Abstract
Steroid hormones are synthesized in the adrenal gland, gonads, placenta and brain and are critical for normal reproductive function and bodily homeostasis. The steroidogenic acute regulatory (StAR) protein regulates the rate-limiting step in steroid biosynthesis, i.e. the delivery of cholesterol from the outer to the inner mitochondrial membrane. The expression of the StAR protein is predominantly regulated by cAMP-dependent mechanisms in the adrenal and gonads. Whereas StAR plays an indispensable role in the regulation of steroid biosynthesis, a complete understanding of the regulation of its expression and function in steroidogenesis is not available. It has become clear that the regulation of StAR gene expression is a complex process that involves the interaction of a diversity of hormones and multiple signaling pathways that coordinate the cooperation and interaction of transcriptional machinery, as well as a number of post-transcriptional mechanisms that govern mRNA and protein expression. However, information is lacking on how the StAR gene is regulated in vivo such that it is expressed at appropriate times during development and is confined to the steroidogenic cells. Thus, it is not surprising that the precise mechanism involved in the regulation of StAR gene has not yet been established, which is the key to understanding the regulation of steroidogenesis in the context of both male and female development and function.
Keywords: StAR gene expression, cAMP signaling, AKAP, transcription, translation
Introduction
Steroidogenic endocrine tissues such as the adrenal gland and the gonads respond to trophic hormones and other external stimuli with a rapid surge in steroid hormone production (Leung and Steele, 1992; Saez, 1994; Cooke, 1999; Ascoli et al., 2002). This acute steroidogenic response is now known to be predominantly dictated by steroidogenic acute regulatory (StAR), a rapidly synthesized labile phosphoprotein whose expression, activation and extinction is regulated by protein kinase A (PKA), PKC, as well as a host of other signaling pathways (reviewed in Stocco and Clark, 1996; Manna and Stocco, 2005). In defining StAR’s role, studies have demonstrated a tight correlation between the synthesis of StAR protein and the synthesis of steroids, and StAR expression is primarily associated with steroidogenic tissues in vertebrates. Consequently, the StAR protein plays a vital role in the regulation of steroid hormones required for life itself, in the case of adrenal steroids, and for maintaining reproductive capacity, in the case of gonadal steroids (Clark et al., 1994; Stocco and Clark, 1996; Manna and Stocco, 2005). The crucial role of StAR in the regulation of steroidogenesis has been obtained from patients suffering from lipoid congenital adrenal hyperplasia (lipoid CAH), an autosomal recessive disorder in which both adrenal and gonadal steroid biosyntheses are severely impaired due to mutations in the StAR gene (Lin et al., 1995; Bose et al., 1996). The targeted disruption of the StAR gene in mouse results in a phenotype that is essentially identical to that found in lipoid CAH in humans (Caron et al., 1997; Hasegawa et al., 2000). To date, dozens of nonsense and deletion mutations have been identified in the StAR gene that cause lipoid CAH in humans, and the incidence of this disease has been shown to be higher in the people of Japanese, Korean and Palestinian ancestry (Bose et al., 1996; Miller and Strauss, 1999; Stocco, 2002). It has been demonstrated that the mutant StAR proteins, in contrast to the wild type, were completely inactive in promoting steroid synthesis (Lin et al., 1995; Bose et al., 1996).
The expression of the StAR protein is predominantly associated with the steroid-producing cells of the adrenal, ovary and testis in the adult (Clark et al., 1995b, Hasegawa et al., 2000; Manna and Stocco, 2005). During development, StAR mRNA has first been detected in mouse embryos at Day 10.5 (E10.5) in the urogenital ridge, a region that ultimately gives rise to the adrenals, gonads and a portion of the kidneys (Clark et al., 1995b). StAR expression is confined in the ovary at puberty and it has been shown to be expressed during various ovulatory phases, in the interstitum, atretic follicles, granulosa and theca and corpora luteal cells (Balasubramanian et al., 1997; Pollack et al., 1997; Ronen-Fuhrmann et al., 1998; Kerban et al., 1999; Sekar et al., 2000; Devoto et al., 2001). In the human ovary, the expression of StAR is regulated throughout the luteal phase and plays a key role in controlling luteal progesterone production during the development and demise of the corpus luteum (Devoto et al., 2002; Kohen et al., 2003; Sierralta et al., 2005). StAR mRNA is detected at E12.5 in the adrenal primordia and later is expressed in adrenal glomerulosa and fasciculata-reticularis (Pollack et al., 1997; Lehoux et al., 1998; Zhang et al., 2003). Moreover, the physiological changes in the synthesis of steroid hormones are closely linked with alterations in StAR expression, suggesting tissue-specific importance of the StAR protein during development as well as in reproduction.
In the adrenal and gonads, acute steroidogenesis is mediated by mechanisms that enhance the transcription, translation or the activity of StAR (Clark et al., 1995b; Manna et al., 2002a, 2006a; Manna and Stocco, 2005). It should further be noted that while the inhibition of the StAR protein at the level of transcription or translation results in a dramatic decrease in steroid biosynthesis in these tissues, ∼10% of steroid production occurs through StAR-independent mechanisms (Lin et al., 1995; Clark et al., 1997; Manna et al., 2001). There is now a wealth of information supporting the concept that the regulation of StAR directs acute steroidogenesis, but we are still far from being able to explain why and how certain tissues express StAR and the precision with which they do so. This review will focus on the current level of understanding concerning the regulation of StAR in light of prospective research and will discuss viewpoints that may help to unravel the mechanisms governing StAR expression and steroidogenesis.
Transcription of the StAR gene
Several lines of evidence demonstrate the involvement of multiple transcription factors and signaling pathways in StAR expression and steroidogenesis (Sugawara et al., 1995; Manna et al., 2003b, 2006a, 2009a, b; Stocco et al., 2005; Martin et al., 2008); however, the regulatory mechanisms involved in cAMP-mediated StAR transcription remain unresolved. Transcriptional regulation, a key control mechanism in fundamental biological processes, is mediated by a family of cAMP-responsive nuclear factors in response to the stimulation of the adenylate cyclase signaling pathway. The transcription of the StAR gene utilizes enhancer elements to ‘switch on’ and silencer elements to ‘switch off’ gene expression (Manna et al., 2003b, 2009a; Hiroi et al., 2004; Silverman et al., 2006; Martin et al., 2008). The activation of transcription by cAMP signaling is classically mediated through the interaction of the cAMP response element (CRE)-binding protein (CREB) with a conserved CRE (TGACGTCA), or a minor variation thereof, found in the promoter region of cAMP-responsive genes (Montminy et al., 1986; Meyer and Habener, 1993; Montminy, 1997; De Cesare and Sassone-Corsi, 2000). The StAR promoter sequences of mouse (Clark et al., 1994), human (Sugawara et al., 1995) and rat (Sandhoff et al., 1998) exhibit extensive homology; however, they lack a consensus CRE and as such resemble the promoters of several steroid hydroxylase genes that are regulated by cAMP signaling (Waterman, 1994), suggesting the involvement of alternate mechanisms in cAMP responsiveness. In lieu of a consensus CRE, a number of positive and negative factors (which bind within the region that is ∼200 bp upstream of the transcription start site) as well as signaling processes have been reported in transcriptional regulation of the StAR gene (Fig. 1) (Zazopoulos et al., 1997; Christenson et al., 2001; Shea-Eaton et al., 2002; Manna et al., 2003a, b, 2004, 2009a; Hiroi et al., 2004; Silverman et al., 2006; Martin et al., 2008).
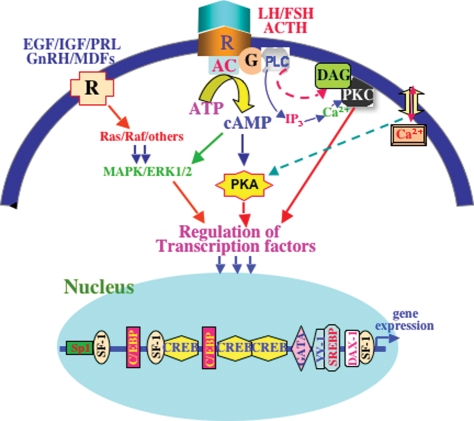
Figure 1.
A schematic model illustrating multiple signaling pathways in regulating StAR gene transcription. The interaction of trophic hormones [luteinizing hormone(LH)/adrenocorticotropic hormone(ACTH)] with specific membrane receptors results in the activation of coupled G proteins (G), which in turn activates membrane-associated adenylyl cyclase (AC) that catalyzes cAMP formation from ATP. cAMP then activates protein kinase A (PKA), which results in the phosphorylation of a number of transcription factors. The concerted action of multiple transcription factors, and their interaction with most, if not all, of the cis-regulatory elements, appears to be involved in regulating StAR gene expression. cAMP-dependent mechanisms predominantly regulate StAR expression and steroidogenesis in steroidogenic tissue. The PKC pathway is involved in regulating transcription of the StAR gene. Ca2+ signaling has been shown to be effectively involved in modulating trophic hormone-stimulated steroidogenesis. Epidermal growth factor (EGF), insulin-like growth factor (IGF), prolactin (PRL), gonadotrophin-releasing hormone (GnRH) and macrophage-derived factors (MDFs) bind to specific membrane receptors (R), activate a cascade of protein kinases (Ras/Raf/others; MAPK/ERK) and have been demonstrated to function in the regulation of StAR expression and steroid biosynthesis.
The cAMP-responsive region of the StAR promoter contains a highly conserved overlapping motif (5′-TGACTGATGA-3′; −81/−72 bp in the mouse) that recognizes the CRE [CREB/CRE modulator (CREM)/activating transcription factor 1], activator protein 1 (AP-1, Fos and Jun) and CCAAT/enhancer-binding protein β (C/EBPβ) families of proteins (Manna et al., 2003a, 2004; Hiroi et al., 2004; Clem et al., 2005; Silverman et al., 2006; Manna and Stocco, 2008; Yivgi-Ohana et al., 2009). These proteins belong to the basic leucine zipper (bZIP) family of transcription factors, which are structurally similar and can interact with themselves, or each other, to form selective dimers that will result in a variety of transcriptional responses (Dwarki et al., 1990; Hai and Curran, 1991; Masquilier and Sassone-Corsi, 1992; Millhouse et al., 1998; Rutberg et al., 1999; Manna et al., 2009b). The expression and activities of these proteins are mediated by a number of extracellular signals and by the phosphorylation of these proteins on several different Ser and Thr residues by multiple kinases (Abate et al., 1993; Chrivia et al., 1993; Roesler, 2001; Wilson et al., 2002; Johannessen et al., 2004; Tang et al., 2005; Manna et al., 2006b). The activity of these bZIP proteins is induced by the transcriptional co-activators, CREB binding protein (CBP) and its functional homolog p300, which possess histone acetyltransferase activity and communicate between the transcription factors and the basal transcription machinery in order to promote StAR gene transcription (Hiroi et al., 2004; Clem et al., 2005; Silverman et al., 2006; Manna and Stocco, 2007). While histone acetylation plays a major role in cAMP-mediated steroidogenesis, the inhibition of histone deacetylase activity by trichostatin A (TSA) is also effective in increasing StAR expression and steroid synthesis in mouse Leydig cells (Manna and Stocco, unpublished results; Clem and Clark, 2006; Liu et al., 2007). In addition, we have observed that TSA markedly enhances (Bu)2cAMP-stimulated StAR expression and steroid synthesis, suggesting a more prominent role for histone deacetylation in steroidogenesis. However, its mechanism of action has yet to be discerned.
CREB/CREM/ATF plays a major role in a variety of physiological functions including growth and development, and is activated upon phosphorylation by a number of extracellular stimuli and environmental conditions (Nantel et al., 1996; Sanyal et al., 2002; Johannessen et al., 2004; Manna et al., 2004; Sassone-Corsi, 2005; Hogeveen and Sassone-Corsi, 2006; Kehat et al., 2006). Specifically, increased transcriptional activity is observed following the phosphorylation of CREB (P-CREB) at Ser133 or CREM at Ser117, events that are indispensable for their interactions with CBP/p300 (Gonzalez and Montminy, 1989; Chrivia et al., 1993; Kwok et al., 1994; Parker et al., 1996; Fimia et al., 1999). In steroidogenic cells, both PKA and PKC signaling can increase the P-CREB in a time-dependent manner, and this event correlates tightly with P-CREB and CBP’s association with the proximal StAR promoter (Hiroi et al., 2004; Clem et al., 2005; Manna et al., 2006b; Silverman et al., 2006; Sugawara et al., 2006; Manna and Stocco, 2007). Transgenic mice expressing a non-phosphorylatable mutant of CREB (CREB-M1; Ser133→Ala) develop somatotroph hypoplasia and dwarfism, demonstrating the physiological relevance of CREB function in the developmental processes (Struthers et al., 1991). Likewise, CREB-M1 has been shown to diminish the steroidogenic response, indicating that the StAR gene is dependent on P-CREB or a similar CRE-binding protein for proper induction. Studies have shown that P-CREB and P-Fos/Jun-DNA interactions result in CBP recruitment to the StAR promoter and are associated with histone acetylase activity that facilitates chromatin remodeling and thus increases StAR transcription (Hiroi et al., 2004; Clem et al., 2005; Manna and Stocco, 2007, 2008).
