Abstract
Background: A high-fat (HF) diet and sedentary lifestyle are implicated in the development of obesity. Controlled feeding studies and measures of short-term resting energy expenditure (REE) have suggested that the type of dietary fat may alter energy expenditure (EE).
Objective: The objective was to examine the effects of an HF diet rich in either monounsaturated or saturated fatty acids (FAs) and of exercise on EE and chronic disease risk factors.
Design: Eight healthy men [age: 18–45 y; body mass index (in kg/m2): 22 ± 3] were randomly assigned in a 2 × 2 crossover design to 1 of 4 treatments: HF diet (50% of energy) with a high amount of saturated fat (22% of energy) plus exercise (SE) or a sedentary (SS) condition or a diet high in monounsaturated fat (30% of energy) plus exercise (UE) or a sedentary (US) condition. The subjects spent 5 d in a metabolic chamber and cycled at 45% of maximal oxygen uptake for 2 h each day during the exercise visits. Respiratory gases and urinary nitrogen were measured to determine 24-h EE. Resting metabolic rate was measured on days 2, 4, and 6.
Results: Average 24-h EE was not different with respect to dietary FA composition (3202 ± 146, 3208 ± 151, 2240 ± 82, and 2270 ± 104 for SE, UE, SS, and US, respectively). Total and LDL cholesterol and blood pressure were significantly greater after the SE and SS treatments than after the UE and US treatments.
Conclusion: Resting metabolic rate and 24-h EE were not significantly different after short-term exposure to an HF diet rich in monounsaturated FAs or after exposure to a diet rich in saturated FAs in healthy, nonobese men.
INTRODUCTION
Background excess energy consumption by humans is stored in the body as fat, which drives the obesity epidemic that already affects more than one-third of American adults. Heart disease, high blood pressure, and diabetes are only a few of many possible diseases that are at least partially attributed to obesity (1, 2). High-fat (HF) diets, which are usually high in energy density, may lead to this excess energy consumption. Recently, however, studies have suggested that the type of fat being consumed differentially affects excess energy storage and energy metabolism (3).
Oleic acid (OA), a monounsaturated FA, and palmitic acid (PA), a saturated FA, are commonly used to study the effects of different FA classes on the body's metabolism. The ratio of unsaturated fats to saturated fats in the diet has been shown to influence energy substrate utilization in humans (4), but the effects on overall energy expenditure (EE) are less well understood. Studies in rats have shown that the type of FA differentially effects the thermic effect of food, ie, dietary thermogenesis is lower in rats fed a diet high in saturated fat than in rats fed a diet high in unsaturated fat (5). Additionally, rodent studies have also indicated that diets rich in saturated FAs lower EE, which may or may not be independent of the differences in diet-induced thermogenesis (5–7); however, neither of these phenomena have been either substantiated or refuted clearly in humans.
Recently, Kien et al (3) showed that body energy stores were greater in human subjects who consumed a diet high in saturated fats (rich in PA) than in subjects who consumed a diet high in unsaturated fats (rich in OA). These results suggest a modest increase in daily EE over a 28-d period with a high-OA diet (3). In that study, however, daily EE was calculated on the basis of energy intake and changes in body stores rather than on the basis of measured EE. Conversely, they found no differences between dietary FA composition in measured REE during both fasting and fed states. On the basis of these observations, the purpose of this study was to compare the effects of HF diets rich in either monounsaturated FAs or saturated FAs on 24-h total EE (24-h EE) and resting metabolic rate (RMR), as measured by indirect calorimetry. Additionally, the effects of acute exercise and sedentary conditions with subjects consuming these HF diets were also examined. On the basis of the findings from Kien et al (3), we hypothesized that 24-h EE would be lower during a diet high in saturated fat than during a diet high in monounsaturated fat.
SUBJECTS AND METHODS
Subjects
Eight healthy men between the ages of 18 and 45 y were recruited to participate in a research study at the University of Wisconsin (UW) hospital's Clinical and Translational Research Core (CTRC). The study was approved by the Institutional Review Board at UW-Madison, and informed written consent was obtained from each participant. For this randomized crossover study, inclusion criteria were men with a body mass index (in kg/m2) between 18 and 30 and a moderately sedentary lifestyle (<3 h/wk of low-to-moderate-intensity exercise and no vigorous exercise). Exclusion criteria included any history of metabolic or pulmonary disease, implanted electrical devices, and claustrophobia. All 8 participants completed all 4 of the study visits.
