Abstract
Background: Saturated fatty acid (SFA) intake increases plasma LDL-cholesterol concentrations; therefore, intake should be reduced to prevent coronary heart disease (CHD). Lower habitual intakes of SFAs, however, require substitution of other macronutrients to maintain energy balance.
Objective: We investigated associations between energy intake from monounsaturated fatty acids (MUFAs), polyunsaturated fatty acids (PUFAs), and carbohydrates and risk of CHD while assessing the potential effect-modifying role of sex and age. Using substitution models, our aim was to clarify whether energy from unsaturated fatty acids or carbohydrates should replace energy from SFAs to prevent CHD.
Design: This was a follow-up study in which data from 11 American and European cohort studies were pooled. The outcome measure was incident CHD.
Results: During 4–10 y of follow-up, 5249 coronary events and 2155 coronary deaths occurred among 344,696 persons. For a 5% lower energy intake from SFAs and a concomitant higher energy intake from PUFAs, there was a significant inverse association between PUFAs and risk of coronary events (hazard ratio: 0.87; 95% CI: 0.77, 0.97); the hazard ratio for coronary deaths was 0.74 (95% CI: 0.61, 0.89). For a 5% lower energy intake from SFAs and a concomitant higher energy intake from carbohydrates, there was a modest significant direct association between carbohydrates and coronary events (hazard ratio: 1.07; 95% CI: 1.01, 1.14); the hazard ratio for coronary deaths was 0.96 (95% CI: 0.82, 1.13). MUFA intake was not associated with CHD. No effect modification by sex or age was found.
Conclusion: The associations suggest that replacing SFAs with PUFAs rather than MUFAs or carbohydrates prevents CHD over a wide range of intakes.
See corresponding editorial on page 1283.
INTRODUCTION
The effect of dietary fat on risk of coronary heart disease (CHD) is at least partially mediated by the effect of dietary fat on plasma lipids (1). National health organizations (2, 3) recommend dietary changes that reduce intakes of saturated fatty acids (SFAs) to prevent CHD. The rationale is to reduce the LDL-cholesterol concentration in plasma. A lower habitual intake of SFAs requires substitution of other macronutrients to maintain the energy balance. The question remains whether a lower intake of SFAs should be replaced with a higher intake of unsaturated fatty acids or a higher intake of carbohydrates.
One follow-up study examined the risk of CHD with a lower energy intake from SFAs and a concomitant higher energy intake from unsaturated fatty acids or carbohydrates (4). The results from that study showed that replacing SFAs with unsaturated fatty acids was inversely associated with risk of CHD, whereas replacing SFAs with carbohydrates was not associated with risk of CHD. In another follow-up study, it was found that sex and age modified the association between intakes of SFAs and risk of CHD (5). In that study, SFA intake was strongly directly associated with risk of CHD among women aged <60 y but not among women aged ≥60 y. Among men, the results indicated a direct association between SFA intake and risk of CHD among men aged <60 y but not among men aged ≥60 y. Effect modification by sex and age may be due to hormonal differences; female sex hormones have estrogenic activity and male sex hormones have androgenic activity. Furthermore, after menopause the female sex hormones decline. Moreover, effect modification by sex and age may be due to differences in the underlying hazard functions.
In the present study, we pooled data from 11 American and European studies included in the Pooling Project of Cohort Studies on Diet and Coronary Disease and investigated associations between energy intakes from unsaturated fatty acids and carbohydrates and the risk of CHD while assessing the potential effect-modifying role of sex and age. Using substitution models, our objective was to clarify whether energy intake from unsaturated fatty acids or carbohydrates should replace energy intake from SFAs to prevent CHD. Pooling data allows study of associations between major types of dietary fat and risk of CHD in different populations with different diets and over a wide range of intakes.
SUBJECTS AND METHODS
Study population
The criteria for inclusion of studies in the Pooling Project of Cohort Studies on Diet and Coronary Disease were as follows: 1) published follow-up study with ≥150 incident coronary events, 2) availability of usual dietary intake, and 3) a validation or repeatability study of the diet-assessment method used (6). Through literature searches and inquiries with experts in the field, 14 American and European cohort studies were identified and investigators of 11 studies agreed to include their data in the project (6). The included studies are presented in Table 1 (4, 5, 7–15). The follow-up of the Nurses' Health Study was divided into 2 periods for analysis to take advantage of the repeated assessments of dietary intake and the long follow-up. Following the underlying theory of survival data, blocks of person-time in different periods are statistically independent, even if derived from the same persons (16). Two of the 11 included studies were randomized primary prevention studies: the Alpha-Tocopherol and Beta-Carotene Cancer Prevention Study (ATBC) and the Women's Health Study (WHS). The treatments were α-tocopherol, β-carotene, or both in the ATBC and α-tocopherol and aspirin in the WHS.
TABLE 1.