The canonical CREB/ATF site (TGACGTCA) differs from the Fos and Jun consensus motif (TGACTCA) by one nucleotide, and thus overlap and/or crosstalk-affecting transcription can occur (Dwarki et al., 1990; Hai and Curran, 1991; Masquilier and Sassone-Corsi, 1992; Millhouse et al., 1998; Rutberg et al., 1999; Manna and Stocco, 2007). Fos and Jun play central roles in proliferation, differentiation and transformation and are regulated in a cell-type-specific manner. The c-Jun proto-oncogene, but not other Fos and Jun members, is critically involved in regulating steroidogenesis in Leydig and adrenal cells (Lehoux et al., 1998; Manna and Stocco, 2008). In accordance with this, mice lacking c-Jun are embryonic lethal with embryos dying between mid and late gestation, underscoring the importance of c-Jun in developmental processes (Hilberg et al., 1993; Johnson et al., 1993). The activation of either the PKA and PKC pathways increases the phosphorylation of c-Jun at Ser63 and c-Fos at Thr325, in turn recruiting CBP to the StAR promoter, and this progression of events serves to regulate StAR gene transcription (Clem et al., 2005; Manna et al., 2006b; Manna and Stocco, 2007, 2008). Phosphorylation of Fos and Jun also alters their capacity to interact with other transcription factor(s), ultimately affecting their dimerization and DNA-binding specificity (Hai and Curran, 1991; Hunter and Karin, 1992; Masquilier and Sassone-Corsi, 1992; Rutberg et al., 1999; Hess et al., 2004). Consequently, crosstalk between CREB and c-Fos/c-Jun has been demonstrated to be associated with both gain-of-function and loss-of-function on a single cis-element in fine tuning the trans-regulatory events involved in StAR gene expression (Manna and Stocco, 2007).
The overlapping CREB/ATF and Fos/Jun binding region in the mouse StAR promoter (−81/−72 bp) is also a target of C/EBPβ, a member of the C/EBP family protein. C/EBPα and C/EBPβ are expressed in steroidogenic cells, and the levels of C/EBPβ in the nucleus are increased by LH and cAMP analogs (Piontkewitz et al., 1996; Nalbant et al., 1998). Also, two putative C/EBP-binding sites have been identified in the human (Christenson et al., 1999) StAR promoter, and the roles of both C/EBPα and C/EBPβ are demonstrated in StAR gene transcription (Christenson et al., 1999; Reinhart et al., 1999; Silverman et al., 1999). In female mice, the disruption of either C/EBPα or C/EBPβ prevents normal reproductive development, leading to reduced or halted ovulation and an inability to form the corpus luteum (Piontkewitz et al., 1996; Sterneck et al., 1997). The phosphorylation of C/EBPβ at Thr325 has been shown to increase the association of C/EBPβ with the proximal StAR promoter (Tremblay et al., 2002; Hsu et al., 2008). Furthermore, CBP/p300 is involved in the trans-activation of C/EBPβ and GATA-4, which also induces StAR gene expression (Silverman et al., 2006). It is tempting to speculate that other factors, in addition to these bZIP proteins, may also bind to the proximal region of the StAR promoter, become phosphorylated in response to cAMP signaling (for example, SF-1, GATA-4) and enhance the recruitment of CBP/p300 to the StAR promoter.
CREB/CREM, Fos/Jun and C/EBPβ interact with each other in addition to a numerous array of transcription factors, and through these interactions, they confer a gradient of effects at the level of StAR gene transcription (Reinhart et al., 1999; Manna et al., 2003a, 2004; Clem et al., 2005; Silverman et al., 2006; Manna and Stocco, 2007). It has been demonstrated that CRE DNA-binding proteins heterodimerize with Fos/Jun and C/EBPs and result in either repression and/or activation of the transcription of several genes (Masquilier and Sassone-Corsi, 1992; Millhouse et al., 1998; Rutberg et al., 1999; Ross et al., 2001; Wilson et al., 2002). We have previously reported that CREB and c-Fos/c-Jun can form heterodimers, bind to the closely related CRE/AP-1 sequence, alter DNA-binding affinity and result in the repression of StAR gene transcription (Manna and Stocco, 2007). On the other hand, CREB/ATF and C/EBP family members compete for binding to the elements that show sequence similarity to either the CRE or CCAAT motifs that are present within a promoter, and direct the transcription of several genes (Bakker and Parker, 1991; Liu et al., 1991; Wilson et al., 2002). It is not presently known whether C/EBPβ acts as an activator or repressor in conjunction with CREB and/or Fos/Jun with respect to StAR gene transcription but further investigation may allow us to draw such a distinction. Regardless of the trans-regulatory mechanisms involved, the 5′-flanking −81/−72 bp region of the StAR gene appears to function as a key element in the complex series of processes regulating StAR gene transcription.
Post-transcriptional processing of StAR mRNA
It is clear that the transcriptional regulation of StAR is important in dictating its tissue-specific expression, and that transcriptional events tightly integrate a number of signals in order to control the steroidogenic output in these tissues. Given that StAR mRNA levels rise rapidly in response to the cellular signals that also drive StAR protein expression and steroidogenesis, it is often presumed that StAR transcription and translation are tightly coupled in response to a single signaling event (Nieschlag et al., 2004; Manna and Stocco, 2007, 2008). However, it is now being discerned how a number of post-transcriptional mechanisms, such as the polyadenylation [poly(A)] of StAR mRNA and the post-translational modification of StAR protein, team together with a growing number of interacting partners to regulate StAR-mediated steroidogenesis (Fig. 2). Studies have suggested that the ability to regulate StAR mRNA stability could serve as a mechanism for maintaining or enhancing persistent levels of StAR mRNA.
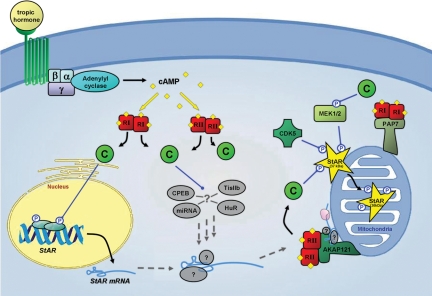
Figure 2.
A model illustrating post-transcriptional and post-translational regulation of StAR. Tropic hormone activation initiates multiple signal transduction pathways known to induce StAR gene expression. Central to these pathways is the second messenger cAMP that binds to the regulatory subunits (RI and RII) of PKA resulting in the release of the active catalytic subunits (C). In addition to directing StAR gene transcription, it is predicted that PKA and other signaling events are likely to coordinate the post-transcriptional regulation of StAR mRNA, potentially through miRNAs and mRNA-binding proteins such as Tis11b, HuR and CPEB. The expression of StAR mRNA as a result of this process appears to be enhanced by A-kinase anchor protein (AKAP)121, which may recruit StAR mRNA, possibly in complex with other proteins, to the outer mitochondrial membrane, allowing it to be translated and activated on site. AKAP121 also tethers type II PKA to the mitochondria, which appears to serve a role in enhancing the activation of StAR at the outer mitochondrial membrane through the phosphorylation of Ser195 in StAR. Similarly, acyl-co-enzyme A-binding domain containing 3 (PAP7; also, ABCD3) has been demonstrated to bind type I PKA at the outer mitochondrial, also increasing steroidogenesis. The pool of AKAP-tethered PKA is likely to serve in activating mitochondrial kinase MEK1/2, which has recently been shown to activate StAR by phosphorylating Ser232. Furthermore, StAR activity in Leydig cells has been linked to its association with and phosphorylation by Cdk5; however, the specific target on StAR for this kinase remains unknown. These post-translational modifications to StAR may serve to enhance its stability or its ability to interact with other proteins necessary for cholesterol transport. Alternatively, these events may serve to prolong the duration it takes for StAR to be withdrawn into the mitochondria. The N-terminal region of full-length (37 kDa) StAR targets the protein for importation into the mitochondria and is proteolytically cleaved following StAR’s translocation. Thus, although the C-terminal domain (30 kDa) of StAR possesses the intrinsic capacity to promote cholesterol transfer, it is possible that the steroidogenic potential of StAR is maximized by events that cause it to dwell longer at the outer mitochondrial membrane in contact with other proteins known to promote steroidogenesis. (Figures and dashed arrows depicted in gray represent putative targets and interactions.)
The StAR gene is transcribed from a sequence ranging 3–7.5 kb in length in vertebrates (Clark et al., 1995a; Bauer et al., 2000; Manna et al., 2001, 2002b; Goetz et al., 2004). This sequence appears to span seven exons that do not appear to be differentially spliced, although an eighth splice junction following the STOP codon of StAR in the Japanese eel may give rise to an alternative 3′ untranslated region (UTR) (Affaitati et al., 2003). Although only one isoform of the StAR protein is observed, northern blots for StAR commonly reveal at least two predominant species of mRNA that, like a large portion of mammalian mRNAs, are derived through the use of one of many different poly(A) sites located in the 3′UTR of the StAR mRNA (Ariyoshi et al., 1998; Devoto et al., 2001; Tian et al., 2005). The stability and subcellular localization of a significant number of mRNAs are strongly influenced by sequences found in their 3′ UTRs, and it is likely that such mechanisms affect the post-transcriptional regulation of StAR (Guhaniyogi and Brewer, 2001; Lutz, 2008). In the rodent, StAR gene alternative poly(A) site usage results in either a shorter, more stable 1.6-kb form or a longer, but more ephemeral 3.5-kb form (Jefcoate et al., 2000). In humans, a 1.7-kb StAR appears as the principle transcript in steroidogenic tissues, whereas longer forms of 2.4 and 4.4 kb are observed in the adrenal and gonads, respectively (Sugawara et al., 1995; Clark and Combs, 1999). In addition, both the 1.7 and 4.4-kb StAR mRNAs show increased expression in the corpus luteum during early and mid-luteal phase, correlating with increased StAR protein expression and progesterone secretion (Devoto et al., 2001). In this manner, StAR appears similar to many other genes where longer 3′UTRs result in reduced protein expression; however, it is interesting to note that the ratio and rates at which these mRNAs are expressed is sensitive to a number of stimuli including cAMP. These observations raise a number of prospects that should be explored, as the ability to control mRNA half-life through mechanisms targeting the 3′UTR could provide steroidogenic cells an extremely sensitive set of tools for controlling StAR protein expression. It has been shown that specific regions in the longer 3′UTR of rodent StAR mRNA are responsible for destabilizing the transcript (Zhao et al., 2005a). Included in this region are several putative AU-rich elements (AREs), which in other acutely regulated genes can recruit factors to the mRNA that target it for rapid degradation (Barreau et al., 2005). Moreover, other mRNA-binding proteins can differentially stabilize such mRNAs in response to stimulation through binding to such elements. This mechanism appears feasible since the stability of the longer StAR mRNA is enhanced through PKA and MAPK pathways (Zhao et al., 2005b, Duan and Jefcoate, 2007). Alternatively, longer 3′UTRs in transcripts frequently possess target sites for microRNAs (miRNAs) that target the mRNA for degradation (Sandberg et al., 2008). Although the impact of miRNAs on StAR expression has yet to be examined, prospective miRNA sites have already been identified in the 3′UTR of rodent StAR mRNA (Duan and Jefcoate, 2007; Griffiths-Jones et al., 2008). Adding another layer of complexity to this is the possibility that the poly(A) sites in the StAR transcript are preferentially utilized in response to cell signaling or tissue-specific events (Lutz, 2008). The relative strength of a poly(A) site can be gauged by how rapidly the cleavage-poly(A) apparatus is assembled, and it is known that downstream sequences within an mRNA in conjunction with mRNA-binding proteins can regulate the usage of particular poly(A) signals (Chao et al., 1999). Therefore, how StAR mRNA stability influences steroidogenesis will be greatly improved by examining the cohort of molecules that can associate with these transcripts.