Protocol
Four treatment conditions were completed in random order: HF diet (50% of energy) with a high amount of saturated fat (22% of energy) plus exercise (SE) or a sedentary (SS) condition or a diet high in monounsaturated fat (30% of energy) plus exercise (UE) or a sedentary (US) condition. Each study visit was separated by ≥2 wk. Before participation, subjects underwent a physical exam and screening. During the screening visit, RMR (kcal/d) was measured for 30 min with the Deltatrac II Metabolic Monitor (VIASYS Health Care Inc, SensorMedics, Yorba Linda, CA) after the subjects fasted overnight. During calibration of the Deltatrac II with 95% O2 and 5% CO2, subjects rested for 30 min in the supine position with the canopy in place. During testing, they were instructed to remain motionless without sleeping while respiratory gases were collected. The 24-h total energy intake of each participant was calculated to equal a predicted EE of 1.35 × RMR during sedentary visits and 1.8 × RMR during exercise study visits. After RMR was measured, the subjects underwent a bicycle (maximal aerobic capacity) test on an Ergoline 800 cycle ergometer (SensorMedics Corp) at the Pulmonary Function Laboratory with a CPX-D rapid gas analyzer (Medical Graphics Corp, St Paul, MN) to measure maximal oxygen uptake (V̇O2 max). Active data were collected for 5 min while the volunteers pedaled with no resistance. When the subjects reached a steady state, the graded V̇O2 max test was initiated at 20 W and increased stepwise by 15 W for 2-min intervals until volitional exhaustion. Criteria for achieving V̇O2 included a V̇O2 plateau or RER (respiratory exchange ratio) >1.1 and heart rate >90% of the age-predicted heart rate max.
After completion of the screening visit, participants were scheduled for the inpatient study visits. For 4 d before their inpatient stay, participants were provided with a lead-in diet that was representative of the standard American diet. All foods contained roughly 55% carbohydrate, 15% protein, and exactly 30% fat. Total caloric intake for the lead-in diet was based on the participant's RMR × 1.65 (1.65 is an average US physical activity value) (8). Additionally, participants were asked to refrain from any vigorous physical activity for 24 h before their inpatient visit.
For each study visit, participants arrived at the CTRC at 1800 to begin the 5-d, 6-night inpatient visit in the metabolic chamber. A urine sample was collected (baseline), and subjects were given 0.025g 18O/kg body weight for the measurement of total body water (TBW) and body composition from urinary 18O. Participants slept from 2300 to 0645 each night, and sleeping during the day was prohibited. Seated blood pressure and fasting blood samples for the measurement of blood lipids (total, HDL, and LDL cholesterol), glucose, and insulin were drawn at 0700 on days 1 and 6. On waking at 0645, participants exited the chamber, and RMR was measured on mornings 2, 4, and 6. Subjects then had 30–45 min for personal hygiene before reentering the chamber at 0815. On day 4 of each study visit (third day of HF diet), no respiratory gases were collected because the participants were in the chamber with the door open to allow for a break from the confinement. However, diet, activity, and sampling on this day were identical to those on the rest of the days. Discharge occurred at 0800 on the sixth morning of each visit.
Diet
During the study visits, the meals were provided by the UW Hospitals and Clinics kitchen. All items were weighed and consumed entirely by the subjects. During the first full day of each visit, the participants remained on a typical American diet consisting of 55% carbohydrate, 15% protein, and 30% fat. On days 2 through 5, participants consumed either a high-saturated-fat or a high-monounsaturated-fat diet. Both HF diets provided 35% of energy from carbohydrate, 15% from protein, and 50% from fat. For the high-saturated-fat treatment, 23% of total energy consumed was saturated, 22% was monounsaturated fat, and 10% was polyunsaturated fat. For the high-monounsaturated-fat treatment, 30% of total energy was monounsaturated fat, 10% of energy was saturated, and 10% was polyunsaturated fat. The subjects received 3 meals each day (breakfast at 0830, lunch at 1200, and dinner at 1900) along with an afternoon (1600) and evening (2200) snack. Individual daily meals consisted of 25%, 25%, 40% minus 50 kcal, and 10% of daily caloric needs for breakfast, lunch, dinner, and afternoon snack, respectively. Additionally, the subjects received a 50-kcal evening snack. Total caloric intake was prescribed to equal 24-h EE. A caloric intake of RMR × 1.35 for sedentary visits was determined from a previous observational experience of participants from multiple cohorts under sedentary conditions while they spent whole days in the metabolic chamber. The actual ratios of energy intake to RMR (based on average RMR data from days 2, 4, and 6) were 1.32 (SS), 1.79 (SE), 1.33 (US), and 1.77 (UE), values that were close to the study design, although all treatments fell slightly below the prescribed ratio.
Exercise
During the exercise visits, the participants rode a stationary bike at 45% of their V̇O2 max twice each day (from roughly 1000–1100 and 2100–2200). The goal for the exercise visits was to raise 24-h EE to RMR × 1.8 based on the study by Smith et al (9). To calculate duration of exercise, the energy cost of cycling at 45% of V̇O2 was estimated from the individual linear equation between V̇O2 and work (in W) generated during each subject's V̇O2 max test. Relative V̇O2 (mL · kg−1 · min−1) at 45% of V̇O2 max was used to calculate the rate of EE (kcal/min) at that intensity (in W), which was used in combination with RMR to estimate the minutes of cycling needed to raise 24-h EE during the exercise study visits. The total number of minutes was then split into the morning and evening sessions of exercise.