Characteristics of the cohort studies included in the Pooling Project of Cohort Studies on Diet and Coronary Disease1
| Median age at baseline (80% central range) | Median follow-up time |
Number of CHD events |
Median dietary fat intake (80% central range) |
||||||||
| Study | Calendar year of inception | Baseline cohort | Fatal and nonfatal CHD events | CHD deaths | Fatal and nonfatal | Deaths | Dietary assessment | SFAs | MUFAs | PUFAs | |
| n | y | y | y | % of energy | |||||||
| AHS (7) | 1977 | FFQ | |||||||||
| F | 13,430 | 57 (39–76) | 6.3 | 6.3 | 75 | 41 | 11.3 (9.4–14.9) | 12.7 (11.0–14.4) | 8.8 (7.2–10.6) | ||
| M | 9212 | 55 (39–74) | 6.3 | 6.3 | 148 | 49 | 10.7 (9.0–14.0) | 12.6 (11.4–14.1) | 9.0 (7.7–10.4) | ||
| ARIC (8) | 1987 | FFQ | |||||||||
| F2 | 6481 | 53 (47–62) | 9.2 | — | 123 | — | 11.8 (8.1–15.7) | 12.5 (8.5–16.3) | 4.9 (3.4–6.9) | ||
| M | 5240 | 54 (47–63) | 9.2 | 9.2 | 269 | 51 | 12.8 (9.0–16.5) | 13.8 (9.8–17.2) | 5.1 (3.6–7.0) | ||
| ATBC (9) | 1985 | FFQ | |||||||||
| M | 21,141 | 57 (51–65) | 6.0 | 6.1 | 1339 | 534 | 19.9 (14.5–25.7) | 13.7 (11.4–16.2) | 4.7 (3.3–9.0) | ||
| FMC (10) | 1966 | DH | |||||||||
| F | 2481 | 49 (38–65) | 10.0 | 10.0 | 162 | 48 | 19.9 (14.5–25.8) | 11.3 (8.7–14.2) | 2.2 (1.7–3.8) | ||
| M | 2712 | 47 (37–63) | 10.0 | 10.0 | 322 | 147 | 21.3 (16.1–26.9) | 11.9 (9.4–15.0) | 2.3 (1.8–3.7) | ||
| GPS (5) | 1974 | DH/7-D | |||||||||
| F | 1666 | 50 (40–60) | 10.0 | 10.0 | 34 | 14 | 19.5 (14.2–24.8) | 15.2 (11.3–18.6) | 6.6 (4.5–9.5) | ||
| M | 1658 | 50 (40–60) | 10.0 | 10.0 | 102 | 38 | 19.7 (14.5–25.0) | 15.8 (12.0–19.5) | 6.6 (4.5–9.5) | ||
| HPFS (11) | 1986 | FFQ | |||||||||
| M | 41,754 | 53 (42–67) | 9.7 | 9.7 | 1273 | 421 | 11.3 (7.8–14.8) | 12.7 (9.1–16.0) | 5.9 (4.3–8.0) | ||
| IIHD (12) | 1963 | FFQ | |||||||||
| M3 | 8272 | 48 (41–59) | — | 10.0 | — | 165 | 9.4 (6.0, 13.2) | 10.2 (7.2, 13.4) | 6.4 (3.6, 9.5) | ||
| IWHS (13) | 1986 | FFQ | |||||||||
| F3 | 30,180 | 61 (56–67) | — | 10.0 | — | 294 | 12.0 (9.1–15.3) | 12.6 (9.4–15.6) | 6.1 (4.3–8.2) | ||
| NHSa (4) | 1980 | FFQ | |||||||||
| F | 81,415 | 47 (38–57) | 6.5 | 6.5 | 397 | 97 | 16.1 (11.5–20.7) | 16.5 (11.6–21.4) | 5.2 (3.6–7.5) | ||
| NHSb (4) | 1986 | FFQ | |||||||||
| F | 61,706 | 52 (43–62) | 10.0 | 10.0 | 696 | 208 | 11.6 (8.6–15.0) | 12.1 (9.1–15.2) | 6.0 (4.3–8.2) | ||
| VIP (14) | 1992 | FFQ | |||||||||
| F2 | 10,555 | 50 (40–60) | 4.1 | — | 23 | — | 14.3 (10.8–18.3) | 11.9 (9.4–14.7) | 4.4 (3.5–6.0) | ||
| M | 9521 | 50 (40–60) | 4.1 | 4.1 | 134 | 38 | 15.7 (11.9–20.1) | 13.1 (10.3–16.3) | 4.7 (3.7–6.4) | ||
| WHS (15) | 1993 | FFQ | |||||||||
| F | 37,272 | 52 (46–64) | 5.3 | 5.3 | 152 | 10 | 10.1 (7.1–13.5) | 11.1 (7.8–14.6) | 5.6 (4.1–7.6) | ||
| All studies4 | 344,696 (71) | 6.5 | 6.5 | 5249 (32) | 2155 (34) | ||||||
AHS, Adventis Health Study; ARIC, Atherosclerosis Risk in Communities Study; ATBC; Alpha-Tocopherol and Beta-Carotene Cancer Prevention Study; CHD, coronary heart disease; DH, dietary history interview; FFQ, food-frequency questionnaire; FMC, Finnish Mobile Clinic Health Study; GPS, Glostrup Population Study; HPFS, Health Professionals Follow-Up Study; IIHD, Israeli Ischemic Heart Disease Study; IWHS, Iowa Women's Health Study; MUFAs, monounsaturated fatty acids; NHSa, Nurses' Health Study 1980; NHSb, Nurses' Health Study 1986; PUFAs, polyunsaturated fatty acids; SFAs, saturated fatty acids; 7-D; 7-d weighed food record; VIP, Västerbotten Intervention Program; WHS, Women's Health Study.
Insufficient events for analysis of coronary deaths among women.
Because IIHD and IWHS had only self-reported data on nonfatal CHD, we used only fatal CHD events from these studies.
Percentages of females in parentheses.
Criteria for exclusion of persons from the population at risk were as follows: age <35 y; history of cardiovascular disease, diabetes, or cancer (other than nonmelanoma skin cancer); and extreme energy intake (ie, > or <3 SDs from the study-specific log-transformed mean energy intake of the population). The final population consisted of 344,696 persons (71% women; Table 1).
Exposure measures
Dietary intake was determined at baseline by using a food-frequency questionnaire or a dietary history interview (Table 1). The validation and repeatability of the diet-assessment methods were evaluated and were found reasonable for population studies of the nutrients of interest. Total energy intake was calculated as the sum of energy intake derived from fat, carbohydrates, and protein. Derived exposure measures were dietary intake of monounsaturated fatty acids (MUFAs; primarily n−9 oleic acid, also known as omega-9 oleic acid), polyunsaturated fatty acids (PUFAs; including n−3 and n−6 fatty acids, also known as omega-3 and omega-6 fatty acids; primarily n−6 linoleic acid), and carbohydrates. The MUFA and PUFA for which the data are reported in the Israeli Ischemic Heart Disease Study (IIHD) were the n−9 MUFA oleic acid and the n−6 PUFA linoleic acid.