Currently, nothing is known regarding proteins that bind to StAR mRNA, although several interesting candidates that might uniquely regulate its abundance and expression can be proposed on the basis of sequences in its 3′UTR. One of the destabilizing AREs in the longer 3′UTR of the StAR gene closely resembles that found in the mRNA encoding vascular endothelial growth factor (VEGF) (Duan and Jefcoate, 2007). In the adrenal cortex, ACTH signaling through cAMP can promote the replacement of TPA-induced sequence 11b (TiS11b) with HuR at this ARE in order to stabilize VEGF transcripts and to promote its expression (Cherradi et al., 2006). Tis11b, a zinc finger RNA-destabilizing protein, and HuR, a nuclear-cytoplasmic mRNA shuttling protein that stabilizes transcripts, are both observed in multiple steroidogenic cells (Duan and Jefcoate, 2007), leading to the speculation of their involvement in regulating StAR gene expression. In addition to its AREs, the rodent 3′UTR in StAR also reveals putative cytoplasmic polyadenylation elements (CPEs) flanking one of the distal poly(A) signals (Dyson et al., unpublished observation). The recruitment of CPE-binding proteins (CPEBs) to cis-elements in the 3′UTR of mRNAs can modulate their translation in response to different stimuli, and plays an indisputable role in regulating gene expression during oogenesis and spermatogenesis (Mendez and Richter, 2001). The observation that CPEB is also expressed in steroid-producing somatic tissues such as the hippocampus, heart and the kidney, however, poses an intriguing link to steroidogenesis and possibly to StAR expression (Gebauer and Richter, 1996; Theis et al., 2003; Zearfoss et al., 2008).
It is also possible that upon exiting the nucleus, fully processed StAR mRNA is targeted by factors that can enhance or repress its translation. Several proteins in steroidogenic cells have been shown to function in this capacity, such as mevalonate kinase, which can bind and repress the translation of the LH receptor mRNA in granulosa cells (Nair et al., 2008). Such a role for this kinase ostensibly allows the integration of signals for cholesterol metabolism with tropic hormone signaling, and it would be of interest to determine whether, through similar mechanisms, it could regulate StAR mRNA as well. DAX-1 has also been shown to shuttle between the nucleus and cytoplasm, where it appears to accompany nascent mRNAs into associations with ribosomes (Lalli et al., 2000). Given the impact that DAX-1 has on StAR transcription, it is tempting to speculate that it could also affect StAR expression post-transcriptionally (Zazopoulos et al., 1997; Lalli et al., 2000; Manna et al., 2009a). We have recently observed that the mitochondrial A-kinase anchoring protein 121 (AKAP121) can enhance StAR expression post-transcriptionally (Dyson et al., 2008). AKAP121 is unique among its family members owing to an N-terminal KH domain through which it is capable of targeting mRNAs to the mitochondria, most often by binding to unique sequences in the 3′UTRs of these mRNAs (Ginsberg et al., 2003). Interestingly, the translation of mRNAs bound to AKAP121 can either be repressed, as is the case for lipoprotein lipase (Unal et al., 2008), or enhanced, which occurs with manganese superoxide dismutase (Ginsberg et al., 2003). In both Leydig and granulosa cell lines, AKAP121 enhances the expression of StAR protein without appearing to alter StAR mRNA levels, and we predict that AKAP121 serves to coordinate and enhance the translation of StAR at the outer mitochondrial membrane (Dyson et al., 2008). The growing evidence that post-transcriptional regulation of mRNAs is necessary to complement transcription, and the ample possibilities by which StAR could be thus regulated, present us with a number of hypotheses and it is hoped that more progress will be made in this area as continued research overcomes the technical challenges arising in the study of RNA interactions in vivo.
Post-translational modification of StAR
The evidence for the post-translational regulation of StAR has become more apparent, as the mechanism by which StAR binds cholesterol and mediates its transfer is subject to multiple factors that can attenuate or enhance the process. At present, a host of proteins including the voltage-dependent anion channel 1 (Bose et al., 2008b), the peripheral benzodiazepine receptor (PBR) (Hauet et al., 2005; Liu et al., 2006), PBR- and PKAR1A-associated protein (PAP7) (Liu et al., 2006), phosphate carrier protein (Bose et al., 2008b) and hormone-sensitive lipase (HSL) (see what follows) (Shen et al., 2003) are thought to functionally or physically interact with StAR. Significant exposition on how these interactions impact steroidogenesis has already been made, and would indicate that the entire complement of proteins involved in this process has not yet been fully defined (Shen et al., 2003; Liu et al., 2006; Bose et al., 2008b). Excellent reviews of these proteins, prospective step-by-step descriptions of how StAR may function and analyses of the physical conformation of StAR have already been made (Baker et al., 2007; Miller, 2007; Papadopoulos et al., 2007; Bose et al., 2008b). To better expand on this, we have herein focused on the specific post-translational modifications that alter StAR itself, and will limit our discussion to the interacting partners pertinent to these post-translational processes.
The most obvious post-translational modification to StAR is its proteolytic processing in the mitochondria. StAR is synthesized as a 37 kDa precursor-protein with an N-terminal mitochondrial-targeting sequence that is cleaved from the remainder of the protein during or after translocation into the mitochondria (Clark et al., 1994; Yamazaki et al., 2006). The N-terminal domain of StAR also destabilizes the protein in the cytoplasm, likely targeting the full-length protein for rapid removal by the proteasome, and contributing to a short half-life of the 37-kDa form (Clark et al., 1994; Stocco and Clark, 1996; Granot et al., 2003). This appears to occur in most cell types without StAR being ubiquitinylated, as inhibitors of the proteasome consistently increase StAR levels (Granot et al., 2007). The cleavage of the leader sequence results in a 30-kDa peptide largely defined by its cholesterol-binding domain, which shows similarity to other lipid-trafficking proteins, and is known as a StAR-related lipid-transfer (START) domain (Alpy and Tomasetto, 2005). The START domain appears responsible for the mechanics of inducing cholesterol transfer and that it functions at the outer mitochondrial membrane even in the absence of the leader sequence (Arakane et al., 1998; Bose et al., 2002b, Miller, 2007). Furthermore, the internalization of StAR into the mitochondria does not appear necessary for the transfer of cholesterol. Studies in non-steroidogenic COS (African green monkey kidney) cells demonstrate that StAR importation and cleavage proceed hand in hand, and that mitochondrial internalization serves to neutralize StAR activity by removing it from the outer mitochondrial membrane (Bose et al., 2002a, Yamazaki et al., 2006). Somewhat at odds with these findings is that the intra-mitochondrial cleavage of StAR appears linked to StAR activity in rat adrenal glands (Artemenko et al., 2001). In addition, the loss of the mitochondrial leader sequence, while increasing the stability of StAR, strongly diminishes its ability to promote gonadal and adrenal steroidogenesis in mice (Granot et al., 2003; Sasaki et al., 2008). Therefore, the impact that the mitochondrial targeting and processing of StAR have on steroidogenesis remains poorly defined.
It is possible that the artificial expression of StAR in non-steroidogenic tissues inaccurately models the mechanism of StAR at the mitochondria; however, the fact that StAR is properly imported and cleaved in COS and other non-steroidogenic cells suggests that any physical properties requiring proteolysis are not limited (Yamazaki et al., 2006; Sasaki et al., 2008). Alternatively, the equilibrium between the cytoplasmic and mitochondrial pathways that target StAR for degradation may be significantly altered in different cell types such that steroidogenic cells more effectively direct StAR to the mitochondria while removing it from other locations in the cell. Notably, cysteine proteases, such as those present in the lysosome, do not appear to affect the degradation of StAR precursor in hormonally responsive granulosa cells, yet they can strongly reduce StAR precursor abundance in COS cells (Tajima et al., 2001; Bose et al., 2008b). Given that StAR lacking its targeting sequence functions equivalently to full-length StAR in COS cells but not in steroidogenic cells, it is feasible that the subcellular distribution and proteolysis of StAR are more tightly controlled in steroidogenic tissues (Arakane et al., 1996; Sasaki et al., 2008). Further research focused on StAR proteolysis in steroidogenic tissues, in particular with regard to the N-terminal domain, should provide insight into how acute steroidogenesis is regulated.
The fundamental research characterizing the acute steroidogenic response to trophic hormone stimulation linked the phosphorylation of newly synthesized proteins to steroid hormone production (Garren et al., 1971). Subsequent research was able to rely on this feature to search for phosphoproteins unique to steroidogenic tissues that were synthesized in response to hormone treatment (Pon et al., 1986; Alberta et al., 1989; Stocco and Sodeman, 1991), and presently StAR appears to be targeted for phosphorylation by several kinases (Fig. 2). Following its discovery, the sequence of StAR confirmed the presence of at least two consensus PKA phosphorylation sites in Ser56/57 and Ser194/195, in murine and human StAR, respectively (Arakane et al., 1997). Of these two sites, the phosphorylation of StAR on Ser194 by PKA is essential in order to render the protein fully active in its capacity to support cholesterol transfer, and mutation of this site strongly reduces the ability of StAR to induce cholesterol transport across the mitochondrial membranes both in vitro and in vivo (Arakane et al., 1997; Manna et al., 2002a, b; Baker et al., 2007). Furthermore, the mutation of Ser195 is one of many point mutations in human StAR that reportedly gives rise to congenital lipoid adrenal hyperplasia (Katsumata et al., 2000). We have recently examined how the different isoforms of PKA regulate StAR phosphorylation (Dyson et al., 2009). Although both principal subtypes of PKA (either type I or type II as defined by the regulatory subunit present) are seen in steroidogenic tissues, type II PKA appears more efficient at phosphorylating StAR. This appears to be at least partly due to the presence of AKAP121, which can tether type II PKA to the outer mitochondrial membrane, and our current prediction is that type II PKA anchored to the mitochondria ensures the efficient phosphorylation of StAR prior to its importation into the mitochondria (Chen et al., 1997; Dyson et al., 2009). Another candidate for regulating the phosphorylation of StAR is PAP7, an AKAP that binds PBR and recruits type I and possibly type II PKA to the mitochondria (Liu et al., 2006). PAP7 strongly enhances hormone-induced steroidogenesis, although its direct effects on StAR expression or phosphorylation have yet to be examined. Interestingly, several protein phosphatases have been reported in complex with AKAP121, such as protein phosphatase 1 and protein tyrosine phosphatase D1 (Steen et al., 2003; Cardone et al., 2004; Bridges et al., 2006). Multiple phosphatases have been implicated in StAR expression and steroidogenesis; thus, it may prove useful to revisit the function of these enzymes were they shown to be in complex at the mitochondria (Sayed et al., 1998; Castillo et al., 2004).
The mechanistic function of StAR’s phosphorylation by PKA is not fully understood, and several prospective theories have been put forth. The ability of StAR to bind cholesterol is not affected by the phosphorylation of Ser194/195, and instead it appears that the action of PKA enhances the capacity of StAR to function at the mitochondria (Baker et al., 2007). Earlier studies suggest that the phosphorylation of StAR may enhance the stability of the protein, and given its short half-life in the cytoplasm, this would be effective (Clark et al., 2001). However, recombinant human StAR bearing an Ser195Ala mutation shows a significantly reduced ability to induce steroidogenesis when added in vitro to isolated mitochondria, suggesting that the intrinsic activity of StAR is affected by its phosphorylation (Baker et al., 2007). Another possibility is that the phosphorylation of StAR by PKA retards its importation, thereby increasing the duration of time it spends at the outer mitochondrial membrane. A similar phenomenon is observed when the mitochondrial leader sequence is lengthened to slow StAR’s import (Bose et al., 2002a). Alternatively, the phosphorylation of StAR may enhance its interaction with other proteins at the mitochondria.