Respiratory chamber
The metabolic chamber at the CTRC was similar in design to the chamber at the Department of Human Biology at Maastricht University in Maastricht, Netherlands (10), and the specifications and diagnostics were described previously (11). Additionally, information on chamber temperature, humidity, airflow, pressure, and data collection instrumentation were described elsewhere (12). Briefly, the composition of air is measured by using carbon dioxide (Hartman and Braun Uras-4) and oxygen (Magnos-6) gas analyzers (Applied Automation, Bartlesville, OK). Values for V̇O2, V̇CO2, RER, and EE were obtained through the use of a spreadsheet macro program (EXCEL, version 2002; Microsoft, Redmond, WA) that was designed for the metabolic chamber.
Confirmation of calibration in the chamber was done by using methanol burns (10) throughout the duration of the study. For each burn, anhydrous methanol (99.8% pure; Fisher Scientific, Pittsburgh, PA) was burned for 8 to 10 h, and the mean (±SD) percentage error in recovery for oxygen and carbon dioxide was calculated. For oxygen and carbon dioxide, the percentage errors in recoveries were 2.8 ± 6.7% and −5.5 ± 1.9%, respectively. The percentage recoveries from each burn were used to develop correction factors for the corresponding chamber data from each study visit.
Calculations of RMR and EE
Energy expenditure was calculated by using equations from Jequier and Schutz (13) from V̇CO2 produced and V̇O2 consumed. Because subjects were not in the chamber for a full 24-h period each day, EE data from 0830 to 0645 (22.25 h) was extrapolated to 24 h to calculate 24-h EE. RMR was measured for 30 min on days 2, 4, and 6 at 0700, and the last 20 min of data collection were used for determining RMR. RMR was calculated by using the full Weir equation (14):
where X is nitrogen excretion.
Sample collection and analysis
For the 18O analysis, 1 mL urine was equilibrated with carbon dioxide at 25°C for 48 h. The 18O enrichment was then measured by using continuous-flow isotope ratio mass spectrometry. A description of the sample analysis was reported elsewhere (12). Body water was calculated by dilution on the assumption that the oxygen dilution space was 1.007 × TBW (15), and fat-free mass was calculated as TBW ÷ 0.73.
Statistical analysis
The SAS version 8.2 statistical package (SAS Institute Inc, Cary, NC) was used for all data analysis, with subjects serving as their own controls because of the randomized crossover design. PROC MIXED was used for repeated-measures analysis to test the effects of the 4 different treatment groups on EE. Post hoc analyses were done by using a Tukey test. Values are presented as mean ± SEM unless otherwise indicated, and statistical significance was set at P < 0.05.
RESULTS
Subjects
Eight healthy men completed all 4 study visits, and subject characteristics are shown in Table 1. Per the eligibility criteria, all subjects were sedentary (<3 h/wk of low-to-moderate exercise), had a body mass index between 18 and 30, and were free of metabolic disease.
TABLE 1.
Subject characteristics1
| Value | |
| Age (y) | 25.10 ± 8.02 |
| Height (cm) | 184.82 ± 6.16 |
| Weight (kg) | 76.51 ± 6.52 |
| BMI (kg/m2) | 22.54 ± 3.34 |
| Body fat (%) | 20.26 ± 2.65 |
| V̇O2 max (mL · kg−1 · min−1)2 | 40.53 ± 5.08 |
All values are means ± SDs.
Maximal oxygen uptake.
Resting metabolic rate
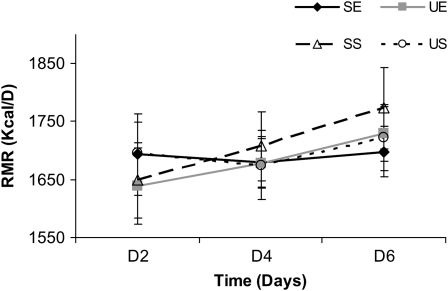
RMR was measured on mornings 2, 4, and 6, which corresponded to the first, third, and fifth days of feeding of the high-saturated-fat or high-monounsaturated-fat diets of each study visit. Mean 20-min RMR values for each day can be found in Figure 1. There was no significant main effect of time or treatment and no time × treatment interaction. Furthermore, there were no differences found between the RMR measured during the screening visit or any that were measured during the 4 study visits.
FIGURE 1.