Outcome measures
The outcome measures were fatal CHD (including sudden death) and nonfatal myocardial infarction. Standardized criteria were used to ascertain events of fatal and nonfatal myocardial infarction (6). Because the IIHD and Iowa Women's Health Study (IWHS) had only self-reported data on nonfatal CHD, we used only fatal CHD events from these studies.
Statistical analyses
Within each study, hazard ratios (HRs) with 95% CIs for the incidence of a coronary event and of mortality from CHD were calculated by using Cox proportional hazards regression with time in study (y) as the time metric. The observation time for each participant was defined as the number of months from the date on which information on diet was obtained until CHD occurrence, death of another cause, disappearance, or end of follow-up, whichever came first. Studies with follow-up periods >10 y were truncated to reduce possible effect modification by time.
Two models were used to investigate whether energy intake from unsaturated fatty acids or carbohydrates should replace the energy intake from SFAs to prevent coronary events. Model 1 included intakes of MUFAs, PUFAs, trans fatty acids (TFAs), carbohydrates, and protein expressed as percentages of total energy intake (as continuous variables) and total energy intake (kcal/d; as a continuous variable). Age at baseline (y) and the calendar year in which the baseline questionnaire was returned were entered into the model through the strata statement; thus, assuming the same effect for the variable of interest but allowing the underlying hazard functions to differ with respect to age and time of collection of dietary information. Model 2 included variables in model 1 and CHD risk factors measured at baseline: smoking (never smokers, former smokers, and current smokers of 1–4, 5–14, 15–24, or ≥25 cigarettes/d), body mass index (in kg/m2; <23, 23 to <25, 25 to <27.5, 27.5 to <30, or ≥30), physical activity (levels 1–5), highest attained educational level (<high school, high school, or >high school), alcohol intake (0, 0 to <5, 5 to <10, 10 to <15, 15 to <30, 30 to <50, or ≥50 g/d), history of hypertension (yes or no), and energy-adjusted quintiles of fiber intake (g/d) and cholesterol intake (mg/d). Every variable was standardized across studies to the extent possible (6). A missing indicator variable was created for each categorical variable. Adjustment for treatment group was done in the ATBC (placebo or nonplacebo), but this was not possible in the ongoing WHS because of confidentiality issues. The estimated HRs for unsaturated fatty acids and carbohydrates can be interpreted as the estimated differences in risk of a 5% lower energy intake from SFAs and a concomitant higher energy intake from unsaturated fatty acids and carbohydrates, respectively. In other words the results may be interpreted as replacing SFAs with unsaturated fatty acids or carbohydrates.
Methods for pooling the study-specific HRs followed those described by Smith-Warner et al (17). The study-specific logs of HRs were weighted by the inverse of their variances, and a pooled (combined) estimate of the HRs was computed by using a random-effects model. Evidence for between-studies heterogeneity among the study-specific HRs was assessed by using the estimated between-studies variance component Q statistic.
To evaluate potential effect modification by age, the study population was divided into 2 age groups in further analyses: <60 y at entry and ≥60 y at entry. Effect modification by sex and age was investigated by including a cross-product interaction term between the exposure variable and sex or age. Pooled P values for the test of interaction were calculated by using squared Wald statistics by pooling the study-specific interaction log HRs and dividing by the square of the SE of the pooled interaction term. The resulting statistic was referred to a chi-square distribution with 1 df.
The proportional hazards assumption was checked by including a cross-product interaction term between the exposure variable and the stratifying variable age (y). We tested the exposure variables for nonlinearity in a spline regression model. The analyses were performed by using SAS statistical software, release 9.1 (SAS Institute Inc, Cary, NC) and Stata statistical software, release 9.0 (Stata Corporation, College Station, TX).
RESULTS
Characteristics of the cohort studies are given in Table 1. During 4–10 y of follow-up, 5249 coronary events and 2155 coronary deaths occurred among 344,696 persons (71% women; Table 1).