Whereas PKA is clearly moderating StAR function at the mitochondria, current evidence suggests that at least two other kinases can directly or indirectly modulate StAR phosphorylation and activity. Studies have shown that StAR itself is a substrate for the extracellular signal-regulated kinases (ERK1/2), which phosphorylate StAR at Ser232 within a highly conserved ERK1/2 docking site and enhance StAR activity at the mitochondria (Poderoso et al., 2008, 2009). Whereas the activation of ERK1/2 can result from PKA activity, the phosphorylation of StAR by either enzyme does not appear contingent upon the other, and at the moment it is possible that ERK1/2 and PKA activities serve analogous roles in influencing StAR. However, one notable difference is that StAR’s phosphorylation by ERK1/2 requires cholesterol (Poderoso et al., 2008). Thus, unlike the phosphorylation of Ser194 by PKA, the action of ERK1/2 likely requires a permissive conformation of StAR to be first induced by its ligand. Hence, it will be interesting to determine whether the phosphorylation at Ser232 alters the affinity of StAR for cholesterol. More recent work examining the role of cyclin-dependent kinase 5 (CDK5) in Leydig cells has suggested that it too may alter StAR phosphorylation (Lin et al., 2009). The presence CDK5 in reproductive tissues has resulted in the description of several novel roles for the kinase outside of cell cycle regulation (Zhang et al., 1997; Musa et al., 2000). In Leydig cells, CDK5 is observed to physically interact with StAR, where it appears to enhance the post-transcriptional expression of StAR (Lin et al., 2009). Furthermore, the inhibition of CDK5 activity reduces StAR phosphorylation but not its expression. Whether CDK5 can directly or indirectly phosphorylate StAR will require additional investigation to elucidate its role in acute steroidogenesis.
Role of HSL in steroidogenesis
HSL is a multifunctional enzyme that is highly expressed in several tissues including adipose tissue, adrenal, gonads, where it is responsible for neutral cholesteryl ester hydrolase (NCEH) activity in steroidogenic cells (Osuga et al., 2000; Kraemer and Shen, 2002; Li et al., 2002; Rao et al., 2003). Targeted disruption of HSL in mice results in a lack of NCEH activity in the adrenal and testis, and males are sterile due to oligospermia, indicating that HSL plays essential role in regulating intracellular cholesterol metabolism (Osuga et al., 2000). Interestingly, the infertility observed in male HSL knockout mice is not associated with abnormal steroid biosynthesis, but is rather a direct consequence of the loss of HSL on spermatogenesis (Osuga et al., 2000; Chung et al., 2001). Several lines of evidence demonstrate that hormonal and neuronal control of HSL activity is mediated by phosphorylation of Ser residues in response to PKA signaling (Manna et al., unpublished results; Osterlund, 2001; Kraemer and Shen, 2002). Importantly, the hydrolysis of cholesteryl esters stored in lipid droplets serves as an important source of cholesterol, and is often necessary for optimal steroid biosynthesis (Fig. 3). Other cholesterol sources are important as well, and it can be synthesized de novo within the cell, or acquired from lipoprotein-derived cholesteryl esters obtained by either receptor-mediated endocytic or selective cellular uptake. In the latter process, circulating lipoproteins [high-density lipoprotein (HDL) or low-density lipoprotein (LDL)] bind to scavenger receptor class B, type 1 (SR-B1) and release cholesterol esters into the cells (Fig. 3) (Gwynne and Strauss, 1982; Temel et al., 1997; Fidge, 1999; Williams et al., 1999; Azhar and Reaven, 2002; Rao et al., 2003). Receptor-mediated endocytic uptake of lipoprotein-derived cholesteryl esters is processed via the LDL receptor in the human systems (Gwynne and Strauss, 1982; Brown and Goldstein, 1986). The roles of the Niemann–Pick C1 and C2 proteins in cholesterol trafficking via LDL receptor-mediated endocytosis and cleavage of cholesteryl esters by lysosomal acid lipase have also been reported (Watari et al., 2000; Gevry and Murphy, 2002).
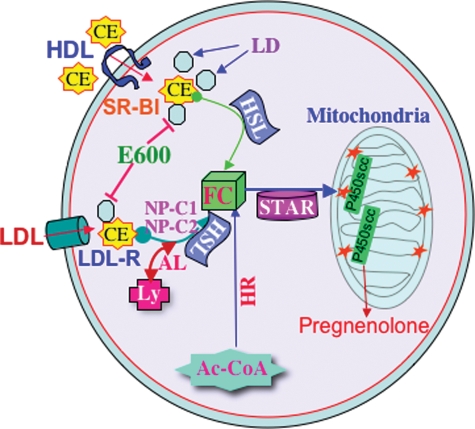
Figure 3.
Metabolism of cholesteryl ester (CE) and role of hormone-sensitive lipase (HSL) in steroidogenesis. The hydrolysis of cholesteryl esters stored in lipid droplets is an important source of cholesterol for optimum steroid biosynthesis. HSL is a multifunctional enzyme that is responsible for neutral cholesteryl ester hydrolase activity. E600 blocks the release of cholesterol from lipid droplets (LD) and thus affects StAR expression and steroid synthesis. Circulating lipoproteins (HDL or LDL) bind to scavenger receptor class B, type 1 (SR-B1) and release cholesteryl esters into the cells. Free cholesterol (FC) for steroid production is mostly obtained in rodents via HDL-mediated cholesteryl ester internalization and followed by cleavage by HSL. However, receptor-mediated uptake of lipoprotein-derived cholesteryl esters is processed via the LDL receptor in the human systems. De novo synthesis of cholesterol from acetyl-co-enzyme A (AC-CoA) provides also FC for steroid synthesis. The StAR protein regulates steroidogenesis by controlling the transport of cholesterol from the outer to the inner mitochondrial membrane, the site of the cytochrome P450scc enzyme. Conversion of cholesterol is the first enzymatic step in steroid hormone biosynthesis. LD, lipid droplets; AL, acid lipase; NP-C1 and C2, Niemann–Pick C1 and C2; Ly, lysosome; HR, HMG-co-enzyme A reductase. HDL, high density lipoprotein; LDL, low density lipoprotein.
Regardless of the source of cholesterol utilized for steroidogenesis, the transport of cholesterol from the outer to the inner mitochondrial membrane is the rate-limiting step in steroid biosynthesis and is mediated by the StAR protein (Lin et al., 1995; Stocco and Clark, 1996; Christenson and Strauss, 2000; Strauss et al., 2003; Miller, 2007). It has previously been shown that higher StAR expression and steroid production in R2C rat Leydig cells under basal conditions are due to constitutive expression of HSL and SR-B1 (Rao et al., 2003). It was proposed that these components are involved in the uptake of cholesterol esters from lipoproteins, thereby increasing the availability of cholesterol for steroidogenesis. Nevertheless, both in vivo and in vitro studies have demonstrated a striking correlation between hormonal induction of SR-B1 expression (associated with enhanced lipid uptake from HDL) and steroid biosynthesis in different steroidogenic cell models (Rigotti et al., 1996; Azhar et al., 1998; Manna et al., 2006b, 2007). Previous studies have demonstrated that the physical interaction of HSL with StAR results in an elevation in hydrolytic activity of HSL and that they both facilitate trafficking of intracellular cholesterol from lipid droplets into the mitochondria (Shen et al., 2003). However, the precise mechanism through which this is accomplished remains obscure. We have recently observed that the activation of PKA pathway increases the phosphorylation but not synthesis of HSL, concomitant with StAR expression and steroidogenesis in both mouse Leydig and adrenal cells (Manna et al., unpublished results). In contrast, the inhibition of HSL activity by E600, which blocks the release of cholesterol from lipid droplets, markedly diminishes StAR expression and steroid biosynthesis. The decrease in the steroidogenic response in connection with HSL was also confirmed by targeted silencing of endogenous HSL using small interfering RNA, further underscoring the importance of HSL in the regulation of StAR expression and steroidogenesis. Whether members of the START domain family, i.e. StarD3 (MLN64), StarD4–StarD6, that are ubiquitously expressed in several tissues (Watari et al., 1997; Soccio et al., 2002; Zhang et al., 2002; Strauss et al., 2003) may play roles in cholesterol mobilization to the mitochondria for steroidogenesis requires additional investigation. Indeed, it has recently been demonstrated that StarD6 possesses a similar hydrophobic sterol-binding pocket and C-terminal extension to those of StAR (StarD1), and that StarD6 is fully capable in inducing StAR-like responsiveness involved in steroidogenesis (Bose et al., 2008a).
In summarizing these studies, we have attempted to describe how multiple signaling pathways, transcription factors and other possible regulatory elements may serve to regulate StAR-mediated steroidogenesis. Whereas considerable progress has been made towards understanding the regulatory events surrounding steroidogenesis, a complete knowledge of the molecular mechanisms involved in StAR gene transcription, translation and activation is required. Consequently, more information will be required before a clear-cut understanding of how the developmental, tissue-specific and hormone-induced StAR expression is obtained that effectively governs the synthesis of steroid hormones in different steroidogenic tissue.
Funding
This review was supported in part by National Institutes of Health grant HD 17481 and with funds from the Robert A. Welch Foundation grant B1-0028.
Acknowledgements
The authors wish to thank our many collaborators and the studies of several research groups whose contribution helped in preparing this review.
References
- Abate C, Baker SJ, Lees-Miller SP, Anderson CW, Marshak DR, Curran T. Dimerization and DNA binding alter phosphorylation of Fos and Jun. Proc Natl Acad Sci USA. 1993;90:6766–6770. doi: 10.1073/pnas.90.14.6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affaitati A, Cardone L, de Cristofaro T, Carlucci A, Ginsberg MD, Varrone S, Gottesman ME, Avvedimento EV, Feliciello A. Essential role of A-kinase anchor protein 121 for cAMP signaling to mitochondria. J Biol Chem. 2003;278:4286–4294. doi: 10.1074/jbc.M209941200. [DOI] [PubMed] [Google Scholar]
- Alberta JA, Epstein LF, Pon LA, Orme-Johnson NR. Mitochondrial localization of a phosphoprotein that rapidly accumulates in adrenal cortex cells exposed to adrenocorticotropic hormone or to cAMP. J Biol Chem. 1989;264:2368–2372. [PubMed] [Google Scholar]
- Alpy F, Tomasetto C. Give lipids a START: the StAR-related lipid transfer (START) domain in mammals. J Cell Sci. 2005;118:2791–2801. doi: 10.1242/jcs.02485. [DOI] [PubMed] [Google Scholar]
- Arakane F, Sugawara T, Nishino H, Liu Z, Holt JA, Pain D, Stocco DM, Miller WL, Strauss JF., III Steroidogenic acute regulatory protein (StAR) retains activity in the absence of its mitochondrial import sequence: implications for the mechanism of StAR action. Proc Natl Acad Sci USA. 1996;93:13731–13736. doi: 10.1073/pnas.93.24.13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakane F, King SR, Du Y, Kallen CB, Walsh LP, Watari H, Stocco DM, Strauss JF., III Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J Biol Chem. 1997;272:32656–32662. doi: 10.1074/jbc.272.51.32656. [DOI] [PubMed] [Google Scholar]
- Arakane F, Kallen CB, Watari H, Foster JA, Sepuri NB, Pain D, Stayrook SE, Lewis M, Gerton GL, Strauss JF., III The mechanism of action of steroidogenic acute regulatory protein (StAR). StAR acts on the outside of mitochondria to stimulate steroidogenesis. J Biol Chem. 1998;273:16339–16345. doi: 10.1074/jbc.273.26.16339. [DOI] [PubMed] [Google Scholar]