Mean (±SEM) resting metabolic rate (RMR) on days 2, 4, and 6 for each of the 4 treatment conditions. No significant differences were found for any of the 4 treatment conditions by repeated-measures ANOVA. SE, high-saturated-fat diet plus exercise; SS, high-saturated-fat diet plus sedentary; UE, high-monounsaturated-fat diet plus exercise; US, high-monounsaturated-fat diet plus sedentary.
24-h Energy expenditure
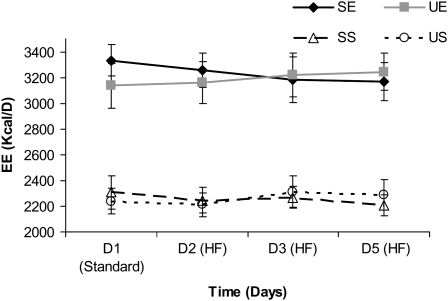
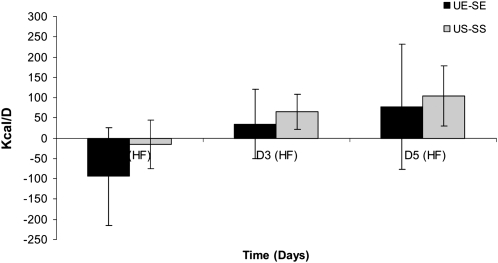
During exercise visits, participants cycled for an average of 119 ± 15 min on the cycle ergometer, which was divided evenly between the morning and evening exercise sessions. The data for 24-h EE for each treatment condition are shown in Figure 2. By design, there was a main effect of treatment (P < 0.01), with both exercise visits being higher than both sedentary visits. There was no main effect of time or exercise × time interaction. With regard to FA composition, there were no differences between UE and SE or between US and SS. Average 24-h EEs for all days for each of the study visits were 2240 ± 82, 3202 ± 146, 2270 ± 104, and 3208 ± 151 kcal/d for SS, SE, US, and UE, respectively. The within-subject difference in 24-h EE between the monounsaturated and saturated fat conditions were also calculated for both exercise and sedentary treatments. The averages for these within-subject differences are shown in Figure 3. For sedentary conditions (US and SS), the values were −94 ± 85, 35 ± 155, and 77 ± 96 kcal/d for days 2, 3, and 5, respectively. For the exercise conditions (UE and SE), the values were −15 ± 44, 66 ± 74, and 104 ± 78 kcal/d for days 2, 3, and 5, respectively. Statistical analysis showed no significant treatment effect (exercise or sedentary conditions), time (day) effect, or treatment × time interaction.
FIGURE 2.
Mean (±SEM) total energy expenditure (EE) on days 1, 2, 3, and 5 of each of the 4 treatment conditions. Repeated-measures ANOVA was conducted between the 4 treatment conditions. There was a main effect of treatment (P < 0.01); EE was higher during both exercise visits than during both sedentary visits. However, no time (day) effect was found; nor were there differences with respect to dietary fatty acid composition. HF, high fat; SE, high-saturated-fat diet plus exercise; SS, high-saturated-fat diet plus sedentary; UE, high-monounsaturated-fat diet plus exercise; US, high-monounsaturated-fat diet plus sedentary.
FIGURE 3.
Mean (±SEM) within-subject differences in 24-h energy expenditure between the monounsaturated fat diet and the saturated fat diet for both the exercise and sedentary treatments on days 2, 3, and 5. Repeated-measures ANOVA was conducted between the 4 treatment conditions. There was no significant treatment effect (exercise or sedentary conditions), time (day) effect, or treatment × time interaction. HF, high fat; SE, high-saturated-fat diet plus exercise; SS, high-saturated-fat diet plus sedentary; UE, high-monounsaturated-fat diet plus exercise; US, high-monounsaturated-fat diet plus sedentary.
Energy balance
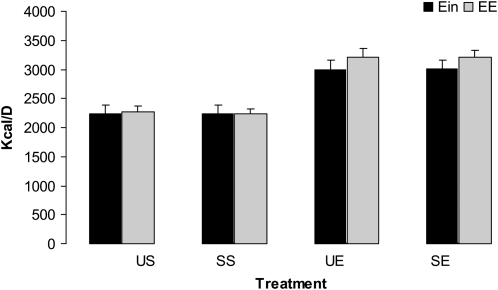
The relation between average energy intake and 24-h EE from the HF-diet days for each treatment is shown in Figure 4. For energy balance (intake versus expenditure), there was a significant main effect of treatment (P < 0.01). There was no main effect of time or exercise × time interaction. Energy balance was achieved during the sedentary visits, whereas 24-h EE was slightly higher than energy intake during the exercise visits, which led to a significant treatment effect. No differences between dietary FA diets existed for either exercise or sedentary conditions.
FIGURE 4.