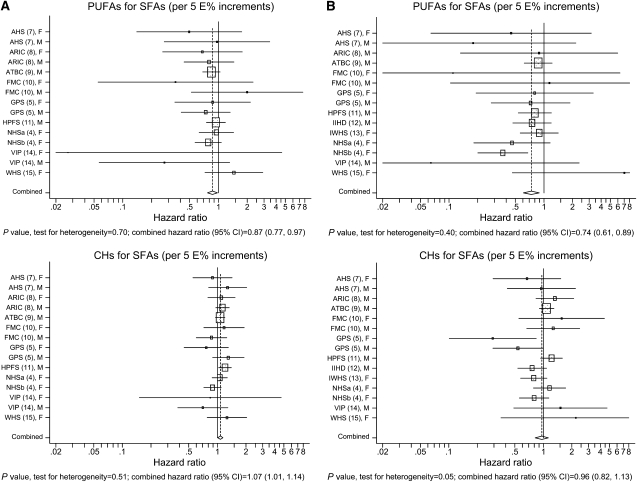
Combined HRs and 95% CIs for coronary events and deaths for a 5% lower energy intake from SFAs and a concomitant higher energy intake from unsaturated fatty acids or carbohydrates are shown in Table 2. There was an indication of an overall direct association between substitution of MUFAs and risk of coronary events (HR: 1.19; 95% CI: 1.00, 1.42), but not between substitution of MUFAs and risk of coronary deaths (HR: 1.01; 95% CI: 0.73, 1.41; Table 2). There was an overall significant inverse association between substitution of PUFAs and risk of coronary events (HR: 0.87; 95% CI: 0.77, 0.97) and between substitution of PUFAs and risk of coronary deaths (HR: 0.74; 95% CI: 0.61, 0.89; Table 2). There was an overall significant direct association between substitution of carbohydrates and risk of coronary events (HR: 1.07; 95% CI: 1.01, 1.14) but not between substitution of carbohydrates and risk of coronary deaths (HR: 0.96; 95% CI: 0.82, 1.13; Table 2). There was no effect modification by sex (Table 2). Study-specific and combined HRs and 95% CIs for coronary events and deaths for a 5% lower energy intake from SFAs and a concomitant higher energy intake from PUFAs or carbohydrates are shown in Figure 1. Combined HRs and 95% CIs for coronary events and deaths among persons aged <60 y at entry and persons aged ≥60 y at entry are shown in Table 3. Among women aged <60 y, there was a borderline significant inverse association between substitution of PUFAs and risk of coronary events (HR: 0.73; 95% CI: 0.53, 1.01); among women aged ≥60 y, the HR was 1.22 (95% CI: 0.84, 1.77; Table 3). Among men aged <60 y, the HR was 0.90 (95% CI: 0.72, 1.12) for PUFAs and coronary events; among men aged ≥60 y, the HR was 0.81 (95% CI: 0.65, 1.01; Table 3). Among women aged <60 y, there was a strong significant inverse association between substitution of PUFAs and coronary deaths (HR: 0.49; 95% CI: 0.29, 0.83); among women aged ≥60 y, the HR was 0.73 (95% CI: 0.48, 1.11; Table 3). Among men aged <60 y, the HR was 0.83 (95% CI: 0.61, 1.13) for PUFAs and coronary deaths; among men aged ≥60 y, the HR was 0.78 (95% CI: 0.54, 1.12; Table 3). There was no effect modification by age among women or men (Table 3). There was no effect modification by sex among persons aged <60 y or ≥60 y (data not shown).
TABLE 2.
Combined hazard ratios (HRs) for coronary events and coronary deaths per 5% increments in energy intake from polyunsaturated fatty acids (PUFAs) or carbohydrates (CHs) in the Pooling Project of Cohort Studies on Diet and Coronary Disease1
| All |
Women |
Men |
|||||
| HR (95% CI) | P value, test for between-studies heterogeneity | HR (95% CI) | P value, test for between-studies heterogeneity | HR (95% CI) | P value, test for between-studies heterogeneity | P value, test for effect modification by sex | |
| Coronary events2 | |||||||
| MUFAs for SFAs | |||||||
| Model 13 | 1.39 (1.20, 1.61) | 1.33 (1.01, 1.74) | 1.47 (1.25, 1.73) | ||||
| Model 24 | 1.19 (1.00, 1.42) | 0.32 | 1.15 (0.84, 1.58) | 0.30 | 1.23 (0.98, 1.55) | 0.32 | 0.49 |
| PUFAs for SFAs | |||||||
| Model 13 | 0.69 (0.59, 0.81) | 0.66 (0.54, 0.81) | 0.68 (0.52, 0.87) | ||||
| Model 24 | 0.87 (0.77, 0.97) | 0.70 | 0.85 (0.68, 1.06) | 0.51 | 0.87 (0.76, 1.01) | 0.61 | 0.84 |
| CHs for SFAs | |||||||
| Model 13 | 1.06 (1.01, 1.12) | 0.98 (0.90, 1.06) | 1.10 (1.05, 1.16) | ||||
| Model 24 | 1.07 (1.01, 1.14) | 0.51 | 1.00 (0.89, 1.12) | 0.72 | 1.11 (1.02, 1.20) | 0.37 | 0.13 |
| Coronary deaths5 | |||||||
| MUFAs for SFAs | |||||||
| Model 13 | 1.16 (0.83, 1.60) | 0.97 (0.62, 1.52) | 1.31 (0.82, 2.09) | ||||
| Model 24 | 1.01 (0.73, 1.41) | 0.18 | 0.88 (0.51, 1.54) | 0.27 | 1.10 (0.71, 1.69) | 0.18 | 0.40 |
| PUFAs for SFAs | |||||||
| Model 13 | 0.57 (0.42, 0.77) | 0.51 (0.29, 0.89) | 0.64 (0.46, 0.90) | ||||
| Model 24 | 0.74 (0.61, 0.89) | 0.40 | 0.61 (0.37, 1.01) | 0.14 | 0.80 (0.64, 0.99) | 0.81 | 0.24 |
| CHs for SFAs | |||||||
| Model 13 | 0.93 (0.82, 1.06) | 0.85 (0.68, 1.07) | 1.00 (0.86, 1.15) | ||||
| Model 24 | 0.96 (0.82, 1.13) | 0.05 | 0.86 (0.65, 1.13) | 0.16 | 1.03 (0.86, 1.24) | 0.14 | 0.08 |
MUFAs, monounsaturated fatty acids; SFAs, saturated fatty acids.
n = 306,244 for all; n = 215,006 for women; and n = 91,238 for men.
Model 1 included intake of MUFAs, PUFAs, trans fatty acids, protein, and CHs expressed as percentages of total energy intake (as continuous variables) and total energy intake (kcal/d) (as a continuous variable). Age at baseline (y) and the calendar year in which the baseline questionnaire was returned were entered into the model through the strata statement. Within each study, HRs with 95% CIs for the incidence of a coronary event and of mortality from coronary heart disease were calculated by using Cox proportional hazards regression with time in study (y) as the time metric. The study-specific logs of HRs were weighted by the inverse of their variances, and a combined estimate of the HRs was computed by using a random-effects model. The estimated HRs for unsaturated fatty acids and CHs can be interpreted as the estimated differences in risk of a 5% lower energy intake from SFAs and a concomitant higher energy intake from unsaturated fatty acids and CHs, respectively.
