to compare groups, along with Spearman correlation to assess the association of age with CPAP adherence. The likelihood that subjects and study personnel could predict whether an individual subject was using therapeutic or sham CPAP was assessed using the Kappa statistic.
Seizure frequencies for the 8-week baseline phase and the 8-week treatment phase (after 2 weeks of acclimation) were tabulated. Fisher exact test was used to compare the proportions of subjects in each group who had obtained a greater than 50% reduction in seizure frequency (responder rate), an established metric in epilepsy clinical trials.17
RESULTS
Subjects.
We screened 865 subjects to enroll 68 subjects meeting criteria into the study (figure 1). The leading reason for ineligibility was not meeting criteria for seizure frequency and quantification (401 subjects), followed by not meeting the SA-SDQ cutoffs (353 subjects). Between the time of enrollment and polysomnography, 23 subjects exited from the study. Compared to those who were retained in the study until the PSG, these subjects did not differ in age, gender, body mass index (BMI), SA-SDQ score, or baseline seizure frequency.
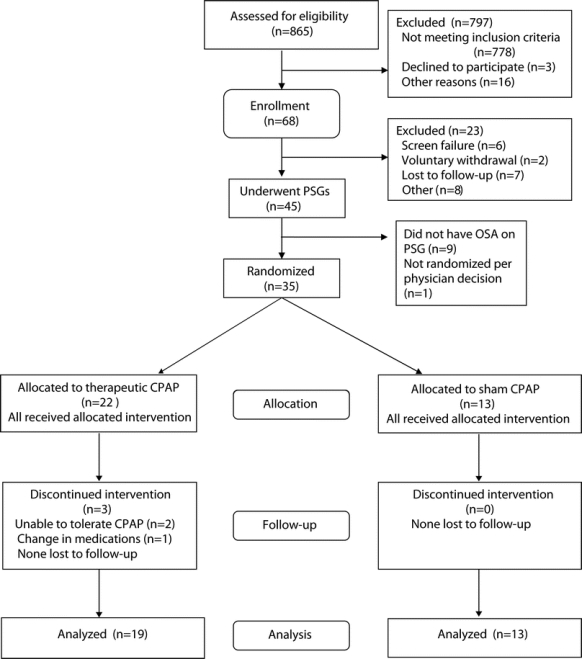
Figure 1 Flow diagram showing screening, enrollment, randomization, and follow-up of subjects
Of 45 subjects undergoing PSG, 36 met PSG criteria for OSA (80%), 35 were randomized (one was not randomized per physician discretion), and 32 completed the study. Only three subjects discontinued the intervention after randomization, all in the therapeutic group. One subject desired admission to an epilepsy monitoring unit for seizure localization, necessitating medication taper. The other two subjects withdrew because they found CPAP cumbersome to use, although they did not experience any adverse effects from the device.
Characteristics of our sample and identification of subjects with PSG-documented OSA.
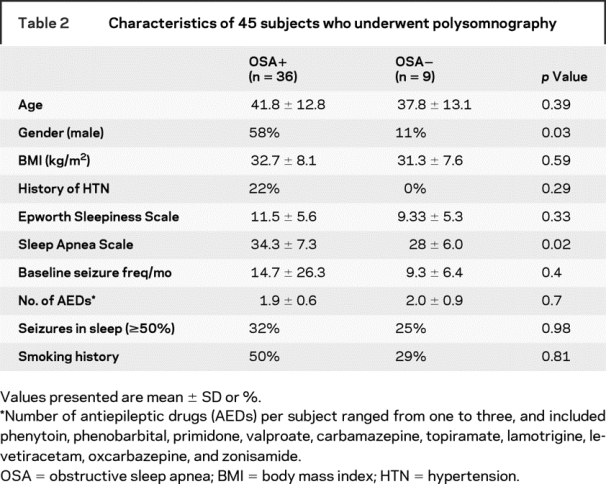
The majority of subjects had partial epilepsy, with generalized epilepsy present in only seven subjects. In comparing the 36 subjects meeting PSG criteria for OSA (AHI ≥ 5) with the 9 subjects who did not meet PSG criteria (table 2), only male gender and the total SA-SDQ score were significantly higher in the subjects with OSA. All subjects with a history of hypertension were in the group with PSG-documented OSA, although the proportion of subjects with hypertension in the two groups did not reach significance.