Mean (±SEM) energy balance [energy intake (Ein) versus energy expenditure (EE)] for each treatment condition. Repeated-measures ANOVA was conducted between the 4 treatment conditions. There was a significant main effect of treatment (P < 0.01); 24-h energy expenditure was slightly higher than energy intake during the exercise visits. No differences between dietary fatty acid diets existed for either the exercise or sedentary condition. No significant effect of time (day) or treatment × time interaction was observed. SE, high-saturated-fat diet plus exercise; SS, high-saturated-fat diet plus sedentary; UE, high-monounsaturated-fat diet plus exercise; US, high-monounsaturated-fat diet plus sedentary.
Chronic disease risk factors
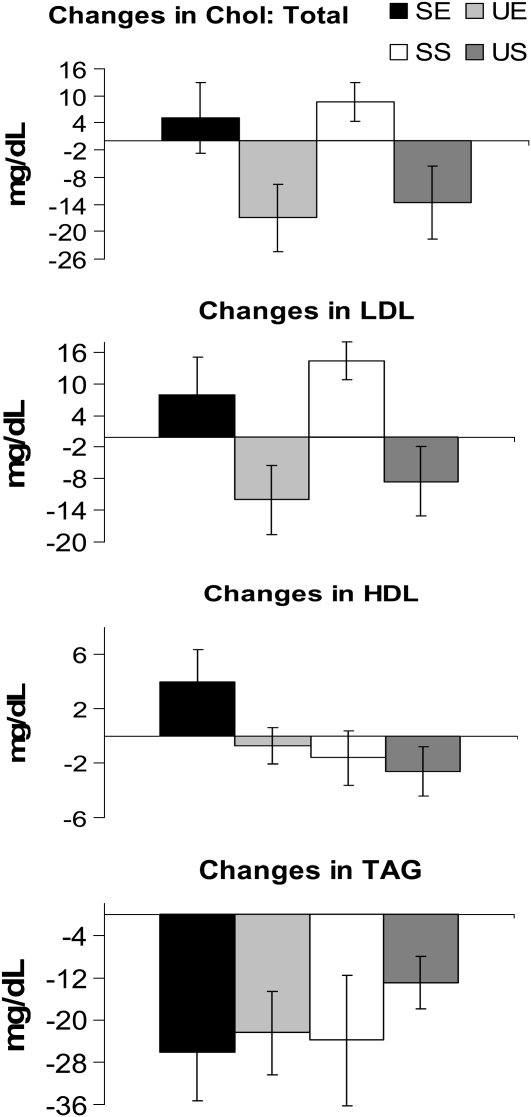
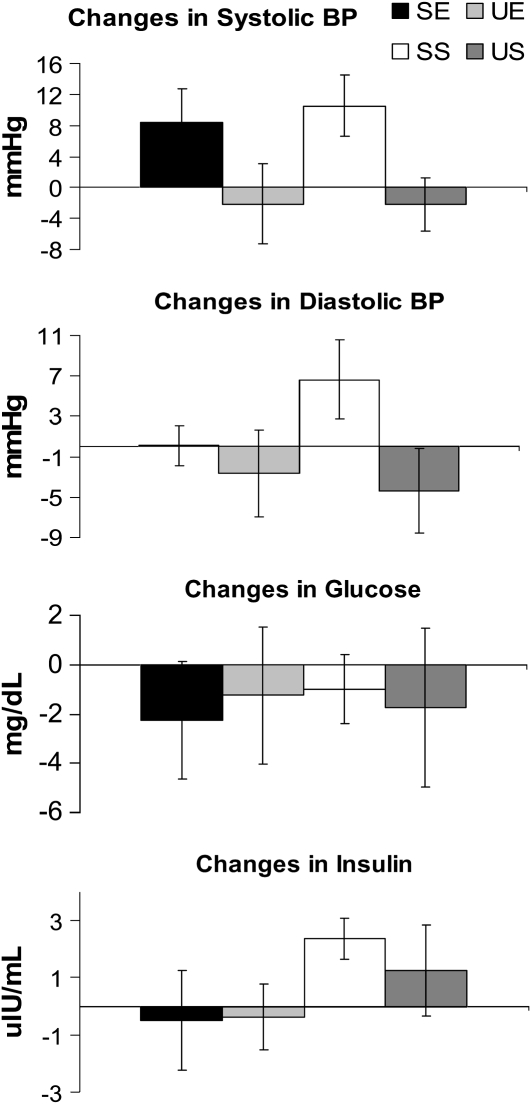
The changes from day 1 to day 6 in several chronic disease risk factors for each treatment condition are shown in Figure 5 and Figure 6. Both systolic and diastolic blood pressures were significantly higher with the saturated fat treatments (SE and SS) than with the monounsaturated fat treatments (UE and US). Furthermore, both total cholesterol and LDL cholesterol were significantly higher with the SE and SS treatments than with the UE and US conditions, whereas HDL cholesterol was greater with the SE than with the UE and US treatments. Plasma triglyceride concentrations showed a similar significant decrease in all groups. Finally, no significant differences between treatments were detected for blood glucose or insulin concentrations.