Model 2 included variables in model 1 and smoking (never smokers, former smokers, or current smoker of 1–4, 5–14, 15–24, or ≥25 cigarettes/d), BMI (in kg/m2; <23, 23 to <25, 25 to <27.5, 27.5 to <30, or ≥30), physical activity (levels 1–5), highest attained educational level (<high school, high school, or >high school), alcohol intake (0, 0 to <5, 5 to <10, 10 to <15, 15 to <30, 30 to <50, or ≥50 g/d), history of hypertension (yes or no), and energy-adjusted quintiles of fiber intake (g/d) and cholesterol intake (mg/d). The estimated HRs for unsaturated fatty acids and CHs can be interpreted as the estimated differences in risk of a 5% lower energy intake from SFAs and a concomitant higher energy intake from unsaturated fatty acids and CHs, respectively.
n = 327,660 for all; n = 228,150 for women; and n = 99,510 for men.
FIGURE 1.
Study-specific and combined hazard ratios and 95% CIs for coronary events (A) (n = 306,244) and coronary deaths (B) (n = 327,660) in the Pooling Project of Cohort Studies on Diet and Coronary Disease. The model included intake of monounsaturated fatty acids, polyunsaturated fatty acids (PUFAs), trans fatty acids, carbohydrates (CHs), and protein expressed as percentages of total energy intake (E%; as continuous variables), total energy intake (kcal/d; as a continuous variable), smoking (never smokers, former smokers, or current smoker of 1–4, 5–14, 15–24, or ≥25 cigarettes/d), BMI (in kg/m2; <23, 23 to <25, 25 to <27.5, 27.5 to <30, or ≥30), physical activity (levels 1–5), highest attained educational level (<high school, high school, or >high school), alcohol intake (0, 0 to <5, 5 to <10, 10 to <15, 15 to <30, 30 to <50, or ≥50 g/d), history of hypertension (yes or no), and energy-adjusted quintiles of fiber intake (g/d) and cholesterol intake (mg/d). Age at baseline (y) and the calendar year in which the baseline questionnaire was returned (y) were entered into the model through the strata statement. Within each study, hazard ratios with 95% CIs for the incidence of a coronary event and of mortality from coronary heart disease were calculated by using Cox proportional hazards regression with time in study (y) as the time metric. The study-specific logs of hazard ratios were weighted by the inverse of their variances, and a combined estimate of the hazard ratios was computed by using a random-effects model. The estimated hazard ratios for PUFAs and CHs can be interpreted as the estimated differences in risk of a 5% lower energy intake from saturated fatty acids (SFAs) and a concomitant higher energy intake from PUFAs and CHs, respectively. The squares and horizontal lines represent the study-specific hazard ratios and 95% CIs, respectively. The area of the squares reflects the study-specific weight (inverse of the variance). The diamonds represent the combined hazard ratios and 95% CI. AHS, Adventis Health Study; ARIC, Atherosclerosis Risk in Communities Study; ATBC, Alpha-Tocopherol and Beta-Carotene Cancer Prevention Study; FMC, Finnish Mobile Clinic Health Study; GPS, Glostrup Population Study; HPFS, Health Professionals Follow-Up Study; IIHD, Israeli Ischemic Heart Disease Study; IWHS, Iowa Women's Health Study; NHSa, Nurses' Health Study 1980; NHSb, Nurses' Health Study 1986; VIP, Västerbotten Intervention Program; WHS, Women's Health Study.
TABLE 3.
Combined hazard ratios (HRs) for coronary events and coronary deaths per 5% increments in energy intake from polyunsaturated fatty acids (PUFAs) or carbohydrates (CHs) among women and men aged <60 y or ≥60 y in the Pooling Project of Cohort Studies on Diet and Coronary Disease1
| Women |
Men |
|||||||||
| <60 y |
≥60 y |
<60 y |
≥60 y |
|||||||
| HR (95% CI) | P value, test for between-studies heterogeneity | HR (95% CI) | P value, test for between-studies heterogeneity | P value, test for effect modification by age | HR (95% CI) | P value, test for between-studies heterogeneity | HR (95% CI) | P value, test for between-studies heterogeneity | P value, test for effect modification by age | |
| Coronary events2 | ||||||||||
| MUFAs for SFAs | ||||||||||
| Model 13 | 1.13 (0.87, 1.45) | 1.42 (0.93, 2.16) | 1.44 (1.06, 1.94) | 1.65 (1.29, 2.11) | ||||||
| Model 24 | 1.03 (0.73, 1.44) | 0.37 | 1.27 (0.74, 2.17) | 0.69 | 0.89 | 1.13 (0.74, 1.72) | 0.09 | 1.43 (1.04, 1.96) | 0.46 | 0.64 |
| PUFAs for SFAs | ||||||||||
| Model 13 | 0.56 (0.44, 0.73) | 0.89 (0.62, 1.29) | 0.73 (0.55, 0.97) | 0.71 (0.57, 0.87) | ||||||
| Model 24 | 0.73 (0.53, 1.01) | 0.34 | 1.22 (0.84, 1.77) | 0.48 | 0.15 | 0.90 (0.72, 1.12) | 0.37 | 0.81 (0.65, 1.01) | 0.55 | 0.80 |
| CHs for SFAs | ||||||||||
| Model 13 | 0.96 (0.83, 1.10) | 1.01 (0.87, 1.17) | 1.09 (1.02, 1.16) | 1.14 (1.05, 1.24) | ||||||
| Model 24 | 0.98 (0.86, 1.12) | 0.42 | 1.09 (0.88, 1.36) | 0.50 | 0.92 | 1.12 (1.00, 1.24) | 0.34 | 1.14 (1.00, 1.29) | 0.85 | 0.85 |
| Coronary deaths5 | ||||||||||
| MUFAs for SFAs | ||||||||||
| Model 13 | 0.70 (0.22, 2.26) | 0.95 (0.57, 1.58) | 1.35 (0.79, 2.30) | 1.25 (0.74, 2.11) | ||||||
| Model 24 | 0.87 (0.32, 2.39) | 0.10 | 0.67 (0.34, 1.32) | 0.94 | 0.72 | 1.09 (0.74, 1.62) | 0.58 | 1.35 (0.80, 2.25) | 0.42 | 0.87 |
| PUFAs for SFAs | ||||||||||
| Model 13 | 0.42 (0.20, 0.86) | 0.57 (0.31, 1.05) | 0.75 (0.57, 0.97) | 0.64 (0.40, 1.02) | ||||||
| Model 24 | 0.49 (0.29, 0.83) | 0.68 | 0.73 (0.48, 1.11) | 0.42 | 0.58 | 0.83 (0.61, 1.13) | 0.39 | 0.78 (0.54, 1.12) | 0.38 | 0.82 |
| CHs for SFAs | ||||||||||
| Model 13 | 0.79 (0.46, 1.35) | 0.82 (0.69, 0.97) | 1.02 (0.84, 1.23) | 0.98 (0.86, 1.12) | ||||||
| Model 24 | 0.91 (0.62, 1.34) | 0.17 | 0.80 (0.61, 1.06) | 0.73 | 0.79 | 1.08 (0.82, 1.43) | 0.09 | 1.03 (0.80, 1.33) | 0.29 | 0.40 |
MUFAs, monounsaturated fatty acids; SFAs, saturated fatty acids.