- Ariyoshi N, Kim YC, Artemenko I, Bhattacharyya KK, Jefcoate CR. Characterization of the rat Star gene that encodes the predominant 3.5- kilobase pair mRNA. ACTH stimulation of adrenal steroids in vivo precedes elevation of Star mRNA and protein. J Biol Chem. 1998;273:7610–7619. doi: 10.1074/jbc.273.13.7610. [DOI] [PubMed] [Google Scholar]
- Artemenko IP, Zhao D, Hales DB, Hales KH, Jefcoate CR. Mitochondrial processing of newly synthesized steroidogenic acute regulatory protein (StAR), but not total StAR, mediates cholesterol transfer to cytochrome P450 side chain cleavage enzyme in adrenal cells. J Biol Chem. 2001;276:46583–46596. doi: 10.1074/jbc.M107815200. [DOI] [PubMed] [Google Scholar]
- Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev. 2002;23:141–174. doi: 10.1210/edrv.23.2.0462. [DOI] [PubMed] [Google Scholar]
- Azhar S, Reaven E. Scavenger receptor class BI and selective cholesteryl ester uptake: partners in the regulation of steroidogenesis. Mol Cell Endocrinol. 2002;195:1–26. doi: 10.1016/s0303-7207(02)00222-8. [DOI] [PubMed] [Google Scholar]
- Azhar S, Nomoto A, Leers-Sucheta S, Reaven E. Simultaneous induction of an HDL receptor protein (SR-BI) and the selective uptake of HDL-cholesteryl esters in a physiologically relevant steroidogenic cell model. J Lipid Res. 1998;39:1616–1628. [PubMed] [Google Scholar]
- Baker BY, Epand RF, Epand RM, Miller WL. Cholesterol binding does not predict activity of the steroidogenic acute regulatory protein, StAR. J Biol Chem. 2007;282:10223–10232. doi: 10.1074/jbc.M611221200. [DOI] [PubMed] [Google Scholar]
- Bakker O, Parker MG. CAAT/enhancer binding protein is able to bind to ATF/CRE elements. Nucleic Acids Res. 1991;19:1213–1217. doi: 10.1093/nar/19.6.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian K, Lavoie HA, Garmey JC, Stocco DM, Veldhuis JD. Regulation of porcine granulosa cell steroidogenic acute regulatory protein (StAR) by insulin-like growth factor I: synergism with follicle- stimulating hormone or protein kinase A agonist. Endocrinology. 1997;138:433–439. doi: 10.1210/endo.138.1.4894. [DOI] [PubMed] [Google Scholar]
- Barreau C, Paillard L, Osborne HB. AU-rich elements and associated factors: are there unifying principles? Nucleic Acids Res. 2005;33:7138–7150. doi: 10.1093/nar/gki1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer MP, Bridgham JT, Langenau DM, Johnson AL, Goetz FW. Conservation of steroidogenic acute regulatory (StAR) protein structure and expression in vertebrates. Mol Cell Endocrinol. 2000;168:119–125. doi: 10.1016/s0303-7207(00)00316-6. [DOI] [PubMed] [Google Scholar]
- Bose HS, Sugawara T, Strauss JF, III, Miller WL. The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. International Congenital Lipoid Adrenal Hyperplasia Consortium. N Engl J Med. 1996;335:1870–1878. doi: 10.1056/NEJM199612193352503. [DOI] [PubMed] [Google Scholar]
- Bose HS, Lingappa VR, Miller WL. Rapid regulation of steroidogenesis by mitochondrial protein import. Nature. 2002;a 417:87–91. doi: 10.1038/417087a. [DOI] [PubMed] [Google Scholar]
- Bose HS, Lingappa VR, Miller WL. The steroidogenic acute regulatory protein, StAR, works only at the outer mitochondrial membrane. Endocr Res. 2002;b 28:295–308. doi: 10.1081/erc-120016800. [DOI] [PubMed] [Google Scholar]
- Bose HS, Whittal RM, Ran Y, Bose M, Baker BY, Miller WL. StAR-like activity and molten globule behavior of StARD6, a male germ-line protein. Biochemistry. 2008;a 47:2277–2288. doi: 10.1021/bi701966a. [DOI] [PubMed] [Google Scholar]
- Bose M, Whittal RM, Miller WL, Bose HS. Steroidogenic activity of StAR requires contact with mitochondrial VDAC1 and phosphate carrier protein. J Biol Chem. 2008;b 283:8837–8845. doi: 10.1074/jbc.M709221200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges D, MacDonald JA, Wadzinski B, Moorhead GB. Identification and characterization of D-AKAP1 as a major adipocyte PKA and PP1 binding protein. Biochem Biophys Res Commun. 2006;346:351–357. doi: 10.1016/j.bbrc.2006.05.138. [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Cardone L, Carlucci A, Affaitati A, Livigni A, DeCristofaro T, Garbi C, Varrone S, Ullrich A, Gottesman ME, Avvedimento EV, et al. Mitochondrial AKAP121 binds and targets protein tyrosine phosphatase D1, a novel positive regulator of src signaling. Mol Cell Biol. 2004;24:4613–4626. doi: 10.1128/MCB.24.11.4613-4626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron KM, Soo SC, Wetsel WC, Stocco DM, Clark BJ, Parker KL. Targeted disruption of the mouse gene encoding steroidogenic acute regulatory protein provides insights into congenital lipoid adrenal hyperplasia. Proc Natl Acad Sci USA. 1997;94:11540–11545. doi: 10.1073/pnas.94.21.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo F, Cano F, Maloberti P, Castilla R, Neuman I, Poderoso C, Paz C, Podesta EJ, Maciel FC. Tyrosine phosphates act on steroidogenesis through the activation of arachidonic acid release. Endocr Res. 2004;30:623–627. doi: 10.1081/erc-200043795. [DOI] [PubMed] [Google Scholar]
- Chao LC, Jamil A, Kim SJ, Huang L, Martinson HG. Assembly of the cleavage and polyadenylation apparatus requires about 10 seconds in vivo and is faster for strong than for weak poly(A) sites. Mol Cell Biol. 1999;19:5588–5600. doi: 10.1128/mcb.19.8.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Lin RY, Rubin CS. Organelle-specific targeting of protein kinase AII (PKAII). Molecular and in situ characterization of murine A kinase anchor proteins that recruit regulatory subunits of PKAII to the cytoplasmic surface of mitochondria. J Biol Chem. 1997;272:15247–15257. doi: 10.1074/jbc.272.24.15247. [DOI] [PubMed] [Google Scholar]
- Cherradi N, Lejczak C, Desroches-Castan A, Feige JJ. Antagonistic functions of tetradecanoyl phorbol acetate-inducible-sequence 11b and HuR in the hormonal regulation of vascular endothelial growth factor messenger ribonucleic acid stability by adrenocorticotropin. Mol Endocrinol. 2006;20:916–930. doi: 10.1210/me.2005-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson LK, Strauss JF., III Steroidogenic acute regulatory protein (StAR) and the intramitochondrial translocation of cholesterol. Biochim Biophys Acta. 2000;1529:175–187. doi: 10.1016/s1388-1981(00)00147-5. [DOI] [PubMed] [Google Scholar]
- Christenson LK, Johnson PF, McAllister JM, Strauss JF., III CCAAT/enhancer-binding proteins regulate expression of the human steroidogenic acute regulatory protein (StAR) gene. J Biol Chem. 1999;274:26591–26598. doi: 10.1074/jbc.274.37.26591. [DOI] [PubMed] [Google Scholar]
- Christenson LK, Osborne TF, McAllister JM, Strauss JF., III Conditional response of the human steroidogenic acute regulatory protein gene promoter to sterol regulatory element binding protein-1a. Endocrinology. 2001;142:28–36. doi: 10.1210/endo.142.1.7867. [DOI] [PubMed] [Google Scholar]
- Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- Chung S, Wang SP, Pan L, Mitchell G, Trasler J, Hermo L. Infertility and testicular defects in hormone-sensitive lipase-deficient mice. Endocrinology. 2001;142:4272–4281. doi: 10.1210/endo.142.10.8424. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Combs R. Angiotensin II and cyclic adenosine 3′,5′-monophosphate induce human steroidogenic acute regulatory protein transcription through a common steroidogenic factor-1 element. Endocrinology. 1999;140:4390–4398. doi: 10.1210/endo.140.10.7085. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR) J Biol Chem. 1994;269:28314–28322. [PubMed] [Google Scholar]
- Clark BJ, Pezzi V, Stocco DM, Rainey WE. The steroidogenic acute regulatory protein is induced by angiotensin II and K+ in H295R adrenocortical cells. Mol Cell Endocrinol. 1995;a 115:215–219. doi: 10.1016/0303-7207(95)03683-0. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Soo SC, Caron KM, Ikeda Y, Parker KL, Stocco DM. Hormonal and developmental regulation of the steroidogenic acute regulatory protein. Mol Endocrinol. 1995;b 9:1346–1355. doi: 10.1210/mend.9.10.8544843. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Combs R, Hales KH, Hales DB, Stocco DM. Inhibition of transcription affects synthesis of steroidogenic acute regulatory protein and steroidogenesis in MA-10 mouse Leydig tumor cells. Endocrinology. 1997;138:4893–4901. doi: 10.1210/endo.138.11.5535. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Ranganathan V, Combs R. Steroidogenic acute regulatory protein expression is dependent upon post-translational effects of cAMP-dependent protein kinase A. Mol Cell Endocrinol. 2001;173:183–192. doi: 10.1016/s0303-7207(00)00410-x. [DOI] [PubMed] [Google Scholar]
- Clem BF, Clark BJ. Association of the mSin3A-histone deacetylase 1/2 corepressor complex with the mouse steroidogenic acute regulatory protein gene. Mol Endocrinol. 2006;20:100–113. doi: 10.1210/me.2004-0495. [DOI] [PubMed] [Google Scholar]
- Clem BF, Hudson EA, Clark BJ. Cyclic adenosine 3′,5′-monophosphate (cAMP) enhances cAMP-responsive element binding (CREB) protein phosphorylation and phospho-CREB interaction with the mouse steroidogenic acute regulatory protein gene promoter. Endocrinology. 2005;146:1348–1356. doi: 10.1210/en.2004-0761. [DOI] [PubMed] [Google Scholar]
- Cooke BA. Signal transduction involving cyclic AMP-dependent and cyclic AMP-independent mechanisms in the control of steroidogenesis. Mol Cell Endocrinol. 1999;151:25–35. doi: 10.1016/s0303-7207(98)00255-x. [DOI] [PubMed] [Google Scholar]
- De Cesare D, Sassone-Corsi P. Transcriptional regulation by cyclic AMP-responsive factors. Prog Nucleic Acid Res Mol Biol. 2000;64:343–369. doi: 10.1016/s0079-6603(00)64009-6. [DOI] [PubMed] [Google Scholar]
- Devoto L, Kohen P, Gonzalez RR, Castro O, Retamales I, Vega M, Carvallo P, Christenson LK, Strauss JF., III Expression of steroidogenic acute regulatory protein in the human corpus luteum throughout the luteal phase. J Clin Endocrinol Metab. 2001;86:5633–5639. doi: 10.1210/jcem.86.11.7982. [DOI] [PubMed] [Google Scholar]
- Devoto L, Kohen P, Vega M, Castro O, Gonzalez RR, Retamales I, Carvallo P, Christenson LK, Strauss JF. Control of human luteal steroidogenesis. Mol Cell Endocrinol. 2002;186:137–141. doi: 10.1016/s0303-7207(01)00654-2. [DOI] [PubMed] [Google Scholar]
- Duan H, Jefcoate CR. The predominant cAMP-stimulated 3 × 5 kb StAR mRNA contains specific sequence elements in the extended 3′UTR that confer high basal instability. J Mol Endocrinol. 2007;38:159–179. doi: 10.1677/jme.1.02153. [DOI] [PubMed] [Google Scholar]
- Dwarki VJ, Montminy M, Verma IM. Both the basic region and the ‘leucine zipper’ domain of the cyclic AMP response element binding (CREB) protein are essential for transcriptional activation. EMBO J. 1990;9:225–232. doi: 10.1002/j.1460-2075.1990.tb08099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson MT, Jones JK, Kowalewski MP, Manna PR, Alonso M, Gottesman ME, Stocco DM. Mitochondrial A-kinase anchoring protein 121 binds type II protein kinase A and enhances steroidogenic acute regulatory protein-mediated steroidogenesis in MA-10 mouse Leydig tumor cells. Biol Reprod. 2008;78:267–277. doi: 10.1095/biolreprod.107.064238. [DOI] [PubMed] [Google Scholar]
- Dyson MT, Kowalewski MP, Manna PR, Stocco DM. The differential regulation of steroidogenic acute regulatory protein-mediated steroidogenesis by type I and type II PKA in MA-10 cells. Mol Cell Endocrinol. 2009;300:94–103. doi: 10.1016/j.mce.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidge NH. High density lipoprotein receptors, binding proteins, and ligands. J Lipid Res. 1999;40:187–201. [PubMed] [Google Scholar]