FIGURE 5.
Mean (±SEM) changes in fasting lipids from day 1 to day 6 for each of the 4 treatment conditions. A one-factor ANOVA was conducted between the 4 treatment conditions. Greater changes in total and LDL cholesterol (Chol) were observed in the high-saturated-fat diet plus exercise (SE) and high-saturated-fat diet plus sedentary activity (SS) conditions than in the high-monounsaturated-fat diet plus exercise (UE) and high-monounsaturated-fat diet plus sedentary activity (US) conditions, whereas greater changes in HDL were observed in the SE condition than in the UE and US conditions. Triglyceride (TAG) concentrations decreased with all 4 treatments, but no treatment differences were observed.
FIGURE 6.
Mean (±SEM) changes in fasting blood pressure (BP), glucose, and insulin from day 1 to day 6 for each of the 4 treatment conditions. A one-factor ANOVA between the 4 treatment conditions showed greater changes in systolic and diastolic blood pressure in the high-saturated-fat diet plus exercise (SE) and high-saturated-fat diet plus sedentary activity (SS) conditions than in the high-monounsaturated-fat diet plus exercise (UE) and high-monounsaturated-fat diet plus sedentary activity (US) conditions. No significant treatment differences were found for changes in glucose or insulin concentrations.
DISCUSSION
Short-term exposure to an HF diet rich in either monounsaturated or saturated FAs did not differentially affect RMR or 24-h EE. Per the study design, exercise treatments were significantly higher in 24-h EE, but were not different from sedentary conditions with respect to RMR. Whereas there was no effect of dietary FAs on energy balance, treatment differences existed because the exercise treatments were both in a slightly negative energy balance but subjects were in energy balance during both sedentary visits.
Our finding of no dietary effect of FAs on 24-h EE was contrary to what Kien et al (3) found in their study of female and male subjects. When their subjects went from a baseline diet to an HF diet for a 28-d period, there was no change in calculated daily EE (DEE) in the high-OA group, whereas the change in the high-PA group was significantly lower than the change in the high-OA group. A more recent study from this group showed that these differences in calculated DEE existed in their male but not in their female subjects (16). They suggested that changes in DEE between the different HF diets could have be due to differences in mitochondrial FA oxidation or function, which would affect the total energy cost of physical activity (3). However, it is important to note that 24-h EE was not measured in their study. Rather, energy intake data and the changes in body stores during the 28-d period were used to indirectly calculate DEE. On the basis of our results, it appears that when EE is measured in subjects consuming a high-OA or high-PA diet, there is no differential effect of these dietary FAs on 24-h EE. Moreover, for an α of 0.05, we had 80% power to detect a 104- and 166-kcal/d difference for sedentary and exercise conditions, respectively. Therefore, we had adequate power to detect the −266-kcal/d difference reported by Kein et al (3). One possible explanation for the differences between our study and that by Kien et al (3) is they did not actually measure DEE; thus, the difference they observed was related to dietary energy.
As part of the study by Kien et al, Borsheim et al (17) measured respiratory gases during 80 min of cycling and for 270 min after exercise in 19 adults after 28 d of a high-OA or high-PA diet. They observed lower oxygen consumption with the high-PA diet than with the high-OA diet during exercise and a trend for a difference in the postexercise period (17), but we found no differences in the 24-h EE response to the dietary FA treatments during the exercise treatments. A possible explanation for these differences could be the composition of the HF diets. Whereas our high monounsaturated fat diet contained 60% of fat kilocalories as OA, Borsheim et al (17) used nearly 80% of fat energy from OA. Additionally, their significant findings during a shorter period of time may have been negated had respiratory gases been measured over an entire 24-h period, as was done in our study. Another possibility is that our feeding trail was shorter than theirs. Whereas we did not detect a time effect from the within-subject differences between dietary FAs in 24-h EE, there was a general trend in 24-h EE over time for the monounsaturated compared with the saturated FA diets. This was apparent for both exercise and sedentary conditions, and it may take a longer period of feeding to see a differential effect on 24-h EE based on dietary FA composition.
The lack of difference in RMR between our treatment conditions is similar to the findings of others. Kien et al (3) also found no differences in REE during either the fed or fasting state between male and female subjects consuming a high-OA or a high-PA diet. However, Kein et al (16) further explored the sex effects of his 28-d study and found that the women in their study actually showed a higher REE with the high-PA diet in the fed, state but not in the fasted state. The male subjects in their study showed no difference in REE with respect to FA composition for either the fasted or fed state, which is what we found in our male subjects. Similarly, Piers et al (18) found no differences between a 4-wk saturated fat–rich diet or a monounsaturated fat–rich diet on RMR in male subjects. Therefore, it is possible that a dietary FA affect on REE in the fed state exists in female but not in male subjects.