Adventis Health Study and Glostrup Population Study had insufficient events for analysis of all coronary events among women aged <60 y. Atherosclerosis Risk in Communities Study, Nurses' Health Study 1980, and Västerbotten Intervention Program had insufficient events for analysis of all coronary events among women aged ≥60 y. n = 176,036 for women aged <60 y; n = 27,026 for women aged ≥60 y; n = 66,405 for men aged <60 y; and n = 24,833 for men aged ≥60 y.
Model 1 included intake of MUFAs, PUFAs, trans fatty acids, protein, and CHs expressed as percentages of total energy intake (as continuous variables) and total energy intake (kcal/d) (as a continuous variable). Age at baseline (y) and the calendar year in which the baseline questionnaire was returned were entered into the model through the strata statement. Within each study, HRs with 95% CIs for the incidence of a coronary event and of mortality from coronary heart disease were calculated by using Cox proportional hazards regression with time in study (y) as the time metric. The study-specific logs of HRs were weighted by the inverse of their variances, and a combined estimate of the HRs was computed by using a random-effects model. The estimated HRs for unsaturated fatty acids and CHs can be interpreted as the estimated differences in risk of a 5% lower energy intake from SFAs and a concomitant higher energy intake from unsaturated fatty acids and CHs, respectively.
Model 2 included variables in model 1 and smoking (never smokers, former smokers, or current smoker of 1–4, 5–14, 15–24, or ≥25 cigarettes/d), BMI (in kg/m2; <23, 23 to <25, 25 to <27.5, 27.5 to <30, or ≥30), physical activity (levels 1–5), highest attained educational level (<high school, high school, or >high school), alcohol intake (0, 0 to <5, 5 to <10, 10 to <15, 15 to <30, 30 to <50, or ≥50 g/d), history of hypertension (yes or no), and energy-adjusted quintiles of fiber intake (g/d) and cholesterol intake (mg/d). The estimated HRs for unsaturated fatty acids and CHs can be interpreted as the estimated differences in risk of a 5% lower energy intake from SFAs and a concomitant higher energy intake from unsaturated fatty acids and CHs, respectively.
Adventis Health Study, Finnish Mobile Clinic Health Study, Glostrup Population Study, and Women's Health Study had insufficient events for analysis of coronary deaths among women aged <60 y. Finnish Mobile Clinic Health Study, Nurses' Health Study 1980, and Women's Health Study had insufficient events for analysis of coronary deaths among women aged ≥60 y. Adventis Health Study had insufficient events for analysis of coronary deaths among men aged <60 y. Atherosclerosis Risk in Communities Study had insufficient events for analysis of coronary deaths among men aged ≥60 y. n = 142,297 for women aged <60 y; n = 37,253 for women aged ≥60 y; n = 68,158 for men aged <60 y; and n = 24,357 for men aged ≥60 y.
DISCUSSION
This study suggests that to prevent CHD, SFA intake should be replaced with PUFA intake rather than MUFA or carbohydrate intake. However, the effects of substitution of carbohydrates may vary depending on the quality of the carbohydrates consumed. Several classifications may be relevant to CHD risk, including 1) dietary fiber content (6), 2) extent of processing (whole compared with refined grain) (18), or 3) glycemic index (19). In this study, only type of fat, not type of carbohydrates, was considered. The estimated HRs for carbohydrate intake in this study, however, may reflect variation in glycemic carbohydrate intake as variation in fiber intake was taken into account in the analyses.
An advantage of the Pooling Project of Cohort Studies on Diet and Coronary Disease is that publication bias is reduced because of the inclusion of cohort studies [Adventis Health Study (AHS), Atherosclerosis Risk in Communities Study (ARIC), Finnish Mobile Clinic Health Study (FMC), IWHS, Västerbotten Intervention Program (VIP), and WHS] from which results on dietary fats and risk of CHD have not been published. Moreover, pooling data allowed studying this topic in different populations with different diets and over a wide range of intakes (Table 1). Other advantages included the ability to evaluate whether the exposure-disease association is modified by other risk factors.