- Fimia GM, De Cesare D, Sassone-Corsi P. CBP-independent activation of CREM and CREB by the LIM-only protein ACT. Nature. 1999;398:165–169. doi: 10.1038/18237. [DOI] [PubMed] [Google Scholar]
- Garren LD, Gill GN, Masui H, Walton GM. On the mechanism of action of ACTH. Recent Prog Horm Res. 1971;27:433–478. doi: 10.1016/b978-0-12-571127-2.50035-3. [DOI] [PubMed] [Google Scholar]
- Gebauer F, Richter JD. Mouse cytoplasmic polyadenylation element binding protein: an evolutionarily conserved protein that interacts with the cytoplasmic polyadenylation elements of c-mos mRNA. Proc Natl Acad Sci USA. 1996;93:14602–14607. doi: 10.1073/pnas.93.25.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevry NY, Murphy BD. The role and regulation of the Niemann–Pick C1 gene in adrenal steroidogenesis. Endocr Res. 2002;28:403–412. doi: 10.1081/erc-120016815. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD, Feliciello A, Jones JK, Avvedimento EV, Gottesman ME. PKA-dependent binding of mRNA to the mitochondrial AKAP121 protein. J Mol Biol. 2003;327:885–897. doi: 10.1016/s0022-2836(03)00173-6. [DOI] [PubMed] [Google Scholar]
- Goetz FW, Norberg B, McCauley LA, Iliev DB. Characterization of the cod (Gadus morhua) steroidogenic acute regulatory protein (StAR) sheds light on StAR gene structure in fish. Comp Biochem Physiol B Biochem Mol Biol. 2004;137:351–362. doi: 10.1016/j.cbpc.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Granot Z, Geiss-Friedlander R, Melamed-Book N, Eimerl S, Timberg R, Weiss AM, Hales KH, Hales DB, Stocco DM, Orly J. Proteolysis of normal and mutated steroidogenic acute regulatory proteins in the mitochondria: the fate of unwanted proteins. Mol Endocrinol. 2003;17:2461–2476. doi: 10.1210/me.2003-0074. [DOI] [PubMed] [Google Scholar]
- Granot Z, Kobiler O, Melamed-Book N, Eimerl S, Bahat A, Lu B, Braun S, Maurizi MR, Suzuki CK, Oppenheim AB, et al. Turnover of mitochondrial steroidogenic acute regulatory (StAR) protein by Lon protease: the unexpected effect of proteasome inhibitors. Mol Endocrinol. 2007;21:2164–2177. doi: 10.1210/me.2005-0458. [DOI] [PubMed] [Google Scholar]
- Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:D154–D158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guhaniyogi J, Brewer G. Regulation of mRNA stability in mammalian cells. Gene. 2001;265:11–23. doi: 10.1016/s0378-1119(01)00350-x. [DOI] [PubMed] [Google Scholar]
- Gwynne JT, Strauss JF., III The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr Rev. 1982;3:299–329. doi: 10.1210/edrv-3-3-299. [DOI] [PubMed] [Google Scholar]
- Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci USA. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, Zhao L, Caron KM, Majdic G, Suzuki T, Shizawa S, Sasano H, Parker KL. Developmental roles of the steroidogenic acute regulatory protein (StAR) as revealed by StAR knockout mice. Mol Endocrinol. 2000;14:1462–1471. doi: 10.1210/mend.14.9.0515. [DOI] [PubMed] [Google Scholar]
- Hauet T, Yao ZX, Bose HS, Wall CT, Han Z, Li W, Hales DB, Miller WL, Culty M, Papadopoulos V. Peripheral-type benzodiazepine receptor-mediated action of steroidogenic acute regulatory protein on cholesterol entry into Leydig cell mitochondria. Mol Endocrinol. 2005;19:540–554. doi: 10.1210/me.2004-0307. [DOI] [PubMed] [Google Scholar]
- Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J Cell Sci. 2004;117:5965–5973. doi: 10.1242/jcs.01589. [DOI] [PubMed] [Google Scholar]
- Hilberg F, Aguzzi A, Howells N, Wagner EF. C-Jun is essential for normal mouse development and hepatogenesis. Nature. 1993;365:179–181. doi: 10.1038/365179a0. [DOI] [PubMed] [Google Scholar]
- Hiroi H, Christenson LK, Chang L, Sammel MD, Berger SL, Strauss JF., III Temporal and spatial changes in transcription factor binding and histone modifications at the steroidogenic acute regulatory protein (StAR) locus associated with stAR transcription. Mol Endocrinol. 2004;18:791–806. doi: 10.1210/me.2003-0305. [DOI] [PubMed] [Google Scholar]
- Hogeveen KN, Sassone-Corsi P. Regulation of gene expression in post-meiotic male germ cells: CREM-signalling pathways and male fertility. Hum Fertil. 2006;9:73–79. doi: 10.1080/14647270500463400. [DOI] [PubMed] [Google Scholar]
- Hsu CC, Lu CW, Huang BM, Wu MH, Tsai SJ. Cyclic adenosine 3′,5′-monophosphate response element-binding protein and CCAAT/enhancer-binding protein mediate prostaglandin E2-induced steroidogenic acute regulatory protein expression in endometriotic stromal cells. Am J Pathol. 2008;173:433–441. doi: 10.2353/ajpath.2008.080199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T, Karin M. The regulation of transcription by phosphorylation. Cell. 1992;70:375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Jefcoate CR, Artemenko IP, Zhao D. Relationship of StAR expression to mitochondrial cholesterol transfer and metabolism. Endocr Res. 2000;26:663–680. doi: 10.3109/07435800009048586. [DOI] [PubMed] [Google Scholar]
- Johannessen M, Delghandi MP, Moens U. What turns CREB on? Cell Signal. 2004;16:1211–1227. doi: 10.1016/j.cellsig.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Johnson RS, van Lingen B, Papaioannou VE, Spiegelman BM. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev. 1993;7:1309–1317. doi: 10.1101/gad.7.7b.1309. [DOI] [PubMed] [Google Scholar]
- Katsumata N, Tanae A, Sato N, Horikawa R, Tanaka M. Adrenal gland. Significance of new StAR gene mutation of S195A-StAR phosphorylation found in congenital adrenal lipoid hyperplasia. Clin Endocrinol. 2000;48:141–143. [Google Scholar]
- Kehat I, Hasin T, Aronheim A. The role of basic leucine zipper protein-mediated transcription in physiological and pathological myocardial hypertrophy. Ann NY Acad Sci. 2006;1080:97–109. doi: 10.1196/annals.1380.009. [DOI] [PubMed] [Google Scholar]
- Kerban A, Boerboom D, Sirois J. Human chorionic gonadotropin induces an inverse regulation of steroidogenic acute regulatory protein messenger ribonucleic acid in theca interna and granulosa cells of equine preovulatory follicles. Endocrinology. 1999;140:667–674. doi: 10.1210/endo.140.2.6499. [DOI] [PubMed] [Google Scholar]
- Kohen P, Castro O, Palomino A, Munoz A, Christenson LK, Sierralta W, Carvallo P, Strauss JF, III, Devoto L. The steroidogenic response and corpus luteum expression of the steroidogenic acute regulatory protein after human chorionic gonadotropin administration at different times in the human luteal phase. J Clin Endocrinol Metab. 2003;88:3421–3430. doi: 10.1210/jc.2002-021916. [DOI] [PubMed] [Google Scholar]
- Kraemer FB, Shen WJ. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J Lipid Res. 2002;43:1585–1594. doi: 10.1194/jlr.r200009-jlr200. [DOI] [PubMed] [Google Scholar]
- Kwok RP, Lundblad JR, Chrivia JC, Richards JP, Bachinger HP, Brennan RG, Roberts SG, Green MR, Goodman RH. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- Lalli E, Ohe K, Hindelang C, Sassone-Corsi P. Orphan receptor DAX-1 is a shuttling RNA binding protein associated with polyribosomes via mRNA. Mol Cell Biol. 2000;20:4910–4921. doi: 10.1128/mcb.20.13.4910-4921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehoux JG, Fleury A, Ducharme L. The acute and chronic effects of adrenocorticotropin on the levels of messenger ribonucleic acid and protein of steroidogenic enzymes in rat adrenal in vivo. Endocrinology. 1998;139:3913–3922. doi: 10.1210/endo.139.9.6196. [DOI] [PubMed] [Google Scholar]
- Leung PC, Steele GL. Intracellular signaling in the gonads. Endocr Rev. 1992;13:476–498. doi: 10.1210/edrv-13-3-476. [DOI] [PubMed] [Google Scholar]
- Li H, Brochu M, Wang SP, Rochdi L, Cote M, Mitchell G, Gallo-Payet N. Hormone-sensitive lipase deficiency in mice causes lipid storage in the adrenal cortex and impaired corticosterone response to corticotropin stimulation. Endocrinology. 2002;143:3333–3340. doi: 10.1210/en.2002-220341. [DOI] [PubMed] [Google Scholar]
- Lin D, Sugawara T, Strauss JF, III, Clark BJ, Stocco DM, Saenger P, Rogol A, Miller WL. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science. 1995;267:1828–1831. doi: 10.1126/science.7892608. [DOI] [PubMed] [Google Scholar]
- Lin H, Chen MC, Ku CT. Cyclin-dependent kinase 5 regulates steroidogenic acute regulatory protein and androgen production in mouse Leydig cells. Endocrinology. 2009;150:396–403. doi: 10.1210/en.2008-0496. [DOI] [PubMed] [Google Scholar]
- Liu JS, Park EA, Gurney AL, Roesler WJ, Hanson RW. Cyclic AMP induction of phosphoenolpyruvate carboxykinase (GTP) gene transcription is mediated by multiple promoter elements. J Biol Chem. 1991;266:19095–19102. [PubMed] [Google Scholar]
- Liu J, Rone MB, Papadopoulos V. Protein–protein interactions mediate mitochondrial cholesterol transport and steroid biosynthesis. J Biol Chem. 2006;281:38879–38893. doi: 10.1074/jbc.M608820200. [DOI] [PubMed] [Google Scholar]
- Liu Q, Merkler KA, Zhang X, McLean MP. Prostaglandin F2alpha suppresses rat steroidogenic acute regulatory protein expression via induction of Yin Yang 1 protein and recruitment of histone deacetylase 1 protein. Endocrinology. 2007;148:5209–5219. doi: 10.1210/en.2007-0326. [DOI] [PubMed] [Google Scholar]
- Lutz CS. Alternative polyadenylation: a twist on mRNA 3′ end formation. ACS Chem Biol. 2008;3:609–617. doi: 10.1021/cb800138w. [DOI] [PubMed] [Google Scholar]
- Manna PR, Stocco DM. Regulation of the steroidogenic acute regulatory protein expression: functional and physiological consequences. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:93–108. doi: 10.2174/1568008053174714. [DOI] [PubMed] [Google Scholar]
- Manna PR, Stocco DM. Crosstalk of CREB and Fos/Jun on a single cis-element: transcriptional repression of the steroidogenic acute regulatory protein gene. J Mol Endocrinol. 2007;39:261–277. doi: 10.1677/JME-07-0065. [DOI] [PubMed] [Google Scholar]
- Manna PR, Stocco DM. The role of JUN in the regulation of PRKCC-mediated STAR expression and steroidogenesis in mouse Leydig cells. J Mol Endocrinol. 2008;41:329–341. doi: 10.1677/JME-08-0077. [DOI] [PubMed] [Google Scholar]
- Manna PR, Kero J, Tena-Sempere M, Pakarinen P, Stocco DM, Huhtaniemi IT. Assessment of mechanisms of thyroid hormone action in mouse Leydig cells: regulation of the steroidogenic acute regulatory protein, steroidogenesis, and luteinizing hormone receptor function. Endocrinology. 2001;142:319–331. doi: 10.1210/endo.142.1.7900. [DOI] [PubMed] [Google Scholar]
- Manna PR, Dyson MT, Eubank DW, Clark BJ, Lalli E, Sassone-Corsi P, Zeleznik AJ, Stocco DM. Regulation of steroidogenesis and the steroidogenic acute regulatory protein by a member of the cAMP response-element binding protein family. Mol Endocrinol. 2002;a 16:184–199. doi: 10.1210/mend.16.1.0759. [DOI] [PubMed] [Google Scholar]
- Manna PR, Huhtaniemi IT, Wang XJ, Eubank DW, Stocco DM. Mechanisms of epidermal growth factor signaling: regulation of steroid biosynthesis and the steroidogenic acute regulatory protein in mouse Leydig tumor cells. Biol Reprod. 2002;b 67:1393–1404. doi: 10.1095/biolreprod.102.007179. [DOI] [PubMed] [Google Scholar]