Differences in saturated fats, such as PA, and monounsaturated fats, such as OA, exist with regard to absorption and metabolism. This is evidenced by the slightly lower absorption of PA (97%) than of OA (>99%) in humans (19). Applying these absorption percentages to our data would predict a very small difference in overall metabolized intake (<11 kcal/d for sedentary conditions and <14 kcal/d for exercise conditions). This would also predict a very small 8-kcal/d difference between the HF diets in Kien et al's study (3). These small differences in absorption between these 2 FAs, however, cannot explain the calculated differences in DEE observed in their study.
Many studies have shown adverse effects of a diet high in saturated fat on blood lipids, insulin, glucose, blood pressure, and coronary heard disease risk (20–23). Conversely, studies have also shown benefits from a diet high in monounsaturated fats on these same variables (23–25). In the present study, we provide additional evidence for the detrimental effects of diets rich in saturated fats on the basis of increases in systolic and diastolic blood pressure and increases in total and LDL cholesterol. Furthermore, our high-monounsaturated-fat diets resulted in decreases in total and LDL cholesterol and in modest decreases in systolic and diastolic blood pressure. Interestingly, most studies have measured the effects of changes in these markers on chronic disease risk over periods of weeks or months; however, we were able to detect metabolic changes after as few as 4 d of an HF diet rich in either monounsaturated or saturated FAs.
Our ability to detect changes in blood lipids and blood pressure are important with regard to the interpretation of our finding of no change in 24-h EE or REE. The studies by Kien et al (3, 16), which reported changes in calculated total EE and REE, were conducted over 28 d. It is therefore possible that our finding of no difference was due to the short nature of our dietary intervention. Very little data are available on the time course of acclimatization to different FAs; however, we showed a number of physiologic effects after 5 d and others have shown that switching to a HF diet can up-regulate lipid metabolism and skeletal muscle gene expression after only 5 d (26). Thus, we believe that our finding of no change in 24-h EE during our dietary fat feeding trial was valid despite the short duration of the intervention. A longer-term study, however, would further strengthen our findings.
Similarly to any study, our study had certain limitations. Our research subjects were primarily young, nonobese, healthy men; therefore, it remains unknown whether these results would be consistent across different demographics and health states. A second limitation of our study was that whereas energy balance was achieved with the sedentary treatments, subjects were in a slight negative energy balance during both exercise visits. However, because the negative energy balance was similar between the 2 exercise visits, it likely did not adversely affect the results with regard to FA composition. Furthermore, no significant changes in body weight were detected for any of the treatments.
In summary, consumption of an HF diet rich in either monounsaturated or saturated FAs does not result in differences in RMR or 24-h EE during either exercise or sedentary conditions, as measured by indirect calorimetry for 5 consecutive days. These results conflict with those previously reported in the literature, in which a difference in calculated DEE was found between diets rich in OA and those rich in PA during a 28-d period (3). Although no apparent differences in 24-h EE were observed during this 5-d study, it remains to be determined whether measured differences would be observed after a longer period of diet consumption. Finally, HF diets rich in saturated FAs showed unfavorable changes, whereas monounsaturated FAs showed favorable changes in chronic disease risk factors compared with the baseline diet.
Acknowledgments
The authors' responsibilities were as follows—JAC: responsible for subject recruitment and screening, data collection and analysis, and manuscript preparation; ACW: responsible for subject recruitment, screening, and data collection; AKA: responsible for subject screening, health and physical exams, and daily checks with each subject during inpatients stays; and DAS: responsible for the study proposal and design, grant funding, data interpretation, and manuscript preparation. None of the authors had a conflict of interest.