Table 2 Characteristics of 45 subjects who underwent polysomnography
Effect of mode of CPAP (therapeutic vs sham) on AHI during titration study.
Subjects randomized to therapeutic vs sham CPAP did not differ in age, gender, BMI, baseline seizure frequency, or AED number (table 3). Three subjects in the sham group and none in the therapeutic group were taking barbiturates or benzodiazepines. The AHI on the baseline studies did not differ in those randomized to therapeutic vs sham CPAP. The second night of CPAP titration demonstrated a significant reduction in the AHI in the therapeutic group as compared to the sham group (p < 0.001; figure 2).
Table 3 Comparison of subjects receiving therapeutic vs sham CPAP
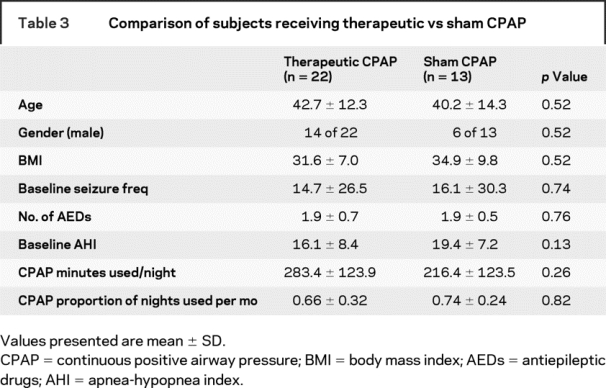
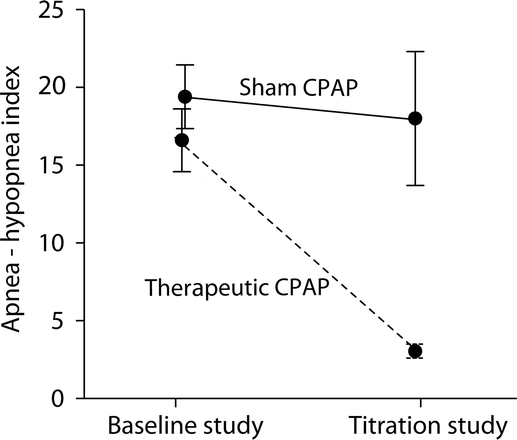
Figure 2 Reduction in apnea-hypopnea index (AHI) between baseline and titration studies
Subjects in the therapeutic continuous positive airway pressure (CPAP) group showed a significant reduction in AHI (reduced to 3.1 ± 2.4 events/hour on CPAP from 16.1 ± 8.4 events/hour at baseline) as compared to the sham group (reduced to 18.0 ± 14.9 events/hour on CPAP from 19.4 ± 7.1 events/hour at baseline). The AHI difference on CPAP in the therapeutic and sham CPAP groups was significant (p < 0.0001).
CPAP tolerance and adherence.
Adverse events were mild, resolved with treatment, and did not result in study discontinuation (details in supplemental data). Overall, the proportion of nights that subjects used CPAP was 0.68 ± 0.30, with average minutes used per night of 265.4 ± 125 (4.4 hours). Adherence was similar in the therapeutic and sham groups (table 3). Older age was associated with increased nightly minutes of CPAP use (r = 0.57; p = 0.002). Subjects 45 years of age and older used their CPAP for 311.1 ± 135.2 minutes/night as opposed to 219.7 ± 98.8 minutes/night in the younger subjects (p = 0.07). Percentage of nights CPAP was used did not correlate with age. Gender did not influence CPAP use.
Blinding.
The likelihood of any group to predict whether a subject was receiving treatment with therapeutic vs sham CPAP after accounting for chance was only in the fair range for subjects (kappa = 0.30), study coordinators (kappa = 0.36), and principal site investigators (kappa = 0.30).
Effect of therapeutic vs sham CPAP on seizure frequency.