- Manna PR, Eubank DW, Lalli E, Sassone-Corsi P, Stocco DM. Transcriptional regulation of the mouse steroidogenic acute regulatory protein gene by the cAMP response-element binding protein and steroidogenic factor 1. J Mol Endocrinol. 2003;a 30:381–397. doi: 10.1677/jme.0.0300381. [DOI] [PubMed] [Google Scholar]
- Manna PR, Wang XJ, Stocco DM. Involvement of multiple transcription factors in the regulation of steroidogenic acute regulatory protein gene expression. Steroids. 2003;b 68:1125–1134. doi: 10.1016/j.steroids.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Manna PR, Eubank DW, Stocco DM. Assessment of the role of activator protein-1 on transcription of the mouse steroidogenic acute regulatory protein gene. Mol Endocrinol. 2004;18:558–573. doi: 10.1210/me.2003-0223. [DOI] [PubMed] [Google Scholar]
- Manna PR, Chandrala SP, Jo Y, Stocco DM. cAMP-independent signaling regulates steroidogenesis in mouse Leydig cells in the absence of StAR phosphorylation. J Mol Endocrinol. 2006;a 37:81–95. doi: 10.1677/jme.1.02065. [DOI] [PubMed] [Google Scholar]
- Manna PR, Chandrala SP, King SR, Jo Y, Counis R, Huhtaniemi IT, Stocco DM. Molecular mechanisms of insulin-like growth factor-I mediated regulation of the steroidogenic acute regulatory protein in mouse Leydig cells. Mol Endocrinol. 2006;b 20:362–378. doi: 10.1210/me.2004-0526. [DOI] [PubMed] [Google Scholar]
- Manna PR, Jo Y, Stocco DM. Regulation of Leydig cell steroidogenesis by extracellular signal-regulated kinase 1/2: role of protein kinase A and protein kinase C signaling. J Endocrinol. 2007;193:53–63. doi: 10.1677/JOE-06-0201. [DOI] [PubMed] [Google Scholar]
- Manna PR, Dyson MT, Jo Y, Stocco DM. Role of dosage-sensitive sex reversal, adrenal hypoplasia congenita, critical region on the X chromosome, gene 1 in protein kinase A- and protein kinase C-mediated regulation of the steroidogenic acute regulatory protein expression in mouse Leydig tumor cells: mechanism of action. Endocrinology. 2009;a 150:187–199. doi: 10.1210/en.2008-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna PR, Dyson MT, Stocco DM. Role of basic leucine zipper proteins in transcriptional regulation of the steroidogenic acute regulatory protein gene. Mol Cell Endocrinol. 2009;b 302:1–11. doi: 10.1016/j.mce.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Boucher N, Brousseau C, Tremblay JJ. The orphan nuclear receptor NUR77 regulates hormone-induced StAR transcription in Leydig cells through cooperation with Ca2+/calmodulin-dependent protein kinase I. Mol Endocrinol. 2008;22:2021–2037. doi: 10.1210/me.2007-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masquilier D, Sassone-Corsi P. Transcriptional cross-talk: nuclear factors CREM and CREB bind to AP-1 sites and inhibit activation by Jun. J Biol Chem. 1992;267:22460–22466. [PubMed] [Google Scholar]
- Mendez R, Richter JD. Translational control by CPEB: a means to the end. Nat Rev Mol Cell Biol. 2001;2:521–529. doi: 10.1038/35080081. [DOI] [PubMed] [Google Scholar]
- Meyer TE, Habener JF. Cyclic adenosine 3′,5′-monophosphate response element binding protein (CREB) and related transcription-activating deoxyribonucleic acid-binding proteins. Endocr Rev. 1993;14:269–290. doi: 10.1210/edrv-14-3-269. [DOI] [PubMed] [Google Scholar]
- Miller WL. StAR search—what we know about how the steroidogenic acute regulatory protein mediates mitochondrial cholesterol import. Mol Endocrinol. 2007;21:589–601. doi: 10.1210/me.2006-0303. [DOI] [PubMed] [Google Scholar]
- Miller WL, Strauss JF., III Molecular pathology and mechanism of action of the steroidogenic acute regulatory protein, StAR. J Steroid Biochem Mol Biol. 1999;69:131–141. doi: 10.1016/s0960-0760(98)00153-8. [DOI] [PubMed] [Google Scholar]
- Millhouse S, Kenny JJ, Quinn PG, Lee V, Wigdahl B. ATF/CREB elements in the herpes simplex virus type 1 latency-associated transcript promoter interact with members of the ATF/CREB and AP-1 transcription factor families. J Biomed Sci. 1998;5:451–464. doi: 10.1007/BF02255935. [DOI] [PubMed] [Google Scholar]
- Montminy M. Transcriptional regulation by cyclic AMP. Annu Rev Biochem. 1997;66:807–822. doi: 10.1146/annurev.biochem.66.1.807. [DOI] [PubMed] [Google Scholar]
- Montminy MR, Sevarino KA, Wagner JA, Mandel G, Goodman RH. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci USA. 1986;83:6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa FR, Takenaka I, Konishi R, Tokuda M. Effects of luteinizing hormone, follicle-stimulating hormone, and epidermal growth factor on expression and kinase activity of cyclin-dependent kinase 5 in Leydig TM3 and Sertoli TM4 cell lines. J Androl. 2000;21:392–402. [PubMed] [Google Scholar]
- Nair AK, Young MA, Menon KM. Regulation of luteinizing hormone receptor mRNA expression by mevalonate kinase—role of the catalytic center in mRNA recognition. FEBS J. 2008;275:3397–3407. doi: 10.1111/j.1742-4658.2008.06490.x. [DOI] [PubMed] [Google Scholar]
- Nalbant D, Williams SC, Stocco DM, Khan SA. Luteinizing hormone-dependent gene regulation in Leydig cells may be mediated by CCAAT/enhancer-binding protein-beta. Endocrinology. 1998;139:272–279. doi: 10.1210/endo.139.1.5663. [DOI] [PubMed] [Google Scholar]
- Nantel F, Monaco L, Foulkes NS, Masquilier D, LeMeur M, Henriksen K, Dierich A, Parvinen M, Sassone-Corsi P. Spermiogenesis deficiency and germ-cell apoptosis in CREM-mutant mice. Nature. 1996;380:159–162. doi: 10.1038/380159a0. [DOI] [PubMed] [Google Scholar]
- Nieschlag E, Behre HM, Bouchard P, Corrales JJ, Jones TH, Stalla GK, Webb SM, Wu FC. Testosterone replacement therapy: current trends and future directions. Hum Reprod Update. 2004;10:409–419. doi: 10.1093/humupd/dmh035. [DOI] [PubMed] [Google Scholar]
- Osterlund T. Structure–function relationships of hormone-sensitive lipase. Eur J Biochem. 2001;268:1899–1907. doi: 10.1046/j.1432-1327.2001.02097.x. [DOI] [PubMed] [Google Scholar]
- Osuga J, Ishibashi S, Oka T, Yagyu H, Tozawa R, Fujimoto A, Shionoiri F, Yahagi N, Kraemer FB, Tsutsumi O, et al. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc Natl Acad Sci USA. 2000;97:787–792. doi: 10.1073/pnas.97.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V, Liu J, Culty M. Is there a mitochondrial signaling complex facilitating cholesterol import? Mol Cell Endocrinol. 2007;265–266:59–64. doi: 10.1016/j.mce.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Parker D, Ferreri K, Nakajima T, LaMorte VJ, Evans R, Koerber SC, Hoeger C, Montminy MR. Phosphorylation of CREB at Ser-133 induces complex formation with CREB-binding protein via a direct mechanism. Mol Cell Biol. 1996;16:694–703. doi: 10.1128/mcb.16.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontkewitz Y, Enerback S, Hedin L. Expression of CCAAT enhancer binding protein-alpha (C/EBP alpha) in the rat ovary: implications for follicular development and ovulation. Dev Biol. 1996;179:288–296. doi: 10.1006/dbio.1996.0258. [DOI] [PubMed] [Google Scholar]
- Poderoso C, Converso DP, Maloberti P, Duarte A, Neuman I, Galli S, Maciel FC, Paz C, Carreras MC, Poderoso JJ, et al. A mitochondrial kinase complex is essential to mediate an ERK1/2-dependent phosphorylation of a key regulatory protein in steroid biosynthesis. PLoS ONE. 2008;3:e1443. doi: 10.1371/journal.pone.0001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poderoso C, Maloberti P, Duarte A, Neuman I, Paz C, Maciel FC, Podesta EJ. Hormonal activation of a kinase cascade localized at the mitochondria is required for StAR protein activity. Mol Cell Endocrinol. 2009;300:37–42. doi: 10.1016/j.mce.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Pollack SE, Furth EE, Kallen CB, Arakane F, Kiriakidou M, Kozarsky KF, Strauss JF., III Localization of the steroidogenic acute regulatory protein in human tissues. J Clin Endocrinol Metab. 1997;82:4243–4251. doi: 10.1210/jcem.82.12.4445. [DOI] [PubMed] [Google Scholar]
- Pon LA, Hartigan JA, Orme-Johnson NR. Acute ACTH regulation of adrenal corticosteroid biosynthesis. Rapid accumulation of a phosphoprotein. J Biol Chem. 1986;261:13309–13316. [PubMed] [Google Scholar]
- Rao RM, Jo Y, Leers-Sucheta S, Bose HS, Miller WL, Azhar S, Stocco DM. Differential regulation of steroid hormone biosynthesis in R2C and MA-10 Leydig tumor cells: role of SR-B1-mediated selective cholesteryl ester transport. Biol Reprod. 2003;68:114–121. doi: 10.1095/biolreprod.102.007518. [DOI] [PubMed] [Google Scholar]
- Reinhart AJ, Williams SC, Clark BJ, Stocco DM. SF-1 (steroidogenic factor-1) and C/EBP beta (CCAAT/enhancer binding protein-β) cooperate to regulate the murine StAR (steroidogenic acute regulatory) promoter. Mol Endocrinol. 1999;13:729–741. doi: 10.1210/mend.13.5.0279. [DOI] [PubMed] [Google Scholar]
- Rigotti A, Edelman ER, Seifert P, Iqbal SN, DeMattos RB, Temel RE, Krieger M, Williams DL. Regulation by adrenocorticotropic hormone of the in vivo expression of scavenger receptor class B type I (SR-BI), a high density lipoprotein receptor, in steroidogenic cells of the murine adrenal gland. J Biol Chem. 1996;271:33545–33549. doi: 10.1074/jbc.271.52.33545. [DOI] [PubMed] [Google Scholar]
- Roesler WJ. The role of C/EBP in nutrient and hormonal regulation of gene expression. Annu Rev Nutr. 2001;21:141–165. doi: 10.1146/annurev.nutr.21.1.141. [DOI] [PubMed] [Google Scholar]
- Ronen-Fuhrmann T, Timberg R, King SR, Hales KH, Hales DB, Stocco DM, Orly J. Spatio-temporal expression patterns of steroidogenic acute regulatory protein (StAR) during follicular development in the rat ovary. Endocrinology. 1998;139:303–315. doi: 10.1210/endo.139.1.5694. [DOI] [PubMed] [Google Scholar]
- Ross HL, Nonnemacher MR, Hogan TH, Quiterio SJ, Henderson A, McAllister JJ, Krebs FC, Wigdahl B. Interaction between CCAAT/enhancer binding protein and cyclic AMP response element binding protein 1 regulates human immunodeficiency virus type 1 transcription in cells of the monocyte/macrophage lineage. J Virol. 2001;75:1842–1856. doi: 10.1128/JVI.75.4.1842-1856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg SE, Adams TL, Olive M, Alexander N, Vinson C, Yuspa SH. CRE DNA binding proteins bind to the AP-1 target sequence and suppress AP-1 transcriptional activity in mouse keratinocytes. Oncogene. 1999;18:1569–1579. doi: 10.1038/sj.onc.1202463. [DOI] [PubMed] [Google Scholar]
- Saez JM. Leydig cells: endocrine, paracrine, and autocrine regulation. Endocr Rev. 1994;15:574–626. doi: 10.1210/edrv-15-5-574. [DOI] [PubMed] [Google Scholar]
- Sandberg R, Neilson JR, Sarma A, Sharp PA, Burge CB. Proliferating cells express mRNAs with shortened 3′ untranslated regions and fewer microRNA target sites. Science. 2008;320:1643–1647. doi: 10.1126/science.1155390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhoff TW, Hales DB, Hales KH, McLean MP. Transcriptional regulation of the rat steroidogenic acute regulatory protein gene by steroidogenic factor 1. Endocrinology. 1998;139:4820–4831. doi: 10.1210/endo.139.12.6345. [DOI] [PubMed] [Google Scholar]
- Sanyal S, Sandstrom DJ, Hoeffer CA, Ramaswami M. AP-1 functions upstream of CREB to control synaptic plasticity in Drosophila. Nature. 2002;416:870–874. doi: 10.1038/416870a. [DOI] [PubMed] [Google Scholar]
- Sasaki G, Ishii T, Jeyasuria P, Jo Y, Bahat A, Orly J, Hasegawa T, Parker KL. Complex role of the mitochondrial targeting signal in the function of steroidogenic acute regulatory protein revealed by bacterial artificial chromosome transgenesis in vivo. Mol Endocrinol. 2008;22:951–964. doi: 10.1210/me.2007-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P. Transcription factors governing male fertility. Andrologia. 2005;37:228–229. doi: 10.1111/j.1439-0272.2005.00701.x. [DOI] [PubMed] [Google Scholar]