REFERENCES
- 1.Bogers RP, Bemelmans WJ, Hoogenveen RT, et al. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med 2007;167:1720–8 [DOI] [PubMed] [Google Scholar]
- 2.Vongpatanasin W. Cardiovascular morbidity and mortality in high-risk populations: epidemiology and opportunities for risk reduction. J Clin Hypertens (Greenwich) 2007;9:11–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kien CL, Bunn JY, Ugrasbul F. Increasing dietary palmitic acid decreases fat oxidation and daily energy expenditure. Am J Clin Nutr 2005;82:320–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones PJ, Schoeller DA. Polyunsaturated:saturated ratio of diet fat influences energy substrate utilization in the human. Metabolism 1988;37:145–51 [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi H, Matsuo T, Tokuyama K, Shimomura Y, Suzuki M. Diet-induced thermogenesis is lower in rats fed a lard diet than in those fed a high oleic acid safflower oil diet, a safflower oil diet or a linseed oil diet. J Nutr 1995;125:920–5 [DOI] [PubMed] [Google Scholar]
- 6.Shimomura Y, Tamura T, Suzuki M. Less body fat accumulation in rats fed a safflower oil diet than in rats fed a beef tallow diet. J Nutr 1990;120:1291–6 [DOI] [PubMed] [Google Scholar]
- 7.Mercer SW, Trayhurn P. Effect of high fat diets on energy balance and thermogenesis in brown adipose tissue of lean and genetically obese ob/ob mice. J Nutr 1987;117:2147–53 [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: The National Academies Press, 2005:184–5 [Google Scholar]
- 9.Smith SR, de Jonge L, Zachwieja JJ, et al. Fat and carbohydrate balances during adaptation to a high-fat. Am J Clin Nutr 2000;71:450–7 [DOI] [PubMed] [Google Scholar]
- 10.Schoffelen PF, Westerterp KR, Saris WH, Ten HF. A dual-respiration chamber system with automated calibration. J Appl Physiol 1997;83:2064–72 [DOI] [PubMed] [Google Scholar]
- 11.Votruba SB, Atkinson RL, Hirvonen MD, Schoeller DA. Prior exercise increases subsequent utilization of dietary fat. Med Sci Sports Exerc 2002;34:1757–65 [DOI] [PubMed] [Google Scholar]
- 12.Hansen KC, Zhang Z, Gomez T, Adams AK, Schoeller DA. Exercise increases the proportion of fat utilization during short-term consumption of a high-fat diet. Am J Clin Nutr 2007;85:109–16 [DOI] [PubMed] [Google Scholar]
- 13.Jequier E, Schutz Y. Long-term measurements of energy expenditure in humans using a respiration chamber. Am J Clin Nutr 1983;38:989–98 [DOI] [PubMed] [Google Scholar]
- 14.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoeller DA, Luke AH. Rapid 18O analysis of CO2 samples by continuous-flow isotope ratio mass spectrometry. J Mass Spectrom 1997;32:1332–6 [DOI] [PubMed] [Google Scholar]
- 16.Kien CL, Bunn JY. Gender alters the effects of palmitate and oleate on fat oxidation and energy expenditure. Obesity (Silver Spring) 2008;16:29–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borsheim E, Kien CL, Pearl WM. Differential effects of dietary intake of palmitic acid and oleic acid on oxygen consumption during and after exercise. Metabolism 2006;55:1215–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piers LS, Walker KZ, Stoney RM, Soares MJ, O'Dea K. Substitution of saturated with monounsaturated fat in a 4-week diet affects body weight and composition of overweight and obese men. Br J Nutr 2003;90:717–27 [DOI] [PubMed] [Google Scholar]
- 19.Baer DJ, Judd JT, Kris-Etherton PM, Zhao G, Emken EA. Stearic acid absorption and its metabolizable energy value are minimally lower than those of other fatty acids in healthy men fed mixed diets. J Nutr 2003;133:4129–34 [DOI] [PubMed] [Google Scholar]
- 20.Straznicky NE, O'Callaghan CJ, Barrington VE, Louis WJ. Hypotensive effect of low-fat, high-carbohydrate diet can be independent of changes in plasma insulin concentrations. Hypertension 1999;34:580–5 [DOI] [PubMed] [Google Scholar]
- 21.Bowman MP, Van DJ, Taper LJ, Thye FW, Ritchey SJ. Effect of dietary fat and cholesterol on plasma lipids and lipoprotein fractions in normolipidemic men. J Nutr 1988;118:555–60 [DOI] [PubMed] [Google Scholar]
- 22.Borkman M, Campbell LV, Chisholm DJ, Storlien LH. Comparison of the effects on insulin sensitivity of high carbohydrate and high fat diets in normal subjects. J Clin Endocrinol Metab 1991;72:432–7 [DOI] [PubMed] [Google Scholar]
- 23.Miller ER, III, Erlinger TP, Appel LJ. The effects of macronutrients on blood pressure and lipids: an overview of the DASH and OmniHeart trials. Curr Atheroscler Rep 2006;8:460–5 [DOI] [PubMed] [Google Scholar]
- 24.Curb JD, Wergowske G, Dobbs JC, Abbott RD, Huang B. Serum lipid effects of a high-monounsaturated fat diet based on macadamia nuts. Arch Intern Med 2000;160:1154–8 [DOI] [PubMed] [Google Scholar]
- 25.Berry EM, Eisenberg S, Friedlander Y, et al. Effects of diets rich in monounsaturated fatty acids on plasma lipoproteins–the Jerusalem Nutrition Study. II. Monounsaturated fatty acids vs carbohydrates. Am J Clin Nutr 1992;56:394–403 [DOI] [PubMed] [Google Scholar]
- 26.Cameron-Smith D, Burke LM, Angus DJ, et al. A short-term, high-fat diet up-regulates lipid metabolism and gene expression in human skeletal muscle. Am J Clin Nutr 2003;77:313–8 [DOI] [PubMed] [Google Scholar]