A 50% or greater reduction in seizures was observed in 28% of the subjects in the therapeutic group as compared to 15% of those in the sham group, although this result was not statistically significant (p = 0.40). Four subjects treated with therapeutic CPAP and one subject treated with sham CPAP became seizure-free. Two of the subjects treated with therapeutic CPAP who had a 50% or greater reduction in seizures had mild sleep apnea, both with baseline AHIs of 5.2 events/hour. Based on these results, in a definitive trial, 150 subjects in each group (therapeutic and sham) would be needed to detect a 13% reduction in seizures, using a χ2 test at 0.05 significance level and 80% power.
DISCUSSION
Our major findings from this pilot study were as follows: 1) subjects could be successfully screened for the trial as evidenced by a high proportion of patients having PSG-documented OSA; 2) subjects given therapeutic and sham CPAP were able to remain in the trial to completion, with few dropouts; 3) sham CPAP functioned as an effective blinding modality, in that the AHI was not reduced in subjects given this modality as compared to those who were titrated with therapeutic CPAP; 4) subjects and study personnel could not consistently distinguish sham from therapeutic CPAP use; 5) subjects treated with therapeutic vs sham CPAP used their devices a similar number of hours/night, with older subjects showing the highest adherence to CPAP; and 6) treatment was associated with few adverse events, which were mild, resolvable, and did not lead to study discontinuation (the two subjects who discontinued CPAP did so because it was cumbersome to use). We were also able to demonstrate that we could conduct a study involving complex and multiple procedures, including sham CPAP, across sites and estimate the magnitude of the potential effect on seizure reduction that we might expect to find in a large scale RCT. A summary of response to AED treatment in medically refractory epilepsy has documented comparable responder rates to ours for active and placebo treatment with AEDs.17
Higher SA-SDQ scores were more likely to be associated with PSG-associated OSA, as was male gender. Of interest, the level of subjective daytime sleepiness was not associated with having PSG-associated OSA, perhaps because patients with epilepsy may be sleepy for a variety of reasons (e.g., seizures, AED use). Prior studies have shown that adult subjects can be successfully identified as having PSG-documented OSA by using screening questionnaires that assessed age, sex, BMI,13,18,19 and, in some instruments, daytime sleepiness.20–22 We previously showed that, in an epilepsy-specific population, the SA-SDQ was a useful screening instrument for OSA.23
BMI was not associated with having PSG-associated OSA. This may reflect that other factors are more tightly associated with having PSG-associated OSA than BMI in the epilepsy population, or that our sample included subjects with a relatively high BMI (the BMI was >30 kg/m2 in the group with and without PSG-associated OSA; table 2).
Sham CPAP has been used in prior trials involving subjects with OSA24–26 as well as in ongoing trials.27 However, with the exception of one study using sham noninvasive ventilation in patients with amyotrophic lateral sclerosis,26 ours is the only trial to 1) focus on patients with a neurologic disorder, and 2) document that subjects could be blinded to treatment received. Our trial also confirmed that sham CPAP does not significantly lower AHI compared to baseline, while therapeutic CPAP does, as had been shown in prior reports.28–30 As in other trials, subjects treated with sham CPAP did not differ in adherence measures from those treated with therapeutic CPAP. In contrast to the expectation that patients with a neurologic disorder might show diminished CPAP adherence in comparison to that reported for other groups, our subjects were adherent with CPAP at rates comparable to these other clinical studies, with CPAP used, on average, 68% of nights and 4.4 hours per night. Older adults were even more likely to use CPAP, although the reason for this improved adherence is uncertain. Given that increasing age was associated with OSA as well as increased adherence, and that OSA in older adults is associated with worsening seizure control or late onset seizures,8 it may be justifiable to target older adults in a future trial of the effects of treating OSA on epilepsy.
Being a pilot study with a relatively small sample size, there were inherent limitations present that a large scale trial will need to address. For example, we were unable to isolate the effects of specific AEDs on the presence of sleep apnea. Some seizure types, such as the idiopathic generalized epilepsies, may be more sensitive to sleep deprivation and therefore improved by treatment of a sleep disorder. The severity of sleep apnea or epilepsy may affect response to treatment. For safety concerns, we excluded patients with severe OSA. This selection may have influenced our results; however, two of our five subjects experiencing a 50% or greater reduction in seizure frequency on therapeutic CPAP had AHIs in the mild range (e.g., five events/hour). If large scale studies show that treatment of even mild OSA results in seizure reductions in patients with epilepsy, that would justify including epilepsy as an associated condition, along with hypertension, cardiac disease, and mood disorders, in determining whether mild OSA should be treated. Such studies would also heighten the awareness among general neurologists and epilepsy specialists that treating OSA may assist in managing a seizure disorder. Our study also relied on seizure calendars to quantify seizures, a methodology which may underestimate seizure frequency,31 especially given that patients with epilepsy with OSA may have more attention and memory problems than patients with epilepsy without OSA. In a large scale trial, obtaining objective monitoring of seizure occurrence may be warranted.