Information bias is unlikely to have affected the present results as diagnoses were established independently of the dietary recalls of the participants. Dietary intake was determined by using a food-frequency questionnaire or a dietary history interview, which may reflect habitual eating pattern. Only baseline information regarding dietary habits was available. The lack of repeated assessment of dietary intake excludes possible analytic approaches to reduce random measurement error. Generally, measurement error leads to underestimation of the true risk and to loss of statistical power for testing associations (under the assumption that measurement error is nondifferential with regard to the outcome). Using calibrated dietary data would provide information on range of absolute intakes by reducing systematic measurement errors across studies in the estimation of total food intake. In this study, however, exposure measures were expressed relative to total energy intake, which substantially reduces errors in the estimation of total food intake. Additional confounding from other CHD risk factors not taken into account remains a possible explanation for the observed associations. In this study, additional adjustment for suggested dietary CHD risk factors (vitamin E, vitamin C, and folic acid) did not change the HRs, but the CIs became slightly wider.
Three randomized trials of dietary fat intake and risk of CHD that evaluated primary preventive interventions have been conducted (20–22). In 2 of these 3 trials, total fat intake was not reduced, but SFA intake was replaced with PUFA intake. In the Los Angeles Veterans Administration Trial (20), the incidence of CHD events manifested by sudden death or myocardial infarction (MI) was lower in the intervention group than in the control group after 8 y of follow-up, although not statistically significant. In the Minnesota Coronary Survey (21), no difference between the intervention and control groups was found for CHD events manifested by sudden death or MI. However, the study was relatively short in duration (4.5 y), and the achieved PUFA intake to SFA intake ratio (1.6) in the intervention group was far below the specified goal (2.5). No randomized trials have reported the effects of replacing SFA intake with carbohydrate intake on CHD risk. The Women's Health Initiative Dietary Modification Trial, however, has reported the effects of reducing total fat intake and increasing vegetable and fruit intake on CHD risk (22). Over 8.1 y, the dietary intervention did not reduce the risk of CHD events manifested by CHD deaths or nonfatal MI. The lack of effect on CHD in that study might have been due to the limited reduction in SFA intake and concomitant reduced intake of unsaturated fatty acids, which resulted in a limited decrease in the LDL-cholesterol concentration. One follow-up study found an inverse association between intake of PUFAs and risk of CHD deaths (23). That study, however, did not address the difference in risk of a lower energy intake from SFAs and a concomitant higher energy intake from PUFAs, but the difference in risk of a higher energy intake from PUFAs, independent of energy intake from SFAs. Another follow-up study examined the risk of CHD for a lower energy intake from SFAs and a concomitant higher energy intake from unsaturated fatty acids or carbohydrates (4). The results from that study showed that replacing SFAs with unsaturated fatty acids was inversely associated with risk of CHD, whereas replacing SFAs with carbohydrates was not associated with risk of CHD. The present study, however, cannot be considered independent of that study because the present study was partly based on the same participants. The beneficial effect of replacing SFAs with unsaturated fatty acids is also in line with time trend data from Poland, where mortality due to CHD has decreased in parallel with an increase in PUFA intake and with a decrease in SFA intake between 1990 and 1999 (24). Thus, the present results that suggest that replacing SFAs with PUFAs may have a greater benefit than replacing SFAs with carbohydrates are in agreement with previous studies.
Substitution of MUFAs for SFAs decreases plasma LDL-cholesterol concentration (25). The indication of an increased risk of CHD associated with a lower intake of SFAs and a concomitant higher energy intake from MUFAs may be due in part to intake of TFAs, which is included in the sum of MUFAs. However, all study-specific HRs of MUFA intake and risk of CHD were adjusted for TFA intake with the exception of the study-specific HRs from the AHS, the Glostrup Population Study, and the IIHD because information on TFA intake was not available for participants from these cohort studies. Furthermore, in analyses only including participants from the 8 cohort studies (ARIC, ATBC, FMC, Health Professionals Follow-Up Study, IWHS, Nurses' Health Study 1980, Nurses' Health Study 1986, VIP, and WHS), for whom information on intake of TFAs was available, adjustment for TFAs did not change the combined HRs (data not shown). The adjustment for TFAs, however, is highly probable to have been incomplete because of industrial modification of the content of TFAs in foods during the time period of the follow-up of the participants. Other mechanisms than reduced LDL-cholesterol concentration, however, may be involved (26). Finally, it should be mentioned that the main source of MUFAs was animal fat, whereby confounding from other dietary components in meat and dairy products cannot be excluded.
It has been suggested that the association between major types of fat and risk of CHD is modified by sex and age (5, 27–30). This study suggests that to prevent CHD, SFAs should be reduced and replaced with PUFAs among all middle-aged and older women and men. However, it cannot be excluded that associations may be stronger in subgroups, but our study only provides a suggestion for these possibilities.
In conclusion, the associations found in this study suggest that replacing SFA intake with PUFA intake rather than MUFA or carbohydrate intake prevents CHD over a wide range of intakes and among all middle-aged and older women and men.
Acknowledgments
The authors' responsibilities were as follows—MAP and AA: study concept and design and data acquisition; MUJ, EJO, BLH, MAP, KB, GEF, UG, GH, PK, SL, PP, DS, JS, JV, WCW, and AA: interpretation of data and critical revision of the manuscript for important intellectual content; MUJ: draft of the manuscript; and MUJ and EJO: statistical analysis. None of the authors had any conflicts of interest.