- Sayed SB, Jones PM, Persaud SJ, Whitehouse BJ. Effects of phosphoprotein phosphatase inhibitors on steroidogenesis and StAR protein expression in Y1 cells. Endocr Res. 1998;24:413–414. doi: 10.3109/07435809809032624. [DOI] [PubMed] [Google Scholar]
- Sekar N, Lavoie HA, Veldhuis JD. Concerted regulation of steroidogenic acute regulatory gene expression by luteinizing hormone and insulin (or insulin-like growth factor I) in primary cultures of porcine granulosa-luteal cells. Endocrinology. 2000;141:3983–3992. doi: 10.1210/endo.141.11.7763. [DOI] [PubMed] [Google Scholar]
- Shea-Eaton W, Sandhoff TW, Lopez D, Hales DB, McLean MP. Transcriptional repression of the rat steroidogenic acute regulatory (StAR) protein gene by the AP-1 family member c-Fos. Mol Cell Endocrinol. 2002;188:161–170. doi: 10.1016/s0303-7207(01)00715-8. [DOI] [PubMed] [Google Scholar]
- Shen WJ, Patel S, Natu V, Hong R, Wang J, Azhar S, Kraemer FB. Interaction of hormone-sensitive lipase with steroidogenic acute regulatory protein: facilitation of cholesterol transfer in adrenal. J Biol Chem. 2003;278:43870–43876. doi: 10.1074/jbc.M303934200. [DOI] [PubMed] [Google Scholar]
- Sierralta WD, Kohen P, Castro O, Munoz A, Strauss JF, III, Devoto L. Ultrastructural and biochemical evidence for the presence of mature steroidogenic acute regulatory protein (StAR) in the cytoplasm of human luteal cells. Mol Cell Endocrinol. 2005;242:103–110. doi: 10.1016/j.mce.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Silverman E, Eimerl S, Orly J. CCAAT enhancer-binding protein beta and GATA-4 binding regions within the promoter of the steroidogenic acute regulatory protein (StAR) gene are required for transcription in rat ovarian cells. J Biol Chem. 1999;274:17987–17996. doi: 10.1074/jbc.274.25.17987. [DOI] [PubMed] [Google Scholar]
- Silverman E, Yivgi-Ohana N, Sher N, Bell M, Eimerl S, Orly J. Transcriptional activation of the steroidogenic acute regulatory protein (StAR) gene: GATA-4 and CCAAT/enhancer-binding protein beta confer synergistic responsiveness in hormone-treated rat granulosa and HEK293 cell models. Mol Cell Endocrinol. 2006;252:92–101. doi: 10.1016/j.mce.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Soccio RE, Adams RM, Romanowski MJ, Sehayek E, Burley SK, Breslow JL. The cholesterol-regulated StarD4 gene encodes a StAR-related lipid transfer protein with two closely related homologues, StarD5 and StarD6. Proc Natl Acad Sci USA. 2002;99:6943–6948. doi: 10.1073/pnas.052143799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen RL, Beullens M, Landsverk HB, Bollen M, Collas P. AKAP149 is a novel PP1 specifier required to maintain nuclear envelope integrity in G1 phase. J Cell Sci. 2003;116:2237–2246. doi: 10.1242/jcs.00432. [DOI] [PubMed] [Google Scholar]
- Sterneck E, Tessarollo L, Johnson PF. An essential role for C/EBPbeta in female reproduction. Genes Dev. 1997;11:2153–2162. doi: 10.1101/gad.11.17.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco DM. Clinical disorders associated with abnormal cholesterol transport: mutations in the steroidogenic acute regulatory protein. Mol Cell Endocrinol. 2002;191:19–25. doi: 10.1016/s0303-7207(02)00048-5. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Sodeman TC. The 30-kDa mitochondrial proteins induced by hormone stimulation in MA-10 mouse Leydig tumor cells are processed from larger precursors. J Biol Chem. 1991;266:19731–19738. [PubMed] [Google Scholar]
- Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 1996;17:221–244. doi: 10.1210/edrv-17-3-221. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Wang X, Jo Y, Manna PR. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol Endocrinol. 2005;19:2647–2659. doi: 10.1210/me.2004-0532. [DOI] [PubMed] [Google Scholar]
- Strauss JF, III, Kishida T, Christenson LK, Fujimoto T, Hiroi H. START domain proteins and the intracellular trafficking of cholesterol in steroidogenic cells. Mol Cell Endocrinol. 2003;202:59–65. doi: 10.1016/s0303-7207(03)00063-7. [DOI] [PubMed] [Google Scholar]
- Struthers RS, Vale WW, Arias C, Sawchenko PE, Montminy MR. Somatotroph hypoplasia and dwarfism in transgenic mice expressing a non- phosphorylatable CREB mutant. Nature. 1991;350:622–624. doi: 10.1038/350622a0. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Holt JA, Driscoll D, Strauss JF, III, Lin D, Miller WL, Patterson D, Clancy KP, Hart IM, Clark BJ, et al. Human steroidogenic acute regulatory protein: functional activity in COS-1 cells, tissue-specific expression, and mapping of the structural gene to 8p11.2 and a pseudogene to chromosome 13. Proc Natl Acad Sci USA. 1995;92:4778–4782. doi: 10.1073/pnas.92.11.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara T, Sakuragi N, Minakami H. CREM confers cAMP responsiveness in human steroidogenic acute regulatory protein expression in NCI-H295R cells rather than SF-1/Ad4BP. J Endocrinol. 2006;191:327–337. doi: 10.1677/joe.1.06601. [DOI] [PubMed] [Google Scholar]
- Tajima K, Babich S, Yoshida Y, Dantes A, Strauss JF, III, Amsterdam A. The proteasome inhibitor MG132 promotes accumulation of the steroidogenic acute regulatory protein (StAR) and steroidogenesis. FEBS Lett. 2001;490:59–64. doi: 10.1016/s0014-5793(01)02138-x. [DOI] [PubMed] [Google Scholar]
- Tang QQ, Gronborg M, Huang H, Kim JW, Otto TC, Pandey A, Lane MD. Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3beta is required for adipogenesis. Proc Natl Acad Sci USA. 2005;102:9766–9771. doi: 10.1073/pnas.0503891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temel RE, Trigatti B, DeMattos RB, Azhar S, Krieger M, Williams DL. Scavenger receptor class B, type I (SR-BI) is the major route for the delivery of high density lipoprotein cholesterol to the steroidogenic pathway in cultured mouse adrenocortical cells. Proc Natl Acad Sci USA. 1997;94:13600–13605. doi: 10.1073/pnas.94.25.13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis M, Si K, Kandel ER. Two previously undescribed members of the mouse CPEB family of genes and their inducible expression in the principal cell layers of the hippocampus. Proc Natl Acad Sci USA. 2003;100:9602–9607. doi: 10.1073/pnas.1133424100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Hu J, Zhang H, Lutz CS. A large-scale analysis of mRNA polyadenylation of human and mouse genes. Nucleic Acids Res. 2005;33:201–212. doi: 10.1093/nar/gki158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay JJ, Hamel F, Viger RS. Protein kinase A-dependent cooperation between GATA and CCAAT/enhancer-binding protein transcription factors regulates steroidogenic acute regulatory protein promoter activity. Endocrinology. 2002;143:3935–3945. doi: 10.1210/en.2002-220413. [DOI] [PubMed] [Google Scholar]
- Unal R, Pokrovskaya I, Tripathi P, Monia BP, Kern PA, Ranganathan G. Translational regulation of lipoprotein lipase in adipocytes: depletion of cellular protein kinase Calpha activates binding of the C subunit of protein kinase A to the 3′-untranslated region of the lipoprotein lipase mRNA. Biochem J. 2008;413:315–322. doi: 10.1042/BJ20071559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watari H, Arakane F, Moog-Lutz C, Kallen CB, Tomasetto C, Gerton GL, Rio MC, Baker ME, Strauss JF., III MLN64 contains a domain with homology to the steroidogenic acute regulatory protein (StAR) that stimulates steroidogenesis. Proc Natl Acad Sci USA. 1997;94:8462–8467. doi: 10.1073/pnas.94.16.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watari H, Blanchette-Mackie EJ, Dwyer NK, Sun G, Glick JM, Patel S, Neufeld EB, Pentchev PG, Strauss JF., III NPC1-containing compartment of human granulosa-lutein cells: a role in the intracellular trafficking of cholesterol supporting steroidogenesis. Exp Cell Res. 2000;255:56–66. doi: 10.1006/excr.1999.4774. [DOI] [PubMed] [Google Scholar]
- Waterman MR. Biochemical diversity of cAMP-dependent transcription of steroid hydroxylase genes in the adrenal cortex. J Biol Chem. 1994;269:27783–27786. [PubMed] [Google Scholar]
- Williams DL, Connelly MA, Temel RE, Swarnakar S, Phillips MC, de la Llera-Moya M, Rothblat GH. Scavenger receptor BI and cholesterol trafficking. Curr Opin Lipidol. 1999;10:329–339. doi: 10.1097/00041433-199908000-00007. [DOI] [PubMed] [Google Scholar]
- Wilson HL, McFie PJ, Roesler WJ. Different transcription factor binding arrays modulate the cAMP responsivity of the phosphoenolpyruvate carboxykinase gene promoter. J Biol Chem. 2002;277:43895–43902. doi: 10.1074/jbc.M203169200. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Matsuoka C, Gendou M, Izumi S, Zhao D, Artemenko I, Jefcoate CR, Kominami S. Mitochondrial processing of bovine adrenal steroidogenic acute regulatory protein. Biochim Biophys Acta. 2006;1764:1561–1567. doi: 10.1016/j.bbapap.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Yivgi-Ohana N, Sher N, Melamed-Book N, Eimerl S, Koler M, Manna PR, Stocco DM, Orly J. Transcription of steroidogenic acute regulatory protein in the rodent ovary and placenta: alternative modes of cyclic adenosine 3′,5′-monophosphate dependent and independent regulation. Endocrinology. 2009;150:977–989. doi: 10.1210/en.2008-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zazopoulos E, Lalli E, Stocco DM, Sassone-Corsi P. DNA binding and transcriptional repression by DAX-1 blocks steroidogenesis. Nature. 1997;390:311–315. doi: 10.1038/36899. [DOI] [PubMed] [Google Scholar]
- Zearfoss NR, Alarcon JM, Trifilieff P, Kandel E, Richter JD. A molecular circuit composed of CPEB-1 and c-Jun controls growth hormone-mediated synaptic plasticity in the mouse hippocampus. J Neurosci. 2008;28:8502–8509. doi: 10.1523/JNEUROSCI.1756-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ML, Yan YC, Sun YP, Koide SS. Identification and expression of epidermal growth factor gene in mouse testis. Cell Res. 1997;7:51–59. doi: 10.1038/cr.1997.6. [DOI] [PubMed] [Google Scholar]
- Zhang M, Liu P, Dwyer NK, Christenson LK, Fujimoto T, Martinez F, Comly M, Hanover JA, Blanchette-Mackie EJ, Strauss JF., III MLN64 mediates mobilization of lysosomal cholesterol to steroidogenic mitochondria. J Biol Chem. 2002;277:33300–33310. doi: 10.1074/jbc.M200003200. [DOI] [PubMed] [Google Scholar]
- Zhang FP, Pakarainen T, Poutanen M, Toppari J, Huhtaniemi I. The low gonadotropin-independent constitutive production of testicular testosterone is sufficient to maintain spermatogenesis. Proc Natl Acad Sci USA. 2003;100:13692–13697. doi: 10.1073/pnas.2232815100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Duan H, Kim YC, Jefcoate CR. Rodent StAR mRNA is substantially regulated by control of mRNA stability through sites in the 3′-untranslated region and through coupling to ongoing transcription. J Steroid Biochem Mol Biol. 2005;a 96:155–173. doi: 10.1016/j.jsbmb.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Zhao D, Xue H, Artemenko I, Jefcoate C. Novel signaling stimulated by arsenite increases cholesterol metabolism through increases in unphosphorylated steroidogenic acute regulatory (StAR) protein. Mol Cell Endocrinol. 2005;b 231:95–107. doi: 10.1016/j.mce.2004.08.006. [DOI] [PubMed] [Google Scholar]