While large-scale randomized multisite clinical trials will be necessary to confirm our results, our findings support a larger evolving literature advocating the treatment of sleep disorders, including OSA, in patients with epilepsy and other neurologic diseases.
ACKNOWLEDGMENT
Drs. Terri Weaver and David Rapoport assisted us in the implementation of the sham CPAP device.
Supplementary Material
Address correspondence and reprint requests to Dr. Beth A. Malow, Vanderbilt University, Department of Neurology, Medical Center North, Room A-0118, 1161 21st Avenue South, Nashville, TN 37232-2551 beth.malow@vanderbilt.edu
Supplemental data at www.neurology.org
Supported by NINDS R01 NS42698 and the General Clinical Research Centers at University of Michigan (M01-RR00042), University of North Carolina-Chapel Hill (M01-RR00146), and Vanderbilt University (M01-RR00095). Respironics, Inc. provided continuous positive airway pressure equipment for the trial. No other support for the trial was provided by Respironics.
Disclosure: The authors report no disclosures.
Received November 11, 2007. Accepted in final form May 7, 2008.
REFERENCES
- 1.Hauser WA, Annegers JF, Kurland LT. The prevalence of epilepsy in Rochester, Minnesota, 1940–1980. Epilepsia 1991;32:429–445. [DOI] [PubMed] [Google Scholar]
- 2.Sander JW. Some aspects of prognosis in the epilepsies: a review. Epilepsia 1993;34:1007–1016. [DOI] [PubMed] [Google Scholar]
- 3.Janz D. The grand mal epilepsies and the sleeping-waking cycle. Epilepsia 1962;3:69–109. [DOI] [PubMed] [Google Scholar]
- 4.Rajna P, Veres J. Correlations between night sleep duration and seizure frequency in temporal lobe epilepsy. Epilepsia 1993;34:574–579. [DOI] [PubMed] [Google Scholar]
- 5.Frucht MM, Quigg M, Schwaner C, Fountain NB. Distribution of seizure precipitants among epilepsy syndromes. Epilepsia 2000;41:1534–1539. [DOI] [PubMed] [Google Scholar]
- 6.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230–1235. [DOI] [PubMed] [Google Scholar]
- 7.Malow BA, Levy K, Maturen K, Bowes R. Obstructive sleep apnea is common in medically refractory epilepsy patients. Neurology 2000;55:1002–1007. [DOI] [PubMed] [Google Scholar]
- 8.Chihorek AM, Abou-Khalil B, Malow BA. Obstructive sleep apnea is associated with seizure occurrence in older adults with epilepsy. Neurology 2007;69:1823–1827. [DOI] [PubMed] [Google Scholar]
- 9.Malow BA, Fromes GA, Aldrich MS. Usefulness of polysomnography in epilepsy patients. Neurology 1997;48:1389–1394. [DOI] [PubMed] [Google Scholar]
- 10.Devinsky O, Ehrenberg B, Barthlen GM, Abramson HS, Luciano D. Epilepsy and sleep apnea syndrome. Neurology 1994;44:2060–2064. [DOI] [PubMed] [Google Scholar]
- 11.Vaughn BV, D’Cruz, OF, Beach R, Messenheimer JA. Improvement of epileptic seizure control with treatment of obstructive sleep apnoea. Seizure 1996;5:73–78. [DOI] [PubMed] [Google Scholar]
- 12.Malow BA, Weatherwax KJ, Chervin RD, et al. Identification and treatment of obstructive sleep apnea in adults and children with epilepsy: a prospective pilot study. Sleep Medicine 2003;4:509–515. [DOI] [PubMed] [Google Scholar]
- 13.Douglass AB, Bornstein R, Nino-Murcia G, et al. The Sleep Disorders Questionnaire. I: Creation and multivariate structure of SDQ. Sleep 1994;17:160–167. [DOI] [PubMed] [Google Scholar]
- 14.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring Systems for Sleep Stages of Human Subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute, 1968. [Google Scholar]