REFERENCES
- 1.Hu FB, Manson JE, Willett WC. Types of dietary fat and risk of coronary heart disease: a critical review. J Am Coll Nutr 2001;20:5–19 [DOI] [PubMed] [Google Scholar]
- 2.Krauss RM, Eckel RH, Howard B, et al. AHA dietary guidelines: revision 2000: a statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation 2000;102:2284–99 [DOI] [PubMed] [Google Scholar]
- 3.Nordic Nutrition Recommendations 2004 Integrating nutrition and physical activity. Copenhagen, Denmark: Nordic Council of Ministers, 2004 [Google Scholar]
- 4.Hu FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med 1997;337:1491–9 [DOI] [PubMed] [Google Scholar]
- 5.Jakobsen MU, Overvad K, Dyerberg J, Schroll M, Heitmann BL. Dietary fat and risk of coronary heart disease: possible effect modification by gender and age. Am J Epidemiol 2004;160:141–9 [DOI] [PubMed] [Google Scholar]
- 6.Pereira MA, O'Reilly E, Augustsson K, et al. Dietary fiber and risk of coronary heart disease: a pooled analysis of cohort studies. Arch Intern Med 2004;164:370–6 [DOI] [PubMed] [Google Scholar]
- 7.Fraser GE, Sabate J, Beeson WL, Strahan TM. A possible protective effect of nut consumption on risk of coronary heart disease. The Adventist Health Study. Arch Intern Med 1992;152:1416–24 [PubMed] [Google Scholar]
- 8.Folsom AR, Arnett DK, Hutchinson RG, Liao F, Clegg LX, Cooper LS. Physical activity and incidence of coronary heart disease in middle-aged women and men. Med Sci Sports Exerc 1997;29:901–9 [DOI] [PubMed] [Google Scholar]
- 9.Pietinen P, Ascherio A, Korhonen P, et al. Intake of fatty acids and risk of coronary heart disease in a cohort of Finnish men. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Epidemiol 1997;145:876–87 [DOI] [PubMed] [Google Scholar]
- 10.Knekt P, Reunanen A, Jarvinen R, Seppanen R, Heliovaara M, Aromaa A. Antioxidant vitamin intake and coronary mortality in a longitudinal population study. Am J Epidemiol 1994;139:1180–9 [DOI] [PubMed] [Google Scholar]
- 11.Ascherio A, Rimm EB, Giovannucci EL, Spiegelman D, Stampfer M, Willett WC. Dietary fat and risk of coronary heart disease in men: cohort follow up study in the United States. BMJ 1996;313:84–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldbourt U, Yaari S, Medalie JH. Factors predictive of long-term coronary heart disease mortality among 10,059 male Israeli civil servants and municipal employees: a 23-year mortality follow-up in the Israeli Ischemic Heart Disease Study. Cardiology 1993;82:100–21 [DOI] [PubMed] [Google Scholar]
- 13.Kushi LH, Folsom AR, Prineas RJ, Mink PJ, Wu Y, Bostick RM. Dietary antioxidant vitamins and death from coronary heart disease in postmenopausal women. N Engl J Med 1996;334:1156–62 [DOI] [PubMed] [Google Scholar]
- 14.Hallmans G, Agren A, Johansson G, et al. Cardiovascular disease and diabetes in the Northern Sweden Health and Disease Study Cohort—evaluation of risk factors and their interactions. Scand J Public Health 2003;31(suppl):18–24 [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Buring JE, Sesso HD, Rimm EB, Willett WC, Manson JE. A prospective study of dietary fiber intake and risk of cardiovascular disease among women. J Am Coll Cardiol 2002;39:49–56 [DOI] [PubMed] [Google Scholar]
- 16.Rothman KJ, Greenland S. Modern epidemiology. 2nd ed Philadelphia, PA: Lippincott Williams & Wilkins, 1998 [Google Scholar]
- 17.Smith-Warner SA, Spiegelman D, Ritz J, et al. Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol 2006;163:1053–64 [DOI] [PubMed] [Google Scholar]
- 18.Jensen MK, Koh-Banerjee P, Hu FB, et al. Intakes of whole grains, bran, and germ and the risk of coronary heart disease in men. Am J Clin Nutr 2004;80:1492–9 [DOI] [PubMed] [Google Scholar]
- 19.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002;287:2414–23 [DOI] [PubMed] [Google Scholar]
- 20.Dayton S, Pearce ML, Goldman H, et al. Controlled trial of a diet high in unsaturated fat for prevention of atherosclerotic complications. Lancet 1968;2:1060–2 [DOI] [PubMed] [Google Scholar]
- 21.Frantz ID, Jr, Dawson EA, Ashman PL, et al. Test of effect of lipid lowering by diet on cardiovascular risk. The Minnesota Coronary Survey. Arteriosclerosis 1989;9:129–35 [DOI] [PubMed] [Google Scholar]
- 22.Howard BV, Van Horn L, Hsia J, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295:655–66 [DOI] [PubMed] [Google Scholar]
- 23.Shekelle RB, Shryock AM, Paul O, et al. Diet, serum cholesterol, and death from coronary heart disease. The Western Electric Study. N Engl J Med 1981;304:65–70 [DOI] [PubMed] [Google Scholar]
- 24.Zatonski WA, Willett W. Changes in dietary fat and declining coronary heart disease in Poland: population based study. BMJ 2005;331:187–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb 1992;12:911–9 [DOI] [PubMed] [Google Scholar]
- 26.McLennan PL. Relative effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on cardiac arrhythmias in rats. Am J Clin Nutr 1993;57:207–12 [DOI] [PubMed] [Google Scholar]
- 27.Xu J, Eilat-Adar S, Loria C, et al. Dietary fat intake and risk of coronary heart disease: the Strong Heart Study. Am J Clin Nutr 2006;84:894–902 [DOI] [PubMed] [Google Scholar]
- 28.Oh K, Hu FB, Manson JE, Stampfer MJ, Willett WC. Dietary fat intake and risk of coronary heart disease in women: 20 years of follow-up of the Nurses' Health Study. Am J Epidemiol 2005;161:672–9 [DOI] [PubMed] [Google Scholar]
- 29.Esrey KL, Joseph L, Grover SA. Relationship between dietary intake and coronary heart disease mortality: lipid research clinics prevalence follow-up study. J Clin Epidemiol 1996;49:211–6 [DOI] [PubMed] [Google Scholar]
- 30.Posner BM, Cobb JL, Belanger AJ, Cupples LA, D'Agostino RB, Stokes J., III Dietary lipid predictors of coronary heart disease in men. The Framingham Study. Arch Intern Med 1991;151:1181–7 [PubMed] [Google Scholar]