- 15.American Sleep Disorders Association. EEG Arousals: Scoring rules and examples. A preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep 1992;15:173–184. [PubMed] [Google Scholar]
- 16.Selwa LM, Marzec ML, Chervin RD, et al. Sleep staging and respiratory events in epilepsy patients: is there a first night effect? Epilepsia 2008 (in press). [DOI] [PMC free article] [PubMed]
- 17.French J, Kanner A, Bautista J. Efficacy and tolerability of the new antiepileptic drugs II: treatment of refractory epilepsy. Neurology 2004;62:1261–1273. [DOI] [PubMed] [Google Scholar]
- 18.Dealberto MJ, Ferber C, Garma L, Lemoine P, Alperovitch A. Factors related to sleep apnea syndrome in sleep clinic patients. Chest 1994;105:1753–1758. [DOI] [PubMed] [Google Scholar]
- 19.Rowley JA, Aboussouan LS, Badr MS. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep 2000;23:929–938. [DOI] [PubMed] [Google Scholar]
- 20.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999;131:485–491. [DOI] [PubMed] [Google Scholar]
- 21.Kump K, Whalen C, Tishler PV, et al. Assessment of the validity and utility of a sleep-symptom questionnaire. Am J Respir Crit Care Med 1994;150:735–741. [DOI] [PubMed] [Google Scholar]
- 22.Pouliot Z, Peters M, Neufeld H, Kryger MH. Using self-reported questionnaire data to prioritize OSA patients for polysomnography. Sleep 1997;20:232–236. [DOI] [PubMed] [Google Scholar]
- 23.Weatherwax KJ, Lin X, Marzec ML, Malow BA. Obstructive sleep apnea in epilepsy patients: the Sleep Apnea scale of the Sleep Disorders Questionnaire (SA-SDQ) is a useful screening instrument for obstructive sleep apnea in a disease-specific population. Sleep Med 2003;4:517–521. [DOI] [PubMed] [Google Scholar]
- 24.Montserrat JM, Ferrer M, Hernandez L, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med 2001;164:608–613. [DOI] [PubMed] [Google Scholar]
- 25.Barbe F, Mayoralas LR, Duran J, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. a randomized, controlled trial. Ann Intern Med 2001;134:1015–1023. [DOI] [PubMed] [Google Scholar]
- 26.Gruis KL, Brown DL, Weatherwax KJ, Feldman EL, Chervin RD. Evaluation of sham non-invasive ventilation for randomized, controlled trials in ALS. Amyotroph Lateral Scler 2006;7:96–99. [DOI] [PubMed] [Google Scholar]
- 27.Kushida CA, Nichols DA, Quan SF. The apnea positive pressure long-term efficacy study (APPLES): rationale, design, methods, and procedures. J Clin Sleep Med 2006;2:288–300. [PubMed] [Google Scholar]
- 28.Henke KG, Grady JJ, Kuna ST. Effect of nasal continuous positive airway pressure on neuropsychological function in sleep apnea-hypopnea syndrome. A randomized, placebo-controlled trial. Am J Respir Crit Care Med 2001;163:911–917. [DOI] [PubMed] [Google Scholar]
- 29.Loredo JS, Ancoli-Israel S, Dimsdale JE. Effect of continuous positive airway pressure vs placebo continuous positive airway pressure on sleep quality in obstructive sleep apnea. Chest 1999;116:1545–1549. [DOI] [PubMed] [Google Scholar]
- 30.Yu BH, Ancoli-Israel S, Dimsdale JE. Effect of CPAP treatment on mood states in patients with sleep apnea. J Psychiatr Res 1999;33:427–432. [DOI] [PubMed] [Google Scholar]
- 31.Hoppe C, Poepel A, Elger CE. Epilepsy: accuracy of patient seizure counts. Arch Neurol 2007;64:1595–1599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


